SUMMARY
Vinculin and its splice isoform metavinculin play key roles in regulating cellular morphology, motility and force transduction. Vinculin is distinct from metavinculin in its ability to bundle filamentous actin (F-actin). To elucidate the molecular basis for these differences, we employed computational and experimental approaches. Results from these analyses indicate that the C-terminus of both vinculin and metavinculin form stable interactions with the F-actin surface. However, the metavinculin tail (MVt) domain contains a 68 amino acid insert, with helix 1 (H1) sequestered into a globular subdomain, which protrudes from the F-actin surface and prevents actin bundling by sterically occluding actin filaments. Consistent with our model, deletion and selective point mutations within the MVt H1 disrupt this protruding structure, and facilitate actin bundling similar to vinculin tail (Vt) domain.
Graphical Abstract

eTOC Blurb
Vinculin and its splice isoform metavinculin differ in their ability to organize F-actin into higher order structures. Krokhotin et al. demonstrate that partial deletions or selective point mutations within the metavinculin tail domain can convert its F-actin reorganization properties to that of vinculin. The authors propose a model to describe this phenomenon.
INTRODUCTION
Vinculin is a cytoskeletal protein found in cell-matrix and cell-cell junctions, where it is involved in anchoring cell surface receptors (e.g. integrins, cadherins) to the actin cytoskeleton (Bays and DeMali, 2017; Parsons et al., 2010; Ziegler et al., 2006). Without vinculin, cells exhibit rounded morphology (Coll et al., 1995), increased motility and resistance to apoptosis and anoikis (Subauste et al., 2004). Additionally, homozygous mice (VCL−/−) experience failure in cardiac and nervous system development, which causes embryonic death at E10 (Xu et al., 1998). Partial deletion of vinculin (VCL+/−) leads to dilated cardiomyopathy (CM) (Zemljic-Harpf et al., 2004).
Metavinculin is a larger splice isoform of vinculin bearing an additional 68 amino acid insert in its tail domain (Koteliansky et al., 1992). While vinculin is ubiquitously expressed, metavinculin is localized to cardiac, skeletal and smooth muscle (Belkin et al., 1988a; Feramisco et al., 1982; Gimona et al., 1987) and expressed at sub-stoichiometric levels relative to vinculin. Notably, metavinculin expression is tightly regulated by external signals such as mechanical load (Belkin et al., 1988b; Witt et al., 2004; Zemljic-Harpf et al., 2004). Deficiency or loss of metavinculin expression is linked to dilated cardiomyopathy and disorganized intercalated discs (Masato et al., 1997), and point mutations in the metavinculin insert are associated with dilated and hypertrophic cardiomyopathies (Olson et al., 2002; Vasile et al., 2006).
One distinct difference between the metavinculin and vinculin lies in the ability to reorganize F-actin filaments. Whereas, F-actin forms thick bundles in presence of vinculin tail (Vt) domain (Tolbert et al., 2013), the tail domain of metavinculin (MVt) does not induce actin bundling (Olson et al., 2002). Moreover, sub-stoichiometric amounts of MVt can inhibit Vt mediated F-actin bundling (Kim et al., 2016; Oztug Durer et al., 2015). Additionally, MVt point mutants, associated with cardiomyopathies, induce mesh-like organization of F-actin, and formation of large assemblies embedded with linear bundles when mixed with Vt (Olson et al., 2002; Sarker et al., 2019). However, it is unclear what the molecular basis is for differences in the ability of Vt and MVt to promote F-actin bundling and other types of F-actin assemblies.
The tail domain of Vt binds F-actin, and consists of an N-terminal strap (NtS) followed by a five-helix bundle (H1-H5) and a C-terminal hairpin (Figure 1A) (Bakolitsa et al., 1999, 2004). The 68 amino acid insert found in MVt is located in between helices 1 (H1) and 2 (H2) within the helix bundle. Part of this insert displaces the NtS and H1 to form a structurally equivalent new NtS’ and H1’ within MVt. The original NtS and H1 are likely disordered or dynamic as NtS, H1 and the rest of the insert are undetectable by X-ray crystallography (Rangarajan et al., 2010).
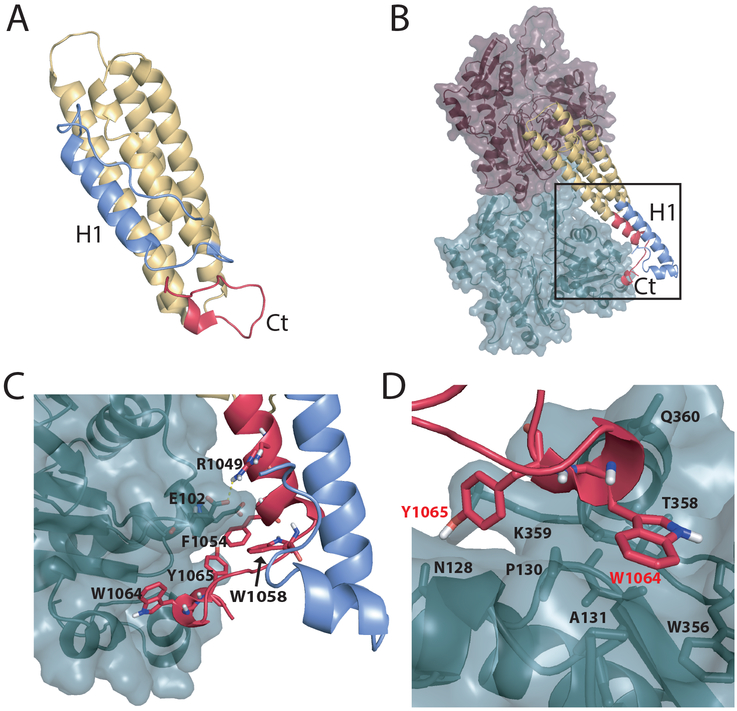
Figure 1:
DMD simulations reveal conformational rearrangement in Vt upon F-actin binding. (A) Vt in isolation (PDB ID 1TR2). N-terminal H1 helix binds to the H2-H5 four-helix bundle within the hydrophobic groove between helices 2 and 5. The C-terminus forms a tightly folded hairpin. (B) Vt bound to F-actin. The binding of Vt to F-actin promotes unfolding of the H1 helix and C-terminus. The Vt C-terminus binds F-actin, while the H1 helix forms an extension of the H2 helix. (C) Zoomed in view of the region highlighted with a black box in (B). (D) Binding interface between Vt Y1065, W1064 and the F-actin surface. The H1 helix and loop between helices 1 and 2 are shown in blue. The C-terminus (Ct) is shown in red.
Upon binding F-actin, Vt and MVt undergo structural rearrangement. H1 (H1’) appears to partition away from Vt (MVt) helix bundle, as it is undetectable by cryo-electron microscopy (EM) (Kim et al., 2016) and shows increased proteolytic cleavage upon actin engagement (Oztug Durer et al., 2015). Additionally, the C-terminal hairpin unfolds, as supported by the missing electron density in cryo-EM structures of Vt (MVt) bound to actin (Kim et al., 2016). This structural rearrangement exposes a cryptic binding surface of Vt enabling Vt homodimerization, which mediates F-actin bundling. H1 likely becomes part of the cryptic interface, as a Vt mutation (M898A) within H1 exhibits binding to F-actin comparable to the wild type but lacks ability to induce bundling (Kim et al., 2016). Given the presence of the insert within MVt, we have recently proposed that MVt undergoes structural rearrangement upon F-actin engagement that promotes formation of an additional protruding subdomain which sterically occludes formation of parallel F-actin bundles (Sarker et al., 2019).
