Abstract
Purpose
Naturally occurring primary canine lung cancers share clinicopathologic features with human lung cancers in never-smokers, but the genetic underpinnings of canine lung cancer are unknown. We have charted the genomic landscape of canine lung cancer and performed functional characterization of novel, recurrent HER2 (ERBB2) mutations occurring in canine pulmonary adenocarcinoma (cPAC).
Experimental Design
We performed multi-platform genomic sequencing of 88 primary canine lung tumors or cell lines. Additionally, in cPAC cell lines, we performed functional characterization of HER2 signaling and evaluated mutation-dependent HER2 inhibitor drug dose response.
Results
We discovered somatic, coding HER2 point mutations in 38% of cPACs (28/74), but none in adenosquamous (cPASC, 0/11) or squamous cell (cPSCC, 0/3) carcinomas. The majority (93%) of HER2 mutations were hotspot V659E transmembrane domain (TMD) mutations comparable to activating mutations at this same site in human cancer. Other HER2 mutations were located in the extracellular domain and TMD. HER2V659E was detected in the plasma of 33% (2/6) of dogs with localized HER2V659E tumors. HER2V659E cPAC cell lines displayed constitutive phosphorylation of AKT and significantly higher sensitivity to the HER2 inhibitors lapatinib and neratinib relative to HER2-wild-type cell lines (IC50s < 200 nM in HER2V659E versus IC50s > 2500 nM in HER2WT).
Conclusions
This study creates a foundation for molecular understanding of and drug development for canine lung cancer. These data also establish molecular contexts for comparative studies in dogs and humans of low mutation burden, never-smoker lung cancer and mutant HER2 function and inhibition.
Keywords: ERBB2, HER2, Neratinib, Naturally occurring canine cancer, Pulmonary adenocarcinoma
Introduction
Naturally occurring primary canine lung cancer is clinically challenging(1) with a disease course and underlying biology that resemble human lung cancer in never-smokers. Human never-smoker lung cancer accounts for 10–25% of lung cancers, causes approximately 26,000 deaths annually, and has a high incidence of erb-B family gene mutations such as those impacting EGFR. While incidence of smoking-related lung cancer is decreasing, lung cancer incidence in never-smokers is increasing (2). Never-smoker lung cancer is primarily non-small cell lung cancer (NSCLC), arising from lung tissue, as opposed to small cell lung cancer (SCLC) arising in bronchi of smokers. NSCLC histologies include adenocarcinoma (AC) and squamous cell carcinoma (SCC). The etiology of never-smoker lung cancer is also distinct from that of smokers. It is associated with factors including environmental exposures (second-hand smoke, radon, asbestos, arsenic, silica, and pollution) as well as age, sex, family history, and genetic loci (3). Unique genomic characteristics of human never-smoker lung cancer include low somatic mutation burden, enrichment for C:G>T:A transitions, and somatic activating point mutations or fusions impacting EGFR (45%), ALK (5–11%), ROS (1.5–6%), HER2 (3–5%), and RET (2%) (4). Five-year overall survival is estimated at 23%, but outcomes are dependent on molecular subtype and treatment regimen. For example, EGFR inhibitors can improve outcomes in EGFR-mutant lung cancers, however 85% of never-smoker lung AC and SCC cases are EGFR wild-type (WT) in the United States. Clinical trials of immune checkpoint inhibitors have recently shown improved outcomes for human lung cancers, but analysis of large phase II immunotherapy trials suggests that benefits are limited in low-mutation-burden (≤ 10 mutations/Mb) cases such as those found in never-smokers (5). A need exists for improved biologic understanding and development of new models to fuel translational research in never-smoker lung cancer.
Lung cancer in pet dogs has limited standard of care beyond surgery and little is known of its molecular underpinnings (1). Primary lung tumors typically arise in older dogs (11 years) and resemble human NSCLC histotypes including canine pulmonary adenocarcinoma (cPAC), adenosquamous carcinoma (cPASC), and squamous cell carcinoma (cPSCC). These subtypes collectively represent 13–15% of primary lung tumors (6,7). Patients are often diagnosed late with lesions incidentally discovered during routine geriatric evaluation or due to nonspecific symptoms including dyspnea (6% to 24%) and cough (52% to 93%) that do not manifest until the tumor is more than 3 cm. The detection of canine lung cancers has significantly increased over the past twenty years as a result of not only improved animal healthcare and diagnostics, but also possibly due to increased companion animal exposures to pollutants. These tumors can be diagnostically challenging. Rates at which ultrasound or CT-guided fine needle aspirates of the pulmonary mass provide cytologic diagnosis range from 38% to 90% of cases, varying broadly based on tumor accessibility and aspirate quality. At diagnosis, 71% of malignant canine lung tumors show signs of invasion and 23% show distant metastasis. Partial or complete lung lobectomy is standard of care, dependent on extent of disease spread. Median survival is 345 days for localized disease without nodal involvement where surgical remission can be achieved, but only 60 days when nodes are involved. Responses to cytotoxic chemotherapy (cisplatin, vindesine, doxorubicin, and mitoxantrone) in the setting of disseminated disease are limited. Targeted small molecules and immune checkpoint inhibitors have not been extensively studied in part because the molecular underpinnings of canine lung cancer remain largely unknown. In naturally occurring canine NSCLC, while comprehensive genomic profiling has been limited, KRAS hotspot mutation prevalence estimates from targeted studies have varied from 0–25% (7–9). We have previously shown that EGFR mutation, overexpression, or phosphorylation is rare in cPAC compared to matched non-affected chemotherapy-naive lung tissue whereas significant overexpression and/or phosphorylation of PDGFRα, ALK, and HER2 are present(10). We now describe for the first time the detailed genetic underpinnings of primary canine lung cancers through multi-platform next-generation sequencing of 88 cases.
Materials and Methods
Sample Collection
Tumors and cell lines from 89 dogs from the Canine Comparative Oncology and Genomics Consortium (CCOGC)(11) and The Ohio State University (OSU) College of Veterinary Medicine Biospecimen Repository were included. Veterinary pathologists board certified by the American College of Veterinary Pathologists (ACVP) confirmed tumor diagnosis based on histopathology. This study was approved by The OSU Institutional Animal Care Use Committee (2010A0015-R2). Tumor and normal tissue samples were flash frozen in liquid nitrogen or formalin-fixed and paraffin-embedded (FFPE). Cell lines were received between 05/29/2017 (all sequenced lines) and 05/18/2018 (OSUK9PAPADRiley), maintained in RPMI 1640 with GlutaMAX™ (Gibco™, Thermo Fisher Scientific #61870036) supplemented with 10% heat inactivated fetal bovine serum at 37°C and 5% CO2 and passaged at 90% confluence. Cell lines with known passage data (except BACA) were sequenced within the first eight passages of derivation, subsequently expanded for phenotypic studies, and authenticated by IDEXX BioResearch (Columbia, MO) using the Cell Check Canine STR Profile and Interspecies Contamination Test on January 30, 2018 or by NkX2 (or TTF-1) RT-PCR. Mycoplasma testing was performed in all lines at time of arrival and every three months of continuous harvest by using the Mycoplasma Detection Kit (ATCC #30–1012K). All samples tested negative at all times with final testing performed 6/21/2019 for Riley and 06/15/2017 for all other lines). Human cell lines included BT474 (ATCC #HTB20, HER2 focal amplification) and H1781 (ATCC #CRL-5894, HER2G776V). Blood for cell-free DNA and germline DNA extraction was collected in 10ml K2 EDTA Blood tubes (ThermoFisher Scientific #22–253-145). Plasma separation was performed at room temperature within 1h (2x serial centrifugation at 2000 rpm x 15 min). Plasma aliquots were stored frozen at −80°C. DNA extraction from plasma, white blood cells and tissue was performed with MagMAX Cell-Free DNA Isolation Kit (Thermo Fisher Scientific #A29319), DNeasy Blood and Tissue Kit (QIAGEN #69504) and Qiagen AllPrep DNA/RNA Mini Kit (QIAGEN #80204), respectively.
Exome Sequencing and Analysis
Informatic tools, versions, and flags are shown in Table S1. We utilized a custom Agilent SureSelect canine exome capture kit with 982,789 probes covering 19,459 genes. Exome libraries were sequenced on the Illumina HiSeq2000 producing paired end reads of 85bp. FASTQ files were aligned to the canine genome (CanFam3.1) using BWA v0.7.8. Aligned BAM files were realigned and recalibrated using GATK v3.3.0 and duplicate pairs were marked with Picard v1.128 (http://broadinstitute.github.io/picard). Somatic copy number variants (CNVs), and structural variants (SVs) were called with tCoNutT (https://github.com/tgen/tCoNuT) and DELLY v0.76 respectively. Somatic single nucleotide variants (SNV) were identified from two or more of the following callers: Seurat v2.6, Strelka v1.0.13 and MuTect v1.1.4. Germline SNVs were called using Haplotype Caller (GATK v3.3.0), Freebayes and samtools-Mpileup. Variant annotation was performed with SnpEff v3.5. The SomaticSignatures R package was used to identify mutation signatures.
