Abstract
Purpose
Somatic gene mutations have been increasingly recognized to impact prognosis following resection of colorectal liver metastases (CLM). We aimed to determine the impact of combinations of somatic mutations on survival in patients undergoing CLM resection.
Experimental Design
We identified patients who underwent initial CLM resection during 2007–2017 and had genetic sequencing data available. Risk factors for overall survival (OS) and recurrence-free survival (RFS) were determined using Cox proportional hazards models.
Results
Of 1460 patients who underwent CLM resection during the study period, 507 met the inclusion criteria. Multigene testing revealed mutation rates greater than 10% for TP53 (mutated in 70.8% of patients), APC (53.5%), RAS (50.7%), PIK3CA (15.8%), and SMAD4 (11.0%). BRAF was mutated in 2.0% of patients. BRAF, RAS, TP53, and SMAD4 mutations were significantly associated with OS, and RAS, TP53, and SMAD4 mutations were significantly associated with RFS. Coexisting mutations in RAS, TP53, and SMAD4 were associated with significantly worse OS and RFS than coexisting mutations in any 2 of these genes and mutations in 1 or none of these genes. Coexisting mutations in 2 genes conferred significantly worse OS and RFS than single mutation or no mutations. OS and RFS did not differ significantly between patients with RAS mutation and wild-type TP53 and SMAD4 and patients with wild-type RAS (P = 0.858 and 0.729, respectively).
Conclusions
RAS mutation status alone is not sufficient for precisely predicting prognosis after CLM resection.
Keywords: molecular marker, somatic mutation, co-existing mutation, triple mutation, colorectal liver metastasis
Introduction
Understanding of tumor biology and risk stratification based on clinicopathologic prognostic factors have improved the selection of patients undergoing resection of colorectal liver metastases (CLM) [1, 2]. Recent studies have focused on response to preoperative chemotherapy [3–5] and molecular alterations in CLM for risk stratification. Specifically, gene mutation analysis has been studied for its utility in identifying patients who may benefit from CLM resection [6–9].
Previous studies have shown that mutations in BRAF and KRAS are associated with a poor outcome after CLM resection [6, 10–14]. Mutations in NRAS, TP53, PIK3CA, and SMAD4 were reported to be potential prognostic factors after CLM resection [15–20]. However, the association between mutations in TP53 or PIK3CA and survival has not been consistent across reported studies [15–18]. Our group previously reported that coexisting mutations in RAS and TP53 were associated with worse prognosis [18]. We hypothesized that co-existence of frequently mutated genes would predict prognosis after CLM resection more precisely than mutation in only 1 of the genes.
To test this hypothesis, we sought to determine the impact of combinations of high-frequency somatic mutations and clinicopathologic factors on survival among patients undergoing liver resection for CLM.
Materials and Methods
Study population
Patients who underwent initial liver resection for CLM with curative intent in the Department of Surgical Oncology at The University of Texas MD Anderson Cancer Center from January 2007 through March 2017 were identified from a prospectively-compiled database. Patients without genetic sequencing data were excluded. Demographic and clinicopathologic characteristics, results of mutational analysis, and outcomes were collected. In order to validate our study, we analyzed the influence of multiple somatic mutations on patients who had unresectable colorectal metastases. Patients who had genetic sequencing data were identified from a prospectively-compiled database of the Department of Medical Oncology between 2005 and 2015. All research was conducted under an appropriate institutional review board protocol (PA18–0295) and waiver of consent from patients. The study was conducted in accordance with the Declaration of Helsinki.
Institutional approach to surgical management of CLM
This approach has been previously published [21]. At our institution, the vast majority of patients with CLM receive preoperative chemotherapy. During preoperative chemotherapy, restaging is performed. CLM are deemed resectable when a hepatectomy can achieve a negative margin while preserving more than 20% to 30% of the total estimated liver volume, sparing 2 contiguous hepatic segments, and maintaining vascular inflow, vascular outflow, and biliary drainage [22]. In the case of disease progression or suboptimal tumor response after first-line chemotherapy, second-line chemotherapy is considered [23]. For patients with synchronous CLM and an intact primary tumor, decisions regarding the treatment sequence (primary tumor resection first, combined, or liver resection first) are discussed at a multidisciplinary conference, where decisions are primarily based on the extent of the primary tumor and CLM [7]. For patients with an anticipated insufficient future liver remnant, preoperative portal vein embolization and staged hepatectomy are proposed. Perioperative chemotherapy is typically administered to complete a total of 12 cycles, including those given preoperatively [24]. Patients are routinely followed after resection with history, physical examination, laboratory evaluation, and axial imaging every 3 to 4 months for the first 2 years and every 4 to 6 months for the subsequent 3 years.
Somatic gene mutation profiling
As previously described [21], tumor DNA was isolated from 5-mm-thick unstained sections from tumor tissue blocks or slides from primary colorectal cancer or CLM specimens. Macrodissection was performed in cases of low tumor cellularity. Next-generation sequencing was performed with an AmpliSeq 50-gene panel related to cancer (Supplementary Table 1) using the Ion Torrent Personal Genome Machine (Life Technologies, CA) in a Clinical Laboratory Improvement Amendment–certified molecular diagnostic laboratory [25].
Definitions
As previously described [21], synchronous metastases were defined as metastases diagnosed within 12 months of primary tumor diagnosis. A positive surgical margin was defined as the presence of tumor cells within 1 mm of the transection line. Pathologic response was defined as less than 50% residual cancer cells remaining. [26] The number and diameter of liver metastases were determined from examination of surgical pathology specimens. T category of the primary tumor was classified according to the staging system in the AJCC Cancer Stating Manual, eighth edition.[27] Preoperative chemotherapy regimens were recorded and further categorized according to whether they included an anti-VEGR or anti-EGFR agent.
