Abstract
Background:
Obesity is a known modifiable risk factor associated with adverse outcomes in children with cancer. We sought to determine if obesity during childhood cancer treatment increases risk for second malignant neoplasms (SMNs).
Methods:
In this case-control study, cases (with SMN) and controls (with a single primary cancer) were selected from the California Cancer Registry who had primary cancer diagnosed <21 years treated at Children’s Hospital Los Angeles (CHLA) between 1988–2014. Controls were matched 3:1 to cases at the registry level by clinical factors. Medical records were abstracted for cancer treatment exposures, cancer predisposition syndrome, body mass index (BMI), BMI Z-score, and BMI category at diagnosis and EOT.
Results:
Fifty-nine cases and 130 controls were included. Median age at primary cancer diagnosis was 6 years, 64.5% were male, median time from primary cancer to SMN was 7.5 years, and 31.7% were obese or overweight. In matched multivariable analyses, there were elevated but non-significant associations between SMN and higher BMI Z-score at diagnosis (OR 1.27 [0.99–1.63]) and higher BMI categories at diagnosis (adjusted OR [aOR] overweight 1.25 [0.55–2.52]; aOR obese 2.51 [1.00–6.29]). There was a significantly increased risk for SMN among patients who were obese at both diagnosis and EOT (aOR 4.44 [1.37–14.34]).
Conclusions:
This study suggests obesity during childhood cancer treatment may be associated with increased risk for SMNs, particularly among those obese throughout therapy.
Impact:
Additional studies to confirm these findings and to develop interventions have the potential to impact SMN development in children with cancer.
INTRODUCTION
Because long-term cancer survival for children and adolescents now exceeds 80% (1), late effects of therapy have assumed great importance for this growing population. Among these, second malignant neoplasms (SMNs) lead to more deaths among 25-year survivors of cancer than any other cause (2). Compared with development of first cancers among the general population, survivors of childhood cancer have a six-fold higher risk of developing SMNs, with a 30-year cumulative incidence of 9.3% (3,4). Known risk factors for SMN include younger age at primary cancer diagnosis, female sex, primary cancer type, genetic predisposition, longer follow-up time, and exposure to alkylators, anthracyclines, epipodophyllotoxins, and ionizing radiation (4–12).
Another potential contributor to SMNs is obesity, a condition associated with increased risk for cancer and adverse outcomes in cancer patients. Among adults, obesity is associated with an increased risk for developing many cancer types (13,14), including esophageal (15), liver (16), gallbladder (17), pancreas (18), breast (19,20), gastric (15), uterine (21), ovarian (21,22), endometrial (23), kidney (24), colorectal cancer (25,26), and meningioma (27,28). Obese adults with breast cancer demonstrate poorer survival (29), and obese children have demonstrated excess chemotherapy toxicity, higher rates of cancer relapse, and lower overall survival compared to patients with normal BMI (29–33). Several mechanisms, such as hormonal changes, pro-inflammatory states, and chemotherapy sequestration have been proposed to explain how obesity may create a microenvironment that protects the tumor from chemotherapeutic agents and promotes cancer cell growth (34–39). These observations offer biologic plausibility for obesity influencing the development of SMNs, perhaps in combination with known oncogenic effects of cancer treatments. With obesity affecting up to 15–30% of childhood cancer survivors (40–42), it is important to evaluate this as a candidate risk factor for developing SMNs, especially since it is potentially modifiable.
We undertook an exploratory study to evaluate the independent impact of obesity during cancer treatment on the development of SMN in pediatric cancer survivors. Our primary aim was to assess BMI at primary cancer diagnosis as a potential risk factor for SMN after controlling for known treatment and host factors. A secondary aim included evaluating BMI at end of therapy (EOT) to determine whether changes in BMI over time could also impact the risk of SMNs. Our general hypothesis was that obesity during cancer treatment is associated with increased risk for SMNs.
METHODS
Case and Control Selection
This case-control study involving medical record review was approved by the Children’s Hospital Los Angeles (CHLA) Institutional Review Board and was in accordance with good research practices. To identify cases and controls, we utilized longitudinal data from the California Cancer Registry (CCR), a large, well-established SEER (Surveillance, Epidemiology and End Results) registry that collects demographic, clinical, treatment, and follow-up vital statistics data on all incident cancers (excluding skin basal and squamous carcinomas) among residents of California.
Cases were defined as patients diagnosed at <21 years old who had a primary diagnosis of invasive cancer (cancer that has spread beyond the tissue in which it develops, ie. not in-situ cancers) at CHLA between January 1988 and December 2014 (date of registry data completion at time of study) and were later diagnosed at any time after primary cancer with a second invasive cancer (SMN) anywhere in California through December 2014. Controls were selected via the registry from the entire cohort of all pediatric cancer patients <21 years old diagnosed at CHLA between January 1988 and December 2014 who did not develop SMN. Controls were matched using registry variables by the following criteria in order to control optimally for other known potential risk factors for SMN: age at diagnosis of primary cancer (exact age if possible, but extended to <1, 1–9, 10–20 years old if matching by exact age yielded insufficient number of matches), sex, cancer site, histology code, radiation exposure for upfront therapy (no/yes), stage (exact stage if possible, but extended to dichotomous localized/regional vs. distant if matching by exact stage yielded insufficient number of matches), year of diagnosis (if possible), and days of follow-up (matched controls had to be followed at least as long as the interval between the case’s primary diagnosis and SMN in order to minimize survival bias). The goal was to match three controls per case with exact matching criteria. If fewer than three controls were available, some characteristics (age, stage, year of diagnosis) were relaxed (as described above) to achieve the three controls. If there were more than three controls found to match per case, controls were selected with the closest year of diagnosis. Two cases did not have any good matches based on all strict matching criteria, however were included in the analysis and matched with controls that were mismatched on only one of the strict criteria (one mismatched on sex, one mismatched on upfront radiation exposure); we confirmed after chart review that these pairings had no impact on chemoradiotherapy treatment exposure.
Chart Abstraction and Definition of Variables
Categorization of race/ethnicity (non-Latino white, Latino white, black, Asian/Pacific Islander, other) was collected at the registry level. Race/ethnicity is reported to the registry by the hospitals and physician offices as documented in the patient’s medical record.