Targeted Amplicon Sequencing and Analysis
We developed a custom canine cancer amplicon sequencing panel consisting of 281 amplicons targeting exons and hotspot regions in 57 genes, with amplicon sizes ranging from 91–271 bp (Table S7). We pooled primers in two multiplexed pools to separate adjacent amplicons and any amplicons that showed cross-amplification using in silico PCR. We prepared sequencing libraries using digital PCR amplification following the manufacturer’s protocols for the ThunderBolts Cancer Panel (RainDance Technologies) with modifications as previously described(12). Sequencing was performed on the Illumina MiSeq generating paired-end 275 bp reads. Sequencing reads were demultiplexed and extracted using Picardtools. Sequencing adapters were trimmed using ea-utils and fastq files were assessed for quality using FASTQC. Sequencing reads were aligned to CanFam3.1 using bwamem-MEM(13). Custom in-house scripts based on samtools were used to create pileups for every sample. Pileups were analyzed in R to call SNVs and indels. For each potential non-reference allele at each targeted locus in a sample, we evaluated the distribution of background noise across all other sequenced samples. To call a variant, we required the observed non-reference allele is an outlier from the background distribution with a Z-score > 5. In addition, we required tumor depth ≥100x, allele frequency ≥10%, number of reads supporting the variation ≥10, and allele fraction in the germline sample <1%. Finally, variant calls were manually curated by visualization in IGV v2.3.71. All next generation sequencing data (exome and amplicon) has been deposited in NCBI repository under accession number PRJNA523624.
Sanger Sequencing
23 primer pairs covering all exons of HER2 were designed using Primer 3 (http://bioinfo.ut.ee/primer3–0.4.0/) including a universal M13 tag. Amplicons were Sanger sequenced at the DNASU sequencing facility at Arizona State University on an ABI 3730XL (Applied Biosystems, Foster City, CA) and analyzed with Mutation Surveyor DNA Variant Analysis Software (SoftGenetics, State College, PA).
HER2 Inhibitor Drug Dose-Response Studies
HER2 inhibitors lapatinib (Selleckchem, #S2111), neratinib (Puma Biotechnology, Los Angeles, CA), and trastuzumab (Selleckchem, #A2007), as well as the EGFR inhibitor erlotinib (Selleckchem, #S1023) were assessed in 10–16-point 72 hr drug-dose response screens (from 2×10−7 nM to 100 μM for small molecules and from 1×10−6 μg/ml to 1000 μg/ml for trastuzumab) with CellTiter-Glo® luminescent cell viability assay (Promega, #G7570) endpoints. Luminescence was read using a Synergy Mx (Biotek) plate reader. Six replicates were performed for each dose. Curve-fitting and IC50 calculations were performed using GraphPad Prism v7.00 (GraphPad Software, La Jolla, CA).
Droplet Digital PCR for cell-free DNA analysis
HER2V659E genotyping was performed on tumor samples and plasma cell-free DNA with droplet digital PCR (ddPCR). PCR amplification was performed as follows: 1 cycle 3 min at 95 °C, 50 cycles 15 sec at 95 °C and 1 min at 60 °C with a 0.5 °C/sec ramping from 95 °C to 60 °C, 1 cycle 98 °C for 10 min and hold at 4 °C. Droplet fluorescence was measured using RainDrop Digital PCR Sense instrument and analyzed using accompanying RainDrop Analyst II Software v.1.0 (RainDance). Primer and probe sequences used for HER2V659E detection in ctDNA were Forward: 5’-CCCACGACCACAGCCA-3’, Reverse: 5’-CCCTGTGACATCCATCATTGC-3’ and Probe: 5’-CAGAATGCCC(T/A)CCACAGC-3’.
qRT-PCR
cDNA was obtained by reverse transcription with iScript (Biorad, #1708891) and samples were subjected to HER2 (target) and HPRT1 (reference) amplification in a QuantStudio™ 6 Flex Real-Time PCR System under standard conditions with Syber Green technology (KapaBiosystems, #KK4602). Primer sequences were: HER2-Forward: 5’-CATCTGCACCATTGATGTCTA-3’, HER2-Reverse: 5’-GGCCCAAGTCTTCATTCTGA-3’, HPRT1-Forward: 5’GCAGCCCCAGCGTCGTGATT-3’, HPRT1-Reverse: 5’CATCTCGAGCAAGCCGCTCAGT-3’. Data was analyzed with Quantstudio Real Time PCR software v1.1. Values for ΔCt, ΔΔCt, and fold changes were calculated as follows: ΔCt=Ct HER2 - Ct HPRT1; ΔΔCt=ΔCt tumor sample–ΔCt average of normal samples; and fold change =2^(-ΔΔCt).
Immunohistochemistry
HER2 protein expression was evaluated on FFPE sections (4 μm) of normal lung and tumor mounted on SuperFrost™ Plus glass slides (Fisher Scientific, #12–550-15). Slides were deparaffinized in xylene and rehydrated in an ethanol gradient. Antigen retrieval was performed with 1mM EDTA adjusted to pH 9.0. An autostainer (Dako, model S3400) was used to carry out immunostaining. A HER2 rabbit monoclonal antibody (Cell Signaling Technology, #4290) was used at 1:400 dilution followed by secondary biotinylated rabbit anti-goat IgG (Vector Laboratories, BA-1000) diluted 1:200. Detection was performed with VECTASTAIN® Elite® ABC System (#PK-6100). IHC positive controls for HER2 tyrosine kinase receptor expression were single tissue samples of two canine complex mammary carcinomas(14). Negative controls were performed on all tissues using a universal rabbit negative isotype control not directed against any known antigen (Dako, #IR600).
Quantitative Image Analysis
Immunostained and control 1×3-inch microscope slides were scanned at 40X on a high-resolution Scanscope XT (Leica Biosystems) at The OSU Comparative Pathology & Mouse Phenotyping Shared Resource. For quantification of immunoreactivity, images were imported into Visiopharm Image Analysis software (Visiopharm, Hørsholm, Denmark version 2017.27.0.3313), segmented into areas of tumor, necrosis, and normal lung tissue using color labels for each tissue type. HER2 connectivity was scored using the modified 10007 - HER2, Breast Cancer APP (Visiopharm). Thresholds were adjusted to match specimen HER2 stain intensities for accurate scoring. Area (μm2) was quantified for each tissue type and percentages derived from specimen total tissue area. Tumor areas were further segmented into staining and non-staining categories and their percentages were calculated based on total tumor area in μm2. Maximum, mean, and minimum intensities were also quantified using a built-in software calculation. Staining is expressed as percentage of stain present with 100% equal to black (maximum dark brown) and 0% equal to white (no stain present). Initial thresholds and tissue types were established and mark-ups reviewed in consultation with a pathologist board-certified by the ACVP to ensure accurate measurements and to differentiate between tissue types.
Immunoblot Analyses
Subconfluent cells were serum starved overnight, then treated with 20 nM neuregulin for 15 min prior to harvest. Cells were lysed in RIPA buffer with cOmplete™ Mini Protease Inhibitor (Roche, #11836153001) and PhosSTOP™ (Roche, # 4906845001) and loaded in Laemmli buffer at 1 μg/μl. Samples were separated on 4–15% SDS-PAGE Criterion Gels (BioRad, #5671085) and transferred to Immobilon-FL PVDF membrane (MilliporeSigma, #IEVH7850). Membranes were blocked for 1 hour in LiCor blocking buffer and incubated with primary antibody at 4oC, overnight, followed by fluorescence-conjugated secondary antibodies. Membranes were scanned using the LiCor Odyssey CLx instrument. Primary antibodies were AKT (CST #4691S, 1:1000), phospho-AKT (CST #4060P, 1:1000), and β-actin (CST #4970S, 1:1000).