As binding of actin to Vt induces formation of heterogenous F-actin bundles, structural elucidation of actin-induced Vt homodimers or potentially existing Vt-MVt heterodimers is intractable to X-ray or Cryo-EM techniques. The only existing Cryo-EM structure of Vt (MVt) in complex with F-actin lacks five residues from the C-terminus (C5) (Kim et al., 2016). While the C5 C-terminal hairpin tail is unstructured in isolated Vt, it is important for Vt homodimerization (Shen et al., 2011). However, the role of the C-terminal hairpin in promoting actin-induced Vt dimerization is unknown. Here we used computational modeling to investigate the role for the C-terminal hairpin in actin induced Vt-dimerization and in MVt inhibition of Vt-mediated actin bundling. We find that the Vt C-terminal hairpin directly binds F-actin and may play a key role in creating an interface for Vt homodimerization. We also reveal that the MVt C-terminus is not part of the protruding subdomain, which forms upon engagement of MVt to F-actin.
Additionally, consistent with previous observations (Rangarajan et al., 2010), we find that truncation of the H1 sequence in MVt completely abrogates its ability to inhibit F-actin bundling, instead the truncated MVt effectively bundles F-actin and, thus, exhibits Vt-like behavior. This behavior is consistent with a formation of a bulky subdomain which is predicted to comprise H1 (Sarker et al., 2019). We also computationally predict point mutations within H1, which significantly reduce the ability of MVt to inhibit Vt-mediated bundling. Our findings are summarized with a structural model, which explains why some MVt mutants (e.g. cardiomyopathy mutants A934V, ΔL954 and R975W) promote formation of large disordered F-actin assemblies while other mutants (e.g. H1 deletion) facilitate F-actin bundling.
RESULTS
Vt conformation represents an ensemble of states with 5 and 4 helix bundles.
In the cryo-EM structure of Vt and MVt in complex with F-actin, the N-terminal strap, H1 helix and C-terminus are not observable (Kim et al., 2016). Moreover, the H1 helix is more susceptible to proteolytic cleavage when Vt is bound to F-actin (Oztug Durer et al., 2015). These observations suggest that the strap, H1 helix and C-terminus becomes unstructured/dynamic upon engagement with F-actin (Kim et al., 2016). To explore details of this conformational transition, we employed discrete molecular dynamics (DMD) simulations (Chen et al., 2008; Ding and Dokholyan, 2006; Dokholyan et al., 1998; Proctor et al., 2011; Shirvanyants et al., 2012). To gain insight into dynamics of an isolated Vt domain, we performed a set of replica-exchange DMD simulations (STAR Methods) using an existing PDB structure (ID 1TR2, chain A, residues 881–1061) as a starting point. The five C-terminal residues (1062–1066) missing in the crystal structure were added using the Pymol built-in tool. The melting curve of isolated Vt domain obtained from replica-exchange DMD simulations (STAR Methods) has a minor secondary peak, which reveals the existence of a stable structural state with an unfolded H1 and C-terminus (Figure S1, and Movie S1). This observation suggests that Vt may exist in a conformational ensemble, in which the five-helix bundle is the dominant state at lower temperatures, whereas the four-helix bundle becomes a prevalent state at higher temperatures. Thus, isolated Vt appears primed for conformational transition such that binding of F-actin shifts the equilibrium between the two states in favor of the four-helix bundle. We also performed DMD replica exchange simulations of Vt bound to F-actin (STAR Methods). As an initial model we used cryo-EM reconstruction (PDB ID 3JBI) of the Vt domain bound to an actin homodimer (the model of F-actin). The missing residues were added to Vt using the Pymol built-in tool. The Vt structure used for simulations of Vt bound to F-actin is the same length (residues 881–1066) as in the simulations performed on the isolated Vt domain (STAR Methods). To preserve the relative orientation of individual actin monomers inside the homodimer, actin atoms were immobilized during the simulation. Our simulations show Vt in two distinct conformations, the five-helix bundle conformation (Figure S2) with similar domain structure to that determined by X-ray crystallography (Bakolitsa et al., 1999), and a conformation where the Vt C-terminus is tightly bound to actin surface while H1 forms an extension of H2 helix (Figure 1B). To compare significantly populated states between an isolated Vt domain and Vt bound to F-actin, we selected 1000 lowest energy structures and clustered these structures (STAR Methods). We find that in the isolated Vt domain, the native 5-helix bundle conformation is present in 85% of the structures, whereas for Vt bound to F-actin, the situation is almost reversed and native-like states accounts only for ~19% of all lowest energy structures.
The Vt C-terminus binds F-actin.
The existing structure of Vt in complex with F-actin obtained by cryo-EM reconstruction, lacks five C-terminal residues (ΔC5) (Kim et al., 2016). This construct was engineered to reduce sample heterogeneity required for structure determination, as the C-terminal truncation impairs Vt dimerization and F-actin bundling (Shen et al., 2011). However, as a structure of the Vt dimer is lacking, it is unclear how the Vt C-terminus promotes conformational changes needed for Vt-mediated actin bundling. Our DMD simulations suggest that the Vt C-terminus binds F-actin via formation of specific interactions between Vt residues W1064 and Y1065 and the actin surface (Figure 1 B,C). In particular, W1064 interacts with W356, A131, P102, P130, T358, and Y1065 interacts with N128 and K359 of actin (Figure 1 D). Moreover, part of Vt C-terminus accommodates a helical conformation and forms an extension of H5. In addition, Vt R1049 forms a salt bridge with actin E102 and contributes to the stability of the binding interface between Vt and F-actin. It was previously shown that the mutation R1049E impairs both F-actin binding and bundling (Jannie et al., 2015). Based on this observation, it was argued that the proposed model of Vt and F-actin binding was incorrect since in that model, obtained by docking F-actin and isolated Vt, R1049 was distal to F-actin surface (Jannie et al., 2015). This contradiction was resolved with the determination of a cryo-EM structure of the Vt-actin complex, which revealed that both the Vt N- and C-termini undergo large conformational changes upon binding F-actin. Although R1049 was not resolved in the cryo-EM reconstruction, it was hypothesized that R1049 interacts with negatively charged residues at the actin surface (Kim et al., 2016). Our DMD simulations are consistent with this hypothesis. We argue that this residue is not resolved in the cryo-EM reconstruction, due to perturbation of key interactions between the Vt C-terminus and F-actin upon ΔC5 deletion. We also observe that the H1 helix undocks from its binding groove within the five-helix bundle and forms an extension of H2, with the Vt C-terminus bound to F-actin providing a resting scaffold for H1.
We further investigated the stability of the interaction between Vt C-terminus and actin by performing DMD simulations at constant temperature for various Vt C-terminal truncations. We observe that fluctuation of C-terminus drastically increases after truncation of 2 residues from the C-terminus (ΔC2) (Figure S3). These observations are consistent with the substantial drop in ability of Vt to bundle F-actin upon ΔC2 truncation (Shen et al., 2011). Fluctuations of C-terminus in ΔC1 mutant (one residue removed from the C-terminus) are comparable to WT Vt, which correlates with ability of the ΔC1 mutant to bundle F-actin comparable to WT Vt (Shen et al., 2011).
We also observed that a Vt Y1065F mutant shows increased Vt C-terminal/actin interaction stability, as ΔΔGY1065F calculated using Eris software (Yin et al., 2007a, 2007b) is equal to −1.51 kcal/mol, consistent with our previous findings that Vt Y1065F greatly enhances F-actin bundling efficiency relative to wild type Vt (Tolbert et al., 2014).
The MVt C-terminus is not essential for inhibition of Vt-mediated actin bundling.
We and others have shown that addition of MVt to Vt inhibits Vt-mediated F-actin bundling (Kim et al., 2016; Oztug Durer et al., 2015), and have recently proposed a mechanism of inhibition (Sarker et al., 2019). We postulated and provided evidence for a model in which F-actin binding to MVt promotes formation of an additional globular subdomain by H1 helix and the insert. This subdomain protrudes from F-actin surface and prevents Vt that bound to different F-actin filaments to come within interaction distance.
