Abstract
Estrogen receptor (ER)-positive breast cancer recurrence is thought to be driven by tumor initiating cells (TICs). TICs are enriched by endocrine therapy (ET) through NOTCH signaling. Side effects have limited clinical trial testing of NOTCH-targeted therapies. Death-associated factor 6 (DAXX) is a newly identified marker whose RNA expression inversely correlates with NOTCH in human ER+ breast tumor samples. In this study, knockdown and overexpression approaches were used to investigate the role of DAXX on stem/pluripotent gene expression, TIC survival in vitro and TIC frequency in vivo, and the mechanism by which DAXX suppresses TICs in ER+ breast cancer. 17β-estradiol (E2)-mediated ER activation stabilized the DAXX protein, which was required for repressing stem/pluripotent genes (NOTCH4, SOX2, OCT4, NANOG, and ALDH1A1), and TICs in vitro and in vivo. Conversely, ET promoted rapid protein depletion due to increased proteasome activity. DAXX was enriched at promoters of stem/pluripotent genes, which was lost with ET. Ectopic expression of DAXX decreased stem/pluripotent gene transcripts to levels similar to E2-treatment. DAXX-mediated repression of stem/pluripotent genes and suppression of TICs was dependent on DNMT1. DAXX or DNMT1 was necessary to inhibit methylation of CpGs within the SOX2 promoter and moderately within the gene body of NOTCH4, NOTCH activation, and TIC survival. E2-mediated stabilization of DAXX was necessary and sufficient to repress stem/pluripotent genes by recruiting DNMT1 to methylate some promoters, and suppress TICs. These findings suggest that a combination of ET and DAXX-stabilizing agents may inhibit ER+ tumor recurrence.
Keywords: Breast Cancer, Estrogen Receptor, DAXX, Tumor initiating cells, Cancer Stem Cells
INTRODUCTION
Recurrent breast cancer during or following ET may arise from a small population of cells referred to as Tumor Initiating Cells (TICs) (1,2). These TICs could be responsible for tumor recurrence during ET due to stem-like properties. These properties include their small proportion in number, ability to self-renew and generate a heterogeneous tumor, and intrinsic resistance to conventional and targeted anti-cancer therapies, including ET (3). Thus, anti-ER therapy effectively target the majority of a breast cancer, but the persisting TICs remain and are able to generate a more aggressive, ET-resistant cancer (4). NOTCH signaling has been reported to promote survival and self-renewal of both normal mammary stem cells and TICs (5–8).
Overexpression of the active form of NOTCH in the mouse mammary gland promotes metastatic breast cancer (9,10). Further, ET increases NOTCH signaling in ER+ breast cancer (11), and this activation is required for TIC survival (4,7). NOTCH receptors (NOTCH1, 2, 3, 4) are expressed as heterodimeric proteins at the cell surface. Activation is initiated upon interaction with their ligands (Delta-like1, 3, 4 and Jagged1, 2) on adjacent cells. Ligand-receptor engagement triggers endocytosis of the extracellular NOTCH domain into the ligand-expressing cell (12–14). The remaining transmembrane domain is cleaved by the metalloproteinase ADAM10 or 17 to form the NOTCH external truncation (NEXT). Finally, the gamma (γ)-secretase complex cleaves NEXT releasing the NOTCH intracellular domain allowing for nuclear translocation and regulation of gene transcription (15). This last process can be inhibited by a γ-secretase inhibitor (GSI), inhibiting NOTCH signaling in breast cancer, and resulting in a reduction of TIC survival, tumorigenesis and tumor recurrence in vitro and in vivo (7,16,17). Numerous GSIs have been developed and clinical trials are ongoing (17). GSI therapy has yet to result in clinical approval for the treatment of breast cancer. This is in part due to gastrointestinal toxicity and skin cancer development (18). Thus, there is a clinical need for a novel target and therapy that is able to inhibit TIC-survival and frequency in ER+ breast cancer and avoid toxicity.
In order to identify new therapeutic NOTCH biomarkers and targets, Albain et al. completed a biomarker presurgical window trial (ClinTrials.gov Identifier: ) (19). Death domain-associated protein 6 (DAXX) was identified as a novel NOTCH target with potential clinical significance in ER+ breast cancer (manuscript in preparation). Its transcript expression was significantly upregulated in human breast cancers treated with ET after a short exposure to a GSI. As NOTCH is required for TIC-survival, and inhibited by GSI, we hypothesized that increased DAXX expression may downregulate TIC-survival. We tested this by determining if DAXX was necessary and/or sufficient to restrict TIC-survival using ER+ breast cancer cells: MCF-7 (wild-type p53) and T47D (mutant p53) in vitro and in vivo and investigated mechanisms by which DAXX regulates TIC-survival.
MATERIALS AND METHODS
Cell Culture
MCF-7, T47D, BT474, MDA-MB-231 and MDA-MB-468 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). The BCM-5097 ER+ PDX tumor was purchased from Dr. Michael Lewis (Baylor College of Medicine, Houston, TX). All cell lines were authenticated December 2018 by short tandem repeat allelic profiling (ATCC, Manassas, VA) and maintained at a low passage number (below 20 passages/cell line). Maintenance of cells in appropriate medium is provided in supplementary materials and methods
Chemicals
The 17β-Estradiol (E2) (Sigma Aldrich, catalogue # E8875), fulvestrant (Selleck chemicals), Cycloheximide (CHX) (gift from Dr. Charles Hemenway, Loyola University Chicago), MG132 (Sigma-Aldrich catalogue # M8699), 5-azacytidine (5-AZA) (Sigma Aldrich) were suspended in 100% ethanol or dimethylsulfoxide (DMSO) to form stocks solution, stored in the dark, and maintained in −20°C. The stock solutions were diluted 1:1000 vol/vol in growth medium to form working concentrations of 5nM (E2), 100nM (fulvestrant), 10μM (CHX), 10μM (MG132), and 10μM (5-AZA).
RNA Interference and Transfection Reagents
A pool of four DAXX small interfering RNAs (siRNAs) were used to knockdown DAXX expression in vitro (Dharmacon GE Life Sciences, Lafayette, CO). Non-targeting scrambled control siRNA (SCBi) was purchased from Qiagen (Germantown, MD). DNMT1 siRNA was purchased from Origene (Catalogue # SR301244). The transfection reagent Lipofectamine RNAiMAX (Catalogue # 13778150) was purchased from Thermo Fisher Scientific (Waltham, MA) and used at a ratio of 1:1 ratio with 10nM of appropriate siRNA according to the manufacturer’s protocol. Cells were incubated in transfection medium for 48 hours.
DAXX overexpression by transfection
A mammalian expression vector, pCMV6-entry containing a human DAXX cDNA was purchased from Origene (Rockville, MD) and used to transiently overexpress DAXX in cell lines.
Western Blot Analysis
The Western blot protocol is described in detail in the Supplementary Materials and Methods. The primary antibodies DAXX (1:1000, Cell Signaling Technology), β-ACTIN (1:2000, Sigma Aldrich), NOTCH4 (1:1000, Santa Cruz Biotechnologies), DNMT1 (1:1000, Santa Cruz Biotechnologies), PARP-1 (1:1000, Santa Cruz Biotechnologies), ER-α (1:1000, Cell Signaling Technologies) were diluted in 5% milk or 20% Roche and added to the membrane and incubated overnight at 4°C with constant agitation.
Real-time PCR
MCF-7 and T47D cells were exposed to specified growth conditions, following which total RNA was extracted according to the manufacturer’s protocol using the RiboPure RNA Purification Kit (Ambion, Austin, TX, Catalogue # AM1924). RNA yield was determined using a NanoDrop Spectrophotometer (Therm Fisher Scientific, Waltham, MA). RNA was reverse transcribed to cDNA using a reverse transcriptase enzyme and kit according to the manufacturer’s instructions (Multiscribe™ Reverse Transcriptase Kit, Applied Biosystems, Foster City, CA, Catalogue # N8080234). The reaction was performed 25°C for 10 min, 48°C for 30 minutes, 95°C for 5 minutes, 25°C for 60 minutes. Real-time PCR was performed using iTaq™ SYBR® Green Supermix (Biorad, Hercules, CA) to detect transcript levels. The PCR conditions were: 10 minutes at 95°C, 40 cycles of 10 second at 95°C then 45 seconds at 60°C. Following this, a melt curve was conducted for 40 cycles as a control as outlined by the manufacturer of the StepOnePlus Real-time PCR machine (Applied Biosystems, Foster City, CA). Hypoxanthine-guanine phosphoribosyltransferase (HPRT) was used as a loading control to normalize Ct expression values for each gene transcript. Relative fold-change in transcript expression between each sample was calculated using the 2(-ΔΔCt) method as outlined: ΔCt Experimental= (Ct value of experimental gene of experimental group-Ct value of HPRT of experimental group), ΔCt control= (Ct value of experimental gene of control group- Ct value of HPRT of control group), ΔΔCt= (ΔCt experimental group-ΔCt control group), relative quantity (RQ) = 2(-ΔΔCt). Primer sequences are listed in Supplemental Table 1A.