Abstraction of medical records for the selected cases and controls was performed by two CHLA physicians (DM and LC) for all treatment received at CHLA using pre-defined variables of interest and strict adherence to a detailed protocol. Information on treatment patients received elsewhere was not available. Medical record data were censored as of the date of SMN for cases or the corresponding follow-up time for controls matched to each case.
Obesity-related variables included BMI (calculated from height in centimeters and weight in kilograms), BMI Z-score, and BMI category at initial diagnosis and EOT. BMI as kg/square meter (m2) and corresponding pediatric BMI Z-score (for patients up to 20 years old) (43) were calculated for each patient at each time point. In order to include comparable BMI Z-scores for twelve measurements of ten patients ≥20 years old (age range, 20.0–22.0 years), Z-score was estimated as if the collected height and weight were for a patient 19.99 years old. A BMI Z-score of 0.0 corresponded to the 50th percentile of BMI, 1.04 to the 85th percentile of BMI (overweight threshold), and 1.64 to the 95th percentile of BMI (obese threshold) for sex and height. For BMI category, BMI percentile for age and sex for patients ≥2 and <20 years old, and weight for length (WFL) for patients <2 years old were obtained and categorized using the United States Centers for Disease Control pediatric calculator and World Health Organization infant calculator, respectively (44,45). Patients were categorized into BMI category as normal/underweight (BMI<85% or WFL<95% for age and sex), overweight (85%≤BMI<95% or WFL ≥95% for age and sex), or obese (BMI≥95% for age and sex) (44,46). For patients ≥20 years old, BMI category was categorized as normal or underweight BMI<25 kg/m2, overweight 25≤BMI<30 kg/m2, and obese BMI≥30 kg/m2.
Genetic predisposition variables included documentation of suspected or diagnosed cancer predisposition syndrome (pre-specified as Down syndrome, BRCA1 or 2 mutation, Li-Fraumeni syndrome, neurofibromatosis type 1 or 2, tuberous sclerosis, Beckwith-Wiedemann syndrome, retinoblastoma, hemihypertrophy, von Hippel Lindau syndrome, or other). To minimize detection bias, cases that were not suspected to have an underlying genetic predisposition syndrome until after SMN diagnosis were categorized as not having an underlying syndrome, since the controls would not have had this additional scrutiny.
The selected matching criteria was selected in order to, as nearly as possible, approximate treatment exposures between cases and controls. To confirm cases and controls had similar treatment exposures, treatment data were obtained from one of the following sources: cumulative chemotherapy and radiation doses documented in CHLA survivorship clinic treatment summaries; chemotherapy and radiation doses indicated in individualized roadmaps; or total doses administered as documented in the patient’s medical record. Treatment-related data included radiation dose and field from the radiation oncology treatment summary, cumulative doses of alkylating agents (in cyclophosphamide-equivalent dose [CED]) (47), anthracyclines (in doxorubicin-equivalent dose) (48), epipodophyllotoxins, platinum-based chemotherapy (5), and EOT date. Treatment doses for controls were censored at follow-up time that the matched case developed SMN. Treatment factors were categorized as follows: upfront radiation exposure as this is collected at the registry level and was a matching criteria (no/yes), radiation for progressive/relapsed disease which is not collected at the registry level and was not a matching criteria (no/yes), cumulative CED (0 mg/m2, 1–4000 mg/m2, >4000 mg/m2), cumulative anthracycline dose (0 mg/m2, 1–169 mg/m2, >169 mg/m2), cumulative epipodophyllotoxin dose (0 mg/m2, 1–1800 mg/m2, >1800 mg/m2), and platinum-based chemotherapy exposure (no/yes). Categorization cutoffs were determined based on sample distribution quartiles.
Statistical Analysis
Conditional logistic regression was performed based on a variable number of controls matched to each case (ranging from one to three). Risk factors for development of SMN were assessed by univariate followed by multivariable analyses for all included matched case-control sets and separately for matched case-control sets that had sufficient EOT data. The final multivariable model for each subset included all factors with p values <0.10 in univariate analysis. Interaction of sex, follow-up time, and BMI category with each candidate predictor variable was assessed. All analyses were performed using pHReg in SAS version 9.4 (Cary, NC). For all analyses, significance was two-sided and set at p<0.05.
RESULTS
Cases and Controls
Seventy-one pediatric cases with SMN were identified by the registry. After exclusion criteria were applied, a total of 64 case-control sets underwent chart review (Figure 1).
Figure 1: Consort Diagram.
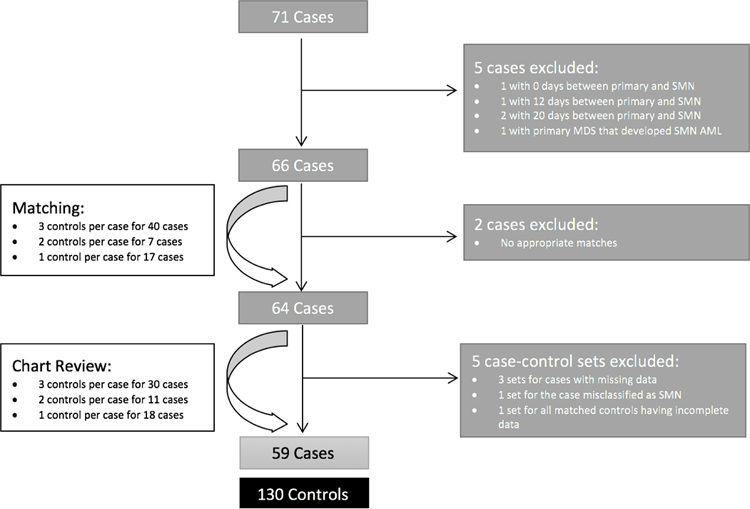
Cases were patients with primary cancer diagnosed at <21 years old that developed SMN. Controls were patients with primary cancer diagnosed at <21 years old that did not develop SMN. SMN=second malignant neoplasm; MDS=myelodysplastic syndrome; AML=acute myeloid leukemia.