Statistical analysis
For statistical analyses, we chose to group KRAS and NRAS mutations in a single category, RAS mutation. This choice is in line with our previously published analyses [9, 18] and is supported by the fact that previous studies by other groups suggest that NRAS mutations are associated with worse survival in patients with metastatic colorectal cancer [19, 28, 29].
Sample size calculation was done based on the method reported by Lakatos [30] using the following parameters: α, 0.05; beta, 0.20; mutation rate, 10%; 5-year OS in RAS wild-type patients, 62%; 5-year OS in RAS mutant patients, 34% [18]. With these assumptions, a sample of 500 patients would be able to detect approximately 18% or more decrease in 5-year OS after CLM resection. Categorical variables were expressed in numbers and percentages and were compared among groups using Fisher’s exact test or the chi-square test, as appropriate. Continuous variables were expressed as median values with the interquartile range. A Cox proportional hazards model analysis initially included the following factors: age (continuous variable), sex, primary tumor location, T category, primary lymph node metastasis, pre-hepatectomy carcinoembryonic antigen level (continuous variable), timing of metastasis (synchronous vs. metachronous), preoperative chemotherapy, extrahepatic disease, postoperative adjuvant chemotherapy, number of CLM (continuous variable), largest liver metastasis diameter (continuous variable), surgical margin status (R1 vs. R0), BRAF mutation, RAS mutation, TP53 mutation, APC mutation, PIK3CA mutation, and SMAD4 mutation. A backward elimination with a threshold P value of 0.05 was used to select variables for the final models. Hazard ratios and 95% confidence intervals were calculated for each factor. The performance of a Cox proportional hazards model was internally validated through a 10-fold cross validation approach [31]. Discrimination was evaluated using a Harrell’s concordance statistic [32]. We estimated the median overall survival (OS) time and survival curves adjusted for covariates by using direct adjusted survival estimation [33, 34]. This method uses the Cox regression model to estimate survival probabilities at each time point for each individual and averages them to obtain an OS estimate. The proportional hazards assumption was tested by using Schoenfeld residuals. P ≤ 0.05 was considered to indicate statistical significance. Statistical analysis was conducted with SAS (SAS Institute, Cary, NC).
Results
Study population
Of 1460 patients who underwent CLM resection during the study period, 507 patients met inclusion criteria (Supplementary Figure 1). Demographic and clinicopathologic characteristics are summarized in Table 1. Prehepatectomy chemotherapy was delivered to 455 (89.7%) patients including 142 (28.0%) with more than 6 cycles, and an anti-EGFR agent was delivered preoperatively to 39 (7.7%) patients. The median duration of follow-up was 3.0 years (interquartile range, 1.9–4.5 years). During the follow-up period, 166 (32.7%) patients died and 397 (78.3%) patients experienced recurrence.
Table 1.
Demographic and clinicopathologic characteristics in 507 patients who underwent resection of CLM
| Characteristic | Value |
|---|---|
| Patient factors | |
| Age, median (IQR), yr | 55 (46–62) |
| Sex, male : female, n | 286 : 221 |
| ASA score ≥ 3, n (%) | 442 (87.2%) |
| Primary lesion factors | |
| Location, colon : rectum, n | 358 : 149 |
| T category ≥ 3, n (%)* | 436 (87.4) |
| Lymph node metastasis, n (%)* | 354 (71.8) |
| Liver metastases clinical factors | |
| Prehepatectomy CEA level, median (IQR), ng/mL | 4.0 (2.2–12.5) |
| Synchronous metastasis, n (%) | 382 (75.3%) |
| Extrahepatic metastasis, n (%) | 83 (16.4%) |
| Prehepatectomy chemotherapy, n (%) | 455 (89.7%) |
| > 6 cycles, n (%) | 142 (28.0%) |
| Oxaliplatin-containing regimen | 374 (73.8%)† |
| Irinotecan-containing regimen | 107 (21.1%)‡ |
| With anti-VEGF agent, n (%) | 356 (70.2%)§ |
| With anti-EGFR agent, n (%) | 39 (7.7%)¶ |
| Liver metastases histopathologic factors | |
| Tumor number, median (IQR) | 2 (1–4) |
| Maximum diameter, median (IQR), cm | 2.5 (1.5–4.0) |
| R1 surgical margin, n (%) | 104 (20.5%) |
Abbreviations: IQR, interquartile range; ASA, American Society of Anesthesiologists; CEA, carcinoembryonic antigen; VEGF, vascular endothelial growth factor; EGFR, epidermal growth factor receptor.
Data not available for T category in 8 patients and lymph node metastasis in 14 patients.
Patients with RAS mutation vs. patients with RAS wild-type.191 (74.3%) vs. 183 (73.2%), P = 0.840.
Patients with RAS mutation vs. patients with RAS wild-type.49 (19.1%) vs. 58 (23.2%), P = 0.277.
Patients with RAS mutation vs. patients with RAS wild-type.183 (71.2%) vs. 173 (69.2%), P = 0.629.
Patients with RAS mutation vs. patients with RAS wild-type.4 (1.6%) vs. 35 (14.0%), P < 0.001.
Mutational analyses
Of the 50 cancer-related genes examined, 13 were mutated in more than 1% of patients (Supplementary Figure 2). Five genes had frequency of somatic mutation higher than 10%: TP53 (mutated in 359 patients; 70.8%), APC (271 patients; 53.5%), KRAS (237 patients; 46.7%), PIK3CA (80 patients; 15.8%), and SMAD4 (56 patients; 11.0%). NRAS was mutated in 22 patients (4.3%), and BRAF was mutated in 10 patients (2.0%). Two patients had concurrent mutations of KRAS and NRAS. RAS (KRAS and/or NRAS) mutation was found in 257 patients (50.7%). RAS mutation and PIK3CA mutation differed significantly by location of colorectal cancer: right colon vs. left colon vs. rectum; RAS mutation, 71.9% vs. 39.2% vs. 51.7%, P < 0.001; PIK3CA mutation, 14.8% vs. 24.0% vs. 10.7%, P = 0.013. TP53 mutation (P = 0.053), APC mutation (P = 0.479), and SMAD4 mutation (P = 0.612) were not significantly different by location of colorectal cancer.