Results
The genomic landscape of naturally occurring primary canine lung cancer
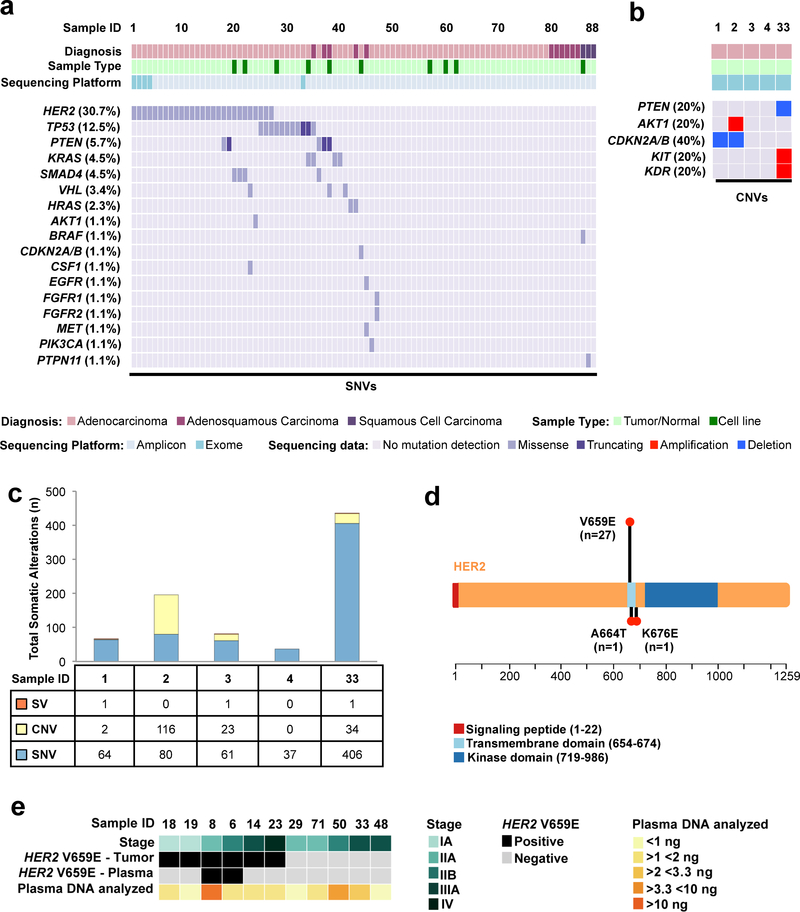
In order to map the genomic landscape of primary canine lung cancer we undertook multi-platform next-generation sequencing of 88 NSCLC cases including 77 tumor/normal matched pairs and 11 cell lines (Table 1). The cohort included 74 cPAC, 11 cPASC, and 3 cPSCC. Labrador retrievers represented the most commonly affected pure breed dog (21%) with mixed breeds (25%) and multiple other single pure breeds. The predominant cPAC subtype was papillary adenocarcinoma (62%). The cohort was gender-balanced (52% females) and primarily neutered/spayed (92%) with a median age at diagnosis of 11 years. Smoking status in the pet’s household was not available. Extended clinical annotation is shown in Table S2 and Figure S1.
Table 1.
Genomic analyses performed in primary canine lung cancer.
| Analysis platform | Sample analyzed* | Type of alteration detected |
|---|---|---|
| Exome | 5 cPAC and matching normal | Germline and somatic SNVs, CNVs, and SVs in coding regions |
| Amplicon | 61 cPAC and matching normal^, 8 cell lines | Germline and somatic SNVs in 53 cancer genes |
| 10 cPASC and matching normal, 1 cell line | Germline and somatic SNVs in 53 cancer genes | |
| 2cPSCC and matching normal, 1 cell line | Germline and somatic SNVs in 53 cancer genes | |
| Total | 78 tumor-normal pairs, 10 cell lines | |
an additional cell line (OSUK9PADRiley) was analyzed by Sanger sequencing
all matching normal samples were available except one (CCB30381)
cPAC: Canine Pulmonary Adenocarcinoma; cPASC: Canine Pulmonary Adenosquamous Carcinoma; cPSCC: canine Pulmonary Squamous Cell Carcinoma.
To identify somatic point mutations, copy number changes, and translocations, we first performed exome sequencing of five cPAC tumors and matching normal samples with a mean target coverage of 298X and 263X respectively (99% of target bases covered ≥ 40X, Table S3) A total of 648 high-confidence somatic SNVs (median 64, range 37–406), 165 focal CNVs (median 19, range 0–116), and 3 SVs (median 1, range 0–1) were identified (Tables S3–S5 and Figure 1A, 1B, and 1C). The average tumor mutation burden (TMB) for somatic point mutations per haploid callable megabase (Mb) in these cases was 2.04 mutations/Mb (range 0.58 – 6.38). Among these somatic variants we identified mutations in genes whose human orthologs have been implicated in human cancer according to COSMIC(15) Tiers 1 and 2 including somatic SNVs (Table S4), focal CNVs (Figure 1B and Table S5), SVs and numerical chromosomal changes (whole chromosome or arm-level gains/losses) (Figure S2). The sole gene bearing recurrent nonsynonymous SNVs was HER2 (80%) with the missense mutation V659E occurring in three cases and the missense mutation K676E in a fourth case (Figure 1D). No HER2 amplifications were detected in these five tumors. We also assessed somatic point mutation signatures according to their trinucleotide context (Figure S3) (16,17). The most common signature in these five cases was the age-associated COSMIC Signature 1A in 4/5 (80%) and COSMIC Signature 2, associated with APOBEC cytidine deaminase activity, in two cases.
Figure 1. The Genomic Landscape of Primary Canine Lung Cancers.
(A) Recurrent likely pathogenic somatic mutations in cancer genes identified in primary canine lung cancers through multi-platform sequencing. SNVs were determined from combined tumor/normal exome and/or amplicon sequencing across 88 total tumors and cell lines. (B) CNVs were determined from tumor/normal exome data in five cPAC cases. (C) Somatic mutation burden (SNVs, CNVs, and SVs) identified by exome sequencing of five tumor/normal cPAC cases. (D) Distribution of somatic HER2 mutations within the HER2 protein identified in primary canine lung cancers. The length of the lollipops is proportional to the number of mutations found. (E) Detection of HER2 hotspot mutations in plasma from 11 canine primary lung cancer cases.
To identify somatic point mutations across a broader cohort of canine lung cancers, we used a custom canine cancer amplicon-based sequencing panel (Table S7) in 73 additional lung tumors (61 cPAC, 10 cPASC, 2 cPSCC), two previously exome-sequenced tumors with matched normal tissue, and 10 cell lines (8 cPAC, 1 cPASC, and 1 cPSCC). These cases were sequenced to an average depth of 3,383x (Table S8). A median of 1 somatic coding point mutation (range 0–3) within sequenced panel regions was identified across all cases. Likely pathogenic recurrent point mutations included HER2 V659E (29.8%), KRAS G12D/V (3.4%), SMAD4 D351Y/G (3.4%) and TP53 R239Q/G (2.2%) (Table S9). Two additional somatic missense mutations in HER2 were identified in single cases (Figure 1D). Overall, recurrently mutated genes containing somatic potentially pathogenic SNVs included TP53 (12.5%), PTEN (5.7%), SMAD4 (4.5%), KRAS (4.5%), VHL (3.4%) and HRAS (2.3%). Finally, based on both exome and amplicon sequencing, we evaluated germline SNPs to identify putatively pathogenic rare variants (i.e. those not previously identified in dogs based on review of presence in dbSNP 151 (18) and/or ≥10% frequency in DogSD(19)) in 81 genes potentially associated with susceptibility to human lung cancer (20). We identified nine rare putatively pathogenic SNPs in five dogs in the genes CHRNA3, CYP1B1, DNAH11 and HER2 (Table S10). Of these SNPs, the only variant with an equivalent in its human orthologous gene was DNAH11 R1460W corresponding to human DNAH11 R1444W (rs1035326227, MAF < 0.01%). The human SNP has not been associated with disease. HER2 V1189I variants occurred in two cases without somatic HER2 tumor mutations. The human orthologous position, V1184, has not shown human variation. The canine variant has been identified in 4% of cases in DogSD and based on functional effect prediction (FATHMM), it is likely neutral. None of the genes bearing rare SNPs showed second hits in tumor tissue.
We additionally performed matched tumor/normal amplicon sequencing to evaluate the genomic landscapes of 11 cPASC and 3 cPSCCs, subtypes which are under-studied entities in dogs and humans, especially in never-smokers (Figure 1A and Table S9). In contrast with cPAC, no HER2 mutations were identified in these tumors. In cPASC, HRAS Q61L and KRAS Q61K each occurred in one case. Thus 18% of cases bore RAS hotspot mutations. PTEN stop gains additionally occurred in 2/11 (18%) of cases at high tumor allele frequencies and were exclusive with RAS mutations. Additional likely pathogenic somatic mutations also occurred in a single cancer gene in a single tumor each including EGFR A726T, MET M1269V, TP53 R147C, and VHL P97L. Finally, while no recurrent mutations were identified in the three cPSCCs, we identified one case with a somatic BRAF V588E and another bearing PTPN11 G503V.