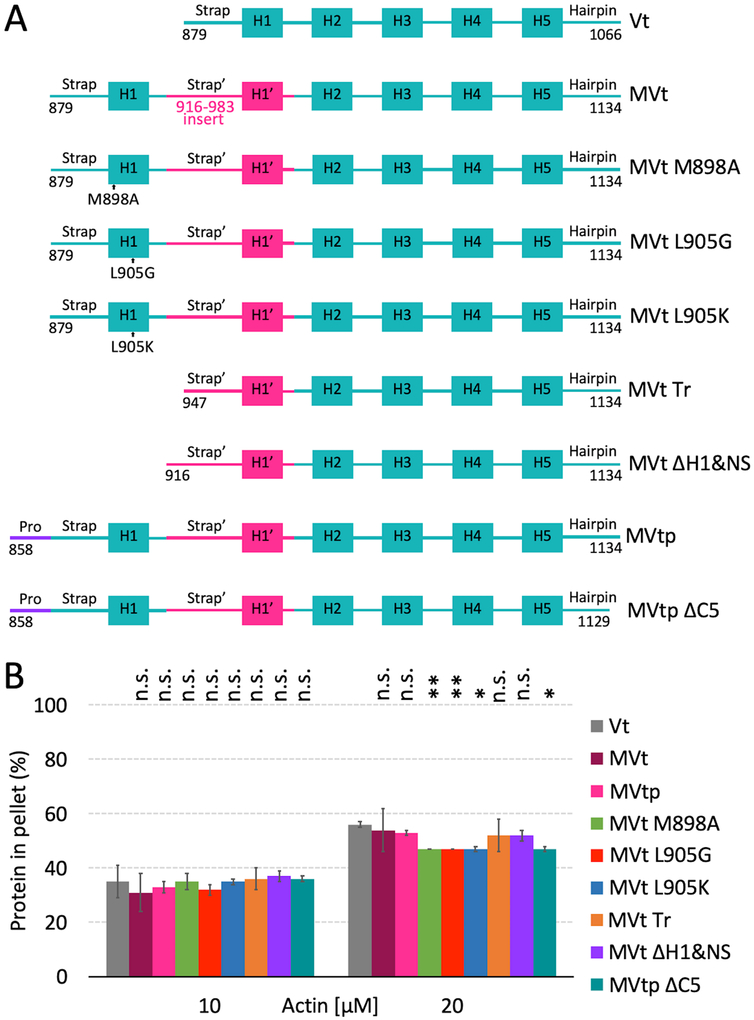
Based on molecular dynamics simulations, we previously proposed two alternative structural models for the protruding MVt subdomain formed upon F-actin binding (Sarker et al., 2019). In the first model, the protruding subdomain is solely formed by the MVt N-terminus, with the MVt C-terminus engaging F-actin (Figure 2A,B and Data S1). Intriguingly, the conformation of MVt C-terminus in this model mirrors the conformation of the Vt C-terminus (Figure S4). In the second model, the protruding subdomain consists of intertwined MVt N- and C-termini (Figure 2C,D and Data S2). To test these models experimentally, we employed actin binding and bundling co-sedimentation experiments to assess how deletion of C-terminal hairpin affects the ability of MVt to bind and inhibit F-actin bundling. For that purpose, we have utilized a construct we denote as MVtp WT, and its C-terminal truncated variant MVtp ΔC5 (Figure 3A). These constructs include the additional 21-residue proline-rich linker preceding the NtS of MVt. We have previously shown that MVt variants with and without proline-rich linker are functionally equivalent (Sarker et al., 2019). We find that MVtp ΔC5 binding to F-actin is unaltered compared to Vt and WT MVtp (Figure 3B) by actin co-sedimentation. In addition, neither MVtp nor the MVtp ΔC5 hairpin deletion are able to induce F-actin assemblies (Figure 4), which suggests that the C-terminus is not engaged in formation of the protruding subdomain, consistent with the first model.
Figure 2:
Two structural models of MVt bound to F-actin. (A) In the first model of MVt, the H1 helix and MVt insert form a protruding structure and the C-terminal hairpin binds F-actin. The C-terminus of Vt is predicted to form similar interactions with F-actin. (B) The protruding MVt structure is highlighted with a black box in (A) with the view rotated by 180 degrees. (C) In the second model, the H1 helix, insert and C-terminus of MVt form a protruding structure. In contrast to Vt, the C-terminus does not directly bind F-actin. (D) The protruding MVt structure highlighted with a black box in (C) with the view rotated by 180 degrees. The MVt H1 helix and insert are colored in blue and the C-terminus in red.
Figure 3:
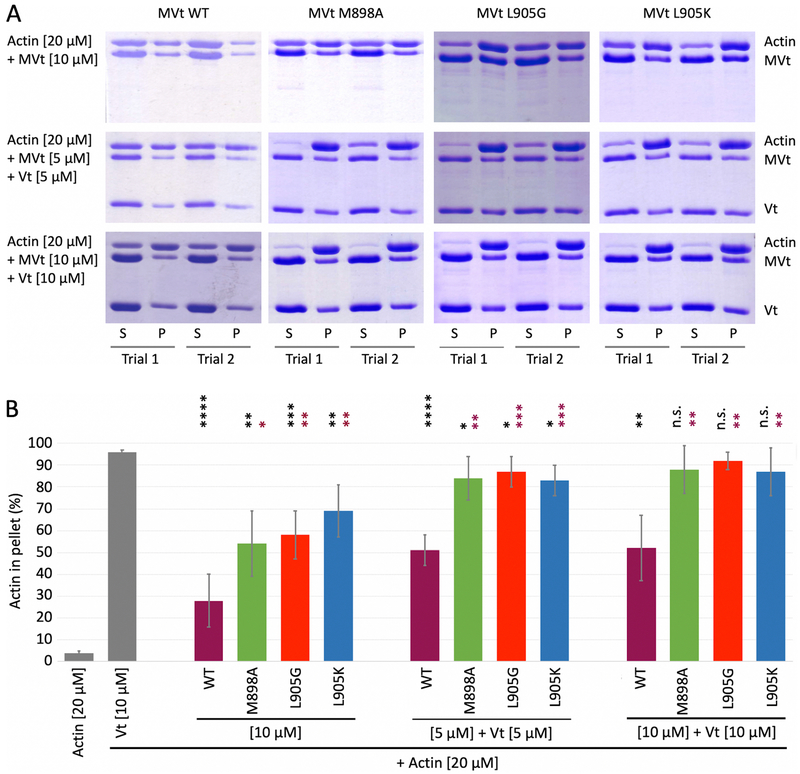
Wild type and mutant MVt and Vt proteins retain binding to F-actin. (A) Schematic representation of Vt and wild type and mutant MVt proteins. (B) Quantification of the Vt or MVt protein fractions present in the pellets, representing F-actin binding by individual proteins, using high-speed co-sedimentation. Concentration of wild type Vt, MVt and MVt mutant proteins is 10 μM. Error bars represent standard deviation (SD) (2 replicates for each system). Statistical significances with respect to Vt are indicated by non-significant (n.s.), p < 0.05 (*), p < 0.01 (**).