Predictors of OS and RFS after CLM resection
Next, we sought to evaluate predictors of OS and RFS after resection of CLM. Specifically, we evaluated the 5 genes with mutational frequency over 10% (TP53, APC, KRAS, PIK3CA, and SMAD4) and known predictors, BRAF and NRAS, together with clinicopathologic factors. As explained above, KRAS and NRAS mutations were grouped together in a single category, RAS mutations. A multivariable Cox proportional hazards model analysis revealed that mutations of BRAF, RAS, TP53, and SMAD4 were independent predictors of OS (Table 2). Additionally, largest liver metastasis diameter and surgical margin status were associated with OS. With respect to risk factors for RFS, mutations of RAS, TP53, and SMAD4 were identified in addition to age, number of CLM, largest liver metastasis diameter, prehepatectomy chemotherapy > 6 cycles, extrahepatic disease, and surgical margin status (Table 2).
Table 2.
Multivariable Cox proportional hazards model analysis for OS and RFS in 490 patients*
| Factor | No. of patients | No. of events | Multivariable HR† | 95% CI | P value |
|---|---|---|---|---|---|
| OS | |||||
| Gene mutation | |||||
| BRAF | 10 | 6 | 2.95 | 1.27–6.86 | 0.012 |
| RAS | 249 | 87 | 2.14 | 1.53–2.99 | < 0.001 |
| TP53 | 347 | 123 | 2.21 | 1.49–3.28 | < 0.001 |
| SMAD4 | 55 | 25 | 1.82 | 1.17–2.83 | 0.008 |
| Clinicopathologic factors | |||||
| Largest liver metastasis diameter | – | – | 1.07 | 1.01–1.13 | 0.033 |
| Surgical margin, positive | 97 | 33 | 1.83 | 1.23–2.72 | 0.003 |
| RFS | |||||
| Gene mutation | |||||
| RAS | 249 | 206 | 1.47 | 1.20–1.82 | < 0.001 |
| TP53 | 347 | 283 | 1.40 | 1.11–1.78 | 0.005 |
| SMAD4 | 55 | 51 | 1.62 | 1.20–2.20 | 0.002 |
| Patients factors | |||||
| Age | 1.01 | 1.00–1.02 | 0.024 | ||
| Clinicopathologic factors | |||||
| Number of CLM | – | – | 1.04 | 1.01–1.07 | 0.006 |
| Largest liver metastasis diameter | – | – | 1.08 | 1.04–1.13 | < 0.001 |
| Prehepatectomy chemotherapy‡ | 134 | 117 | 1.45 | 1.16–1.82 | 0.001 |
| Extrahepatic disease | 79 | 74 | 1.70 | 1.31–2.21 | < 0.001 |
| Surgical margin, positive | 97 | 80 | 1.43 | 1.11–1.85 | 0.006 |
Abbreviations: HR, hazard ratio; CI, confidence interval, CLM, colorectal liver metastasis.
Of the 507 patients, 490 patients were analysed because data were unavailable for T category in 8 patients and lymph node metastasis in 14 patients.
The Cox proportional hazards model analysis initially included age (continuous variable), sex, primary tumor location, T category, primary lymph node metastasis, prehepatectomy carcinoembryonic antigen level (continuous variable), timing of metastasis (synchronous vs. metachronous), prehepatectomy chemotherapy, extrahepatic disease, number of CLM (continuous variable), largest liver metastasis diameter (continuous variable), surgical margin status (R1 vs. R0), BRAF mutation, RAS mutation, TP53 mutation, APC mutation, PIK3CA mutation, and SMAD4 mutation. A backward elimination with a threshold P value of 0.05 was used to select variables for the final models.
> 6 cycles vs. ≤ 6 cycles or no prehepatectomy chemotherapy.