Cycloheximide (CHX) Chase Assay
MCF-7 cells were grown in 0 or 5nM E2 for 24 hours followed by addition of 10μM CHX in the presence or absence of 10μM MG132. Cells were incubated for their specified times (0–20 hours) and total protein was extracted and detected as described in the Western Blot analysis methods.
Bulk Cell Proliferation
MCF-7 or T47D cells at a density of 100,000 cells were plated in separate wells of a 6-well plate for 24 hours. Cells were then washed with PBS 2X, and specified growth medium was added containing 0 or 5nM E2. The growth medium was changed daily up to 7 days. Cells were trypsinized and total viable cells were counted using a Countess Cell Counter. Fold increase in live cell number was calculated by dividing the final viable cells at day 7 by the number of cells initially plated at day 0.
Cell Cycle Analysis
MCF-7 or T47D cells at a density of 100,000 cells were plated in separate wells of a 6-well plate for 24 hours. Cells were washed with PBS 2X and specified growth medium was added to each well. Growth medium was changed daily up to 7 days. Cells were fixed in 100% ethanol and stained with propidium iodide. Cell cycle analysis was conducted using flow cytometry according to the manufacturer’s instructions (Cell Signaling Technology).
Mammosphere Forming Assay
The protocol used was adapted from Shaw et al. (20). Briefly, DMEM-F12 medium (Gibco, Catalogue # 11039021) was heated to 60°C and 2g of methylcellulose was added to the solution. The contents was then continuously stirred at 60°C for 2.5hrs until the methylcellulose was uniformly dissolved. The medium was then stirred overnight at 4°C. The following day, 4 mL of B-27 supplement and 4μL of recombinant human epidermal growth factor (hEGF, Sigma Aldrich, Catalogue # E-9644) was added and the solution was stirred for 30 minutes at 4°C. The medium was then transferred to centrifuge tubes and centrifuged at 9500 rpm in a Beckman rotor at 4°C for 30 minutes to remove any precipitate. The solution (mammosphere medium) was transferred into 50mL conical tubes and stored at −20°C until ready for use. Images were then taken after 7 days and transferred to Powerpoint and the mammospheres for each field were counted based on the scale of the image using a size cut off of 50μm. The Mammosphere Forming Efficiency (MFE) was calculated using the following equation: %MFE= [(total number of mammospheres counted) × (dilution factor)] / (50,000 cells) × 100. Detailed protocol is provided in Supplementary Materials and Methods.
Tumor Initiating Potential
The protocol for this animal study was approved by Loyola University Chicago’s Institutional Animal Care and Use Committee (IACUC). MCF-7 cells were transfected with SCBi or DAXXi siRNA for 2 days. Cells were injected into the mammary fat pad of female, ovariectomized FoxN1 nu/nu athymic nude mice (Harlan Sprague-Dawley, Madison, WI) with 5 animals/ cell dilution. Cells were injected at varying dilutions including 10,000, 100,000 or 1,000,000 cells/animal. Animals were also implanted with a silastic-release capsule containing E2, allowing for a slow, sustained release of E2 that mimics peri/post-menopausal levels (83.8 pg/mL) for up to 8 weeks (21). Cells were implanted into nude mice at a 1:1 ratio with Matrigel® (Corning). After 8 weeks, the number of mice that developed tumors-designated as having a tumor area greater than or equal to 40mm2 were counted as mice-bearing tumors. The estimated frequency of TICs based on the number of tumors that formed at each cell dilution was determined using Extreme Limiting Dilution Analysis (ELDA) software from Walter Eliza Hall Institute of Medical Research (http://bioinf.wehi.edu.au/software/elda/).
Cellular Fractionation
Cells were grown in their specified experimental conditions for 3 days, trypsinized, and the protein within each cell compartment (cytoplasmic or nuclear) was isolated using the SubCellular Protein Fractionation Kit (Thermo Fisher Scientific, Waltham, MA) using the manufacturer’s protocol. Protein levels were then visualized by Western Blotting using Actin as a cytoplasmic control and PARP-1 as a nuclear control.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were conducted using SimpleChIP© Plus Kit (Cell Signaling Technology, Danvers, MA, Cat # 9005). Briefly, cells were trypsinized, cross-linked with a 1% formaldehyde solution (Sigma Aldrich, St. Louis, MO, Catalogue # F8775) for 30 mins and then quenched with 1.25M glycine followed by sonication using the Dismembrator (Model 100, Thermo-Fisher Scientific, Waltham MA). Samples for sonicated for 20 seconds at 30% power, placed on ice for 1 min, and re-sonicated 6 times. Chromatin was isolated following the manufacturer’s protocol. Isolated chromatin was incubated with 2μg of a DAXX-specific antibody (S-20, Santa Cruz Biotechnology, Santa Cruz, CA) or 2μg of a nonspecific rabbit IgG overnight at 4°C. Following DNA fragment isolation, quantitative PCR was performed using immunoprecipitated DNA with primers designed to flank promoter regions at AP-1 consensus sequences of selected genes. Primer sequences are provided in Supplementary Table 1B.
Bisulfite Sequencing and CpG Methylation Status
MCF-7 cells were transfected with siRNA (SCBi, DAXXi, or DNMT1i) and plated in growth conditions (0 or 5nM E2) for 24 hours. DNA was isolated from cells using the Qiagen DNeasy® Blood and Tissue Kit (Qiagen, Germantown, MD). DNA was converted to bisulfite-treated DNA using the EZ DNA Methylation-Gold Kit (Zymo Research) following the manufacturer’s protocol. DNA was then amplified with primers specific to CpG islands within the SOX2 promoter or within intron 29 and exon 29 of the NOTCH4 gene (SOX2: Forward: 5’-AAAGATTTTAATAAGAGAGTGGAAGGAA-3’, Reverse: 5’-CCAAAACCCAAAAAAATAATTTTAAC-3’; NOTCH4: Forward: 5’TTTGGTTTTTAATTGGGGT-AATAATT’3, Reverse: 5’TAACCCCTATCCCTTCAAACTTTA’3) under the following reaction conditions: 95°C 30 seconds, 35 cycles (95°C 20 seconds, 54°C 45 seconds, 68°C 30 seconds), 68°C 5 minutes, using the EpiMark® Hot Start Taw DNA Polymerase enzyme kit (New England BioLabs, Ipswich, MA). The PCR product was then cleaned and purified using the UltraClean® PCR Clean-Up Kit (Mo Bio Laboratories, Carlsbad, CA) and sequenced by Sanger Sequencing by ACGT Inc.
KMplotter Analysis
KMplot.com is a publically available website with software that has compiled the RNA expression of human breast cancer based on tissue microarray and associated clinical outcomes such as recurrence free survival (RFS) (22,23). RFS of patients with ER+ breast cancer were interrogated based on high versus low DAXX RNA expression. Median or quartile transcript expression was analyzed. From this database, RFS rates of ER+ breast cancer patients using parameters offered by the software were obtained. The parameters used to generate each survival curve and specific probe beeswarm plot are provided in Supplementary Table 1C.
Patient-derived Xenografts (PDXs)
The protocol for this animal study was approved by Loyola University Chicago’s Institutional Animal Care and Use Committee (IACUC). The ER+ PDX BCM-5097 was derived from a Caucasian female post treatment with metastatic disease. It is ER and progesterone receptor (PR) positive and negative for overexpression of human epidermal growth receptor 2 (HER2) (24). This tumor was passaged in female, ovariectomized NOD/SCID mice implanted with an E2-containing capsule for two passages. Tumor bits (0.2mm2) were implanted into ovariectomized FoxN1 nu/nu athymic nude mice-containing an E2 capsule. After 8 weeks, a new capsule was implanted to a subset of animals, while the remaining animals were maintained on E2-deprived conditions for 19 days. Tumors were then removed and total protein was isolated. ERα, DAXX and NOTCH4 protein levels were detected by Western blot analysis as described previously.