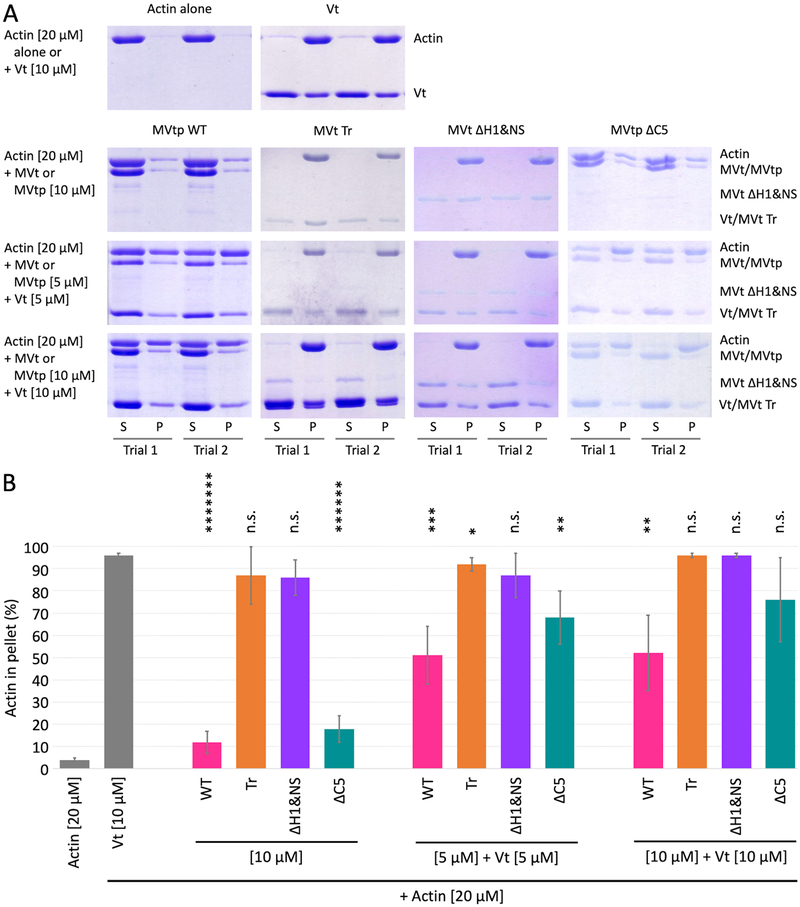
Figure 4:
MVt deletion mutants demonstrate variable F-actin assembly. (A) Representative SDS-PAGE analysis of low speed F-actin co-sedimentation assays incubated with Vt or MVt constructs alone and at 1:1 ratio Vt:MVt at indicated concentrations (S – supernatant, P – pellet). (B) Quantification of actin fractions present in pellets representing higher-order F-actin assemblies that include F-actin bundles in case of Vt. Error bars represent SD (n=2, 2 replicates for each n). Statistical significances with respect to Vt alone are indicated by non-significant (n.s.), p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), etc.
The MVtp ΔC5 variant partially restores bundling at a 1:1 ratio of MVt:Vt. However, the fraction that pellets with F-actin is still less in presence of MVtp ΔC5 compared to Vt alone (Figure 4). These observations may be indicative of a conformational ensemble between the two states indicated by models 1 and 2, whereby deletion of ΔC5 shifts the equilibrium in favor of the first model with protruding subdomain formed by N-terminus alone (Figure 2B).
Our model also explains why sub-stoichiometric ratios of MVt:Vt are sufficient to inhibit actin bundling. Given the internal stiffness of F-actin, a single MVt may prevent dimerization of two distinct actin filament associated Vt domains within certain distance from the MVt binding site. Our estimate shows that this distance (L) is about 300 Å. To get this estimate we employed a simplistic model with two parameters: lateral size of protruding subdomain, R ~ 20 Å, and persistence length of F-actin, P ~ 17 mm (Mameren et al., 2009) (STAR Methods and Figure S5).
Deletion of H1 helix converts actin reorganization properties of MVt to Vt.
The N-terminal region of MVt, which includes the NtS, H1 helix and part of the MVt insert, is likely to be dynamic or disordered (Kim et al., 2016; Rangarajan et al., 2010). Intriguingly, truncation of MVt to include this disordered region and 4 residues from the C-terminus enables MVt (residues 959–1,130) to bundle F-actin similar to Vt (Rangarajan et al., 2010). It is tempting to speculate that NtS’-H1’ of MVt are functional substitutes for Vt NtS-H1, as this is the only region that differs in sequence between the MVt truncation variant (residues 959–1,130) and Vt (Figure S6). However, distinct from the MVt variant, which bundles F-actin with 4 residues truncated from its C-terminus, the Vt C-terminal truncation completely abrogates its bundling properties (Shen et al., 2011). Thus, the difference between NtS’-H1’ and NtS-H1 somehow compensates for the lack of C-terminal residues in MVt.
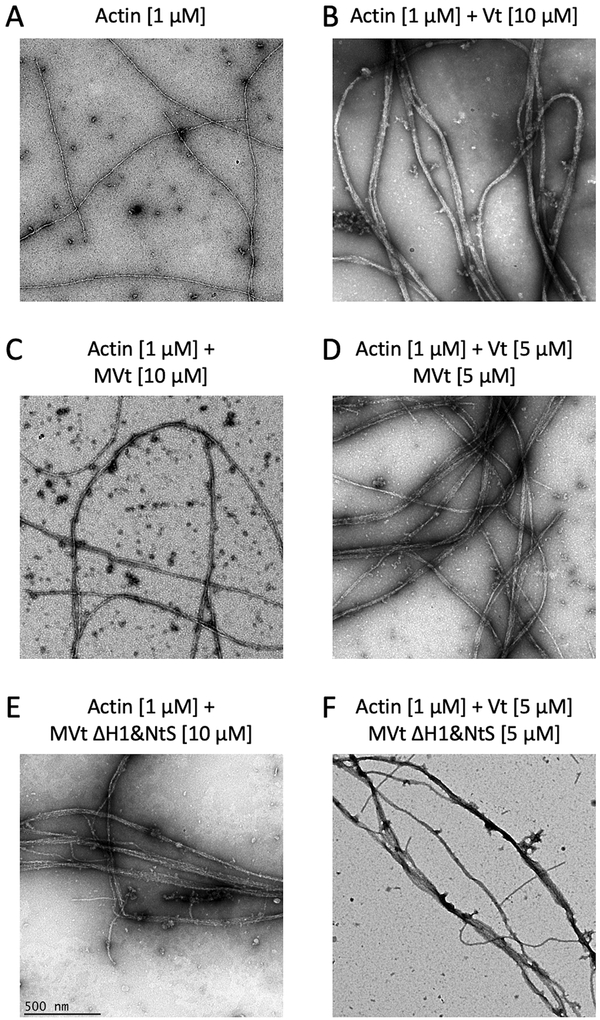
To further explore how truncation of the MVt N-terminus affects the ability of MVt to inhibit Vt-mediated F-actin bundling, we assessed the capacity of MVt with truncated NtS and H1 helix to bind and bundle F-actin (Figure 3A, construct MVt ΔH1&NS, residues 916–1,134). This is a smaller deletion compared that previously used (residues 959–1,130) (Rangarajan et al., 2010). While we find that binding of MVt ΔH1&NS to F-actin is similar to WT MVt (Figure 3B), MVt ΔH1&NS induces F-actin assemblies as revealed by the high fraction of F-actin found in the pellet using a low speed co-sedimentation assay (Figure 4). By use of negative stain electron microscopy, we also confirmed that F-actin forms thick linear bundles in presence of MVt ΔH1&NS (Figure 5). Further truncation of the MVt N-terminus (Figure 3A, construct MVt Tr) does not enhance MVt ability to bundle F-actin, as the MVt Tr construct exhibits bundling activity comparable to WT Vt (Figure 4). This behavior is consistent with both of our models, as H1 forms a key part of the hydrophobic core of the protruding subdomain structure (Figure 2 B,D). The deletion of H1 destabilizes this structure, thereby promoting a plausible MVt dimerization and actin filament crosslinking. These findings, however, are inconsistent with an alternative hypothesis that a disordered/longer linker sequence can generate a large exclusion volume around F-actin fibers preventing them from approaching each other. If this were the case, we would expect to see a gradual enhancement of F-actin bundling with deletion of the N-terminus sequence. Instead, deletion of H1, restores actin bundling properties of MVt to that of Vt, which is more consistent with unfolding of a globular structural element.