After chart review, five additional case-control sets were excluded (three sets for cases having incomplete data, one set for all controls having incomplete data, and one set because the case was later determined to be a testicular relapse misclassified as SMN). Thus, the final sample included 59 cases matched to 130 controls. Three controls were matched per case for 30 cases, two controls per case for 11 cases, and one control per case for 18 cases (Figure 1). The sample of case-control sets with EOT BMI data included 50 cases matched to 108 controls. For the EOT sample, three controls were matched per case for 22 cases, two controls per case for 14 cases, and one control per case for 14 cases.
Demographic and Treatment Characteristics
In aggregate unmatched analysis, our sample of cases and controls was 64.5% male with a median age of 6.0 years at primary cancer diagnosis (Table 1). The most common primary cancer diagnosis was acute lymphocytic leukemia (ALL) (36.5%), followed by Ewing sarcoma/primitive neuroectodermal tumor (PNET) (11.6%), and brain glioma (World Health Organization Grades I-III) (11.6%) (Table 1). Fifty-six percent had distant stage disease at diagnosis (Table 1). Although 34 (18%) patients did not complete therapy at CHLA, and therefore only partial treatment information was able to be extracted, these were equally distributed between cases and controls. As a result of the matching criteria, cases and controls had similar distribution by sex, age at diagnosis, year of diagnosis, radiation exposure as upfront therapy, cancer diagnosis, and stage of disease. Radiation therapy for relapse/progressive disease, chemotherapy exposures and underlying cancer predisposition syndrome distributions were also similar between cases and controls (Table 1).
Table 1:
Demographics, cancer features, treatment factors, and BMI data for cases and controls included in 59 matched sets
| Cases | Controls | P value | |
|---|---|---|---|
| Number of individuals | 59 | 130 | |
| Male | 35 (59.3%) | 85 (65.4%) | 0.42 |
| Median age at diagnosis in years | 5.0 | 7.0 | 0.54 |
| SD [range] | 5.7 [0–20] | 5.3 [0–20] | |
| Age group at diagnosis (in years) | 0.64 | ||
| <1 year old | 4 (6.8%) | 11 (8.5%) | |
| 1–9 years old | 37 (62.7%) | 72 (55.4%) | |
| 10–20 years old | 18 (30.5%) | 47 (36.2%) | |
| Race/Ethnicity | 0.21* | ||
| Non-Latino white | 21 (35.6%) | 40 (30.8%) | |
| Latino white | 29 (49.2%) | 67 (51.5%) | |
| Black | 0 (0.0%) | 8 (6.2%) | |
| Asian/Pacific Islander | 9 (15.2%) | 13 (10.0%) | |
| Unknown | 0 (0.0%) | 2 (1.5%) | |
| Suspected/Confirmed Syndrome at Primary Cancer | 0.26 | ||
| No | 52 (88.1%) | 121 (93.1%) | |
| Yes | 7 (11.9%) | 9 (6.9%) | |
| Year of Diagnosis | 0.37 | ||
| 1988–1997 | 31 (52.5%) | 54 (41.5%) | |
| 1998–2007 | 22 (37.3%) | 60 (46.2%) | |
| 2008–2013 | 6 (10.2%) | 16 (12.3%) | |
| Diagnosis | 0.99* | ||
| Acute lymphocytic leukemia | 18 (30.5%) | 51 (39.2%) | |
| Acute myeloid leukemia | 1 (1.7%) | 3 (2.3%) | |
| Acute leukemia | 1 (1.7%) | 1 (0.8%) | |
| Hodgkin lymphoma | 3 (5.1%) | 9 (6.9%) | |
| Non-Hodgkin lymphoma | 2 (3.4%) | 3 (2.3%) | |
| Ewing sarcoma/PNET | 8 (13.6%) | 14 (10.8%) | |
| Osteosarcoma/Malignant fibrous histiosarcoma | 4 (6.8%) | 9 (6.9%) | |
| Soft tissue sarcoma/Rhabdomyosarcoma | 3 (5.1%) | 4 (3.1%) | |
| Neuroblastoma | 4 (6.8%) | 9 (6.9%) | |
| Ovarian germ cell tumor | 1 (1.7%) | 1 (0.8%) | |
| Retinoblastoma | 1 (1.7%) | 3 (2.3%) | |
| Wilm’s tumor/Nephroblastoma | 2 (3.4%) | 5 (3.8%) | |
| Brain glioma | 7 (11.9%) | 13 (10.0%) | |
| Brain ependymoma | 1(1.7%) | 1 (0.8%) | |
| Medulloblastoma/PNET | 3 (5.1%) | 4 (3.1%) | |
| Stage | 0.057 | ||
| Localized/Regional | 23 (39.0%) | 56 (43.1%) | |
| Distant | 32 (54.2%) | 73 (56.2%) | |
| Unknown | 4 (6.8%) | 1 (0.8%) | |
| Radiation Exposure (Upfront Therapy) | 0.41 | ||
| No | 33 (55.9%) | 81 (62.3%) | |
| Yes | 26 (44.1%) | 49 (37.7%) | |
| N | 59 | 130 | |
| Radiation Exposure (Progression/Relapse) | 0.20 | ||
| No | 51 (86.4%) | 120 (92.3%) | |
| Yes | 8 (13.6%) | 10 (7.7%) | |
| Cumulative Cyclophosphamide Equivalent Dose (CED): | 0.13 | ||
| 0 | 13 (22.0%) | 21 (16.2%) | |
| 1–4000 | 14 (23.7%) | 50 (38.5%) | |
| 4001+ | 32 (54.2%) | 59 (45.4%) | |
| Cumulative Anthracycline Dose: | 0.68 | ||
| 0 | 14 (23.7%) | 27 (20.8%) | |
| 1–169 | 15 (25.4%) | 41 (31.5%) | |
| 170+ | 30 (50.8%) | 62 (47.7%) | |
| Cumulative Epipodophyllotoxin Dose: | 0.063 | ||
| 0 | 30 (50.8%) | 89 (68.5%) | |
| 1–1800 | 11 (18.6%) | 14 (10.8%) | |
| 1801+ | 18 (30.5%) | 27 (20.8%) | |
| Platinum-based Chemotherapy Exposure | 0.39 | ||