Frequent HER2 mutation in canine pulmonary adenocarcinoma (cPAC)
HER2 was the most frequently mutated gene in our multi-platform next generation sequencing cohort, with missense mutations occurring exclusively in cPACs (27/74, 36.5%, two mutations occurring in a single patient) (Figure 1A). No HER2 insertions were identified. We additionally identified a HER2 mutation in the cell line OSUK9PAPADRiley solely by Sanger sequencing of the codon 659 locus. We thus identified 29 total HER2 mutations overall (Figure 1D). In 24 cases, the HER2 variants were evaluated on at least two platforms including exome sequencing, amplicon sequencing, Sanger sequencing, and/or droplet digital PCR (ddPCR, Table S11). The HER2 variant tumor allele fraction (AF) median by amplicon sequencing was 21.3% (range 8.4 – 51.9%). All low AF (< 20%) cases identified by amplicon sequencing were also validated by Sanger and/or ddPCR. Notably, one cell line, OSUK9PAPADOscar, contained a low AF HER2 V659E variant (AFs of 15% by amplicon and 16% by ddPCR) during early short-term culture (passage 4) that was no longer detectable by Sanger sequencing or ddPCR in later passages (passage 15). Importantly, passage 15 was utilized for all functional studies described below and it was thus considered HER2WT in this setting. Overall, V659E missense mutations located in the HER2 TMD occurred in 93.3% of HER2-mutant cases. Additional HER2 mutations included A664T (OSU419040) and K676E (CCB050354), which have not been previously described in orthologous human HER2 regions. In some cases, HER2 mutations co-occurred with mutations in TP53, SMAD4, PTEN, VHL, AKT1 or KDR.
Detection of HER2 mutations in canine plasma DNA
Cell-free tumor DNA (ctDNA) in plasma has been increasingly used for noninvasive genotyping in human cancer patients (21). To develop a canine blood test that could rapidly identify dogs with HER2-mutant lung cancer, we investigated whether cPAC HER2 hotspot mutations are detectable in ctDNA. We evaluated plasma from 11 dogs, 5 with HER2-wild-type tumors and 6 with HER2V659E tumors using droplet digital PCR (ddPCR) (Table S12). In order to evaluate assay performance (specificity), we first analyzed wild-type tumor samples, plasma DNA from unrelated commercially available canine plasma samples and template-free controls to establish assay specificity (BioreclamationIVT #BGLPLEDTA-100-P-m). Using uniform gating for all experiments, we found 1/7 template-free samples showed 1 wild-type droplet and no samples showed any evidence of mutant DNA amplification. In wild-type tumor and plasma DNA samples, 2/8 samples showed 1 mutant droplet each. Based on these results, we required at least 3 mutant droplets to confidently detect HER2V659E. To confirm mutation detection and quantitative performance, we analyzed and detected HER2V659E in 6/6 positive control tumor DNA samples where we had previously identified V659E mutations using amplicon sequencing. In these samples, we observed a high correlation between allele fractions measured using amplicon sequencing and ddPCR (Pearson’s r 0.976, p=0.0008, Figure S4). In 11 plasma samples from dogs with cPAC tested using ddPCR, median total cell-free DNA concentration was 3.7 ng/mL plasma (range 0.7–23.0) (Table S12). Requiring at least three mutant droplets to support mutation detection and testing cfDNA equivalent to 435 μL plasma, the median limit of detection (LoD) for mutation allele fraction was calculated at 0.61% (range 0.10%−3.11%). HER2V659E mutations were detected in 2/6 plasma samples from dogs with HER2V659E-positive tumors at 1.9% and 2.3% allele fractions. HER2V659E was not detected in any plasma samples from dogs with HER2WT tumors, confirming assay specificity (Figure 1E). Sensitivity for mutation detection in this cohort may be limited due to low total cfDNA concentration and amounts analyzed.
HER2 expression in primary canine lung cancer
In human cancers, HER2 bears activating point mutations, copy number gains/amplifications, and RNA and protein overexpression. Amplification and overexpression are typically mutually exclusive with point mutations. HER2 copy number was first determined in the five exome-sequenced cases (Figure S2 and Table S5). No numerical CFA9 gains or focal HER2 amplifications were detected. However, these cases predominantly bore somatic putatively activating point mutations and might not be expected to contain concomitant gains. Therefore, we evaluated HER2 RNA and protein expression by qRT-PCR and IHC. RNA samples from 49 lung tumors (nine HER2-mutant) were evaluated alongside 14 normal lung tissue samples distal to tumor areas but from the same lung lobe. Median HER2 expression fold-change relative to expression of the house-keeping gene HPRT in normal lung samples was 1.06 (range 0.28–4.11) and in tumors was 0.85 (range 0.07–4.50) (Figure S5). No significant difference in relative HER2 expression was observed between tumor and normal or HER2-mutant and HER2WT groups.
Additionally, in order to quantify HER2 protein expression in cPAC, digital image analysis was performed on eight tumors from FFPE. Three of the samples bore the HER2V659E hotspot mutations, one bore HER2A664T, and four were HER2WT. All cases were positive for HER2 staining with homogeneous and diffuse staining of tumor cell cytoplasm and cell membrane, but no staining in adjacent stroma or vasculature (Figure S6). Positive staining was observed in bronchial epithelium of the adjacent non-affected lung in all cases. Consistent with absence of observed HER2 amplifications, no significant differences (mean ± SEM) were detected in the tumor positivity percentage for HER2 (47±5.4 and 35±5.1) between the WT and HER2-mutant groups, respectively. No significant differences in HER2 staining were present for percent minimum (51±2.9 WT vs. 55±5.1, HER2 mutations) or percent maximum (97±0.31 WT vs. 96±0.47 HER2 mutations) stain intensity (Table S13). Overall, most tumors showed moderate expression of HER2 based on qRT-PCR and IHC with some variability, but levels were typically consistent with those seen in normal tissue and did not vary based on HER2 mutation status.
Constitutive HER2 activation in HER2V659E cPAC cell lines
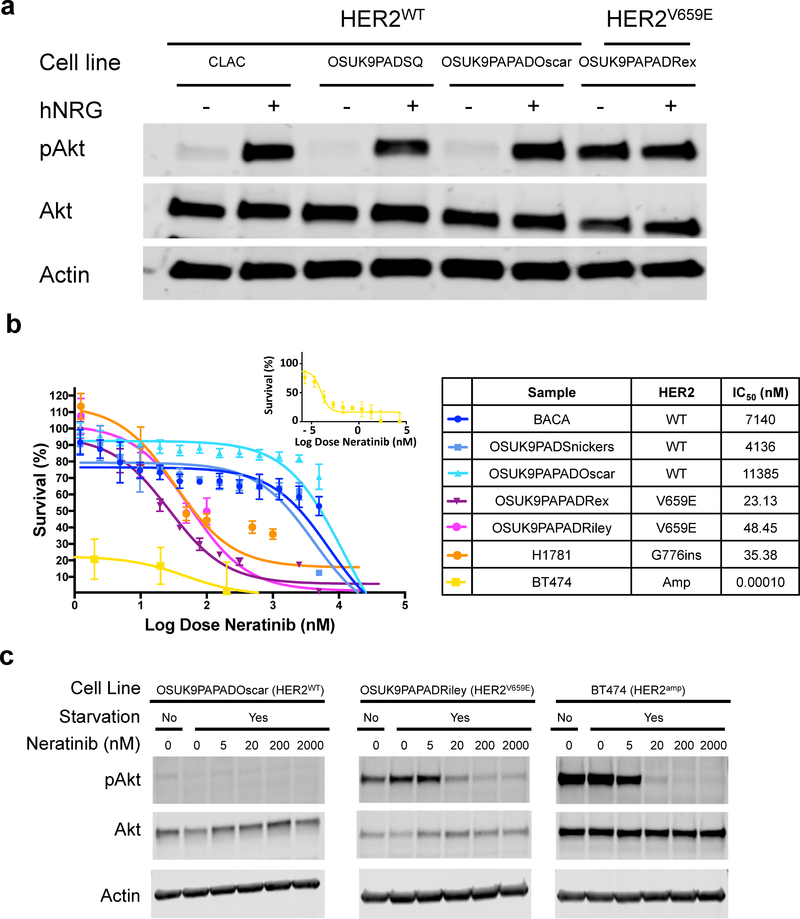
HER2 is a transmembrane receptor tyrosine kinase typically activated by homo- or hetero-dimerization with other HER family receptors. HER2 mutations or overexpression drive constitutive downstream signaling. In human cancers, HER2 V659E mutations stabilize dimers to increase HER2 autophosphorylation, EGFR phosphorylation, activation of phosphatidyl-inositol-3-kinase (PI3K), and activation of mitogen-activated-protein-kinase (MAPK) pro-survival signaling pathway members (e.g. AKT and ERK) relative to wild-type HER2 (22,23). To determine whether HER2V659E constitutively activates downstream signaling in cPAC, we first validated HER2 genotype in seven canine lung cancer cell lines through amplicon sequencing, ddPCR or Sanger sequencing of the V659 locus, Sanger sequencing of all HER2 coding regions, and/or aCGH to determine HER2 copy number status as previously published (Table S11) (24). One cell line, OSUK9PAPADOscar, bore a low allele frequency HER2V659E mutation when sequenced by amplicon panel at low passage (passage 4) as a primary culture, but had lost this allele in later established passages (passage 15) characterized by Sanger sequencing and ddPCR. The latter passages were utilized for functional studies. We thus evaluated HER2 activation in one HER2V659E cPAC cell line, OSUK9PAPADRex, and three HER2WT cell lines (two cPAC – CLAC and OSUK9PAPAPADOscar – and one cPASC – OSUK9PADSQ) by Western blotting for total and phospho-AKT in the presence and absence of the ErbB ligand, neuregulin (hNRG) post-serum starvation. Only the HER2V659E line, OSUK9PAPADRex, showed constitutively high AKT phosphorylation post-starvation even in the absence of hNRG stimulation (Figure 2A).