Co-Expression Analysis of METABRIC DATA for DAXX
The Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) cohort has 1980 women with primary, stages I and II invasive breast cancers (25). There were 1518 ER+ breast cancer patients including node-negative, node–positive, HER2- and HER2+ disease. Of the patients with ER+ breast cancer, 1088 received adjuvant hormonal therapy. Four hundred thirty patients received no adjuvant therapy. The METABRIC cohort was interrogated for co-expression of DAXX and TIC/pluripotent RNAs (ALDH1A1) in ER+ breast cancer using the Breast Cancer Integrative Platform (http://www.omicsnet.org/bcancer/database) (26).
Statistical Analysis
All experiments were conducted in triplicate at a minimum and repeated three independent times, with results reported as Mean ± Standard Deviation (S.D.). Comparisons between experimental groups were performed using ANOVA with a post-hoc Tukey’s test using Graphpad Prism 6 software. The Kaplan-Meier curve was generated using Graphpad and statistical differences between the two experimental groups was calculated by the Log-rank, Mantel-Cox test. Differences in TIC-frequency between SCBi and DAXXi MCF-7 tumors were calculated by the Chi-squared test. Linear regression was used to determine the estimated half-life of DAXX for each individual CHX chase experiment. All three half-life calculations were then pooled together for each group and statistical differences between groups was determined by ANOVA.
RESULTS
Stabilization of the DAXX protein requires ER activation
The role of DAXX on TIC-survival in ER+ breast cancer is not known. We first determined if the DAXX transcript and/or protein expression is modulated by E2 or ET. E2 treatment increased DAXX protein expression while downregulating NOTCH4 protein in three ER+ breast cancer cell lines (MCF-7, BT474 and T47D) (Figure 1A). ET treatment either by E2 deprivation or fulvestrant resulted in decreased DAXX protein but increased NOTCH4 protein expression as compared to E2 treatment (Figure 1A). This suggested that DAXX protein expression was dependent on ER activation and inversely correlated with NOTCH4. This ER-dependence of DAXX was further supported by Western blot results indicating DAXX protein levels did not change in triple negative breast cancer cell lines MDA-MB-231 or MDA-MB-468, in response to E2 (Figure 1B). Additionally, patient derived ER+ xenografts (PDX BCM-5097) grown in the presence of physiologic E2 resulted in increased DAXX but decreased NOTCH4 protein expression which were reversed upon depletion of E2 for 19 days (Figure 1C). To assess if the DAXX-NOTCH4 protein expression was detectable in TICs, protein expression from bulk cells and mammospheres were quantified and compared. While E2 treatment increased DAXX but decreased NOTCH4 protein in bulk cells, mammospheres were enriched for NOTCH4 protein and had little detectable DAXX protein (Figure 1D). However, E2 treatment modestly increased DAXX and decreased NOTCH4 proteins in mammospheres (Figure 1D). These findings suggest that ER activation by E2 inhibits TICs by inducing the DAXX protein to repress NOTCH4.
Figure 1. E2-mediated ER activation is required for DAXX protein expression.
A. MCF-7, BT474 and T47D cells were treated with ethanol (0nM E2), 5nM E2, or E2 + fulvestrant (Fulv) (100nM) for 3 days. ERα, NOTCH4, and DAXX protein levels were detected by Western blotting. B. MDA-MB-231 (231) and MDA-MB-468 (468) cells were treated with ethanol (0nM E2), 5nM E2 for 3 days followed by Western blotting for DAXX. C. PDX BCM-5097 tumors were harvested for detection of ERα, NOTCH4, and DAXX protein levels. D. MCF-7 and T47D cells were transfected with non-specific (SCBi) or DAXX-specific (DAXXi) siRNA for 2 days and then grown for 3 days with 0nM E2, 5nM E2, or 5nM E2 + 100nM fulvestrant followed by Western blotting to detect NOTCH4 and DAXX proteins. After 7 days, mammospheres were harvested by pooling together three wells and NOTCH4 and DAXX proteins were detected by Western blotting.
To determine whether E2-induced DAXX protein was due to increased RNA transcript levels, real-time PCR was performed to detect DAXX transcripts. Levels of DAXX transcripts did not change in response to increasing concentrations of E2 (0, 0.50, 5.0, or 50nM) (Figure 2A). To confirm that ER is functional in both cell lines, transcript expression of a classical ER-inducible gene, TIF1 (PS2) was measured in response to increasing concentrations of E2. PS2 transcript levels increased in response to E2 peaking at 5nM (Figure 2A). Based on these results, E2-mediated activation of the ER may increase DAXX levels by stabilizing the protein. The proteasome inhibitor, MG132, was used to assess whether proteasome activity is required for the decrease in DAXX protein in response to ET. In the absence of MG132 (DMSO), DAXX protein expression was low in the absence of E2 and increased in the presence of 5nM E2 in both cell lines (Figure 2B). In the presence of MG132, DAXX protein expression is higher with ET and remains relatively unchanged with E2 treatment (Figure 2B), suggesting ET promotes degradation of DAXX protein by the proteasome. In order to determine whether the rate of DAXX protein stability is increased by E2 and attenuated by ET, CHX pulse-chase experiments were conducted in the absence or presence of MG132 in a time-dependent manner. E2 deprivation or fulvestrant resulted in a rapid decrease in DAXX protein levels compared to E2 in MCF-7 cells (Figure 2C). This destabilization of DAXX protein is delayed by E2 or is prevented by MG132 treatment (Figure 2C). To achieve a more accurate half-life of DAXX protein decay by ET, a shorter time course of 0 to 4 hours was used as shown in Figure 2C. A DAXX protein decay curve summarized results of 3 independent experiments and was used to calculate the average half-life (t1/2) of the DAXX protein by linear regression analysis (Figure 2D). In summary, the half-life of the DAXX protein is 1.73 hours by E2 deprivation or 1.59 hours by fulvestrant. E2 treatment significantly increases the half-life of DAXX to 10.28 hours (Figure 2D). This suggested DAXX requires E2-mediated ER activation for its protein stabilization, and ET deplete DAXX protein rapidly in ER+ breast cancer cells.
Figure 2. DAXX protein stabilization is mediated by E2-mediated ER Activation.
A. Real-time PCR was performed to measure relative transcript levels of DAXX and PS2 in MCF-7, BT474, and T47D cells after 3 days of treatment with 0 or 5nM E2. Bar graphs show mean ± standard deviation (S.D.) values normalized to HPRT and compared to 0nM E2 based on three independent experiments using the 2−ΔΔCt calculation. A One-way ANOVA was performed on ΔCt values after normalization to HPRT. Symbols denote statistical significance: #= P< 0.05, $= P< 0.01, @= P< 0.001. B. The same cells were treated with 0 or 5nM E2 for 3 days, 12 hours prior to harvesting, cells were treated with vehicle (DMSO) or 10μM MG132. Western blotting was performed to detect DAXX protein levels. C. MCF-7 cells were grown in 0nM E2, 5nM E2, or E2 + 100nM fulvestrant for 24 hours, following which cells were treated with 10μM cycloheximide (CHX) for the indicated times. Western blotting was performed to detect DAXX protein levels. MCF-7 cells treated with 0nM E2 or 5nM E2 + 100nM fulvestrant were treated with10μM CHX for a shorter time course. Western blotting was performed to detect DAXX protein levels. Blots are representative of three independent experiments. D. ImageJ was used to measure densitometry of DAXX protein levels as a ratio to β-ACTIN. The estimated half-life (t1/2) of the DAXX protein for each independent experiment was calculated by linear regression analysis. Means of DAXX protein half-life ± S.D were statistically compared by a One-way ANOVA. @= P< 0.001.