Figure 5:
Negative stain EM images of actin filaments show states of F-actin networks in the presence of Vt, MVt and MVt deletion mutant. Images are acquired at the same magnification (scale bar = 500 nm). Crosslinking or bundling of actin filaments (A) by Vt generates thick F-actin fibers (B). In contrast, MVt does not promote actin filament bundling (C), rather significantly reduces Vt-induced actin bundling (D). Removal of MVt H1 and N-terminal strap (ΔH1&NtS) promotes F-actin bundling similar to Vt (E), and in the presence of Vt shows similar F-actin bundling to Vt alone (F).
We also explored whether strategically designed point mutants can recapitulate the effect of H1 helix deletion. We focused on two residues, M898 and L905, which are located inside H1 helix. According to our model, these residues participate in forming a hydrophobic core associated with protruding MVt subdomain that is induced upon actin engagement (Figure 2 B,D). Calculations of protein stability change (ΔΔG) performed using Eris software (Yin et al., 2007a, 2007b), revealed that M898A, L905G, L905K mutants destabilize the protruding MVt subdomain in each of two structural models. For the first model of the protruding subdomain (Figure 2 A,B), ΔΔG values of 2.1, 9.2 and 12.6 kcal/mol were obtained for M898A, L905G and L905K, respectively, and ΔΔG values 2.6, 9.2 and 9.7 kcal/mol were obtained for the second model (Figure 2 C,D). Consistent with these calculations, our bundling assay showed a dramatic increase of F-actin in the pellet fraction (Figure 6), which further supports our model of protruding globular subdomain formed upon MVt engagement to F-actin.
Figure 6:
Strategically designed single point mutations in MVt demonstrate variable F-actin assembly. (A) Representative SDS-PAGE analysis of low speed F-actin co-sedimentation assays incubated with Vt or MVt constructs alone and at 1:1 ratio Vt:MVt at indicated concentrations (S – supernatant, P – pellet). (B) Quantification of actin fractions present in pellets representing higher-order F-actin assemblies. Error bars represent SD (n=2, 2 replicates for each n). Statistical significance with respect to Vt alone are indicated by black stars, and MVt WT within each set by maroon stars, non-significant (n.s.), p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), etc.
DISCUSSION
Vinculin is an essential cytoskeletal protein found in focal adhesions and adherens junctions. Its splice isoform, metavinculin, has an additional insert in the tail domain and co-expresses with vinculin in cardiac, skeletal and smooth muscles. While complete loss of vinculin is lethal, its partial loss is associated with dilated cardiomyopathy. Loss of metavinculin, as well as point mutations in the metavinculin insert, leads to dilated and hypertrophic cardiomyopathies. Among three mutations in the metavinculin tail domain associated with various cardiomyopathies, two are rare (ΔL954, R975W) and one (A934V) represents a common polymorphism found in 1.2% of population on average, reaching particular high numbers for East Asian (7.5%) and Latino (3.8%) populations (Lek et al., 2016). While rare mutations can be disabling, the effect of common polymorphisms on protein function is usually modest. Nevertheless, common polymorphisms confer higher potential risks for the population due to widespread appearance. Rare mutations are more challenging to discover and interpret, due to lack of statistics corelating them to the disease. Assessing potential risks for common and rare mutations can be accomplished with knowledge of protein structure and dynamics and their relationship to protein functions.
Elucidation of structures associated with Vt and MVt-mediated higher order F-actin assemblies is challenging, as dense heterogeneous F-actin networks organized by Vt and MVt are hard to visualize using modern techniques such as X-ray crystallography and cryo-EM. Consequently, existing structures of Vt and MVt in complex with actin were obtained with truncated C-terminus to prevent formation of F-actin networks. This truncation impairs protein structure and dynamics and limits potential scientific benefits of structural analysis. Here, using combination of molecular dynamics simulation and experimental approaches, we found that C-terminus of Vt and MVt interacts with hydrophobic pocket on F-actin surface and that the MVt H1 helix is a key component of a protruding subdomain, which forms upon MVt engagement to F-actin. This structural element occludes F-actin fibers from forming tight parallel bundles.
While MVt is unable to produce linearly bundled actin filaments, three MVt mutations identified in cardiomyopathies (A934V, ΔL954, R975W) show distinct properties in that they promote formation of larger/distinct actin networks (Olson et al., 2002; Sarker et al., 2019). In contrast, deletion of NtS and H1 in MVt directs formation of linear F-actin bundles. Here, we propose a model, which explains such differential behavior of the MVt variants.
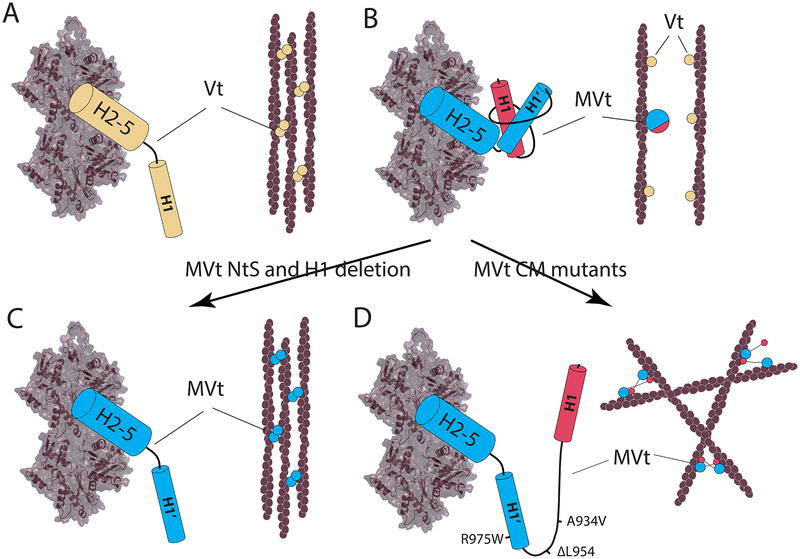
The first building block of this model lies in the ability of Vt to mediate F-actin bundling. It was previously shown that actin binding causes a conformational change in Vt, which exposes a surface, likely encompassing the H1 helical region, that facilitates dimerization of Vt and subsequent actin filament bundling (Johnson and Craig, 2000; Kim et al., 2016). Although the dimer interface is still unknown, we hypothesize that two Vt domains self-associate and align F-actin fibers parallel to each other, which then further promote formation of additional Vt dimers, possibly through domain swapping (Ding and Dokholyan, 2005; Ding et al., 2006). Thus, F-actin bundling may occur through a cooperative “zipper” mechanism. Once dimerization process starts, it can then propagate in both directions from the start site (Figure 7A). This process is perturbed for MVt, as actin binding promotes formation of an additional globular subdomain that breaks the “zipper” by keeping F-actin fibers at a distance beyond the dimerization range (Figure 7B). Deletion or mutation of specific residues within the MVt H1 helix destabilizes the protruding subdomain, which releases the H1’ helix, enables MVt dimerization and leads to F-actin bundling (Figure 7C) in a process similar to Vt-mediated bundling (Figure 7C). Mutations within the MVt insert (such as the cardiomyopathy mutations A934V, ΔL954 and R975W) also destabilize the protruding structure. However, in this case, the H1 helix remains functional. We hypothesize that the H1 helix can make transient interactions with another MVt or Vt, for example by associating with four-helix bundle in place of their cognate H1/H1’ helix. However, this interaction does not promote parallel alignment of F-actin filaments since the H1 helix is connected to its cognate MVt through a longer linker (MVt insert). This process induces a mesh-like organization of F-actin fibers (Figure 7D). It should be noted that two of the cardiomyopathy mutations (A934V, ΔL954) do not affect the H1’ helix, and may still form actin bundles, possibly through formation of homo- or hetero-dimers. However, because the H1 helix is connected to its cognate MVt by a long flexible linker, it is able to explore larger area in the search for its binding partner than the H1’ helix, thus potentiating crosslinking with higher frequency than bundling. Our model is consistent with theoretical and experimental predictions for organization of F-actin networks using linkers of different sizes and various flexibilities, as it has been previously shown that short and stiff linkers organize F-actin into bundles through a highly cooperative process (Shin et al., 2009), while long linkers lead to highly nonhomogeneous F-actin networks (Borukhov et al., 2005; Wagner et al., 2006). Interestingly, the R975W mutant exhibits the most sever disease phenotype (Olson et al., 2002; Vasile et al., 2006) and leads to the most drastic changes in morphology of F-actin networks.