| No | 41 (69.5%) | 98 (75.4%) | |
| Yes | 18 (30.5%) | 32 (24.6%) | |
| Completed therapy at CHLA | 0.80 | ||
| No | 10 (16.9%) | 24 (18.5%) | |
| Yes | 49 (83.0%) | 106 (81.5%) | |
| Records Available at CHLA until Censored | 0.027 | ||
| No | 27 (45.8%) | 38 (29.2%) | |
| Yes | 32 (54.2%) | 92 (70.8%) | |
| N | 59 | 130 | |
| Mean BMI Z-Score Diagnosis (SD) | 0.60 (1.34) | 0.17 (1.34) | 0.043 |
| BMI Category at Diagnosis | 0.16 | ||
| Underweight | 3 (5.1%) | 12 (9.2%) | |
| Normal | 33 (55.9%) | 81 (62.3%) | |
| Overweight | 11 (18.6%) | 25 (19.2%) | |
| Obese | 12 (20.3%) | 12 (9.2%) | |
| N | 50 | 108 | |
| Mean BMI Z-Score EOT (SD) | 0.39 (1.67) | 0.38 (1.50) | 0.95 |
| BMI Category at EOT | 0.041 | ||
| Underweight | 5 (10.0%) | 8 (7.4%) | |
| Normal | 26 (52.0%) | 60 (55.6%) | |
| Overweight | 4 (8.0%) | 24 (22.2%) | |
| Obese | 15 (30.0%) | 16 (14.4%) | |
| BMI Category Change Diagnosis to EOT | 0.066 | ||
| Not Obese to Not Obese | 33 (66.0%) | 89 (82.4%) | |
| Not Obese to Obese | 6 (12.0%) | 10 (9.2%) | |
| Obese to Not Obese | 2 (4.0%) | 3 (2.8%) | |
| Obese to Obese | 9 (18.0%) | 6 (5.6%) | |
P values for categorical variables were calculated using Pearson chi square, p values for continuous variables derived from Wilcoxon rank sum.
Fisher’s exact test. SD= standard deviation; CHLA=Children’s Hospital Los Angeles; PNET=primitive neuroectodermal tumor; EOT=end of therapy.
Characteristics of SMNs
The median time from primary cancer diagnosis to SMN was 7.5 years (standard deviation [SD] 5.6 years, range 0.5–25.3 years). The most common primary cancer was ALL, while the most common SMN was acute myeloid leukemia (AML) (Table 2). Whereas thyroid cancer was the second most common SMN, there were no thyroid cancers as first primaries (Table 2).
Table 2:
Distribution of primary and secondary cancer site/histology for 59 cases by cancer sequence
| Cancer Site/Histology | Primary Cancer n (%) | SMN n (%) |
|---|---|---|
| Acute lymphocytic leukemia | 18 (30.5) | 3.3 (2) |
| Acute myeloid leukemia | 1 (1.7) | 15 (25.4) |
| Acute leukemia, mixed phenotypic | 1 (1.7) | 0 (0.0) |
| Hodgkin lymphoma | 3 (5.1) | 0 (0.0) |
| Non-Hodgkin lymphoma | 2 (3.3) | 1 (1.7) |
| Brain glioma | 7 (11.9) | 9 (15.2) |
| Brain ependymoma | 1 (1.7) | 0 (0.0) |
| Medulloblastoma/PNET | 3 (5.1) | 0 (0.0) |
| Neuroblastoma | 4 (5.6) | 0 (0.0) |
| Ewing sarcoma/PNET | 8 (13.6) | 4 (6.8) |
| Osteosarcoma/Malignant fibrous histiosarcoma | 4 (6.8) | 6 (10.1) |
| Soft tissue sarcoma/Rhabdomyosarcoma | 3 (5.1) | 4 (6.8) |
| Wilm’s tumor/Nephroblastoma | 2 (3.3) | 0 (0.0) |
| Renal Cell Carcinoma | 0 (0.0) | 1 (1.7) |
| Renal Papillary Adenocarcinoma | 0 (0.0) | 1 (1.7) |
| Ovarian germ cell tumor | 1 (1.7) | 0 (0.0) |
| Testicular germ cell tumor | 0 (0.0) | 1 (1.7) |
| Retinoblastoma | 1 (1.7) | 0 (0.0) |
| Thyroid | 0 (0.0) | 10 (16.9) |
| Salivary gland carcinoma | 0 (0.0) | 2 (3.3) |
| Melanoma | 0 (0.0) | 1 (1.7) |
| Rectal adenocarcinoma | 0 (0.0) | 1 (1.7) |
| Breast carcinoma | 0 (0.0) | 1 (1.7) |
SMN=second malignant neoplasm; PNET=primitive neuroectodermal tumor.
BMI Status
In aggregate unmatched analysis, 19% of all cases and controls combined were overweight, and 12.7% were obese at diagnosis (Table 1). For cases compared with controls, a higher proportion of cases were obese at diagnosis (20.3% vs. 9.2%). The mean BMI Z-score was higher for cases than for controls at the time of diagnosis (0.60 vs. 0.17), however the groups had similar mean Z-scores at EOT (Table 1). Similarly, compared to controls, a higher proportion of cases were obese at EOT (30.0% vs. 14.4%), and at both diagnosis and EOT (18.0% vs. 5.6%) (Table 1).
Relationship of BMI and Obesity with Development of SMN
Using conditional logistic regression for matched groups, on univariate analysis we found statistically significant associations between SMN and higher pediatric BMI Z-score at diagnosis (OR 1.30, 95%CI 1.02–1.66, p=0.038), BMI category at diagnosis (OR 2.58 for obese, 95%CI 1.06–6.38, p=0.039, compared to normal/underweight), and moderate epipodophyllotoxin exposure (OR 2.95, 95%CI 1.00–8.76, p=0.051 for 1–1800 mg/m2 compared to 0 mg/m2) (Table 3). There were no associations between race/ethnicity, cumulative CED, cumulative anthracycline dose, or platinum-based chemotherapy exposure and SMN. For the subset of 50 matched groups who had EOT BMI data, there was also an association between BMI category over time and SMN. For patients who were obese at both diagnosis and EOT compared with those who were not obese at both timepoints, the OR for SMN was 4.24 (95%CI 1.35–13.34, p=0.014) (Table 3).