DAXX is required to inhibit NOTCH4, NOTCH activation, and TIC-survival in vitro
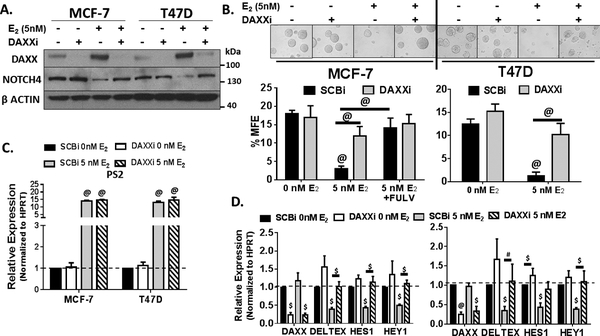
As NOTCH expression and activity have been shown to be repressed by ER and this repression is reversed by ET (4,7,11,27), we hypothesized that E2-mediated ER activation may restrict NOTCH and TIC-survival by increasing DAXX. To test this hypothesis, expression of NOTCH4 protein, canonical NOTCH gene targets (DELTEX1, HES1, and HEY1) and mammosphere forming efficiency (MFE) were measured to assess NOTCH activity and TIC-survival upon DAXX knockdown. MCF-7 and T47D cells were depleted of DAXX using a SmartPool of four DAXX-specific siRNA sequences. These sequences were first individually tested for their effects on DAXX protein expression by Western blot (Suppl Figure 1A). Each individual siRNA in the SmartPool efficiently decreased DAXX protein with the pooled sample being the most efficient. The SmartPool was therefore used for subsequent experiments. E2-mediated ER activation increased DAXX protein but decreased NOTCH4 protein (Figure 3A). E2-mediated decrease in NOTCH4 expression was reversed upon DAXX knockdown (Figure 3A). Further, E2-mediated decrease in %MFE as a measure of TIC-survival was almost completely rescued by DAXX knockdown using the SmartPool (Figure 3B) or individual DAXX siRNAs (Suppl Figure 1B). Two forms of ET, E2-deprivation or fulvestrant significantly increased %MFE compared to E2 treatment (Figure 3B). To assess whether changes in NOTCH4 expression and TIC-survival by DAXX were due to alterations in classical E2-mediated ER activation, expression of PS2 transcripts were measured. Figure 3C showed that while E2 induced PS2 transcripts, this expression was not dependent on DAXX. Also, DAXX knockdown had little effect on E2-induced total bulk cell proliferation (Suppl Figure 2A) and cell cycle progression (Suppl Figure 2B). The effects of E2-mediated DAXX expression and TIC survival were most probably due DAXX-mediated repression of canonical NOTCH gene targets as shown in Figure 3D. Expression of NOTCH direct target genes, DELTEX1, HES1, and HEY1 transcripts were decreased upon E2 treatment and this was reversed when DAXX was knocked down (Figure 3D).
Figure 3. DAXX is required to inhibit NOTCH4 and TIC-survival.
A. MCF-7 and T47D cells were transfected with a non-specific (SCBi) or DAXX-specific (DAXXi) siRNA for 2 days and then treated with 0 or 5nM E2 for 3 days. DAXX and NOTCH4 proteins were detected by Western blotting. B. 50,000 cells were plated into an ultra-low attachment plate containing methylcellulose mammosphere forming medium. After 7 days, mammospheres were imaged at 20X magnification, harvested, and %MFE calculated. Representative images of mammospheres taken by light microscopy are shown. Scale bars = 100μm. Bar graphs show %MFE ± S.D. from three independent experiments. Statistical significance was calculated using a two-way ANOVA with a Tukey post-hoc test for multiple comparisons. Symbols denote statistical significance between 5nM and 0nM E2, SCBi and DAXXi, and E2 and E2+ fulvestrant (FUL): @= P< 0.001. C-D. Real-time PCR was used to detect transcript levels of PS2 and DAXX (Fig. 1C) and NOTCH targets DELTEX, HES1, and HEY1 (Fig. 1D). Bar graphs show mean values ± S.D. of relative transcript expression normalized to HPRT and compared to SCBi + 0nM E2 conditions from three independent experiments using the 2−ΔΔCt calculation. A Two-way ANOVA was performed on ΔCt values after initial normalization to HPRT. Symbols denote statistical significance between SCBi and DAXXi and 0 and 5nM E2: #= P< 0.05, $= P< 0.01, @= P< 0.001.
DAXX is required to suppress TIC-frequency in vivo
The in vitro results indicated that E2-mediated ER activation stabilizes DAXX to repress NOTCH signaling and suppress TIC-survival. To determine whether DAXX is necessary to suppress TIC-survival in vivo, an extreme limiting dilution assay (ELDA) was performed using cells-expressing or depleted for DAXX. DAXX protein expression was efficiently knocked down as shown in Figure 4A. An E2-containing silastic-release capsule was implanted for sustained release of physiologic E2 for up to 8 weeks (21). Tumor incidence was higher in each dilution group when DAXX was depleted compared to control (Figure 4B). A Kaplan-Meier curve of % tumor free mice demonstrated that mice developed tumors faster from DAXX-depleted cells compared to DAXX-expressing cells (Figure 4C). Kaplan-Meier curves for each individual cell dilution show statistical significance at the 1X106 cell dilution group (Suppl Figure 3A–C). Estimated TIC-frequency based on overall tumor frequency was determined for each dilution group using ELDA software (http://bioinf.wehi.edu.au/software/elda), comparing animals injected with control versus DAXX-depleted cells. Analysis indicated an estimated TIC-frequency of 1/589,536 for DAXX-expressing cells versus a frequency of 1/65,072 for DAXX-depleted cells under E2 treatment conditions (Figure 4D). This was an over 9-fold increase in TIC-frequency when DAXX was depleted.
Figure 4. DAXX is necessary to restrict TIC-frequency in vivo.
MCF-7 cells were transfected with a non-specific (SCBi) or DAXX-specific (DAXXi) siRNA for 2 days. 10,000 (1 × 104), 100,000 (1 × 105), or 1,000,000 (1 × 106) cells were resuspended into Matrigel solution (1:1 Matrigel:PBS) and injected into the mammary fat pads of 5 female, athymic, nude mice/dilution. Silastic capsules containing E2 were also implanted for up to 8 weeks. A. DAXX protein levels were detected by Western blotting. B. Tumor area was measured weekly using Vernier calipers. Total # of tumors per group were counted. Tumor images were taken after 8 weeks. C. Kaplan-Meier curve represents the rate of tumor incidence across all cell dilutions. Statistical significance was calculated by the Log-rank (Mantel-Cox) test. D. Estimate of TIC-frequency for each cell dilution group was calculated using the Extreme Limiting Dilution Analysis Software. SCBi and DAXXi TIC-frequency was compared by the Chi-squared test to determine statistical significance. E. Using Kmplotter.com, Recurrence Free Survival (RFS) for women with ER+ breast cancer was compared in DAXX high expressing tumors vs. DAXX low expressing tumors by “best cutoff” as determined by the kmplotter software. The data represent the mean of two probes using the multigene analyzer. Two cohorts of patients were analyzed: systemically untreated (left graph) and endocrine therapy only (right graph) (excluded for chemotherapy). All parameters used to generate these curves are summarized in Supplementary Table 1C. F. Co-expression analysis of the METABRIC cohort comparing DAXX RNA expression vs. ALDH1A1 in ER+ breast cancer patients. DAXX vs. ALDH1A1 RNA expression was evaluated by correlation coefficient (CC) and Q-value.
These in vivo results suggested that DAXX is required for E2-mediated suppression of TIC-frequency, and that ER-targeted therapies deplete DAXX levels allowing for enrichment of TICs. As TICs maybe a primary contributor to cancer recurrence, we used the KMplotter software (22,23) to determine whether DAXX RNA expression correlated with RFS in patients with ER+, breast cancer. Patients were stratified by high or low median DAXX transcript expression separated using the best cutoff as defined by the KMplotter software, and compared the length of time of RFS of two cohorts of patients: those who were systemically untreated (Figure 4E, left graph) or those treated with endocrine therapy, excluding chemotherapy (Figure 4E, right graph). The data presented are the mean of two DAXX probes (201763_s_at and 216038_x_at) using the multigene analyzer. There were no differences in RFS for t systemically untreated patients (Figure 4E, left graph). However, patients with tumors that expressed low DAXX RNA had a significantly shorter RFS compared to high DAXX RNA levels with endocrine therapy treatment alone (Figure 4E, right graph).