Figure 7:
F-actin assemblies mediated by Vt and MVt. (A) Vt promotes formation of linear F-actin bundles (B) WT MVt inhibits F-acting bundling and crosslinking. H1 and MVt insert form a protruding subdomain, which sterically occludes actin filaments from coming into interaction distance. (C) Removal of H1 from MVt, disrupts protruding structure and promotes F-actin bundling. (D) MVt cardiomyopathy mutations perturb formation of the insert-mediated ‘protruding’ structural element. Released H1 forms transient interactions with H2–5 four helix bundle of other Vt/MVt causing F-actin crosslinking. H2–5 is a four-helix bundle formed by helices 2-3-4-5 of MVt. H1 is the first helix of the five-helix bundle in Vt. H1’ is the first helix of the five-helix bundle of MVt.
While we and others have shown that MVt negatively regulates formation of F-actin bundles and higher order assemblies (Kim et al., 2016; Oztug Durer et al., 2015), MVt has been observed to crosslink F-actin fibers into mesh-like structures (Olson et al., 2002). A possible explanation to this apparent contradiction, is that the experiments were conducted at different temperatures or at different pH conditions, which affect stability of the protruding MVt subdomain thus exposing H1 helix and inducing crosslinking of F-actin, a hypothesis subject to an additional study beyond the scope of this work.
While vinculin is ubiquitously expressed, metavinculin is co-expressed with vinculin only in highly contractile tissues and its expression correlates with the degree of mechanical load (Belkin et al., 1988b). This observation suggests that the role of the metavinculin is to modulate vinculin function providing the mechanism for disassembly of F-actin bundles when needed. Either failure to produce metavinculin or introduction of disabling mutations, perturbs this regulatory mechanism potentially leading to diseases such as various cardiomyopathies. Thus, elucidating the structural basis for metavinculin regulatory functions would aid in understanding disease conditions associated with these cell adhesion proteins and potential treatment options.
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Nikolay V. Dokholyan (dokh@psu.edu)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Escherichia coli strain BL21(DE3) cells were transformed with respective plasmids for protein over-expression. A single colony from an agar plate containing appropriate antibiotics was used to prepare an overnight starter culture in Lysogeny broth (LB) media at 37 °C. Full culture containing the same 1 L LB media was inoculated by the starter culture and cells were first grown at 37 °C to an optical density of 0.6–0.8 (600 nm). Protein over-expression was then initiated by addition of isopropyl-D-1-thiogalactopyranoside (IPTG). Cells were then grown at 18 °C overnight and harvested by centrifugation (4.5k rpm, 30 min).
METHOD DETAILS
Protein Expression and Purification
Vinculin tail (Vt) containing residues 879–1066 of the chicken sequence was cloned into pQlinkH vector (Addgene, Cambridge, MA). Metavinculin tail (MVt) containing residues 879–1134 and metavinculin tail plus proline-rich linker (MVtp) containing residues 858–1134 of the human sequence were cloned into 2HR-T vector (Addgene, Cambridge, MA). Plasmids for the MVt and MVtp mutants, namely MVt Tr (equal in size to Vt counting from the C-terminus with a point mutation Q1086K), MVt ΔH1&NtS (MVt sequence with deletion of H1 and N-terminal strap region), MVtp ΔC5 (MVtp sequence with deletion of C-terminal 5 residues), MVt M898A (MVt sequence with a point mutation M898A), MVt L905G (MVt sequence with a point mutation L905G) and MVT L905K (MVt sequence with a point mutation L905K) were prepared by GenScript (Piscataway, NJ) from our supplied MVt and MVtp plasmids. Protein expression and purification were performed as described previously (Sarker et al., 2019).
Actin co-sedimentation
The actin binding and bundling (cross-linking) properties of individual Vt and MVt/MVtp proteins as well as their mixtures were investigated using an adapted actin co-sedimentation assay previously reported (2008 J. Biol. Chem. 283, 6222–6231). High speed co-sedimentation (100,000 RCF for 30 min at 23 °C) for actin binding assay and low speed co-sedimentation for actin bundling assay (12,000 RCF for 15 min at room temperature) were performed as described previously (Sarker et al., 2019). For both binding and bundling co-sedimentation, the supernatant and pellet were separated, resuspended to equal volumes, and analyzed by 15% SDS-PAGE. Actin binding properties were calculated by determining the fractions of Vt/MVt/MVtp proteins present in pellets using the densities of the pellet and supernatant bands. Actin bundling properties were calculated by determining the fractions of actin present in pellets using the densities of the pellet and supernatant bands. Densitometry was performed using ImageJ (C.A. Schneider, W.S. Rasband, K.W. Eliceiri, NIH Image to ImageJ: 25 years of image analysis, Nat. Methods 9 (2012) 671–675). Statistical significances (p values) of the measurements were determined using the Microsoft Excel t-Test function.
Negative stain EM
Actin (1 μM) without or with Vt/MVt/MVtp (10 μM) was incubated in actin polymerization buffer (10 mM Tris, 100 mM KCl, 10 mM imidazole, 2.5 mM MgCl2, 1 mM EGTA, 2 mM DTT, pH 7.5) for 15 minutes. Samples were absorbed directly onto glow-discharged carbon-coated 400 mesh copper grids for 3 minutes and then stained with 2% (w/v) uranyl acetate in water. Transmission electron microscopy (TEM) images were obtained using a FEI Tecnai 12 electron microscope at 80 kV and captured on a Gatan First Light CCD camera using Gatan Digital Micrograph software (Gatan, Pleasanton, CA).
Discrete Molecular Dynamics (DMD) simulations
The initial structure for simulations of the isolated Vt domain was prepared from PDB ID 1TR2 (chain A, residues 881–1061). The missing residues (residues 1062–1066) were added using the Pymol built-in tool. The initial structure was subjected to short DMD simulation (10,000 steps) to remove clashes. This simulation was run at constant temperature T=0.5 kcal/(mol kB), and heat exchange coefficient equal 10, with Andersen thermostat used (Andersen, 1980) for simulations.
The replica-exchange DMD simulation (Ding et al., 2008; Proctor et al., 2011) for the isolated Vt domain was run using set of 10 temperatures (0.330, 0.360, 0.390, 0.420, 0.450, 0.480, 0.510, 0.540, 0.570, 0.600 kcal/(mol kB)) for one million steps. Replicas were exchanged every 1000 steps. Heat exchange coefficient was equal to 0.1. Following the simulations 1000 lowest energy structures were selected and clustered by Root Mean Square Deviation (RMSD) of carbon-alpha backbone atoms using hierarchical clustering algorithms and a clustering cutoff 9 Å. Melting profile was obtained using Weighted Histogram Analysis Method (WHAM) (Kumar et al., 1992).
Constant temperature simulations, used for analysis of the interaction stability between Vt C-terminus and F-actin, were performed at T=0.55 kcal/(mol kB) and run for one million steps. Truncated Vt variants (ΔC1, ΔC2 and ΔC5) were obtained by removing 1, 2 or 5 residues from Vt C-terminus using the built-in Pymol tool. Constant temperature simulation for Movie S1 was performed at T=0.5 kcal/(mol kB) and run for one million steps.