Multivariable hazard ratio of OS and RFS by mutation status of RAS, TP53, and SMAD4
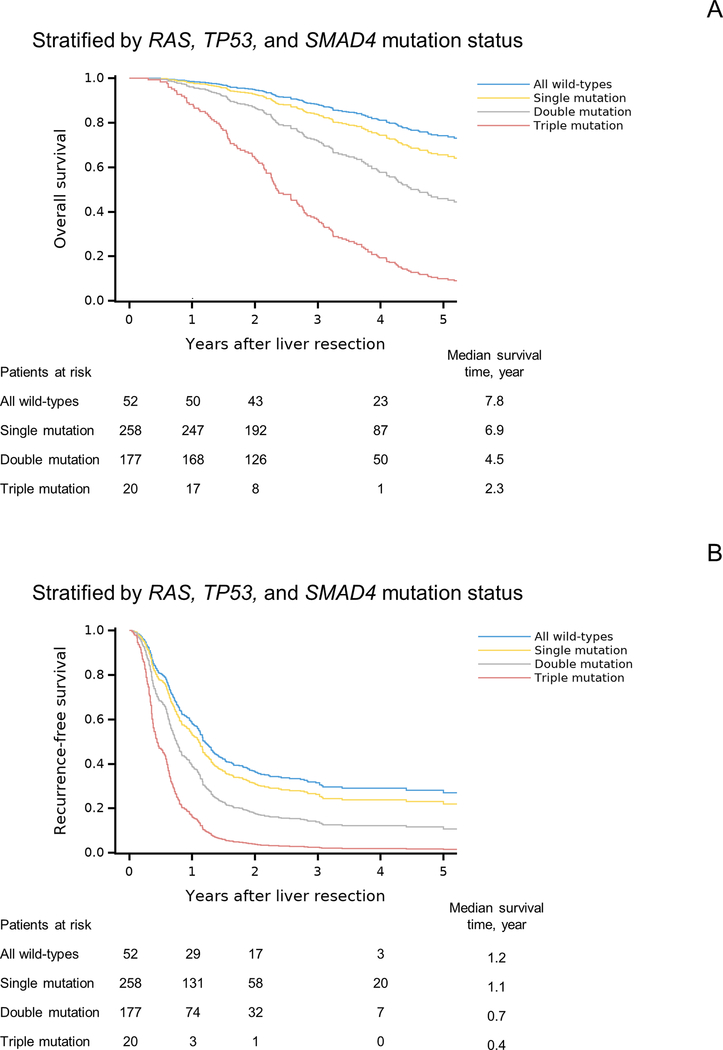
Because somatic mutations of RAS, TP53, and SMAD4 were independently associated with OS and RFS, we calculated multivariable hazard ratios for mutations in all 3, 2, or 1 of these genes (Table 3). Co-existence of mutations in all 3 genes (“triple mutation”) was significantly associated with worse OS and RFS than co-existence of mutations in any 2 of the genes (“double mutation”), mutation in only 1 of the genes (“single mutation”), and no mutation in any of the genes (“wild-type status”). Double mutation was also significantly associated with worse OS and RFS than single mutation and wild-type status. OS and RFS did not differ between patients with single mutation and those with wild-type status. Results of the internal validation revealed little overoptimism in the predictive discrimination of our Cox models for OS and RFS. The correction to the mean Harrell’s c-statistic that determined by cross validation was 0.02 (reducing the value from 0.672 to 0.652) in the Cox model for OS and 0.009 (reducing the value from 0.636 to 0.627) in the Cox model for RFS.
Table 3.
Multivariable hazard ratios (HRs) for OS and RFS by mutation status of KRAS, TP53, and SMAD4 in 507 patients
| KRAS, TP53, and SMAD4 mutation status | Reference | Multivariable HR | 95% CI | P value |
|---|---|---|---|---|
| OS* | ||||
| Triple mutation | vs. Double mutation | 3.21 | 1.72–5.99 | < 0.001 |
| vs. Single mutation | 6.04 | 3.21–11.3 | 0.001 | |
| vs. All wild-type | 8.61 | 3.80–19.5 | < 0.001 | |
| Double mutation | vs. Single mutation | 1.88 | 1.35–2.63 | < 0.001 |
| vs. All wild-type | 2.68 | 1.45–4.97 | 0.002 | |
| Single mutation | vs. All wild-type | 1.43 | 0.77–2.63 | 0.256 |
| RFS† | ||||
| Triple mutation | vs. Double mutation | 2.06 | 1.28–3.29 | 0.003 |
| vs. Single mutation | 3.17 | 1.97–5.07 | < 0.001 | |
| vs. All wild-type | 3.72 | 2.14–6.46 | < 0.001 | |
| Double mutation | vs. Single mutation | 1.54 | 1.24–1.91 | < 0.001 |
| vs. All wild-type | 1.81 | 1.25–2.61 | 0.002 | |
| Single mutation | vs. All wild-type | 1.18 | 0.82–1.68 | 0.378 |
Abbreviations: CI, confidence interval.
Multivariable HR was assessed after adjustment for the following risk factors: BRAF mutation status, largest liver metastasis diameter, and surgical margin status (R1 vs. R0).
Multivariable HR was assessed after adjustment for the following risk factors: age, number of CLM, largest liver metastasis diameter, prehepatectomy chemotherapy (> 6 cycles vs. ≤ 6 cycles or no prehepatectomy chemotherapy), extrahepatic disease, and surgical margin status (R1 vs. R0).
Although use of anti-EGFR agent in perioperative chemotherapy differed significantly among patients with triple mutation, double mutation, single mutation, and wild-type status (5.0% vs. 2.3% vs. 11.2% vs. 9.6%, P = 0.002), other regimens of perioperative chemotherapy were not significantly different: oxaliplatin-containing regimen, 70.0% vs. 75.1% vs. 72.1% vs. 78.9%, P = 0.703; irinotecan-containing regimen, 35.0% vs. 18.1% vs. 21.7% vs. 23.1%, P = 0.358; anti-VEGF agent, 80.0% vs. 71.8% vs. 66.3% vs. 80.8%, P = 0.108. Pathological response did not differ significantly among the groups: 60.0% vs. 45.2% vs. 49.6% vs. 50.0%, P = 0.780.
OS and RFS estimates by mutation status of RAS, TP53, and SMAD4
OS and RFS curves after adjustment for other prognostic factors are shown in Figure 1. Triple mutation was associated with shorter OS and RFS than double mutation, single mutation, and wild-type status. Also, double mutation was associated with shorter OS and RFS than single mutation and wild-type status. The rates of OS and RFS were not significantly different between patients with single mutation and those with wild-type status.
Figure 1. Overall survival (OS) and recurrence-free survival (RFS) by RAS, TP53, and SMAD4 mutation status.
(A) OS curves after adjustment for BRAF mutation status, largest liver metastasis diameter, and surgical margin status.
(B) RFS curves after adjustment for age, number of CLM, largest liver metastasis diameter, prehepatectomy chemotherapy (> 6 cycles vs. ≤ 6 cycles or no prehepatectomy chemotherapy), extrahepatic disease, and surgical margin status.