Table 3:
Odd Ratios (ORs) associated with development of SMN based on univariate analysis of matched sets
|
|
Univariate Analysis |
|||
|---|---|---|---|---|
| N matched sets | OR | 95% CI | p value | |
| BMI Z-Score at Diagnosis | 59 | 1.30 | 1.02–1.66 | 0.038 |
| BMI Category at Diagnosis | 59 | |||
| Normal/Underweight | 1.00 | Ref | - | |
| Overweight | 1.24 | 0.56–2.74 | 0.60 | |
| Obese | 2.58 | 1.05–6.38 | 0.039 | |
| Global Null Hypothesis Test | - | - | 0.12 | |
| BMI Z-Score EOT | 50 | 1.06 | 0.82–1.36 | 0.68 |
| BMI Category at EOT | 50 | |||
| Normal/Underweight | 1.00 | Ref | - | |
| Overweight | 0.40 | 0.10–1.46 | 0.16 | |
| Obese | 2.37 | 0.93–6.06 | 0.071 | |
| Global Null Hypothesis Test | - | - | 0.033 | |
| BMI Category Change Diagnosis to EOT | 50 | |||
| Not Obese to Not Obese | 1.00 | Ref | - | |
| Not Obese to Obese | 1.98 | 0.60–6.56 | 0.26 | |
| Obese to Not Obese | 1.34 | 0.20–8.90 | 0.76 | |
| Obese to Obese | 4.24 | 1.35–13.34 | 0.014 | |
| Global Null Hypothesis Test | - | - | 0.09 | |
| Suspected/Confirmed Syndrome at Primary Cancer | 59 | |||
| No | 1.00 | Ref | - | |
| Yes | 2.86 | 0.75–10.98 | 0.12 | |
| Race/Ethnicity | 59 | |||
| Non-Latino white | 1.00 | Ref | - | |
| Black, Latino white, Asian/Pacific Islander, or other | 0.68 | 0.30–1.54 | 0.36 | |
| Radiation Exposure (Progression/Relapse) | 59 | |||
| No | 1.00 | Ref | - | |
| Yes | 1.61 | 0.53–4.89 | 0.40 | |
| Cumulative Cyclophosphamide Equivalent Dose (CED) | 59 | |||
| 0 | 1.00 | Ref | - | |
| 1–4000 mg/m2 | 0.31 | 0.07–1.33 | 0.12 | |
| 4001+ mg/m2 | 0.58 | 0.19–1.78 | 0.34 | |
| Global Null Hypothesis Test | - | - | 0.29 | |
| Cumulative Anthracycline Dose | 59 | |||
| 0 | 1.00 | Ref | - | |
| 1–169 mg/m2 | 1.78 | 0.28–11.43 | 0.54 | |
| 170+ mg/m2 | 2.16 | 0.31–15.16 | 0.44 | |
| Global Null Hypothesis Test | - | - | 0.73 | |
| Cumulative Epipodophyllotoxin Dose | 59 | |||
| 0 | 1.00 | Ref | - | |
| 1–1800 mg/m2 | 2.95 | 1.00–8.76 | 0.051 | |
| 1801+ mg/m2 | 1.99 | 0.68–5.83 | 0.21 | |
| Global Null Hypothesis Test | - | - | 0.13 | |
| Platinum-based Chemotherapy Exposure | 59 | |||
| No | 1.00 | Ref | - | |
| Yes | 1.23 | 0.38–4.01 | 0.73 | |
no significant interactions between sex and BMI Z-score at diagnosis, sex and each predictor, BMI category and each predictor, follow-up time and BMI Z-score at diagnosis; SMN=second malignant neoplasm; EOT=end of therapy.
On multivariable analysis, after controlling for epipodophyllotoxin dose (the only treatment factor with association on univariate analysis p<0.10), there were elevated but non-significant associations of higher BMI Z-score at diagnosis and higher BMI categories with SMN, but they did not reach statistical significance (Table 4). However, in a multivariable model that included the 50 case-control matched sets with EOT BMI data available, after controlling for epipodophyllotoxin dose, there was a significantly increased risk of SMN for patients who were obese at both diagnosis and EOT compared to those who were not obese at both timepoints (aOR 4.44, 95%CI 1.37–14.34, p=0.013) (Table 5). Those who were not obese at diagnosis and then became obese at EOT had an elevated risk of SMN, though this did not reach statistical significance. Conversely, patients who were obese at diagnosis but not EOT did not display an increased risk of SMN, but the numbers were small (Table 5).
Table 4:
Adjusted ORs (aORs) associated with development of SMN based on multivariable analysis: all matched sets, n=59
| Multivariable Model 1 |
Multivariable Model 2 |
|||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | p value | aOR | 95%CI | p value | |
| BMI Z-score at Diagnosis | 1.27 | 0.99–1.63 | 0.06 | |||
| BMI Category at Diagnosis | ||||||
| Normal/Underweight | 1.00 | Ref | - | |||
| Overweight | 1.25 | 0.55–2.82 | 0.60 | |||
| Obese | 2.51 | 1.00–6.29 | 0.05 | |||
| Global Null Hypothesis Test | - | - | 0.15 | |||
| Cumulative Epipodophyllotoxin Dose | ||||||
| 0 | 1.00 | Ref | - | 1.00 | Ref | - |
| 1–1800 mg/m2 | 2.59 | 0.85–7.92 | 0.09 | 2.80 | 0.92–8.48 | 0.07 |
| 1801+ mg/m2 | 2.00 | 0.67–5.97 | 0.21 | 2.06 | 0.69–6.12 | 0.20 |
| Global Null Hypothesis Test | - | - | 0.21 | - | - | 0.16 |
Factors with p <0.10 in univariate analysis included in multivariable model. SMN=second malignant neoplasm.