Figure 2. HER2V659E constitutively activates downstream HER2 signaling and is associated with response to HER2 inhibitors in primary canine pulmonary adenocarcinoma (cPAC) cell lines.
(A) HER2 signaling activation in canine lung cancer cell lines. Phospho-AKT and AKT levels were assessed by Western blot under serum starvation in the presence and absence of EGFR activation by hNRG in HER2V659E and HER2WT cPAC cell lines. (B) Neratinib drug-dose-response studies in primary lung cancer cell lines. Five canine cell lines (three HER2WT and two HER2V659E) and two human lung cancer positive controls with known HER2 activating mutations (BT474 – HER2-amplified, and H1781 – HER2G776V) and HER2 inhibitor responses were treated with 14 neratinib doses ranging from 100 μM to 5.5×10−2 nM for 72 hours with CellTiterGlo viability endpoints. Survival is shown relative to DMSO vehicle control.
Selective sensitivity of HER2V659E cPAC cell lines to HER2 inhibitors
To determine the potential efficacy of anti-HER2 agents for treatment of HER2V659E cPAC, we performed dose-response studies of selected tyrosine kinase inhibitors (TKIs: neratinib, lapatinib, and erlotinib) and a humanized HER2 recombinant monoclonal antibody (mAb), trastuzumab, which binds the extracellular juxtamembrane domain IV of HER2. We first assessed differential sensitivity of HER2V659E and HER2WT canine lung cancer cell lines to lapatinib (HER2 and EGFR inhibitor) and neratinib (HER2, HER4, and EGFR inhibitor). Five cPAC cell lines (two HER2V659E and three HER2WT) were treated with neratinib and four (one HER2V659E and three HER2WT) with lapatinib for 72 hours. Two HER2-mutant human cancer cell lines – BT474 (HER2AMP) and H1781 (kinase domain HER2G776ins) – were treated as positive drug controls (22,25) (Figures 2B and S7). Significant differences in viability were observed between HER2V659E and HER2WT cPAC cell lines for both TKIs (IC50s < 200 nM in HER2V659E versus IC50s > 2500 nM in HER2WT). All HER2-mutant cell lines were sensitive to neratinib with IC50s < 50 nM (Figure 2B), significantly lower than those observed for HER2WT cells (IC50s > 2.7 μM, p=0.0079). We additionally observed a neratinib dose-dependent decrease in p-AKT in the HER2-mutant cell lines OSUK9PAPADRiley (HER2V659E) and BT474 (HER2amp) whereas p-AKT levels in OSUK9PAPADOscar (HER2WT) were low at all treatment levels (Figure 2C). Given that HER2 receptors dimerize with EGFR, we then evaluated differential sensitivity of the five canine cell lines and the human BT474 control line to the EGFR inhibitor, erlotinib. Not surprisingly, HER2V659E IC50s were greater than those of neratinib at 220 nM and 1.17 μM. HER2WT IC50s ranged from 1.10 – 15.38 μM (Figure S8). Finally, we evaluated the HER2-mutation-dependent effects of trastuzumab in the five cPAC cell lines described above (two HER2V659E and three HER2WT) and the positive control cell line, BT474, at doses ranging from 1×10−6 μg/ml to 1 mg/ml for 72 hours. While trastuzumab did not decrease viability below 50% for any of the cell lines at 72 hours, the greatest dose-dependent responses were observed in the BT474 control line (65% viability at 10 μg/ml, consistent with prior publications) and in the HER2V659E line OSUK9PAPADRex (74% viability at 10 μg/ml). More limited responses were observed in OSUK9PAPADOscar (HER2V659E) and OSUK9PADSnickers (HER2WT) and no responses at any dose in Riley (HER2V659E) and BACA (HER2WT) (Figure S9). Overall, these studies support that HER2V659E in cPAC is an activating event that stabilizes HER2 homo- and hetero-dimers, confers dependency on downstream signaling, and confers sensitivity to targeted HER2 tyrosine kinase inhibition.
Discussion
Through multi-platform next-generation sequencing of 88 naturally occurring primary canine NSCLC cases (77 tumors and 11 cell lines), we describe for the first time the detailed genomic underpinnings of this cancer. The cohort included major NSCLC subtypes occurring in dogs and humans: cPAC (n=74, cPASC (n=11), and cPSCC (n=3) (Table S2, Figure S1). Although lung cancer may be over-represented in Doberman pinschers, Australian shepherds, Irish setters, and Bernese mountain dogs(7), Labrador retrievers comprised the largest pure breed in this cohort (21%) followed by mixed breeds (25%). The cohort was gender-balanced (52% females), primarily neutered/spayed (92%), and bore a median age at diagnosis of 11 years. Given that dogs are companion and service animals that typically share the same environment with humans, they may have a role to play as sentinels for human lung cancer environmental risk factors. Some data suggests that environmental risks are shared across species. For example, an increased risk of developing cPAC (OR:2.4, CI 95%:0.7–7.8; p – not given) trends towards association with having a smoker in the home in dogs with short (brachycephalic) or medium length (mesocephalic) noses, such as Labrador retrievers (26). Although second-hand smoke exposure in the dogs in our cohort is possible given that exposure was not recorded, genomic landscapes of human lung cancers in never-smokers have not been shown to differ based on exposure to second-hand smoke (27). Exposure to other environmental carcinogens such as air pollutants may also play a role in development of lung cancers. For example, increased lung cancer risk may be present in dogs with higher amounts of carbon deposits known as anthracosis (OR:2.1, CI95%: 1.20–3.70; p<0.01) (28), although in humans anthracosis has been commonly observed in normal lungs as well as tumors and lymph nodes. In this cohort, anthracosis was recorded in 15 cases and pneumoconiosis (lung disease associated with pollutant exposure) in one case. However, no associations between anthracosis annotation and genetic features of these cases were observed. Overall, our studies included broad representation of lung cancer across histologic subtypes, breeds, ages, and pollutant exposures reflective of primary canine lung cancer diversity seen in the clinical setting in the United States. Overall, support exists for shared etiologies between canine and human never-smoker lung cancer including secondhand smoke, organic dusts, and outdoor and indoor air pollution, suggesting that study of canine lung cancer can be informative for understanding human lung cancer risks and etiologies. Genomic characterization of canine lung cancers is a first major step towards understanding variables influencing lung cancer development in pet dogs.
Unique genomic characteristics of human never-smoker lung cancer include low somatic mutation burden, C:G>T:A enrichment, and activating mutations or fusions impacting EGFR (45%), ALK (5–11%), ROS (1.5–6%), HER2 (3–5%), and RET (2%)(4,29). Here, we also observed a low somatic burden of SNVs, CNVs, and SVs through exome sequencing in five matched tumor/normal cPAC pairs. We additionally observed that the most common mutation signature in these five cases was the age-associated COSMIC Signature 1A in 4/5 (80%) similar to the enrichment seen in human NSCLC (Figure S3). This signature is associated with age in many human cancers, putatively the result of spontaneous deamination of 5-methyl-cytosine. COSMIC Signature 2, associated with APOBEC cytidine deaminase activity, was also present in two cases. This signature, is most prominently associated with cervical and bladder cancers, but is also commonly found in lung adenocarcinoma and squamous cell carcinoma. While these signatures are sometimes associated with APOBEC gene variants in human cancers (30), no putatively pathogenic germline or somatic APOBEC mutations were observed. Based on our studies, primary canine lung cancers bear a low mutation burden (TMB mean of 2.04 mutations/Mb) and mutation signatures reflective of those seen in human never-smoker lung cancers.