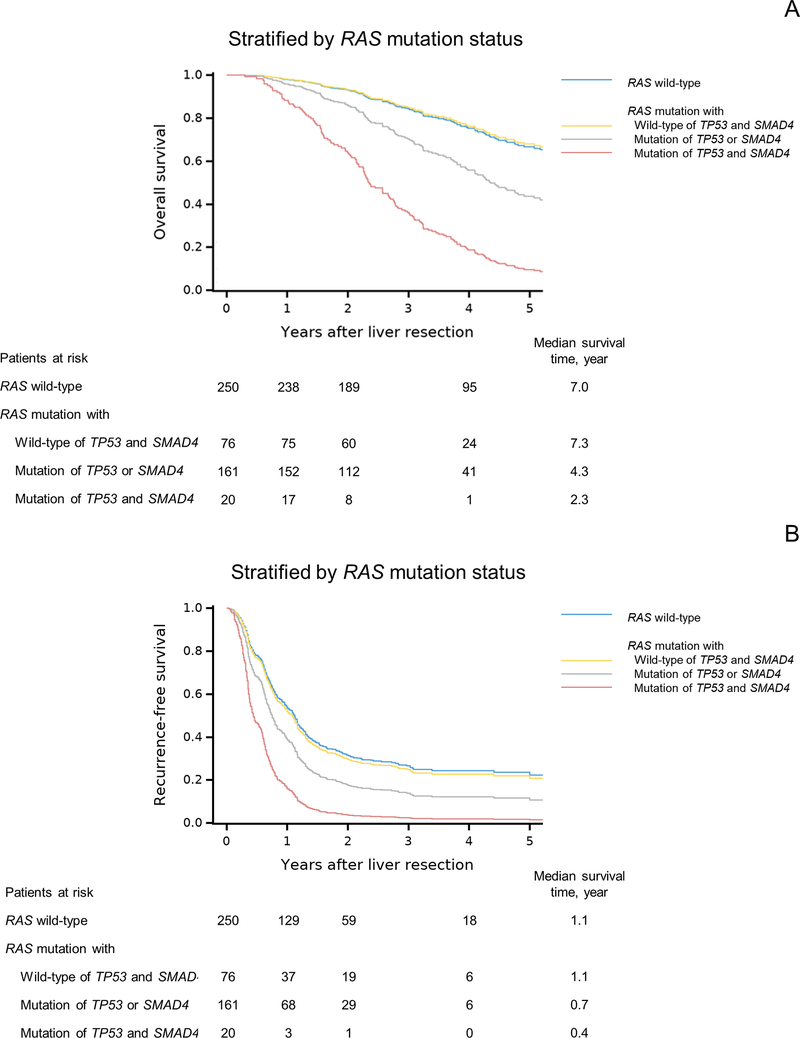
We then evaluated the impact of RAS mutation in combination with TP53 and/or SMAD4 mutations. Whereas OS and RFS were significantly worse in patients with RAS mutation than in patients with wild-type RAS (Supplementary Figure 3), OS and RFS did not differ significantly between patients with RAS mutation and wild-type TP53 and SMAD4 and patients with wild-type RAS (Figure 2 and Supplementary Table 2). Patients with RAS mutation with TP53 or SMAD4 mutation (double mutation) and patients with RAS mutation with mutation of TP53 and SMAD4 (triple mutation) had shorter OS and RFS than patients with wild-type RAS. Similar results of OS and RFS were found when stratified by TP53 mutation in combination with RAS and/or SMAD4 mutations. OS and RFS did not differ significantly between patients with TP53 mutation and wild-type RAS and SMAD4 (n=178) and patients with wild-type TP53 (n=148): hazard ratio (95% confidence interval); OS, 1.12 (0.73–1.72), P = 0.614; RFS, 0.97 (0.75–1.25), P = 0.793. We did not repeat this analysis by SMAD4 mutation because the number of patients with SMAD4 mutation and wild-type RAS and TP53 was small (n = 4).
Figure 2. Overall survival (OS) and recurrence-free survival (RFS) by RAS mutation status.
(A) OS curves after adjustment for BRAF mutation status, largest liver metastasis diameter, and surgical margin status.
(B) RFS curves after adjustment for age, number of CLM, largest liver metastasis diameter, prehepatectomy chemotherapy (> 6 cycles vs. ≤ 6 cycles or no prehepatectomy chemotherapy), extrahepatic disease, and surgical margin status.
Patient characteristics and survival in validation cohort
Supplementary Table 3 shows demographic and clinicopathologic characteristics of a validation cohort including 313 patients with unresectable colorectal metastases. Median (IQR) age was 52 (43–61) years. Site of first metastasis was liver for 97 patients (31.0%), lung for 32 patients (10.2%), peritoneum for 44 patients (14.1%), and multiple organ metastases for 124 patients (39.6%). The median duration of follow-up was 2.3 years (IQR, 1.5–3.5 years). During the follow-up period, 272 (86.9%) patients died. Supplementary Figure 4 shows OS curves after adjustment for site of first metastasis. Double mutation was significantly associated with shorter OS than single mutation. OS did not differ between patients with wild-type status and those with single mutation and between patients with triple mutation and those with double mutation.
Discussion
We demonstrated in this study that multiple somatic mutations in RAS, TP53, and SMAD4 were associated with worse prognosis than mutation in only 1 or none of these genes after resection of CLM. This finding was confirmed in a separate validation cohort of patients with unresectable colorectal metastases. We also found that RAS mutation in patients with wild-type TP53 and SMAD4 was not associated with worse prognosis than wild-type RAS. Our findings indicate that although mutant RAS is a prognostic factor for survival and recurrence, RAS mutation status alone is not sufficient for predicting prognosis in patients undergoing resection of CLM.