Table 5:
Adjusted ORs (aORs) associated with development of SMN based on multivariable analysis: all matched sets with data on BMI at end of therapy (EOT), n=50
| Multivariable Model 3 |
Multivariable Model 4 |
|||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | p value | aOR | 95%CI | p value | |
| BMI Category at EOT | ||||||
| Normal/Underweight | 1.00 | Ref | - | |||
| Overweight | 0.46 | 0.12–1.74 | 0.25 | |||
| Obese | 2.65 | 0.99–7.09 | 0.051 | |||
| Global Null Hypothesis Test | - | - | 0.03 | |||
| BMI Category Change Diagnosis to EOT | ||||||
| Not Obese to Not Obese | 1.00 | Ref | - | |||
| Not Obese to Obese | 2.23 | 0.63–7.89 | 0.21 | |||
| Obese to Not Obese | 1.05 | 0.15–7.53 | 0.96 | |||
| Obese to Obese | 4.44 | 1.37–14.34 | 0.013 | |||
| Global Null Hypothesis Test | - | - | 0.079 | |||
| Cumulative Epipodophyllotoxin Dose | ||||||
| 0 | 1.00 | Ref | - | 1.00 | Ref | - |
| 1–1800 mg/m2 | 2.90 | 0.92–9.14 | 0.07 | 3.03 | 0.96–9.61 | 0.059 |
| 1801+ mg/m2 | 1.89 | 0.59–6.04 | 0.28 | 2.10 | 0.65–6.77 | 0.21 |
| Global Null Hypothesis Test | - | - | 0.19 | - | - | 0.15 |
Factors with p <0.10 in univariate analysis included in multivariable model. SMN=second malignant neoplasm; EOT=end of therapy.
Given the high proportion of AML as SMN in this cohort, we performed sub-analyses on case-control sets with SMN limited to AML diagnosis (n=15) and those with SMN excluding AML (n=44) after controlling for epipodophyllotoxin dose. We found similar associations between higher BMI category SMN whether the SMN was AML (BMI Z-score at diagnosis OR 1.24, 95%CI 0.75–2.05 p=0.41; OR for obese 6.00, 95%CI 0.67–53.87, p=0.11 compared to normal/underweight) or excluded AML (BMI Z-score at diagnosis aOR 1.27, 95%CI 0.94–1.73, p=0.12; aOR for obese 2.00, 95%CI 0.60–6.60, p=0.26 compared to normal/underweight).
DISCUSSION
In this case-control study of childhood cancer survivors, we found evidence suggesting that obesity at diagnosis and EOT might be associated with an increased risk for SMN. After controlling for other patient and treatment factors, the risk for developing SMN was elevated for patients who had been obese or overweight at start of treatment, with indications of greater risk for those who were obese, indicating a potential dose-response relationship with BMI category. Most strikingly, for patients with BMI data available from both diagnosis and EOT, those who had been obese at both time points exhibited a significant >4-fold higher risk of SMN compared to those who were not. We observed some indication that among those patients who were obese at diagnosis but not EOT, the degree of risk of SMN was reduced, but the sample size was small. These findings provide a preliminary signal that obesity at diagnosis and EOT might be associated with a predisposition for SMN development among childhood cancer survivors. Even though the underlying mechanism linking obesity during chemoradiotherapy exposure to SMNs has yet to be elucidated, the burgeoning work done examining the relationship between obesity and cancer provide several biological pathways that explain why obese patients may harbor a microenvironment that promotes genetic alterations and cancer cell growth during chemoradiotherapy exposure, including obesity-induced hormonal derangements, heightened inflammation, and altered pharmacodynamics of chemotherapy (34–39). The findings of this study are clinically significant because SMNs are an important source of substantial added morbidity and mortality, and also because obesity is potentially modifiable through targeted interventions.
Our findings add to the growing list of known concerns for obese children treated for cancer, which include poorer disease response, inferior survival, and increased treatment-related toxicity. In a cohort of pediatric leukemia patients, those with higher BMI had increased risk of residual leukemia after induction chemotherapy than their counterparts with normal BMI (32). Similar to the increased risk of residual disease in obese patients, being overweight or obese has also been associated with increased relapse rates in children after treatment for leukemia (32,33). Obesity and overweight status has also been associated with increased treatment related toxicity and decreased survival in children with ALL when compared to patients with normal BMI (30,31,33,49).
Excess weight is an important health factor affecting a large proportion of pediatric cancer patients and survivors (25,27,37). In our study which included all invasive cancer diagnoses, 39% of cases and 28% of controls were overweight or obese at the time of diagnosis. Since higher BMI is known to increase the risk for multiple serious late effects and life-limiting comorbidities, such as heart disease, metabolic syndrome, and diabetes (41,50,51), our study suggests that SMN should also be regarded as another potential obesity-related late-effect among survivors.
In this study, the most common SMN diagnosed was AML, followed by thyroid cancer, and brain glioma. This is in contrast to the Childhood Cancer Survivor Study, the British Childhood Cancer Survivor Study, and the Dutch Childhood Oncology Group Late Effect Registry cohorts of 5-year cancer survivors which observed that the most frequent SMNs were breast, thyroid, and central nervous system tumors (4,52,53). Our study differs from these cohorts in that it included SMN at any time after first cancer diagnosis rather than only after 5 years. In our sample, 22 of the 59 SMNs were diagnosed <5 years from primary cancer diagnosis. For the 37 SMNs diagnosed >5 years from diagnosis, the most common were differentiated thyroid cancer (n=9), brain glioma/astrocytoma (n=6), and AML (n=5); we had no breast cancer cases. This study’s shorter duration of median follow-up time and more recent treatment era with reduced chest irradiation may explain the different distributions of SMN cancer types among these different study groups.
Since matching criteria controlled for confounders upfront (including host and cancer related factors which functioned as surrogates for chemotherapy and radiotherapy exposures), the only treatment related variable that was found to be predictive of SMN in this sample was moderate epipodophyllotoxin dose. Somewhat surprisingly, we did not find that an underlying cancer predisposition syndrome at the time of the first cancer diagnosis was associated with SMN (see Supplemental Table 1 for distribution of underlying predisposition syndrome diagnoses of cases and controls). The overall prevalence of genetic predisposition syndromes has been reported to be 8.5% of pediatric cancer patients (54). This is the same percentage that we found at the time of diagnosis of the primary cancer based on the combination of cases and controls. We identified an additional four cases whose underlying syndrome was not identified until the time of the SMN (and did not code them as having a syndrome at diagnosis to avoid detection bias). However, even when these four cases were included in the multivariable model as having an underlying syndrome, the independent effect of obesity and SMN was unchanged.