Individual RFS data for each probe is provided in Supplementary Figure 4. Both the best median cut off and quartile RFS data are shown along with the Beeswarm plots for each probe (Suppl Figure 4A and 4B). RFS using individual DAXX probes showed similar results (Supple Figure 4) as the mean of the two probes (Figure 4E, right graph). In addition, as nodal status alone is prognostic for RFS, RFS was also determined for patients with ER+ breast cancer based on DAXX RNA expression and nodal status. DAXX using median or quartile RNA expression in ER+, node-negative patients was modestly prognostic for RFS using one probe (Suppl Figure 5A and 5B). Median or quartile expression of DAXX RNA was prognostic for better RFS in patients with ER+, node-positive breast cancer using one probe (201763_s_at) (Suppl Figure 5C), but not for the second probe (216038_x_at) (Suppl Figure 5D). All parameters used to generate these survival curves are summarized in Supplementary Table 1C.
These findings are in agreement with expression of other TIC-associated markers including ALDH1A1 (Figure 4F). DAXX RNA expression inversely correlates with ALDH1A1 expression when analyzing the METABRIC ER+ breast cancer cohort for DAXX vs. ALDH1A1 RNA expression (Figure 4F). These results are in agreement with the current experimental data that indicate high DAXX expression suppresses TICs and may be a clinical biomarker for low rates of ER+ tumor recurrence following ET.
DAXX represses expression of developmental genes
To assess whether DAXX is a transcriptional repressor in ER+ breast cancer, RNA sequencing was performed in E2-treated MCF-7 cells-expressing or depleted for DAXX. Initial comparison of two independent experiments indicates high degree of similarity under parameters of FKPM=10, fold increase > or = 1.5, and p< 0.05 (Suppl Figure 6A–6C). Specifically, 18 genes are shown to be downregulated and 51 genes were upregulated (Suppl Figure 6A) upon DAXX depletion compared to control. Metscape Pathway Analysis identified a number of pathways that were downregulated and upregulated (Suppl Figure 6B) upon DAXX knockdown. Downregulated pathways include response to antineoplastic agents and response to steroid hormone pathways, both of clinical concern for the treatment of ER+ breast cancer (Suppl Figure 6B). Three pathways-significantly upregulated by DAXX depletion include the embryological process of gastrulation, Pre-NOTCH expression, and processing and the NOTCH signaling pathway (Suppl Figure 6B). Further analysis of the genes upregulated in the gastrulation pathway indicated a large number of genes associated with TIC-survival, self-renewal, and cancer recurrence, including SOX2 (28,29), OCT4 (30,31), NANOG (32), ALDH1A1 (33), and NOTCH4 (6,7). These results are summarized in a heat map outlining all of the genes upregulated in the gastrulation pathway upon DAXX depletion (Suppl Figure 6C). The raw and calculated RNA-sequencing data were deposited into the GEO repository with the accession number: GSE134919. These results support a hypothesis that DAXX represses the NOTCH signaling pathway (Suppl Figure 6C). Additionally, DAXX represses expression of pluripotent stem genes (Suppl Figure 6C) to suppress TIC-survival.
DAXX is enriched on TIC-associated gene promoters and is required for gene repression
To determine if DAXX is a nuclear regulator of pluripotent gene transcripts, cell fractionation was conducted to determine the cellular location of DAXX in response to E2 treatment. Fractionation studies show that the DAXX protein is mostly localized in the nucleus of MCF-7 and T47D cells, and this localization increases in response to E2 treatment (Figure 5A). To test if DAXX is recruited to promoter regions of pluripotent genes that promote TIC-survival, DAXX ChIP assays were conducted on genes identified by RNA-sequencing. A transcription factor desert region previously identified was used as a negative control (NOTCH4 Negative) (34) as well as an isotype IgG control. Primer regions flanking regulatory regions of TIC-associated genes are provided in Supplementary Figure 7A. Activator Protein 1 (AP-1) consensus regions were selected due to previous reports that AP-1 drives NOTCH4 transcription (34,35) and that DAXX binds AP-1 to repress AP-1-driven transcription (36). In the absence of E2, when DAXX expression is low, little detectable DAXX is enriched on promoter regions of NOTCH4, SOX2, OCT4, and NANOG in either cells (Figure 5B). Conversely, E2 stimulates enrichment of DAXX on these promoter regions from 40 to nearly 400 fold over IgG (Figure 5B). To test if this enrichment of DAXX at the promoter of these genes is associated with changes in transcript levels, and to confirm RNA-sequencing results, real-time PCR was performed on RNA-extracted from cells treated without and with E2 and when DAXX was expressed or depleted. In both cell lines, E2 significantly decreased SOX2, OCT4, NOTCH4, NANOG, and ALDH1A1 transcripts (Figure 5C). This decrease by E2 was almost completely prevented when DAXX was depleted similar to levels of E2 deprivation (Figure 5C). Additionally, transcript levels of luminal/epithelial (FOXA1 and E-Cadherin) and mesenchymal (N-Cadherin, SNAIL and SLUG) markers were measured by real-time PCR. DAXX was required for expression of epithelial markers FOXA1 and E-Cadherin (CDH1), while E2 deprivation or DAXX depletion increased RNA expression of mesenchymal markers (N-Cadherin, SNAIL, and SLUG) in MCF-7 and T47D cells (Suppl Figures 7B and 7C). Together, these results indicate that DAXX is a critical transcriptional repressor of pluripotent TIC and mesenchymal genes and suppressor of TIC-survival when ER+ breast cancer cells are exposed to E2.
Figure 5. Nuclear DAXX is enriched on TIC gene promoters and is necessary to repress TIC-genes.
A. MCF-7 (left panels) and T47D (right panels) cells were treated with 0 or 5nM E2 for 3 days. DAXX protein was detected by Western blot in total lysates or cellular fractions. Actin was used as a cytosolic control and PARP1 as a nuclear control. B. MCF-7 and T47D cells were treated with 0 or 5nM E2 for 1 day. Cells were fixed, chromatin was isolated and probed using either a non-specific IgG or DAXX-specific antibody (2μg of antibody for each condition), and DNA fragments were isolated and purified. Fold DAXX enrichment as compared to IgG at TIC-associated gene promoters was quantified by real-time PCR. Fold-enrichment was determined by 2−ΔCt calculation. Bar graphs show mean ± S.D. values of fold-enrichment for each gene promoter measured. ΔCt values for each gene promoter from cells treated with 0nM E2 vs. 5nM E2 were compared and statistical significance was calculated using a non-paired, two-sided Student’s T-test. Symbols denote statistical significance between 0nM and 5nM E2: @= P< 0.001. C. MCF-7 and T47D cells were transfected with a non-specific (SBCi) or DAXX-specific siRNA for 2 days. Cells were treated with 0 or 5nM E2 for 1 day. Transcript levels of SOX2, OCT4, NOTCH4, NANOG, and ALDH1A1 (ALDH) were detected by real-time PCR from MCF-7 and T47D cells. Bar graphs show mean ± S.D. values of relative transcript expression normalized to HPRT and compared to SCBi 0nM +E2 conditions from three independent experiments using the 2−ΔΔCt calculation. A Two-way ANOVA was performed using ΔCt values after initial normalization to HPRT. Symbols denote statistical significance between 0nM and 5nM E2 and SCBi and DAXXi groups: #= P< 0.05, $= P< 0.01, @= P< 0.001.
DAXX requires DNMT1 to restrict TIC-survival and TIC-gene expression
To determine if DAXX-mediated repression of TIC-gene expression and TIC-survival required DNMT1, DNMT1 protein expression was first detected by Western blot in the absence or presence of E2. DNMT1 protein expression was high in both ER+ breast cancer cell lines regardless of E2 treatment (Figure 6A). To assess whether CpG methylation or DNMT1 was required for TIC-survival or TIC-gene expression, 5-Azacytidine (AZA) treatment or DNMT1 knockdown was conducted followed by measurement of %MFE or real-time PCR. E2 decreased %MFE as a measure of TIC-survival (Suppl Figure 8A and 8B) and expression of TIC-gene transcripts (Suppl Figure 8C and 8D). AZA treatment almost completely prevented the decreased TIC survival and TIC-associated gene expression by E2 (Suppl Figure 8A and 8C). Similarly, knockdown of DNMT1 by siRNA reversed E2-mediated decrease in %MFE and expression of TIC-gene transcripts (Suppl Figure 8B and 8D). These results indicated that DNMT1 protein levels were abundant in ER+ breast cancer cells and DNMT1 was necessary to suppress TIC-survival and repress TIC-genes in response to E2. To determine if DAXX required DNMT1 to inhibit TIC-survival and associated gene expression, DAXX was overexpressed under conditions where DNMT1 was expressed or depleted. Dual transfection was effective at both increasing DAXX expression and decreasing DNMT1 expression (Figure 6B). Ectopic expression of DAXX was sufficient to restrict TIC-survival when both cell lines were deprived of E2 similar to levels measured in the presence of E2 (Figure 6C). Conversely, ectopic DAXX expression suppressed TIC-survival when DNMT1 was depleted (Figure 6C), suggesting that DAXX required DNMT1 to suppress TIC-survival. In agreement, ectopic DAXX expression decreased TIC-gene transcripts in the absence of E2 similar to levels detected in response to E2 (Suppl Figure 9A). The decreased TIC-gene transcripts by ectopic DAXX expression was reversed by DNMT1 knockdown (Suppl Figure 9A). These results suggest that DNMT1 is required for DAXX-mediated suppression of TIC-survival and TIC-gene expression.