In this era of increasingly personalized therapeutic strategies for patients with CLM, ascertainment of individual tumor biology is paramount for accurate prediction of prognosis. For example, it has been established that RAS mutation status stratifies patients with respect to outcomes after resection of CLM following various systemic and locoregional therapies [35, 36] in addition to implying lack of response to anti-EGFR therapy [37]. We previously reported the importance of RAS mutation status in combination with TP53 mutations classified as high or low risk according to the evolutionary action score [18]. In the work reported here, we built upon our prior work with analysis of 5 frequently mutated genes and BRAF and found that TP53 mutation status and SMAD4 mutations status were independent prognostic factors, together with RAS and BRAF mutation status.
The main strength of the current study is the comprehensive assessment of the 5 most frequently mutated genes in CLM, along with BRAF and clinicopathologic factors, to predict survival and recurrence in patients with CLM who underwent resection. In contrast, prior publications have focused on the prognostic value of single gene mutations or mutations in only 2 genes of interest rather than more broadly evaluating molecular markers. The findings of our study revealed that mutations of RAS, TP53, and SMAD4 were independently associated with prognosis after adjustment for other prognostic factors.
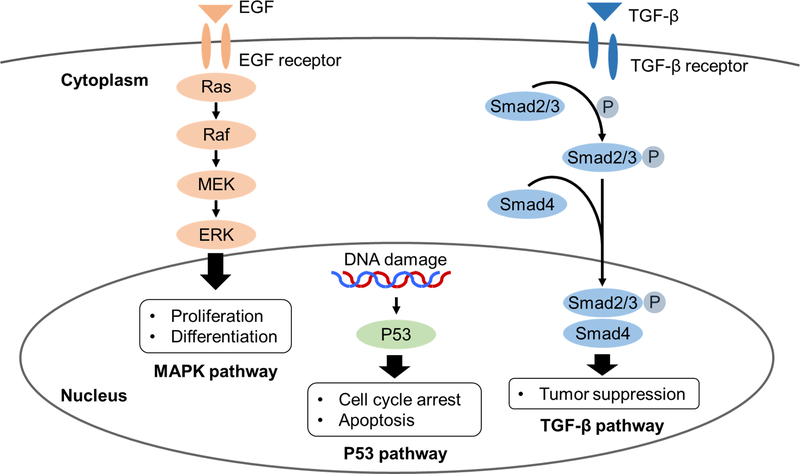
Interestingly, these genes are member of independent signaling pathways (Figure 3). The RAS oncogene is a key member of the MAPK (mitogen-activated protein kinase) pathway and contributes to the deregulation of tumor-cell growth, programmed cell death and invasiveness, and ability to induce new blood-vessel formation [38]. TP53 is a tumor suppressor gene in the P53 pathway and prevents cells from progressing through the cell cycle under conditions that could generate or perpetuate DNA damage [39]. SMAD4 is also a tumor suppressor gene in the transforming growth factor-β pathway and regulates cell proliferation, differentiation, morphogenesis, and apoptosis [40]. Our data can not explain the interaction of pathway alterations although studies reported crosstalk between the signaling pathways [41–43]. It may be that mutation of any 1 of these genes exerts a deleterious effect on survival through the corresponding signaling pathway and that the hazard for survival increases from single to double mutation and from double to triple mutation in a stepwise fashion. Our findings that patients with triple mutation of RAS, TP53, and SMAD4 had the worst survival following resection suggest that these patients may deserve careful consideration for resection and may be considered for alternative strategies for systemic and local therapy. Additionally, our findings may help clinicians tailor the surveillance intensity after CLM resection and change decisions regarding repeat resection vs. medical therapy after recurrence. Lastly, the data presented here represents a clinical demonstration of the complex interplay of independent pathways within tumors leading to worse outcomes for our patients.
Figure 3. Overview of three signaling pathways involved in colorectal cancer.
Abbreviations: EGF, epidermal growth factor; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; TGF-β, transforming growth factor-β; P, phosphorylation.
BRAF mutation is a well-known predictor of prognosis after CLM resection [10–14]. However, RAS and BRAF mutations are almost always mutually exclusive [44], the number of patients with BRAF mutation in our study was small, and BRAF mutation was not identified as a risk factor for RFS in a Cox proportional hazards model. Thus, we focused on RAS, TP53, and SMAD4 mutations, which were independently associated with prognosis, and assessed multivariable hazard ratios for OS and curves of OS after adjusting for BRAF mutation status together with other risk factors.
Another interesting finding of our study was that a subset of patients with RAS mutation has the same long-term outcomes as patients with RAS wild-type. Similar to previous studies [6–8, 11, 13], our study showed that RAS mutation was associated with worse survival than wild-type RAS. However, OS and RFS in patients with RAS mutation and wild-type TP53 and SMAD4 were not significantly different from OS and RFS in patients with RAS wild-type. This finding likely explains the fact that some studies demonstrated worse OS and RFS in patients with RAS mutation than in patients with wild-type RAS [6–8, 11], whereas others failed to confirm a significant difference between these patient groups in OS and RFS [10, 13, 15] or in RFS [45, 46] using a multivariable Cox model. The survival analysis of these studies may have been influenced by other gene mutations hiding behind RAS mutation. It should be noted that this finding needs to be understood in the context of type II error as more than 18,000 events would be required to detect a true difference which may exist according to the sample size analysis. Nonetheless, our findings from the current study, taken together with the findings from previous studies of the prognostic impact of RAS mutation, suggest that testing of a multigene mutation profile, rather than simply testing RAS and BRAF, is needed to precisely predict prognosis on the basis of molecular markers.