The initial structure for simulations of Vt bound to F-actin was prepared from the existing cryo-EM reconstruction (PDB ID 3JBI) of Vt bound to actin homodimer (the model of F-actin). The missing Vt residues (881–916) and (1048–1066) were added using the Pymol built-in tool. The initial structure was subjected to short DMD simulation (10,000 steps) to remove clashes. The simulation was run at constant temperature T=0.5 kcal/(mol kB), and heat exchange coefficient equal 10. The positions of actin atoms were fixed during the simulations. To prevent Vt from dissociating from F-actin, constraints were applied to a set of Vt backbone atoms N, CA, C of residues R976, I977, N980, R987, P990, I991, Q994, I997, Q1018, E1021, M1022, H1025, N1026, E1036, R1039, E1040, A1043, I1046, which were allowed to move within 1 Å from their initial positions. These residues were chosen because of their location in the binding interface between Vt and F-actin.
To model Vt in complex with F-actin we performed two independent replica exchange simulations for 2,5 and 3,5 million steps respectively. The set of 10 temperatures was used 0.330, 0.360, 0.390, 0.420, 0.450, 0.480, 0.510, 0.540, 0.570, 0.600 kcal/(mol kB). Heat exchange coefficient was equal to 0.1. Following the simulations, trajectories from two simulations were merged, 1000 lowest energy structures were selected and clustered by RMSD of carbon-alpha backbone atoms using hierarchical clustering algorithm and a clustering cutoff 9 Å.
Estimate of the inhibitory effect of MVt binding on dimerization of nearby Vt molecules.
In a polymer, with a persistence length P, an expectation value for the cosine of the angle between tangent to the polymer at position 0 and tangent to the polymer at distance L is (Landau and Lifshitz, 1980)
In our simplified model we treat Q as an angle between F-actin fibers in a parallel bundle and an F-actin fiber deflected by MVt (Figure S5). cos Θ can be expressed as
Hence,
and
Numerical solution of the last equation, with P ~ 13 μm (Mameren et al., 2009) and R ~ 20 Å (size of the protruding subdomain) (Sarker et al., 2019)) provides an estimate for L ~ 300 Å. These calculations indicate that Vt dimerization is perturbed within about 300 Å from the MVt binding site.
QUANTIFICATION AND STATISTICAL ANALYSIS
Actin co-sedimentation data were expressed as mean ± SD. Two experimental replications were performed with two independent sets (n-=2) for each condition. The statistical significances (p values) of the measurements were determined using the Microsoft Excel t-Test function for two-tailed independent sample assuming equal variance. The p values corresponded to non-significant (n.s.), p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), etc. No data were excluded from analysis.
DATA AND CODE AVAILABILITY
The published article includes all datasets generated or analyzed during this study. No new code was generated.
Supplementary Material
Supplementary Data 1, related to Figure 2: Model of MVt bound to F-actin. The protruding subdomain formed by the MVt N-terminus.
Supplementary Data 2, related to Figure 2: Model of MVt bound to F-actin. The protruding subdomain consists of intertwined MVt N- and C-termini.
Supplementary Movie 1, related to Figure 1: A single trajectory of Vt produced at constant temperature T=0.5 kcal/(mol kB) highlighting retention of the Vt 4 helix bundle and unfolding of H1.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Bacterial and Virus Strains | ||
| Escherichia coli strain BL21(DE3) | Sarker et al. 2019 | N/A |
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Actin (rabbit muscle acetone powder) | Pel-Freez Biologicals, Rogers, AR | N/A |
| Critical Commercial Assays | ||
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Oligonucleotides | ||
| Recombinant DNA | ||
| Vt - pQlinkH vector | Shen et al., 2011 | N/A |
| MVt - 2HR-T vector | Kim et al., 2016 | N/A |
| MVtp - 2HR-T vector | Kim et al., 2016 | N/A |
| MVtp ΔC5 – 2HR-T vector | Kim et al., 2016 | N/A |
| MVt Tr - 2HR-T vector | This paper | N/A |
| MVt ΔH1&NtS - 2HR-T vector | This paper | N/A |
| MVt M898A - 2HR-T vector | This paper | N/A |
| MVt L905G - 2HR-T vector | This paper | N/A |
| MVT L905K - 2HR-T vector | This paper | N/A |
| Software and Algorithms | ||
| ImageJ | Schneider et al., 2012 | https://imagej.nih.gov/ij/ |
| t-Test function | Microsoft Excel | N/A |
| DMD | Shirvanyants et al., 2012 | https://dokhlab.med.psu.edu/dokhlab/#/ |
| Eris | Yin et al., 2007a | https://dokhlab.med.psu.edu/eris/login.php |
| Other | ||
| 1st model of MVt bound to F-actin | This paper | N/A |
| 2nd model of MVt bound to F-actin | This paper | N/A |
Highlights.
The Vt and MVt C-terminal hairpin forms direct interactions with the F-actin surface
The MVt C-terminus is not essential for inhibition of Vt-mediated actin bundling
Deletion of MVt H1 helix converts actin reorganization properties of MVt to Vt
ACKNOWLEDGEMENTS
This work was supported by the NIH (RO1GM115597, PI: S.C.), (GM31819 and ES013773, PI: J.G.), and (RO1GM114015 and RO1GM123247, PI: N.V.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Andersen HC (1980). Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys 72, 2384–2393. [Google Scholar]
- Bakolitsa C, Pereda M. De, Bagshaw CR, Critchley DR, Liddington RC, and Le L (1999). Crystal Structure of the Vinculin Tail Suggests a Pathway for Activation. 99, 603–613. [DOI] [PubMed] [Google Scholar]
- Bakolitsa C, Cohen DM, Bankston LA, Bobkov AA, Dadwell GW, Jennings L, Crithcley DR, Craig SW, and Liddington RC (2004). Structural basis for vinculin activation at sites of cell adhesion. Nature 430, 583–586. [DOI] [PubMed] [Google Scholar]
- Bays JL, and DeMali KA (2017). Vinculin in cell–cell and cell–matrix adhesions. Cell. Mol. Life Sci. 74, 2999–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin AM, Ornatsky OI, Glukhova MA, and Koteliansky VE (1988a). Immunolocalization of meta-vinculin in human smooth and cardiac muscles. J. Cell Biol. 107, 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin AM, Ornatsky OI, Kabakov AE, Glukhova MA, and Kotelianskys VE (1988b). Diversity of Vinculin/meta-Vinculin in Human Tissues and Cultivated Cells. Biochemistry 6631–6635. [PubMed] [Google Scholar]
- Borukhov I, Bruinsma RF, Gelbart WM, and Liu AJ (2005). Structural polymorphism of the cytoskeleton: a model of linker-assisted filament aggregation. Proc. Natl. Acad. Sci. USA 102, 3673–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ding F, Nie H, Serohijos AW, Sharma S, Wilcox KC, Yin S, and Dokholyan NV (2008). Protein folding: Then and now. Arch. Biochem. Biophys 469, 4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll JL, Ben-Ze’ev a, Ezzell RM, Rodríguez Fernández JL, Baribault H, Oshima RG, and Adamson ED (1995). Targeted disruption of vinculin genes in F9 and embryonic stem cells changes cell morphology, adhesion, and locomotion. Proc. Natl. Acad. Sci. U. S. A 92, 9161–9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, and Dokholyan NV (2005). Simple but predictive protein models. Trends Biotechnol. 23, 450–455. [DOI] [PubMed] [Google Scholar]
- Ding F, and Dokholyan NV (2006). Emergence of Protein Fold Families through Rational Design. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, Prutzman KC, Campbell SL, and Dokholyan NV (2006). Topological Determinants of Protein Domain Swapping. Structure 14, 5–14. [DOI] [PubMed] [Google Scholar]
