Abstract
Objective:
Although pediatric obstructive sleep apnea (OSA) is estimated to affect 2–3% of the general population, its prevalence in sickle cell disease (SCD) is much higher, with research suggesting a prevalence rate of upwards of 40%. Despite the similar underlying pathophysiological mechanisms of neurocognitive effects in pediatric OSA and SCD, there is a scarcity of information on how these two conditions interact. The aim of the present study was to better understand the contribution of sleep apnea to neurocognitive deficits in children diagnosed with SCD.
Method:
The present study assessed cognitive function in 26 children with comorbid SCD and OSA, 39 matched comparisons with SCD only, and 59 matched comparison children without a chronic health condition.
Results:
There were significant differences on measures of processing speed and reading decoding, with children without a chronic health condition scoring better than both chronic health condition groups. Additionally, the no chronic health condition group performed better on a test of quantitative knowledge and reasoning and a test of visual-spatial construction than the SCD only group. Contrary to our hypotheses, there were no between group differences suggesting an additive impact of OSA on cognition. Exploratory analyses revealed associations within the group that had OSA showing that more severe OSA correlated with lower performance on measures of processing speed and quantitative knowledge/reasoning.
Conclusion:
Children with comorbid OSA and SCD do not present with greater deficits in cognitive functioning than children with SCD alone. However, severe OSA may confer additional risk for neurocognitive impairments.
Keywords: anemia, sickle cell, neurocognitive, neuropsychologic, pediatric chronic illness, oxygen desaturation, sleep-disordered breathing
Pediatric obstructive sleep apnea (OSA) and sickle cell disease (SCD) are conditions with high co-morbidity that are also associated with problems in oxygen delivery to the brain and mild neurocognitive syndromes. The ways in which these conditions may interact is not well understood. OSA is a severe form of sleep disordered breathing that is estimated to affect 2–3% of children in the general population (Gottlieb et al., 2003; Tauman & Gozal, 2011). However, its prevalence among youth with SCD, a collection of genetic disorders marked by the production of abnormal hemoglobin, is much higher. The high-risk genotypes of SCD (e.g., HbSS, HbSβ0) account for approximately 65% of patients and are associated with more severe complications, while lower risk genotypes (e.g., HbSC, HbSβ+) typically experience milder symptoms. Recent estimates place the prevalence rate of OSA at 41% among children with sickle cell anemia and approximately 10–15% in children with less severe genotypes (Katz et al., 2018; Rosin et al., 2014). This significant increased risk of developing OSA in pediatric SCD underscores the need for a greater understanding of the relationship between the pathophysiology of OSA and SCD, and its associated cognitive impacts.
OSA is characterized by recurrent upper airway obstruction during sleep, resulting in intermittent hypoxia and sleep fragmentation (Tauman & Gozal, 2011). Typical immediate symptoms include labored and noisy breathing, daytime sleepiness, moodiness, and morning headaches (Kheirandish & Gozal, 2006). In the long-term, oxygen deprivation affects brain function, including possible tissue death (cerebral infarction), and behavioral and neurocognitive deficits (Sforza & Roche, 2012). The most common behavioral problems include hyperactivity, attention deficits, and aggression (Gottlieb et al., 2003). In addition, there are numerous areas of cognitive impairments, with studies showing poorer executive functioning, impaired memory skills, and lower general intelligence in children with sleep-related breathing disorders (Beebe et al., 2004; Gottlieb et al., 2004; Gozal, Kheirandish-Gozal, Bhattacharjee, & Spruyt, 2010; Naismith, Winter, Gotsopoulos, Hickie, & Cistulli, 2004). The cognitive deficits are at least partially reversible with early, effective treatment; but with no treatment or late treatment cognitive deficits are likely to persist (Friedman et al., 2003; Gozal & Pope, 2000; Montgomery-Downs, Crabtree, & Gozal 2005). The duration of exposure to sleep apnea, however, is often unclear because the precise onset is unknown.