This study is limited by its retrospective design and the fact that it was conducted at a single, albeit a high-volume, institution. Because comprehensive multigene testing was rarely performed prior to 2012, the majority of the patients studied underwent liver resection after 2012 and had relatively short follow-up. Mutational analysis was performed on either primary tumor tissue or CLM depending on availability. We do not believe this is likely to have impacted the results as many studies suggest a high rate of concordance for RAS, TP53, APC, PIK3CA, and SMAD4 mutations between primary tumors and metastases [20, 46–49]. We did not assess gene mutations occurring in fewer than 10% of patients because the small numbers may weaken statistical power. However, the genes that we studied—KRAS and NRAS (analyzed together as RAS), TP53, APC, PIK3CA, and SMAD4—are active in 5 different major signaling pathways, and the result is a more representative prognostic sample of cellular drivers associated with oncogenesis and/or tumor suppression. The prognostic effect of RAS on OS may be influenced by the use of anti-EGFR therapy. However, the prognostic effect of RAS on RFS is probably not biased by the use of anti-EGFR therapy because anti-EGFR therapy is not used in the adjuvant setting [50].
In conclusion, double or triple gene mutations in RAS, TP53, and SMAD4 are associated with worse survival and recurrence than mutation in only 1 or none of these genes after resection of CLM. Thus, RAS mutation status alone is not sufficient for predicting survival and recurrence after CLM resection. These findings may be useful for clinical decision making in patients with tumor characteristics associated with poor prognosis and for risk stratification of patients in future clinical studies.
Supplementary Material
Translational Relevance.
Somatic gene mutations have been increasingly recognized to impact prognosis following resection of colorectal liver metastases (CLM). We studied the association of 5 frequently mutated genes (TP53, APC, RAS, PIK3CA, and SMAD4) and BRAF with survival in patients who underwent CLM resection. We found that multiple somatic mutations in RAS, TP53, and SMAD4 were associated with worse prognosis than mutation in only 1 or none of these genes. Additionally, RAS mutation in patients with wild-type TP53 and SMAD4 was not associated with worse prognosis than wild-type RAS. Thus, RAS mutation status alone is not sufficient for predicting prognosis in patients undergoing CLM resection. Our findings may be useful for clinical decision making in patients with tumor characteristics associated with poor prognosis and for risk stratification of patients in future clinical studies.
Acknowledgments
The authors thank Dr. Elena Panettieri for reviewing the data used in the study, Ms. Ruth Haynes for administrative support in the preparation of this manuscript and Ms. Stephanie Deming, an employee of the Department of Scientific Publications at MD Anderson Cancer Center, for copyediting the manuscript.
Grant Support: This research was supported in part by the National Institutes of Health through MD Anderson Cancer Center Support Grant, CA016672.
Abbreviations
- CLM
colorectal liver metastases
- OS
overall survival
- RFS
recurrence-free survival
Footnotes
Disclosures: Nothing to disclose.
Previous communication to a society or meeting: None.
References
- 1.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P , et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996;77:1254–1262. [PubMed] [Google Scholar]
- 2.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg 2004;240:1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruenberger B, Scheithauer W, Punzengruber R, Zielinski C, Tamandl D, Gruenberger T. Importance of response to neoadjuvant chemotherapy in potentially curable colorectal cancer liver metastases. BMC Cancer 2008;8:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun YS, Vauthey JN, Boonsirikamchai P, Maru DM, Kopetz S, Palavecino M, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 2009;302:2338–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nash GM, Gimbel M, Shia J, Nathanson DR, Ndubuisi MI, Zeng ZS, et al. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol 2010;17:572–578. [DOI] [PubMed] [Google Scholar]
- 7.Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg 2013;258:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karagkounis G, Torbenson MS, Daniel HD, Azad NS, Diaz LA Jr., Donehower RC, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer 2013;119:4137–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vauthey JN, Kopetz SE. From multidisciplinary to personalized treatment of colorectal liver metastases: 4 reasons to consider RAS. Cancer 2013;119:4083–4085. [DOI] [PubMed] [Google Scholar]
- 10.Teng HW, Huang YC, Lin JK, Chen WS, Lin TC, Jiang JK, et al. BRAF mutation is a prognostic biomarker for colorectal liver metastasectomy. J Surg Oncol 2012;106:123–129. [DOI] [PubMed] [Google Scholar]
- 11.Umeda Y, Nagasaka T, Mori Y, Sadamori H, Sun DS, Shinoura S, et al. Poor prognosis of KRAS or BRAF mutant colorectal liver metastasis without microsatellite instability. J Hepatobiliary Pancreat Sci 2013;20:223–233. [DOI] [PubMed] [Google Scholar]
- 12.Yaeger R, Cercek A, Chou JF, Sylvester BE, Kemeny NE, Hechtman JF, et al. BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer 2014;120:2316–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schirripa M, Bergamo F, Cremolini C, Casagrande M, Lonardi S, Aprile G, et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br J Cancer 2015;112:1921–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg 2015;102:1175–1183. [DOI] [PubMed] [Google Scholar]
- 15.Isella C, Mellano A, Galimi F, Petti C, Capussotti L, De Simone M, et al. MACC1 mRNA levels predict cancer recurrence after resection of colorectal cancer liver metastases. Ann Surg 2013;257:1089–1095. [DOI] [PubMed] [Google Scholar]