Our study has several strengths, including the ability to ascertain comparably matched cases and controls at the registry level, the ability to match to one to three controls to each case on multiple factors to control efficiently for potential confounding factors with a small sample size, all cases and controls receiving treatment at one facility and standardized medical records available from one facility, incorporation of accurate chemotherapy and radiation data into the analysis, absence of recall bias given chart abstraction of an objective exposure variable, minimization of detection bias by following a strict protocol of chart review, and evaluation of BMI category over time. Limitations include a heterogeneous group of primary cancers and SMNs, inability to obtain sufficient BMI data at time of SMN or corresponding censor date, a relatively small sample, and inability to abstract non-CHLA treatment data for patients not followed at CHLA until censor date. Despite the small sample size, we found a consistent association between obesity and SMN that was particularly significant in patients who were obese both at diagnosis and EOT. From this observational study, it appears that obesity at diagnosis and EOT are likely highly correlated. Without interventions in place, patients who are obese at the start of therapy are most likely to be obese at EOT, and likely throughout their lifetimes. While we were unable to control for all potential external environmental and genetic factors that contribute to a patient’s BMI category, this study provides support for continuing to study obesity and the effects of weight reduction in survivors of childhood cancer. If these findings are replicated, this would provide important justification for developing strategies to reduce obesity after diagnosis and prevent obesity during cancer treatment. These efforts may be helpful for preventing a variety of obesity-related sequelae in cancer survivors, including SMN.
Supplementary Material
*Fisher’s exact test. NF1=Neurofibromatosis type 1.
Acknowledgments:
Diana J. Moke was supported by grant T32 CA09659 from the National Institutes of Health.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800010I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute, Cancer Registry of Greater California. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Franklin Soriano provided invaluable assistance in procuring archived medical records for abstraction.
Abbreviation Table
- aOR
Adjusted odds ratio
- ALL
Acute lymphocytic leukemia
- AML
Acute myeloid leukemia
- BMI
Body mass index
- CCR
California Cancer Registry
- CCSS
Childhood Cancer Survivorship Study
- CED
Cyclophosphamide equivalent dose
- CHLA
Children’s Hospital Los Angeles
- CI
Confidence interval
- EOT
End of therapy
- OR
Odds ratio
- PNET
Primitive neuroectodermal tumor
- SD
Standard deviation
- SEER
Surveillance, Epidemiology, and Ends Results
- SMN
Second malignant neoplasm
- WFL
Weight for length
Footnotes
The authors declare no potential conflicts of interest.
REFERENCES
- 1.National Cancer Institute.. Childhood Cancer by the International Classification of Childhood Cancer (ICCC), Cancer Statistics Review, 1975–2004. Table XXIX [Google Scholar]
- 2.Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 2008;100:1368–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis R, Freedman D, Ron E, Ries L, Hacker D, Edwards B, et al. New malignancies among cancer survivors: SEER cancer registries, 1973–2000. NIH Pub 2006;No. 05–5302 [Google Scholar]
- 4.Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol 2009;27:2356–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neglia J, Friedman D, Yasui Y, Mertens A, Hammond S, Stovall M, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst 2001;93:618–29 [DOI] [PubMed] [Google Scholar]
- 6.Ng A, Kenney L, Gilbert E, Travis L. Secondary malignancies across the age spectrum. Semin Radiat Oncol 2010;20:67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turcotte LM, Liu Q, Yasui Y, Arnold MA, Hammond S, Howell RM, et al. Temporal Trends in Treatment and Subsequent Neoplasm Risk Among 5-Year Survivors of Childhood Cancer, 1970–2015. JAMA 2017;317:814–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turcotte LM, Neglia JP, Reulen RC, Ronckers CM, van Leeuwen FE, Morton LM, et al. Risk, Risk Factors, and Surveillance of Subsequent Malignant Neoplasms in Survivors of Childhood Cancer: A Review. J Clin Oncol 2018;36:2145–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev 2006;15:2020–6 [DOI] [PubMed] [Google Scholar]
- 10.Travis LB, Rabkin CS, Brown LM, Allan JM, Alter BP, Ambrosone CB, et al. Cancer survivorship--genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Inst 2006;98:15–25 [DOI] [PubMed] [Google Scholar]
- 11.Hawkins MM, Wilson LM, Stovall MA, Marsden HB, Potok MH, Kingston JE, et al. Epipodophyllotoxins, alkylating agents, and radiation and risk of secondary leukaemia after childhood cancer. BMJ 1992;304:951–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkinson HC, Hawkins MM, Stiller CA, Winter DL, Marsden HB, Stevens MC. Long-term population-based risks of second malignant neoplasms after childhood cancer in Britain. Br J Cancer 2004;91:1905–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78 [DOI] [PubMed] [Google Scholar]
- 14.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:872–8 [DOI] [PubMed] [Google Scholar]
- 16.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer 2007;97:1005–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsson SC, Wolk A. Obesity and the risk of gallbladder cancer: a meta-analysis. Br J Cancer 2007;96:1457–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer 2007;120:1993–8 [DOI] [PubMed] [Google Scholar]
- 19.Feola A, Ricci S, Kouidhi S, Rizzo A, Penon A, Formisano P, et al. Multifaceted Breast Cancer: The Molecular Connection With Obesity. J Cell Physiol 2016 [DOI] [PubMed] [Google Scholar]
- 20.Chen GC, Chen SJ, Zhang R, Hidayat K, Qin JB, Zhang YS, et al. Central obesity and risks of pre- and postmenopausal breast cancer: a dose-response meta-analysis of prospective studies. Obes Rev 2016 [DOI] [PubMed] [Google Scholar]