SCD primarily affects individuals of African descent, with approximately 1:400 African Americans newborns having the disease (Hassell, 2010). The most common symptoms include pain episodes, acute chest syndrome, and neurological complications such as stroke and silent cerebral infarction (Berkelhammer et al., 2007). As life expectancy has increased in recent years, more attention has been paid to the serious neurocognitive complications of SCD and their impact on quality of life (McClellan, Schatz, Sanchez, & Roberts, 2008; Panepinto, O’Mahar, DeBaun, Loberiza, & Scott, 2005). Although research has revealed cognitive deficits in 75% of children that experience silent infarcts, cognitive impairment is not limited to such cases (Schatz, Brown, Pascual, Hsu, & DeBaun, 2001). In fact, recent studies have found deficits in executive functioning, attention, and working memory in children with no known brain insults (Berkelhammer et al., 2007; Schatz et al., 2002; Schatz & McClellan, 2006). These observed cognitive deficits in the absence of overt strokes or silent infarcts may be linked to the insufficient delivery of oxygen and glucose to the brain, resulting in impaired brain function without observable structural damage on clinical imaging studies (Powars et al., 1999; Reed, Jagust, Al-Mateen, & Vichinsky, 1999). Volumetric changes have also been observed in the absence of visible infarcts, providing further evidence that the presence of brain insults alone may not reflect total brain injury (Baldeweg et al., 2006; Schatz & Buzan, 2006; Steen et al., 2005; Kawadler et al., (2013)).
The high rate of comorbidity of OSA and SCD is cause for concern, particularly in light of a common pathophysiological mechanism (hypoxia), and the potential impact of both disorders on cognitive functioning. Although research on the link between SCD and OSA in the study of neurocognitive functioning is limited, the few existing studies suggest that having both disorders increases risk for impaired blood flow to the brain (e.g., stroke, hypoxemia), which is typically a contributor to cognitive deficits in SCD (Kirkham et al., 2001; Robertson et al., 1988). In addition, recent research has identified nocturnal oxygen desaturation associated with sleep-related disordered breathing as a possible contributing factor to executive dysfunction in pediatric SCD (Hollocks et al., 2012). However, the overall paucity of research highlights the need for further studies.
An unpublished study conducted as part of a doctoral dissertation reported on cognitive functioning for 41 children with SCD and OSA compared with 49 children with SCD and negative screenings for OSA (Katz, 2015). This sample with cognitive testing was part of a larger group of children with SCD and OSA who were studied to understand medical morbidity associated with OSA (Katz, Schatz, & Roberts, 2018). The results indicated comparable cognitive functioning between these two groups, suggesting no added impact of OSA on cognitive deficits in SCD. However, the analyses employed a somewhat liberal matching procedure between groups, raising two large concerns. First, analyses included children with OSA who may have received cognitive testing before the onset of OSA symptoms. Second, the groups were matched on health insurance status, which represents a relatively crude measure of socioeconomic status. The current study includes a subset of participants from this larger dataset but employs a more rigorous matching procedure to address these two concerns.
In the present study, we examined the additive effect of having OSA and SCD by retrospectively comparing children with comorbid SCD and OSA to matched comparison groups (SCD with negative screenings for OSA, demographically matched children without a chronic health condition). We hypothesized that there would be greater cognitive impairment on tests tapping into working memory and processing speed in children with both SCD and OSA than in the comparison groups. All cognitive measures are part of a larger developmental screening program that includes measures that have been previously identified as sensitive to SCD-related disease processes (Schatz, Puffer, Sanchez, Stancil, & Roberts, 2009b). We also explored the relationship between severity of sleep apnea and cognitive deficits using the same sleep parameters associated with cognitive deficits identified by Hollocks and colleagues (2012).
Methods
Participants
Study procedures were approved by the medical center institutional review board. Data collection spanned from April 2002 to April 2013. Participants were 124 children between the ages of four and eight years. Twenty-six children were diagnosed with comorbid SCD and OSA (SCD+OSA; age range: 4.64 – 8.15), 39 children with SCD only (age range: 4.92 – 8.08), and 59 children without a chronic health condition (no CHC; age range: 5.10 – 8.15). All participants self-identified as African-American. Participants from the SCD+OSA and SCD only groups were seen at a pediatric hematology/oncology outpatient clinic in the southeastern USA that serves youth from birth through 21 years of age. The clinic provides medical care to approximately 450 SCD patients each year, representing approximately 90% of all known SCD cases in the catchment area. The larger developmental screening program was offered to all children seen at the sickle cell clinic at ages 1, 3, 5, and 7 years of age. One child was tested prior to reaching five years of age due to an error in calculating the child’s age and children who presented with pain or fatigue were tested at their next appointment, resulting in children being tested as early as four years, nine months or as late as eight years, three months (Puffer, Schatz, & Roberts, 2009).
Participants from both the SCD+OSA and SCD only groups were selected from a larger cohort of 136 children with SCD and OSA and 136 matched children with SCD and negative OSA screenings described previously (Katz et al., 2018). These two cohorts were matched on age, gender, SCD genotype severity, and hemoglobin level from the most recent routine complete blood count (CBC) at the time of the medical record review. We identified 26 children with SCD and OSA from this cohort who had: (a) completed cognitive testing as part of routine cognitive screening exams administered at the start of elementary school, (b) received this testing within two years of their OSA diagnosis, and (c) completed testing after a positive screening for clinical symptoms of sleep apnea.