- 16.Loes IM, Immervoll H, Sorbye H, Angelsen JH, Horn A, Knappskog S, et al. Impact of KRAS, BRAF, PIK3CA, TP53 status and intraindividual mutation heterogeneity on outcome after liver resection for colorectal cancer metastases. Int J Cancer 2016;139:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankel TL, Vakiani E, Nathan H, DeMatteo RP, Kingham TP, Allen PJ, et al. Mutation location on the RAS oncogene affects pathologic features and survival after resection of colorectal liver metastases. Cancer 2017;123:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chun YS, Passot G, Yamashita S, Nusrat M, Katsonis P, Loree JM, et al. Deleterious Effect of RAS and evolutionary high-risk TP53 double mutation in colorectal liver metastases. Ann Surg 2017;269:917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cercek A, Braghiroli MI, Chou JF, Hechtman JF, Kemeny N, Saltz L, et al. Clinical Features and Outcomes of Patients with Colorectal Cancers Harboring NRAS Mutations. Clin Cancer Res 2017;23:4753–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuno T, Cloyd JM, Vicente D, Omichi K, Chun YS, Kopetz SE, et al. SMAD4 gene mutation predicts poor prognosis in patients undergoing resection for colorectal liver metastases. Eur J Surg Oncol 2018; [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi Y, Lillemoe HA, Panettieri E, Chun YS, Tzeng CD, Aloia TA, et al. Conditional recurrence-free survival after resection of colorectal liver metastases: Persistent deleterious association with RAS and TP53 co-mutation. J Am Coll Surg 2019; 10.1016/j.jamcollsurg.2019.04.027: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540–548. [DOI] [PubMed] [Google Scholar]
- 23.Chan AO, Jim MH, Lam KF, Morris JS, Siu DC, Tong T, et al. Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. JAMA 2007;298:1412–1419. [DOI] [PubMed] [Google Scholar]
- 24.Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 2011;29:1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh RR, Patel KP, Routbort MJ, Reddy NG, Barkoh BA, Handal B, et al. Clinical validation of a next-generation sequencing screen for mutational hotspots in 46 cancer-related genes. J Mol Diagn 2013;15:607–622. [DOI] [PubMed] [Google Scholar]
- 26.Blazer DG 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 2008;26:5344–5351. [DOI] [PubMed] [Google Scholar]
- 27.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017;67:93–99. [DOI] [PubMed] [Google Scholar]
- 28.Summers MG, Smith CG, Maughan TS, Kaplan R, Escott-Price V, Cheadle JP. BRAF and NRAS Locus-Specific variants have different outcomes on survival to colorectal cancer. Clin Cancer Res 2017;23:2742–2749. [DOI] [PubMed] [Google Scholar]
- 29.Schirripa M, Cremolini C, Loupakis F, Morvillo M, Bergamo F, Zoratto F, et al. Role of NRAS mutations as prognostic and predictive markers in metastatic colorectal cancer. Int J Cancer 2015;136:83–90. [DOI] [PubMed] [Google Scholar]
- 30.Lakatos E Sample Sizes Based on the Log-Rank Statistic in Complex Clinical-Trials. Biometrics 1988;44:229–241. [PubMed] [Google Scholar]
- 31.Steyerberg EW, Eijkemans MJ, Harrell FE Jr., Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med 2000;19:1059–1079. [DOI] [PubMed] [Google Scholar]
- 32.Harrell FE Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 33.Makuch RW. Adjusted survival curve estimation using covariates. J Chronic Dis 1982;35:437–443. [DOI] [PubMed] [Google Scholar]
- 34.Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, et al. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA 2001;286:1494–1497. [DOI] [PubMed] [Google Scholar]
- 35.Odisio BC, Yamashita S, Huang SY, Harmoush S, Kopetz SE, Ahrar K, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg 2017;104:760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brudvik KW, Jones RP, Giuliante F, Shindoh J, Passot G, Chung MH, et al. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann Surg 2017; [DOI] [PubMed] [Google Scholar]
- 37.Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006;66:3992–3995. [DOI] [PubMed] [Google Scholar]
- 38.Downward J Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 2003;3:11–22. [DOI] [PubMed] [Google Scholar]
- 39.Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer 2006;6:909–923. [DOI] [PubMed] [Google Scholar]
- 40.Massague J TGFbeta signalling in context. Nat Rev Mol Cell Biol 2012;13:616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grusch M, Petz M, Metzner T, Ozturk D, Schneller D, Mikulits W. The crosstalk of RAS with the TGF-beta family during carcinoma progression and its implications for targeted cancer therapy. Curr Cancer Drug Targets 2010;10:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elston R, Inman GJ. Crosstalk between p53 and TGF-beta Signalling. J Signal Transduct 2012;2012:294097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buganim Y, Solomon H, Rais Y, Kistner D, Nachmany I, Brait M, et al. p53 Regulates the Ras circuit to inhibit the expression of a cancer-related gene signature by various molecular pathways. Cancer Res 2010;70:2274–2284. [DOI] [PubMed] [Google Scholar]
- 44.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 2002;418:934. [DOI] [PubMed] [Google Scholar]
- 45.Margonis GA, Spolverato G, Kim Y, Karagkounis G, Choti MA, Pawlik TM. Effect of KRAS mutation on long-term outcomes of patients undergoing hepatic resection for colorectal liver metastases. Ann Surg Oncol 2015;22:4158–4165. [DOI] [PubMed] [Google Scholar]
- 46.Kemeny NE, Chou JF, Capanu M, Gewirtz AN, Cercek A, Kingham TP, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer 2014;120:3965–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knijn N, Mekenkamp LJ, Klomp M, Vink-Borger ME, Tol J, Teerenstra S, et al. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer 2011;104:1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baas JM, Krens LL, Guchelaar HJ, Morreau H, Gelderblom H. Concordance of predictive markers for EGFR inhibitors in primary tumors and metastases in colorectal cancer: a review. Oncologist 2011;16:1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vakiani E, Janakiraman M, Shen R, Sinha R, Zeng Z, Shia J, et al. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J Clin Oncol 2012;30:2956–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Primrose J, Falk S, Finch-Jones M, Valle J, O’Reilly D, Siriwardena A, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol 2014;15:601–611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.