- 21.Brinton LA, Sakoda LC, Frederiksen K, Sherman ME, Kjaer SK, Graubard BI, et al. Relationships of uterine and ovarian tumors to pre-existing chronic conditions. Gynecol Oncol 2007;107:487–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandera EV, Qin B, Moorman PG, Alberg AJ, Barnholtz-Sloan JS, Bondy M, et al. Obesity, weight gain, and ovarian cancer risk in African American women. Int J Cancer 2016;139:593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev 2002;11:1531–43 [PubMed] [Google Scholar]
- 24.Golabek T, Bukowczan J, Szopinski T, Chlosta P, Lipczynski W, Dobruch J, et al. Obesity and renal cancer incidence and mortality--a systematic review of prospective cohort studies. Ann Agric Environ Med 2016;23:37–43 [DOI] [PubMed] [Google Scholar]
- 25.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556–65 [DOI] [PubMed] [Google Scholar]
- 26.Lee DH, Keum N, Giovannucci EL. Colorectal Cancer Epidemiology in the Nurses’ Health Study. Am J Public Health 2016:e1–e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellur SN, Chandra V, Anderson RJ. Association of meningiomas with obesity. Ann Neurol 1983;13:346–7 [DOI] [PubMed] [Google Scholar]
- 28.Wiedmann M, Brunborg C, Lindemann K, Johannesen TB, Vatten L, Helseth E, et al. Body mass index and the risk of meningioma, glioma and schwannoma in a large prospective cohort study (The HUNT Study). Br J Cancer 2013;109:289–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karatas F, Erdem GU, Sahin S, Aytekin A, Yuce D, Sever AR, et al. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast 2016 [DOI] [PubMed] [Google Scholar]
- 30.Orgel E, Genkinger J, Aggarwal D, Sung L, Nieder M, Ladas E. Association of body mass index and survival in pediatric leukemia: a meta-analysis. American Journal of Clinical Nutrition 2016;103:808–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orgel E, Sposto R, Malvar J, Seibel NL, Ladas E, Gaynon PS, et al. Impact on survival and toxicity by duration of weight extremes during treatment for pediatric acute lymphoblastic leukemia: A report from the Children’s Oncology Group. J Clin Oncol 2014;32:1331–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orgel E, Tucci J, Alhushki W, Malvar J, Sposto R, Fu C, et al. Obesity is associated with residual leukemia following induction therapy for childhood B-precursor acute lymphoblastic leukemia. Blood 2014;124:3932–8 [DOI] [PubMed] [Google Scholar]
- 33.Amankwah E, Saenz A, Hale G, Brown P. Association between body mass index at diagnosis and pediatric leukemia mortality and relapse: a systematic review and meta-analysis. Leukemia Lymphoma 2016;57:1140–8 [DOI] [PubMed] [Google Scholar]
- 34.Iyengar N, Hudis C, Dannenberg A. Obesity and cancer: local and systemic mechanisms. Annu Rev Med 2015;66:297–309 [DOI] [PubMed] [Google Scholar]
- 35.Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, Inflammation, and Cancer. Annu Rev Pathol 2016;11:421–49 [DOI] [PubMed] [Google Scholar]
- 36.Kolb R, Sutterwala FS, Zhang W. Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol 2016;29:77–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright C, Simone N. Obesity and tumor growth: inflammation, immunity, and the role of a ketogenic diet. Curr Opin Clin Nutr Metab Care 2016;19:294–9 [DOI] [PubMed] [Google Scholar]
- 38.Howe L, Subbaramaiah K, Hudis C, Dannenberg A. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res 2013;19:6074–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheng X, Mittelman S. The role of adipose tissue and obesity in causing treatment resistance of acute lymphoblastic leukemia. Front Pediatr 2014;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown A, Lupo P, Danysh H, Okcu M, Scheurer M, Kamdar K. Prevalence and Predictors of Overweight and Obesity Among a Multiethnic Population ofPediatric Acute Lymphoblastic Leukemia Survivors: A Cross-Sectional Assessment. Journal of Pediatric Hematology Oncology 2016;38:429–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meacham LR, Gurney JG, Mertens AC, Ness KK, Sklar CA, Robison LL, et al. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer 2005;103:1730–9 [DOI] [PubMed] [Google Scholar]
- 42.Lin MH, Wood JR, Mittelman SD, Freyer DR. Institutional adherence to cardiovascular risk factor screening guidelines for young survivors of acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2015;37:e253–7 [DOI] [PubMed] [Google Scholar]
- 43.Children’s Hospital of Philadelphia Research Institute.. Pediatric Z-Score Calculator. https://zscore.research.chop.edu/index.php.
- 44.Centers for Disease Control and Prevention. BMI Percentile Calculator for Child and Teen https://nccd.cdc.gov/dnpabmi/calculator.aspx
- 45.World Health Organization. Infant Weight for Length Chart https://www.infantchart.com/infantweightlength.php.
- 46.Centers for Disease Control and Prevention. Clinical Growth Charts https://www.cdc.gov/growthcharts/clinical_charts.htm
- 47.Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2014;61:53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, version 4.0 Monrovia, CA: Children’s Oncology Group; 2013. [Google Scholar]
- 49.Eissa HM, Zhou Y, Panetta JC, Browne EK, Jeha S, Cheng C, et al. The effect of body mass index at diagnosis on clinical outcome in children with newly diagnosed acute lymphoblastic leukemia. Blood Cancer J 2017;7:e531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hudspeth VR, Gold SH, Clemmons DR. Diagnosing and Monitoring Endocrine Dysfunction, Diabetes, and Obesity in a Cohort of Adult Survivors of Childhood Cancer. Endocr Pract 2017;23:1394–401 [DOI] [PubMed] [Google Scholar]
- 51.Gunn HM, Emilsson H, Gabriel M, Maguire AM, Steinbeck KS. Metabolic Health in Childhood Cancer Survivors: A Longitudinal Study in a Long-Term Follow-Up Clinic. J Adolesc Young Adult Oncol 2016;5:24–30 [DOI] [PubMed] [Google Scholar]
- 52.Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 2010;102:1083–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhatia S, Armenian SH, Armstrong GT, van Dulmen-den Broeder E, Hawkins MM, Kremer LC, et al. Collaborative Research in Childhood Cancer Survivorship: The Current Landscape. J Clin Oncol 2015;33:3055–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Walsh M, Wu G, Edmonson M, Gruber T, Easton J, et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. Volume 373. New England Journal of Medicine 2015. p 2336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
*Fisher’s exact test. NF1=Neurofibromatosis type 1.