- Ding F, Tsao D, Nie H, and Dokholyan NV (2008). Ab Initio Folding of Proteins with All-Atom Discrete Molecular Dynamics. Structure 16, 1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokholyan NV, Buldyrev SV, Stanley HE, and Shakhnovich EI (1998). Discrete molecular dynamics studies of the folding of a protein-like model. Fold. Des 3, 577–587. [DOI] [PubMed] [Google Scholar]
- Feramisco JR, Helfman DM, Smart JE, Burridge K, and Thomas GP (1982). Coexistence of vinculin-like protein of higher molecular weight in smooth muscle. J. Biol. Chem 257, 11024–11031. [PubMed] [Google Scholar]
- Gimona M, Fürst DO, and Small JV (1987). Metavinculin and vinculin from mammalian smooth muscle: Bulk isolation and characterization. J. Muscle Res. Cell Motil. 8, 329–341. [DOI] [PubMed] [Google Scholar]
- Jannie K, Ellerbroek S, Zhou D, Chen S, Crompton D, García A, and DeMali K (2015). Vinculin-dependent actin bundling regulates cell migration and traction forces. Biochem. J 465, 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RP, and Craig SW (2000). Actin activates a cryptic dimerization potential of the vinculin tail domain. J. Biol. Chem 275, 95–105. [DOI] [PubMed] [Google Scholar]
- Kim LY, Thompson PM, Lee HT, Pershad M, Campbell SL, and Alushin GM (2016). The Structural Basis of Actin Organization by Vinculin and Metavinculin. J. Mol. Biol 428, 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteliansky VE, Ogryzko EP, Zhidkova NI, Weller PA, Critchley DR, Vancompernolle K, Vandekerckhove J, Strasser P, Way M, Gimona M, et al. (1992). An additional exon in the human vinculin gene specifically encodes meta-vinculin-specific difference peptide: Cross-species comparison reveals variable and conserved motifs in the meta-vinculin insert. Eur. J. Biochem 204, 767–772. [DOI] [PubMed] [Google Scholar]
- Kumar S, Bouzida D, Swendsen RH, Kollman PA, and Rosenbergl JM (1992). The Weighted Histogram Analysis Method for Free-Energy Calculations on Biomolecules. I. The Method. 13, 1011–1021. [Google Scholar]
- Landau LD, and Lifshitz EM (1980). Statistical physics, part 1: Volume 5 (course of theoretical physics, volume 5). Publ. Butterworth-Heinemann; 3. [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, et al. (2016). Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameren J. Van, Vermeulen KC, Gittes F, and Schmidt CF (2009). Leveraging Single Protein Polymers To Measure Flexural Rigidity †. J. Phys. Chem. B 113, 3837–3844. [DOI] [PubMed] [Google Scholar]
- Masato M, Emma H, Brian L, Scott V, and D. BR(1997). Dilated Cardiomyopathy Associated With Deficiency of the Cytoskeletal Protein Metavinculin. Circulation 95, 17–20. [DOI] [PubMed] [Google Scholar]
- Olson TM, Illenberger S, Kishimoto NY, Huttelmaier S, Keating MT, and Jockusch BM (2002). Metavinculin mutations alter actin interaction in dilated cardiomyopathy. Circulation 105, 431–437. [DOI] [PubMed] [Google Scholar]
- Oztug Durer ZA, McGillivary RM, Kang H, Elam WA, Vizcarra CL, Hanein D, De La Cruz EM, Reisler E, and Quinlan ME (2015). Metavinculin Tunes the Flexibility and the Architecture of Vinculin-Induced Bundles of Actin Filaments. J. Mol. Biol 427, 2782–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Horwitz AR, and Schwartz MA (2010). Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11, 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor EA, Ding F, and Dokholyan NV (2011). Discrete molecular dynamics. Wiley Interdiscip. Rev. Comput. Mol. Sci 1, 80–92. [Google Scholar]
- Rangarajan ES, Lee JH, Yogesha SD, and Izard T (2010). A helix replacement mechanism directs metavinculin functions. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker M, Lee HT, Mei L, Krokhotin A, Espinosa de los Reye S, Yen L, Costantini LM, Griffith J, Dokholyan NV, Alushin GM, et al. (2019). Cardiomyopathy Mutations in Metavinculin Disrupt Regulation of Vinculin-induced F-actin Assemblies. J. Mol. Biol in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Tolbert CE, Guilluy C, Swaminathan VS, Berginski ME, Burridge K, Superfine R, and Campbell SL (2011). The vinculin C-terminal hairpin mediates F-actin bundle formation, focal adhesion, and cell mechanical properties. J. Biol. Chem 286, 45103–45115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Drew KRP, Bartles JR, Wong GCL, and Grason GM (2009). Cooperativity and Frustration in Protein-Mediated Parallel Actin Bundles. Phys. Rev. Lett 103, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvanyants D, Ding F, Tsao D, Ramachandran S, and Nikolay V (2012). Discrete Molecular Dynamics : an efficient and versatile simulation method for fine protein characterization. J. Phys. Chem. B 8375–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subauste MC, Pertz O, Adamson ED, Turner CE, Junger S, and Hahn KM (2004). Vinculin modulation of paxillin-FAK interactions regulates ERK to control survival and motility. J. Cell Biol. 165, 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert CE, Burridge K, and Campbell SL (2013). Vinculin regulation of F-actin bundle formation: What does it mean for the cell? Cell Adhes. Migr 7, 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert CE, Thompson PM, Superfine R, Burridge K, and Campbell SL (2014). Phosphorylation at Y1065 in vinculin mediates actin bundling, cell spreading, and mechanical responses to force. Biochemistry 53, 5526–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasile VC, Will ML, Ommen SR, Edwards WD, Olson TM, and Ackerman MJ (2006). Identification of a metavinculin missense mutation, R975W, associated with both hypertrophic and dilated cardiomyopathy. 87, 169–174. [DOI] [PubMed] [Google Scholar]
- Wagner B, Tharmann R, Haase I, Fischer M, and Bausch AR (2006). Cytoskeletal polymer networks : The molecular structure of cross-linkers determines. [DOI] [PMC free article] [PubMed]
- Witt S, Zieseniss A, Fock U, Jockusch BM, and Illenberger S (2004). Comparative biochemical analysis suggests that vinculin and metavinculin cooperate in muscular adhesion sites. J. Biol. Chem 279, 31533–31543. [DOI] [PubMed] [Google Scholar]
- Xu W, Baribault H, Adamson ED, Magram J, Sato TN, Dieterlen-Lievre F, and Huber P (1998). Vinculin knockout results in heart and brain defects during embryonic development. Development 125, 327–337. [DOI] [PubMed] [Google Scholar]
- Yin S, Ding F, and Dokholyan NV (2007a). Eris: an automated estimator of protein stability. Nat. Methods 4, 466–467. [DOI] [PubMed] [Google Scholar]
- Yin S, Ding F, and Dokholyan NV (2007b). Modeling Backbone Flexibility Improves Protein Stability Estimation. Structure 15, 1567–1576. [DOI] [PubMed] [Google Scholar]
- Zemljic-Harpf AE, Ponrartana S, Avalos RT, Jordan MC, Roos KP, Dalton ND, Phan VQ, Adamson ED, and Ross RS (2004). Heterozygous inactivation of the vinculin gene predisposes to stress-induced cardiomyopathy. Am. J. Pathol 165, 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler WH, Liddington RC, and Critchley DR (2006). The structure and regulation of vinculin. Trends Cell Biol. 16, 453–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data 1, related to Figure 2: Model of MVt bound to F-actin. The protruding subdomain formed by the MVt N-terminus.
Supplementary Data 2, related to Figure 2: Model of MVt bound to F-actin. The protruding subdomain consists of intertwined MVt N- and C-termini.
Supplementary Movie 1, related to Figure 1: A single trajectory of Vt produced at constant temperature T=0.5 kcal/(mol kB) highlighting retention of the Vt 4 helix bundle and unfolding of H1.
Data Availability Statement
The published article includes all datasets generated or analyzed during this study. No new code was generated.