Figure 6. DAXX requires DNMT1 to restrict TIC-survival and transcript expression.
A. MCF-7 and T47D cells were treated with 0 or 5nM E2 for 3 days. DNMT1 and DAXX protein levels were detected by Western blot analysis using Actin as a loading control. B-C. MCF-7 and T47D cells were transfected with a mock vector (EV) or the human DAXX cDNA (DAXX) for 2 days and then re-transfected with the non-specific (SCBi) or DNMT1-specific (DNMT1i) siRNA for an additional day. B. DAXX and DNMT1 proteins were detected by Western blotting. C. After 7 days, mammospheres were imaged, isolated, measured, and %MFE was calculated. Bar graphs show mean ± S.D. %MFE from three independent experiments. Statistical significance was calculated using two-way ANOVA with a Tukey post-hoc test for multiple comparisons. Symbols denote statistical significance between EV and DAXX, SCBi and DNMT1 under DAXX conditions, and 0nM and 5nM E2: #= P< 0.05, @= P< 0.001. D. MCF-7 cells were transfected with a non-specific (SCBi), DAXX-specific (DAXXi), or DNMT1-specific (DNMT1i) siRNA for 2 days. Cells treated with 0 or 5nM E2 for 1 day. Total DNA was isolated and subjected to bisulfite treatment. Bisulfite converted DNA was amplified using SOX2 promoter-specific primers or NOTCH4-CpG island-specific primers that anneal to bisulfite-treated DNA. The PCR product was purified and sent for DNA sequencing. CpG sites that were read as “T” were considered unmethylated and sites read as “C” were considered methylated. Bar graphs show mean (total number of methylated CpG sites)/ (total CpG sites read × 100) ± S.D. from five independent experiments. Statistical significance was calculated using two-way ANOVA with a Tukey post-hoc test for multiple comparisons. Symbol denotes statistical significance between 0nM and 5nM E2 and SCBi and DAXXi/DNMT1i groups: @= P< 0.001.
E2-mediated hypermethylation of the SOX2 promoter and the NOTCH4 gene is dependent on DAXX or DNMT1
To determine whether DAXX or DNMT1 was necessary for hypermethylation of a pluripotent TIC-gene region such as SOX2 and NOTCH4 in response to E2, cells were depleted of DAXX or DNMT1 by siRNA in the absence or presence of E2 followed by bisulfite treatment and DNA sequencing of the SOX2 promoter region or within the body of the NOTCH4 gene containing 9 and 13 CpG sites, respectively. Representative images of each sequencing read of bisulfite-converted DNA are shown in Supplementary Figure 9B. For SOX2, in the absence of E2, approximately 26% of the 9 CpG sites were methylated (Figure 6D, Suppl Figure 9B). Upon E2 treatment, nearly 90% of the 9 CpG sites were methylated (Figure 6D, Suppl Figure 9B). DAXX depletion reduced the methylation status to less than 40% (Figure 6D, Suppl Figure 9B). DNMT1 depletion reduced methylation to 11% (Figure 6D, Suppl Figure 9B). For NOTCH4, E2 deprivation resulted in nearly 35% of the 13 CpG sites being methylated compared to 5nM E2 conditions in which 55% of the sites were methylated (Figure 6D, Suppl Figure 9B). Upon DAXX or DNMT1 depletion, methylation decreased to nearly 45% with DAXX knockdown and to 20% with DNMT1 knockdown, respectively (Figure 6D, Suppl Figure 9B). Of note, DAXX depletion resulted in almost complete hypomethylated CpGs within intron 29, but did not affect CpGs within exon 29 (Suppl Figure 9B). Thus, DAXX may be required for partial methylation of this region of the NOTCH4 gene while DNMT1 was required for most of the region. Together, these results suggested that DAXX or DNMT1 was necessary to hypermethylate the SOX2 promoter in response to E2, while the epigenetic status of the NOTCH4 CpG island was partially regulated by DAXX.
DAXX is sufficient to restrict NOTCH signaling and TIC survival
We hypothesized that if DAXX can be increased under ET conditions, then TIC-survival will be inhibited. To test this hypothesis, DAXX was ectopically expressed using a mammalian expression vector (pCMV6-Entry) and then grown in the absence or presence of E2. Ectopic expression of DAXX was maintained in the absence of E2 (Figure 7A). Upon DAXX expression, NOTCH4 protein was decreased in the absence of E2 to similar levels detected in response to E2 (Figure 7A). Ectopic expression of DAXX significantly inhibited TIC-survival as measured by %MFE under E2 deprived conditions when TIC-survival is normally high in both MCF-7 and T47D cells (Figure 7B). This restriction of TIC-survival was similar to E2 treatment (Figure 7B). Conversely, ectopic expression of DAXX had no significant effect on total bulk cell proliferation regardless of E2 treatment (Suppl Figure 10A), suggesting DAXX was a critical suppressor of TIC-survival and not overall bulk cell survival. Similarly, DAXX overexpression had little effect on the classical ER-responsive gene, PS2 (Suppl Figure 10B), while significantly decreasing NOTCH target gene transcripts, DELTEX1, HES1, and HEY1 (Suppl Figure 10B) and TIC-associated gene transcripts, SOX2, OCT4 (Figure 7C), NOTCH4, NANOG, and ALDH1A1 (Figure 7D). Further, overexpression of DAXX was sufficient to induce expression of epithelial markers [FOXA1 and E-Cadherin (CDH1)], while inhibiting expression of mesenchymal transcripts including N-Cadherin (CDH2) and SLUG in MCF-7 and T47D cells (Suppl Figure 11). Overall these findings suggest that DAXX is a novel breast cancer TIC suppressor by potentially recruiting DNMT1 to hypermethylate some TIC-associated gene regions thus repressing their expression.
Figure 7. DAXX is sufficient to restrict NOTCH4 and TIC-survival in response to ET.
A. MCF-7 and T47D cells were transfected with the EV or DAXX-containing vector using Polyethylenimine (PEI) for 2 days and then treated with 0 or 5nM E2 for 3 additional days. DAXX and NOTCH4 protein levels were detected by Western blot analysis using β-Actin as a loading control. B-C. 50,000 MCF-7 and T47D cells were plated in ultra-low attachment plates containing mammosphere forming medium for 7 days. Mammospheres were imaged, isolated, counted, and %MFE calculated from three independent experiments. Bar graphs show mean %MFE ± S.D. from three independent experiments. Statistical significance was calculated using two-way ANOVA with a Tukey post-hoc test for multiple comparisons: Symbols denote statistical significance between EV and DAXX and 0nM and 5nM E2 groups: $= P< 0.01, @= P< 0.001. C-D. MCF-7 (C) and T47D (D) cells were transfected with the empty vector (EV) or DAXX-containing vector for 2 days and then treated with 0 or 5nM E2 for 1 day. SOX2, OCT4, NOTCH4, NANOG, and ALDH1A1 transcripts were detected by real-time PCR. Bar graphs show mean values ± S.D. of relative transcript expression normalized to HPRT and compared to SCBi 0nM +E2 from three independent experiments using the 2−ΔΔCt calculation. Symbols denote statistical significance between EV and DAXX and 0nM and 5nM E2: #= P< 0.05, $= P< 0.01, @= P< 0.001. A Two-way ANOVA was performed on ΔCt values after initial normalization to HPRT. E. Working model that endocrine therapy selects for TICs by depleting DAXX protein levels. DAXX depletion results in de-repression of TIC-genes promoting TIC survival. TICs contribute to heterogeneous and resistant recurrent tumors. Therapies that stabilize DAXX may prevent TIC survival and prevent ER+ breast cancer recurrence.