The most common recurrently mutated genes containing somatic potentially pathogenic SNVs in the full cohort included HER2 (31.5%), TP53 (12.5%), PTEN (5.7%), SMAD4 (4.5%), KRAS (4.5%), VHL (3.4%) and HRAS (2.3%). Recurrent CDKN2A/B focal deletions were also observed in 2/5 (40%) cases (Figure 1A, 1B) along with a homozygous missense mutation, G50R, equivalent to human codon G101 mutations. CDKN2A deletions were the most common alteration by frequency, occurring at rates comparable to those in human NSCLC. Two focal deletions were observed out of five exome-sequenced cases with signs of larger-scale CFA11 losses in remaining cases (Figure S2). CDKN2A is mutated in ~30% of all human NSCLC, primarily via homozygous deletion, and this number is reduced to around 25% in never-smokers. The next most common alterations after CDKN2A and HER2 were TP53 missense and truncating mutations comparable to DNA binding domain mutations in human TP53. Similar to human NSCLC, we observed a reduced burden of TP53 mutations (12.4%, two stop gains and nine likely pathogenic missense mutations) relative to human smoker NSCLC in which more than half of tumors are mutated. PTEN mutations were the next most common at 5.6%. PTEN is mutated in ~9% of human NSCLC, but only ~2% of never-smoker NSCLC. We additionally identified four somatic mutations in the tumor suppressor SMAD4, mutated in ~5% of human NSCLC at comparable rates in smoker and never-smoker cancer. KRAS mutations are the most common oncogenic mutations in human smoker NSCLC (~30–40% of cases), but occur at reduced frequencies in never-smoker lung cancer (0–7%). KRAS mutations in our cohort were rare (2 G12V, 1 G12D, and 1 Q61K), but comparable to human hotspots. Canine HRAS missense mutations were also located in human-equivalent hotspots (Q61L, F78S). Additional likely pathogenic somatic mutations included individual cases of AKT1 amplification, KIT/KDR amplification, EGFR A726T (human A755), MET M1269V (human M1268), and VHL P97L (human P97). WWTR1, the only COSMIC gene bearing a somatic translocation in exome-sequenced cases, has been shown to undergo translocation with CAMTA1 in human epithelioid hemangioendothelioma (31). We identified a WWTR1 translocation of unknown consequence with ATP5F1. Although we identified translocations occurring in coding regions in five exome-sequenced tumors, it remains possible that, as in human never-smoker lung cancer, EML4-ALK fusions, ROS1 fusions, RET fusions, and other fusions may also be present in canine lung cancer.
In addition to charting the landscape of cPAC, we have found recurrent KRAS and TP53 mutations in cPASC and provide a view of possible drivers in cPSCC. In cPASC, HRAS Q61L and KRAS Q61K each occurred in one case. Finally, while no recurrent mutations were identified in the three cPSCCs, we identified one case with somatic BRAF V588E (equivalent to the human V600E hotspot) and another bearing PTPN11 G503V (equivalent to the human G503V hotspot).
HER2 contained the most somatic mutations with hotspot mutations occurring solely in cPAC (37.8%). HER2 is a well-characterized human oncogene and drug target mutated in ~6% of all cancers based on cBioPortal query of 10,967 cases in the TCGA pan-cancer atlas (32,33). Most alterations are focal amplifications, but activating point mutations and insertions are also common. In human NSCLC, HER2 mutations are oncogenic drivers in ~1–4% of cases with mutations and insertions mostly in exon 20 at codon 776 resulting in constitutive HER2 kinase domain activation and downstream signaling through PI3K and MAPK pathways (29,34,35). HER2 may also be more commonly mutated in human never-smoker lung cancer, with point mutations at frequencies reported at 3–5%(36), predominately in female never-smokers who carry a median OS of ~2 years (35). HER2 TMD polar mutations (HER2V659E/D, HER2G660D) are present in 0.18% of human lung adenocarcinomas and are exclusive with HER2 kinase domain mutations (37). Amplicon analysis capable of identification of point mutations and small insertions or deletions covered canine HER2 exons 8 and 17–22 including transmembrane and kinase domains. Additionally, Sanger sequencing of all exons in five canine cell lines with wild-type HER2 based on amplicon sequencing (OSUK9PAD, BACA, CLAC, K9PADSQ and OSULSCC1) found no somatic HER2 mutations in other sites (Table S11). It is nonetheless possible that somatic mutations occurring in other regions of HER2 were not identified in amplicon-sequenced samples even though data facilitating functional interpretation of these variants would be limited.
In addition to point mutations, HER2 amplification has also been identified in ~1% of human NSCLC (29), with enrichment in EGFR-inhibitor-resistant tumors (38). Protein overexpression is reported in 6–35% of tumors including up to 42% of adenocarcinomas and correlates with poor prognosis (39–42). We detected no somatic HER2 focal amplifications or numerical CFA9 gains in five exome-sequenced cases (Figure 1B and S2) or two previously aCGH-profiled cell lines. However, four of these seven cases contained somatic, putatively activating HER2 SNVs. Given that HER2 amplification/overexpression and SNVs are typically mutually exclusive, it remains possible that our broader amplicon cohort contained undetected HER2 amplifications. We therefore utilized qRT-PCR and IHC studies to more broadly assess HER2 overexpression and did not find evidence for significant tumor-specific HER2 overexpression (Figures S5 and S6 and Table S13). Thus, it is unlikely that HER2 is frequently amplified in canine lung cancer.
Overall, though we observed a similar mutation spectrum in canine lung cancer relative to human never-smoker NSCLC, the notable exception is abundance of HER2 mutations and lack of EGFR mutations. EGFR mutations occur at low frequency in human smoker lung cancers (0–7%), but are enriched in human never-smokers (~45%). Canine HER2 shares normal and oncogenic roles with human HER2 based on sequence conservation (92.2% protein identity) and prior study of its role in canine cell signaling. HER2V659E occurs at a highly conserved residue (100% identity in the TMD from amino acids 654–674) and to the neu (rat HER2) variant identified in a rat glioblastoma cell line that originally led to discovery of HER2’s oncogene status (43). HER2 has previously been implicated in canine cancers via overexpression by IHC and qRT-PCR in canine mammary tumors (44), through its utility as a vaccine target in canine osteosarcoma (45), and through downstream signaling activation in canine lung cancer (10). Thus, HER2 sequence and pathway biology is conserved, so the predominance of HER2 mutations as erbB signaling activators in lieu of EGFR mutations in cPAC may be the result of cell-of-origin and genetic background influences. Cell of origin determination in canine lung cancers is challenging because the pulmonary adenocarcinoma diagnosis includes tumors arising from primary, secondary and tertiary bronchioles and thus topographic origin can be difficult to determine. However, evidence supports that HER2 is broadly important for canine pulmonary epithelium. For example, neuregulin-stimulated HER2 increases proliferation in pulmonary epithelial cells by activation of the JAK-STAT pathway. Further, when HER2 activation is blocked via antibodies to neuregulin or HER2 in a scratch wound-healing assay of pulmonary epithelial cells, wound closure is significantly delayed, suggesting HER2 activation is necessary for epithelial proliferation (46). We have also found that IHC of canine normal lung showed stronger HER2 staining of all bronchioalveolar regions when compared to EGFR staining of normal adult canine lung. These data suggest that HER2 may play a more central role than EGFR in canine alveolar and airway epithelial cells during chronic lung injury and for general proliferative processes. Prolonged activation could lead to cellular transformation and neoplasia. Further, EGFR mutations have been associated with particular histotypes – i.e. they are frequent in lepidic and acinar patterns and infrequent in mucinous patterns in female Asian never-smoker PAC. In this population, the most frequent adenocarcinoma histotype was acinar (142 cases, 71.7%), followed by papillary (18 cases, 9.1%), solid (17 cases, 8.6%), lepidic (9 cases, 4.5%), and micropapillary (1 case, 0.5%). Interestingly, our canine cohort had predominantly papillary morphology (69%) with only 5% acinar (5%). Therefore, differences in cell of origin in both species could account for the differences in EGFR mutation frequencies. Background genetic context likely also plays a primary role in shaping enrichment for HER2 mutations in cPAC. This is also true in human lung cancer where EGFR mutation frequency varies by more than 3-fold between different human populations. The Asian population has a very high rate of EGFR mutation among the never-smoking population, up to 51.4% overall and as high as 64% in some populations such as the Kinh, versus about 20% in Caucasians (47–49). These differences in human populations suggest a sensitivity of EGFR mutations to genetic context. Conversely, HER2 mutations are found in all human populations at about the same frequency, suggesting that HER2 mutations in humans may not be as sensitive to genetic background.