Summary of Results (Figure 7E)
New findings showed that the DAXX protein restricted TIC-survival and thus represents a novel target with agents that can stabilize the DAXX protein. The DAXX protein is stabilized E2-mediated ER activation, and conversely ET rapidly destabilized the DAXX protein. ET-mediated depletion of DAXX increased TIC-survival, while having little effect on bulk cell proliferation. Molecular analysis indicated DAXX restricted TIC-survival by potentially binding promoters of pluripotent TIC-associated genes, facilitating hypermethylation of the SOX2 promoter and partly the NOTCH4 gene, and repressing TIC-transcript expression. Endocrine therapy depleted the DAXX protein, resulting in de-repression of TIC-associated genes, enrichment of TICs and possibly poor prognosis, as reflected in worse RFS in patients treated with ET whose ER+ tumors expressed low DAXX transcript expression.
DISCUSSION
In ER+ breast cancer, TICs are thought to contribute to the development of recurrent and/or treatment resistant breast cancer (1,2). This is due to the intrinsic properties of these small percentage of cells, including resistance to ET and their ability to regenerate a heterogeneous tumor (1,2,4,8,37,38). One of the mechanisms by which the ER is thought to reduce TICs is through regulation of TIC-associated genes (SOX2, NANOG, OCT4) and inhibition of the NOTCH signaling pathway (1,2,4,8,28,37,38). ER has been shown to inhibit NOTCH signaling, which is required for TIC-survival and self-renewal in breast cancer (8,39). Anti-Notch therapies such as GSIs are often associated with gastrointestinal toxicity and skin cancer (40). Thus, there is a critical need for new therapeutic targets to reduce TICs in ER+ breast cancer to prevent resistance, tumor recurrence, tumor and severe side effects. Through a biomarker clinical trial (ClinTrials.gov Identifier: ), we identified DAXX as a novel gene whose transcript is upregulated after short exposure to a GSI during ET preceding definitive surgery in a window biomarker trial (19). From publically available expression data (kmplot.com), high DAXX RNA correlates with a longer recurrence free survival in women with ER+ breast cancer following ET (Figure 4E), suggesting it may be a potential positive prognostic factor and a novel therapeutic target.
DAXX was initially characterized as a pro-apoptotic factor by interacting with the receptor tumor necrosis factor receptor superfamily member 6 (FAS, CD95) (41). Initial studies showed that DAXX-mediated activation of JUN N-Terminal Kinase (JNK) was necessary to initiate caspase-independent apoptosis (42). Recently, DAXX has been shown to regulate promyelocytic leukemia protein (PML), to maintain the heterochromatin state, and act as a transcriptional repressor (43). In hormone-dependent prostate cancer, DAXX was shown to recruit DNMT1 for epigenetically silencing of its target genes (44). Further, Morozov et al. showed that epigenetic silencing of the Mesenchymal Epithelial Transition (c-MET) requires DAXX (45). Together these findings suggest that DAXX functions as a transcription repressor through recruitment of DNMT1.
Results from the current study indicate that E2-mediated ER activation stabilizes the DAXX protein. Stable DAXX protein binds to regulatory regions of pluripotent and other stem cell genes, possibly recruiting DNMT1 to hyper-methylate promoter or gene body regions resulting in repression of gene transcription. Thus, ER-mediated DAXX protein stability is potentially responsible for restricting TIC-survival and frequency. Targeted inhibition of ER results in rapid depletion of the DAXX protein, loss of DAXX enrichment at regulatory regions of stem cell genes, hypo-methylation of the SOX2 promoter and partly the NOTCH4 gene, and increased TIC-survival. DAXX could be a novel TIC suppressor as ectopic expression of DAXX reduced pluripotent and stem-gene expression, NOTCH signaling, and TIC-survival when cells were treated with ET. Overall, these findings provide rationale to identify novel DAXX promoting therapeutics that enhance or maintain DAXX levels to selectively target TICs in ER+ breast cancer with the purpose of preventing tumor recurrence.
This notion that ER activation may have beneficial effects in regards to cancer recurrence while paradoxical, is not surprising. E2-mediated ER activation drives both proliferation and differentiation of human mammary stem and progenitor cells (39). The problem is that luminal cells become tumorigenic and grow in response to ER activation (46). This is why initially most patients with ER+ breast tumors respond favorably to ET, and is why ET is considered one of the most effective and least toxic anti-cancer therapies to date. A potential unintended consequence of inhibiting the ER long term in a subset of patients however is the loss of its beneficial properties, namely its ability to drive luminal cell differentiation (47). This has been reported by a number of groups demonstrating that the ER restricts TICs in part by preventing the expression of TIC-associated pluripotency genes and signaling pathways, including NOTCH (5,48). The exact mechanism by which ER restricts pluripotency is not fully understood, but it is thought to be due in part to regulation of epigenetic modifiers. This is demonstrated from findings by Bloushtain-Qimron et al. that indicated cell-fate of normal breast and cancer cells is predominantly determined by their genome-wide methylation pattern (49). Furthermore, they showed that TICs have a similar methylation pattern to that of healthy adult mammary stem cells, which included areas of hypomethylation near genes associated with stem-ness or pluripotency (49). This would suggest that epigenetic modifiers are critical for maintaining a stem-like cell fate or a differentiated state and their dysregulation may be a characteristic of TICs. The current findings support a novel mechanism in which DAXX functions as a repressor of TIC-genes and mediates the actions of ER to suppress TIC survival. The exact mechanism by which ER stabilizes the DAXX protein is not known but currently under active investigation.
Although DAXX RNA is unchanged in response to E2 treatment, DAXX RNA expression in human ER+ tumors is prognostic. The data suggest that protein expression might be a better predictor of response to therapy or recurrence. It is our future goal to screen tumor tissue using a validated DAXX IHC protocol to assess response and recurrence. However, there may be a threshold of DAXX RNA expression that is sufficient to overcome DAXX protein destabilization by endocrine therapy. It is possible that some ER+ tumors-expressing high DAXX RNA levels may be able to maintain DAXX protein levels and avoid selection for TICs. Conversely a subset of patients (as demonstrated by the KMplotter data) may not reach this threshold, thus DAXX protein levels are decreased and this allows for selection of TICs. If this is correct, it would provide further evidence that it may be in fact better to compare the upper quartile (top 25%) and lower quartiles (bottom 25%) in terms of DAXX RNA expression in terms of recurrence free survival in patients.
In translating these results to the clinical, the therapeutic challenge will be to test novel agents that stabilize the DAXX protein under ET conditions to suppress TIC-survival but yet not stimulate tumor growth. The goal will be eliminating the resistant TIC sub-population while debulking the tumor to prevent tumor recurrence during or after ET.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
Estradiol-mediated stabilization of DAXX is necessary and sufficient to repress genes associated with stemness, suggesting that the combination of endocrine therapy and DAXX-stabilizing agents may inhibit tumor recurrence in ER+ breast cancer.
Acknowledgements
Funding was provided by the Breast Cancer Research Foundation (to K.S. Albain and C. Osipo) and National Institute of Health T32 (AI007508) (to K.L. Knight), and the National Science Foundation (MCB1716431) (to A.K. Dingwall).