We have additionally shown that HER2 hotspot mutations can be detected in the plasma of dogs bearing HER2V659E cPACs even at early disease stages (Figure 1E and Table S12). In human NSCLC, ctDNA has been shown to be significantly enriched in plasma relative to controls with key genetic features identifiable via liquid biopsy. Associations have been found between ctDNA levels and tumor stage, grade, lymph node status, metastatic sites, response, and survival (50,51). The first FDA-approved liquid biopsy test was the cobas EGFR Mutation Test v2, a real-time PCR assay utilized in NSCLC for the detection of EGFR exon 18–21 mutations in tissue or plasma to guide EGFR inhibitor treatment assignment (52,53). Our proof-of-principle study supports that ctDNA is also detectable in primary canine lung cancer patient plasma. A non-invasive HER2V659E assay will enable genotyping patients when tumor tissue is limited and may have a role in treatment monitoring or detection of minimal residual disease. This assay will also facilitate prospective analysis of HER2V659E’s diagnostic and prognostic value.
In human cancers, HER2 TMD mutations constitutively activate pro-survival HER2 signaling (37) and are associated with HER2 inhibitor responses (23). We have confirmed in this study that, similar to human HER2 TMD mutants, canine HER2V659E cell lines constitutively activate downstream signaling through AKT and are selectively sensitive to the HER2 TKI inhibitors neratinib and lapatinib in vitro (Figure 2 and S7). In order to further assess the role of dimerization in HER2 activation in cPAC, we also performed drug dose response studies for erlotinib and trastuzumab (Figures S8 and S9). One of two HER2-mutant cell lines showed erlotinib sensitivity. Trastuzumab responses were poor overall and did not correlate with HER2 status, although dose-response relationships were observed in three of five cell lines. Trastuzumab’s human binding site is highly conserved in canine (only a single amino acid difference) and trastuzumab has been shown to bind canine HER2 and inhibit proliferation of HER2-overexpressing canine cancer cell lines (54). However, even the human HER2-amplified cell line, BT474, did not show viability reduction below 50% in our hands. It is likely that the effects of trastuzumab on CellTiterGlo viability are broadly muted at the 72-hour timepoints we utilized. Overall, these studies indicate that HER2V659E in cPAC is an activating event that stabilizes HER2 homo- and hetero-dimers, confers dependency on downstream signaling, and confers sensitivity to targeted HER2 tyrosine kinase inhibition. We have charted the genomic landscape of primary canine lung cancers including the NSCLC subtypes cPAC, cPASC, and cPSCC. We have identified recurrent HER2 mutations in these cancers and present, to our knowledge, the first complete suite of evidence supporting an oncogenic role for and dependency on constitutively activating mutations in HER2 in a canine cancer. Further work is needed to exhaustively profile these tumors, particularly according to variation across breeds and through integration of additional data types including epigenomics, RNA sequencing, and proteomics. However, these data nonetheless offer significant immediate diagnostic and therapeutic opportunities for dogs with primary lung cancer and aid comparative understanding of never-smoker and HER2-mutant lung cancer. These findings set the stage for HER2 inhibitor toxicity, dose-finding, and efficacy studies in dogs that will guide utilization of HER2 inhibitors in the veterinary clinic.
Supplementary Material
Statement of significance.
We have discovered recurrent somatic HER2 (ERRB2) point mutations in 38% of canine pulmonary adenocarcinomas (cPAC) which confer both constitutive activation of proliferative signaling and also sensitivity to the HER2 inhibitors lapatinib and neratinib. These findings have relevance for veterinary oncology, comparative oncology, and the study of mutant HER2 inhibition.
Acknowledgments
This study was supported by National Canine Cancer Foundation GL150H-005 (G. Lorch), the Bisgrove Scholars Program (M. Murtaza), philanthropic support to the TGen Foundation (J. Trent, W. Hendricks), CTSA UL1TR001070 (G. Lorch), NCI P30 CA016058 (G. Lorch, J. Trent, W. Hendricks), Brooke’s Blossoming Hope (J. Trent, W. Hendricks). We thank Dr. Amy LeBlanc, Director of the NCI’s Comparative Oncology Program (COP), and Christina Mazcko, COP Program Manager, for their assistance with CCOGC sample procurement and annotation. Additionally, we thank Dr. Holly Borghese of the OSU CVM Veterinary Biospecimen Repository for her expertise in sample procurement and Dr. Kurtis Yearsley of the OSU Pathology Imaging Core for his expertise in quantitative image analysis. We thank Drs. Alshad Lalani, Irmina Diala, and Lisa Eli of Puma Biotechnology for fruitful discussion of HER2 inhibition and provision of neratinib for this study.
Competing interests: WPDH has performed consulting work for The One Health Company, received research funding from Ethos Discovery, and received travel support from Pathway Vet Alliance. WPDH, GL, and MM have filed a provisional patent (62/778,282) that describes these findings.
Abbreviations list
- cPAC
canine pulmonary adenocarcinoma
- cPASC
canine pulmonary adenosquamous carcinoma
- cPSCC
canine pulmonary squamous cell carcinoma
- TMD
transmembrane domain
- NSCLC
non-small cell lung cancer
- SCLC
small cell lung cancer
- AC
adenocarcinoma
- WT
wild-type
- CCOGC
Canine Comparative Oncology and Genomics Consortium
- OSU
Ohio State University
- ACVP
American College of Veterinary Pathologists
- CNVs
formalin-fixed and paraffin-embedded (FFPE); copy number variants
- SVs
structural variants
- SNV
single nucleotide variants
- ddPCR
droplet digital PCR
- ctDNA
cell-free tumor DNA
- AF
allele fraction
References
- 1.Wilson DW. Tumors of the Respiratory Tract In: Meuten DJ, editor. Tumors in domestic animals. 5th ed: John Wiley & Sons; 2017. p 467–98. [Google Scholar]
- 2.Clément-Duchêne C, Wakelee H. Lung Cancer Incidence in Never Smokers. European Journal of Clinical & Medical Oncology 2010;2(2). [Google Scholar]
- 3.Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res 2009;15(18):5626–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012;150(6):1121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378(22):2093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn FF, Muggenburg BA, Griffith WC, editor. Primary lung cacer in the longevity study/control population of the ITRI beagle dog colony. Springfield, VA: National Technical Information Service; 1992. 133–6 p. [Google Scholar]
- 7.Griffey SM, Kraegel SA, Madewell BR. Rapid detection of K-ras gene mutations in canine lung cancer using single-strand conformational polymorphism analysis. Carcinogenesis 1998;19(6):959–63. [DOI] [PubMed] [Google Scholar]
- 8.Kraegel SA, Gumerlock PH, Dungworth DL, Oreffo VI, Madewell BR. K-ras activation in non-small cell lung cancer in the dog. Cancer Research 1992;52(17):4724–7. [PubMed] [Google Scholar]
- 9.Tierney LA, Hahn FF, Lechner JF. p53, erbB-2 and K-ras gene alterations are rare in spontaneous and plutonium-239-induced canine lung neoplasia. Radiation Research 1996;145(2):181–7. [PubMed] [Google Scholar]
- 10.Mariotti ET, Premanandan C, Lorch G. Canine pulmonary adenocarcinoma tyrosine kinase receptor expression and phosphorylation. BMC veterinary research 2014;10:19 doi 10.1186/1746-6148-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon I, Paoloni M, Mazcko C, Khanna C. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS medicine 2009;6(10):e1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murtaza MDS, Pogrebniak K, Rueda OM, Provenzano E, Grant J, Chin SF, Tsui DW, Marass F, Gale D, Ali HR, Shah P, Contente-Cuomo T, Farahani H, Shumansky K, Kingsbury Z, Humphray S, Bentley D, Shah SP, Wallis M, Rosenfeld N, Caldas C. Multifocal clonal evolution characterized using circulating tumour DNA in a case of metastatic breast cancer. Nat Commun 2015;4(6):8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009;25(16):2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu WL, Huang HM, Liao JW, Wong ML, Chang SC. Increased survival in dogs with malignant mammary tumours overexpressing HER-2 protein and detection of a silent single nucleotide polymorphism in the canine HER-2 gene. Veterinary Journal 2009;180(1):116–23. [DOI] [PubMed] [Google Scholar]
- 15.Forbes SA, Beare D, Boutselakis H, Bamford S, Bindal N, Tate J, et al. COSMIC: somatic cancer genetics at high-resolution. Nucleic acids research 2017;45(D1):D777–D83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature 2013;500(7463):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gehring JS, Fischer B, Lawrence M, Huber W. SomaticSignatures: inferring mutational signatures from single-nucleotide variants. Bioinformatics 2015;31(22):3673–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherry ST, Ward M-H, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. 2001;29(1):308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai B, Zhao W-M, Tang B-X, Wang Y-Q, Wang L, Zhang Z, et al. DoGSD: the dog and wolf genome SNP database. 2014;43(D1):D777–D83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Cui H, Gu D, Zhang M, Fang Y, Chen S, et al. Genetic polymorphisms and lung cancer risk: Evidence from meta-analyses and genome-wide association studies. 2017;113:18–29. [DOI] [PubMed] [Google Scholar]
- 21.Perdigones N, Murtaza M. Capturing tumor heterogeneity and clonal evolution in solid cancers using circulating tumor DNA analysis. Pharmacol Ther 2017;174:22–6. [DOI] [PubMed] [Google Scholar]
- 22.Suzawa K, Toyooka S, Sakaguchi M, Morita M, Yamamoto H, Tomida S, et al. Antitumor effect of afatinib, as a human epidermal growth factor receptor 2-targeted therapy, in lung cancers harboring HER2 oncogene alterations. Cancer Sci 2016;107(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ou SI, Schrock AB, Bocharov EV, Klempner SJ, Haddad CK, Steinecker G, et al. HER2 Transmembrane Domain (TMD) Mutations (V659/G660) That Stabilize Homo- and Heterodimerization Are Rare Oncogenic Drivers in Lung Adenocarcinoma That Respond to Afatinib. J Thorac Oncol 2017;12(3):446–57. [DOI] [PubMed] [Google Scholar]
- 24.Clemente-Vicario F, Alvarez CE, Rowell JL, Roy S, London CA, Kisseberth WC, et al. Human genetic relevance and potent antitumor activity of heat shock protein 90 inhibition in canine lung adenocarcinoma cell lines. PloS one 2015;10(11):e0142007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canonici A, Gijsen M, Mullooly M, Bennett R, Bouguern N, Pedersen K, et al. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget 2013;4(10):1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reif JS, Dunn K, Ogilvie GK, Harris CK. Passive smoking and canine lung cancer risk. Am J Epidemiol 1992;135(3):234–9. [DOI] [PubMed] [Google Scholar]
- 27.Couraud S, Debieuvre D, Moreau L, Dumont P, Margery J, Quoix E, et al. No impact of passive smoke on the somatic profile of lung cancers in never-smokers. Eur Respir J 2015;45(5):1415–25. [DOI] [PubMed] [Google Scholar]
- 28.Bettini G, Morini M, Marconato L, Marcato PS, Zini EJTVJ. Association between environmental dust exposure and lung cancer in dogs. 2010;186(3):364–9. [DOI] [PubMed] [Google Scholar]
- 29.Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. 2016;48(6):607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nik-Zainal S, Wedge DC, Alexandrov LB, Petljak M, Butler AP, Bolli N, et al. Association of a germline copy number polymorphism of APOBEC3A and APOBEC3B with burden of putative APOBEC-dependent mutations in breast cancer. 2014;46(5):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, et al. A novel WWTR1‐CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. 2011;50(8):644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. 2013;6(269):pl1–pl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinstein JN, Collisson EA, Mills GB, Shaw KRM, Ozenberger BA, Ellrott K, et al. The cancer genome atlas pan-cancer analysis project. 2013;45(10):1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazieres J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013;31(16):1997–2003. [DOI] [PubMed] [Google Scholar]
- 35.Mazieres J, Barlesi F, Filleron T, Besse B, Monnet I, Beau-Faller M, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol 2016;27(2):281–6. [DOI] [PubMed] [Google Scholar]
- 36.Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Research 2005;65(5):1642–6. [DOI] [PubMed] [Google Scholar]
- 37.Kanika Bajaj Pahuja TTN, Jaiswal Bijay S., Prabhash Kumar, Thaker Tarjani M.,, Kate Senger SC, Kljavin Noelyn M., Antony Aju, Phalke Sameer, Kumar Prasanna, Mravic Marco, Stawiski Eric W., Vargas Derek, Durinck Steffen, Gupta Ravi, Khanna-Gupta Arati, Trabucco Sally E., Sokol Ethan S., Hartmaier Ryan J., Singh Ashish, Chougule Anuradha, Trivedi Vaishakhi, Dutt Amit, Patil Vijay, Joshi Amit, Noronha Vanita, Ziai James, Banavali Sripad D., Ramprasad Vedam, DeGrado William F., Bueno Raphael, Jura Natalia, and Seshagiri Somasekar. Actionable Activating Oncogenic ERRB2/HER2 Transmembrane and Juxtamembrane DomainMutations. Cancer Cell 2018(34):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takezawa K, Pirazzoli V, Arcila ME, Nebhan CA, Song X, de Stanchina E, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer discovery 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellegrini C, Falleni M, Marchetti A, Cassani B, Miozzo M, Buttitta F, et al. HER-2/Neu alterations in non-small cell lung cancer: a comprehensive evaluation by real time reverse transcription-PCR, fluorescence in situ hybridization, and immunohistochemistry. Clinical cancer research 2003;9(10):3645–52. [PubMed] [Google Scholar]
- 40.Rouquette I, Lauwers-Cances V, Allera C, Brouchet L, Milia J, Nicaise Y, et al. Characteristics of lung cancer in women: importance of hormonal and growth factors. Lung Cancer 2012;76(3):280–5. [DOI] [PubMed] [Google Scholar]
- 41.Langer CJ, Stephenson P, Thor A, Vangel M, Johnson DH. Trastuzumab in the treatment of advanced non-small-cell lung cancer: is there a role? Focus on Eastern Cooperative Oncology Group study 2598. Journal of clinical oncology 2004;22(7):1180–7. [DOI] [PubMed] [Google Scholar]
- 42.Lara PN Jr, Laptalo L, Longmate J, Lau DH, Gandour-Edwards R, Gumerlock PH, et al. Trastuzumab plus Docetaxel in HER2/neu–Positive Non–Small-Cell Lung Cancer: A California Cancer Consortium Screening and Phase II Trial. Clinical lung cancer 2004;5(4):231–6. [DOI] [PubMed] [Google Scholar]
- 43.Bargmann CI, Hung MC, Weinberg RA. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell 1986;45(5):649–57. [DOI] [PubMed] [Google Scholar]
- 44.Gama A, Alves A, Schmitt FJVA . Identification of molecular phenotypes in canine mammary carcinomas with clinical implications: application of the human classification. 2008;453(2):123–32. [DOI] [PubMed] [Google Scholar]
- 45.Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, et al. Immunotherapy with a HER2-targeting listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. 2016;22(17):4380–90. [DOI] [PubMed] [Google Scholar]
- 46.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, et al. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 2003;422(6929):322–6. [DOI] [PubMed] [Google Scholar]
- 47.Yatabe Y, Kerr KM, Utomo A, Rajadurai P, Tran VK, Du X, et al. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J Thorac Oncol 2015;10(3):438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97(5):339–46. [DOI] [PubMed] [Google Scholar]
- 49.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9(2):154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nie K, Jia Y, Zhang XJTB. Cell-free circulating tumor DNA in plasma/serum of non-small cell lung cancer. 2015;36(1):7–19. [DOI] [PubMed] [Google Scholar]
- 51.Jiang T, Ren S, Zhou CJLC. Role of circulating-tumor DNA analysis in non-small cell lung cancer. 2015;90(2):128–34. [DOI] [PubMed] [Google Scholar]
- 52.Kwapisz DJAotm. The first liquid biopsy test approved. Is it a new era of mutation testing for non-small cell lung cancer? 2017;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Y-L, Zhou C, Liam C-K, Wu G, Liu X, Zhong Z, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. 2015;26(9):1883–9. [DOI] [PubMed] [Google Scholar]
- 54.Singer J, Weichselbaumer M, Stockner T, Mechtcheriakova D, Sobanov Y, Bajna E, et al. Comparative oncology: ErbB-1 and ErbB-2 homologues in canine cancer are susceptible to cetuximab and trastuzumab targeting. Molecular immunology 2012;50(4):200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.