Footnotes
Conflict of Interest Statement: The authors declare no potential conflicts of interest
REFERENCES
- 1.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A 2009;106(33):13820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 2008;100(9):672–9. [DOI] [PubMed] [Google Scholar]
- 3.Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab 2004;15(5):193–7. [DOI] [PubMed] [Google Scholar]
- 4.Harrison H, Simoes BM, Rogerson L, Howell SJ, Landberg G, Clarke RB. Oestrogen increases the activity of oestrogen receptor negative breast cancer stem cells through paracrine EGFR and Notch signalling. Breast Cancer Res 2013;15(2):R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res 2004;6(6):R605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res 2010;70(2):709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simoes BM, O’Brien CS, Eyre R, Silva A, Yu L, Sarmiento-Castro A, et al. Anti-estrogen Resistance in Human Breast Tumors Is Driven by JAG1-NOTCH4- Dependent Cancer Stem Cell Activity. Cell Rep 2015;12(12):1968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simoes BM, Piva M, Iriondo O, Comaills V, Lopez-Ruiz JA, Zabalza I, et al. Effects of estrogen on the proportion of stem cells in the breast. Breast Cancer Res Treat 2011;129(1):23–35. [DOI] [PubMed] [Google Scholar]
- 9.Gallahan D, Jhappan C, Robinson G, Hennighausen L, Sharp R, Kordon E, et al. Expression of a truncated Int3 gene in developing secretory mammary epithelium specifically retards lobular differentiation resulting in tumorigenesis. Cancer Res 1996;56(8):1775–85. [PubMed] [Google Scholar]
- 10.Hu C, Dievart A, Lupien M, Calvo E, Tremblay G, Jolicoeur P. Overexpression of activated murine Notch1 and Notch3 in transgenic mice blocks mammary gland development and induces mammary tumors. Am J Pathol 2006;168(3):973–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzo P, Miao H, D’Souza G, Osipo C, Song LL, Yun J, et al. Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res 2008;68(13):5226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van de Walle I, Waegemans E, De Medts J, De Smet G, De Smedt M, Snauwaert S, et al. Specific Notch receptor-ligand interactions control human TCR-alphabeta/gammadelta development by inducing differential Notch signal strength. J Exp Med 2013;210(4):683–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiteman P, de Madrid BH, Taylor P, Li D, Heslop R, Viticheep N, et al. Molecular basis for Jagged-1/Serrate ligand recognition by the Notch receptor. J Biol Chem 2013;288(10):7305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sierra RA, Trillo-Tinoco J, Mohamed E, Yu L, Achyut BR, Arbab A, et al. Anti-Jagged Immunotherapy Inhibits MDSCs and Overcomes Tumor-Induced Tolerance. Cancer Res 2017;77(20):5628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopan R Notch signaling. Cold Spring Harb Perspect Biol 2012;4(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandya K, Meeke K, Clementz AG, Rogowski A, Roberts J, Miele L, et al. Targeting both Notch and ErbB-2 signalling pathways is required for prevention of ErbB-2-positive breast tumour recurrence. Br J Cancer 2011;105(6):796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsauskas-Kuprys R, Zlobin A, Osipo C. Gamma secretase inhibitors of Notch signaling. Onco Targets Ther 2013;6:943–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol 2012;30(19):2307–13. [DOI] [PubMed] [Google Scholar]
- 19.Albain KS ZA, Convington KR, Gallagher BT, Hilsenbeck SG, Czerlanis CM, Lo S, Robinson PA, Gaynor ER, Godellas C, Bova D, Czaplicki K, Busby B, Stiff PJ, Fuqua SAW, Miele L, and Osipo C. Identification of a Notch-driven breast cancer stem cell gene signature for anti-Notch therapy in an ER+ presurgical window model. 2015; San Antonio, TX: American Association for Cancer Research; p 4. [Google Scholar]
- 20.Shaw FL, Harrison H, Spence K, Ablett MP, Simoes BM, Farnie G, et al. A detailed mammosphere assay protocol for the quantification of breast stem cell activity. J Mammary Gland Biol Neoplasia 2012;17(2):111–7. [DOI] [PubMed] [Google Scholar]
- 21.O’Regan RM, Cisneros A, England GM, MacGregor JI, Muenzner HD, Assikis VJ, et al. Effects of the antiestrogens tamoxifen, toremifene, and ICI 182,780 on endometrial cancer growth. J Natl Cancer Inst 1998;90(20):1552–8. [DOI] [PubMed] [Google Scholar]
- 22.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat 2010;123(3):725–31. [DOI] [PubMed] [Google Scholar]
- 23.Nagy A, Lanczky A, Menyhart O, Gyorffy B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep 2018;8(1):9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Lewis MT. Establishment of Patient-Derived Xenograft (PDX) Models of Human Breast Cancer. Curr Protoc Mouse Biol 2013;3(1):21–9. [DOI] [PubMed] [Google Scholar]
- 25.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012;486(7403):346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2(5):401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao L, Rizzo P, Osipo C, Pannuti A, Wyatt D, Cheung LW, et al. Notch-1 activates estrogen receptor-alpha-dependent transcription via IKKalpha in breast cancer cells. Oncogene 2010;29(2):201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung EY, Askarian-Amiri ME, Sarkar D, Ferraro-Peyret C, Joseph WR, Finlay GJ, et al. Endocrine Therapy of Estrogen Receptor-Positive Breast Cancer Cells: Early Differential Effects on Stem Cell Markers. Front Oncol 2017;7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domenici G, Aurrekoetxea-Rodriguez I, Simoes BM, Rabano M, Lee SY, Millan JS, et al. A Sox2-Sox9 signalling axis maintains human breast luminal progenitor and breast cancer stem cells. Oncogene 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu T, Liu S, Breiter DR, Wang F, Tang Y, Sun S. Octamer 4 small interfering RNA results in cancer stem cell-like cell apoptosis. Cancer Res 2008;68(16):6533–40. [DOI] [PubMed] [Google Scholar]
- 31.Kim RJ, Nam JS. OCT4 Expression Enhances Features of Cancer Stem Cells in a Mouse Model of Breast Cancer. Lab Anim Res 2011;27(2):147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang ZJ, You J, Luo WY, Chen BS, Feng QZ, Wu BL, et al. Reduced tumorigenicity and drug resistance through the downregulation of octamer-binding protein 4 and Nanog transcriptional factor expression in human breast stem cells. Mol Med Rep 2015;11(3):1647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1(5):555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clementz AG, Rogowski A, Pandya K, Miele L, Osipo C. NOTCH-1 and NOTCH-4 are novel gene targets of PEA3 in breast cancer: novel therapeutic implications. Breast Cancer Res 2011;13(3):R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Iwata F, Grass JA, Osborne CS, Elnitski L, Fraser P, et al. Molecular determinants of NOTCH4 transcription in vascular endothelium. Mol Cell Biol 2005;25(4):1458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cermak L, Simova S, Pintzas A, Horejsi V, Andera L. Molecular mechanisms involved in CD43-mediated apoptosis of TF-1 cells. Roles of transcription Daxx expression, and adhesion molecules. J Biol Chem 2002;277(10):7955–61. [DOI] [PubMed] [Google Scholar]
- 37.Jordan CT. Cancer stem cells: controversial or just misunderstood? Cell Stem Cell 2009;4(3):203–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piva M, Domenici G, Iriondo O, Rabano M, Simoes BM, Comaills V, et al. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol Med 2014;6(1):66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaMarca HL, Rosen JM. Estrogen regulation of mammary gland development and breast cancer: amphiregulin takes center stage. Breast Cancer Res 2007;9(4):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purow B Notch inhibition as a promising new approach to cancer therapy. Adv Exp Med Biol 2012;727:305–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Khosravi-Far R, Chang HY, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 1997;89(7):1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang HY, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 1998;281(5384):1860–3. [DOI] [PubMed] [Google Scholar]
- 43.Salomoni P, Khelifi AF. Daxx: death or survival protein? Trends Cell Biol 2006;16(2):97–104. [DOI] [PubMed] [Google Scholar]
- 44.Puto LA, Benner C, Hunter T. The DAXX co-repressor is directly recruited to active regulatory elements genome-wide to regulate autophagy programs in a model of human prostate cancer. Oncoscience 2015;2(4):362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morozov VM, Massoll NA, Vladimirova OV, Maul GG, Ishov AM. Regulation of c-met expression by transcription repressor Daxx. Oncogene 2008;27(15):2177–86. [DOI] [PubMed] [Google Scholar]
- 46.Osborne CK. Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat 1998;51(3):227–38. [DOI] [PubMed] [Google Scholar]
- 47.Ito T, Sato N, Yamaguchi Y, Tazawa C, Moriya T, Hirakawa H, et al. Differences in stemness properties associated with the heterogeneity of luminal-type breast cancer. Clin Breast Cancer 2015;15(2):e93–103. [DOI] [PubMed] [Google Scholar]
- 48.Farnie G, Clarke RB. Mammary stem cells and breast cancer--role of Notch signalling. Stem Cell Rev 2007;3(2):169–75. [DOI] [PubMed] [Google Scholar]
- 49.Bloushtain-Qimron N, Yao J, Snyder EL, Shipitsin M, Campbell LL, Mani SA, et al. Cell type-specific DNA methylation patterns in the human breast. Proc Natl Acad Sci U S A 2008;105(37):14076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.