The SCD only comparison group was selected by identifying children from the cohort with negative sleep apnea screenings who had completed routine cognitive testing as part of the same cognitive screening program (n = 49). Initial examination of group demographics indicated potentially meaningful differences in the distribution with more females, children with family incomes in the $10,000–$30,000 categories, and lower-risk genotypes for children with negative OSA screenings. To improve the matching, we selected at random eight males (out of 11 total) with lower-risk genotypes and two females (out of seven total) with lower-risk genotypes from those with family incomes in the $10,000 – $30,000 range, resulting in a final sample of 39 children for the SCD only comparison group. In all cases, random selection was performed by drawing participant numbers at random from a bag.
The demographically matched comparison group (no CHC) were children recruited from after school programs and summer care programs with predominantly African American children from Richland and Sumter counties in South Carolina. Sixty-seven children participated following informed consent from a parent for the study procedures. The no CHC status was determined by parent-report on a structured list of a wide range of medical and developmental conditions and an open-ended question to solicit any conditions not listed. Participants were excluded if any neurodevelopmental disorders (e.g., ADHD, autism) or any major health conditions (e.g., history of cancer) were endorsed that could impact cognitive functioning. To account for differences in age, gender, and income between the pool of participants for the no CHC group and the SCD+OSA group, a total of eight participants from the no CHC group were dropped from analyses. Seven of these participants (out of eight total) were selected at random from among those tested at eight years of age (to better match for age) while the final participant that was dropped was selected at random from the original 10 females in the highest family income group to provide a better match on gender and family income.
OSA screening and diagnosis
Overview.
Inclusion in the SCD+OSA group was determined using a two-step procedure: (1) initial screening by the treating hematologist and (2) an overnight polysomnography. This screening approach was found to have high sensitivity to OSA in the larger cohort studied and identified group differences in medical morbidity. Specifically, 95% of children identified with OSA symptoms using this screening method were diagnosed with OSA using polysomnography and the SCD+OSA group demonstrated higher rates of medical complications, including lung morbidity and infections (Katz et al., 2018).
Initial Screening.
OSA screenings for patients were conducted by the treating hematologist at all routine health maintenance appointments, which range from one to four times per year depending primarily on the child’s age and genotype. The hematologist asked parents about OSA-related behaviors including the presence of snoring and early morning headaches and/or early morning pain. If snoring was endorsed, the hematologist asked follow-up questions on whether the snoring is loud and if it is a concern for the parents. Referrals to further evaluate OSA via overnight polysomnography were made if snoring was reported in conjunction with early morning headache or pain or parent concern regarding the snoring.
Polysonomography.
Children that met the aforementioned criteria were referred for overnight polysomnography in a dedicated sleep lab using the Cadwell Sleep System (Cadwell Laboratories, Inc. Kennewick, WA). The precise protocol varied over time due to changes in instrumentation in the lab. Monitoring included, at minimum, four electroencephalography channels, two electrooculography channels, and two leg electromyography channels used to determine wake/sleep state and arousals. Additional measures were channels for electrocardiography measuring heart rate changes and potential arrhythmias, a snoring microphone, oxygen saturation, oronasal flow thermistor (or nasal pressure sensors), abdominal Sleep Breath respiratory movement, chest respiratory movement, chin electromyography, and plethysmography. In the record review, OSA diagnosis from medical record diagnostic codes was confirmed in the sleep study report. There was variability in the use of the apnea/hypopnea index (AHI) or the respiratory disturbance index (RDI) across clinicians with most reporting only one of these values. Eighteen of the diagnoses for the SCD+OSA group were confirmed with AHI scores and eight were confirmed with RDI scores. We confirmed that the AHI or RDI score was at least 1.0 per hour to indicate that objective criteria supported the clinician diagnosis, which is the approach used by other studies assessing OSA in children (Kuhle, Urschitz, Eitner, & Poets, 2009). The AHI or RDI score, mean oxygen level, and lowest oxygen saturation point (NADIR) were recorded for all participants.
Medical record review
Medical record reviews were conducted for patients seen for routine health maintenance visits between April 2002 and April 2013. Positive screenings for OSA symptoms based on clinical history result in assignment of medical codes for snoring in the medical record and positive exams for OSA based on overnight polysomnography result in the assignment of medical codes for OSA. As such, SCD medical records were examined for diagnostic codes for snoring and/or OSA. Medical record reviews were conducted to confirm OSA overnight polysomnography results, assess descriptive information, and document SCD treatment history for children with SCD who had positive screenings for OSA. A total of 19 participants from the SCD+OSA group received surgical treatment (i.e., adenoid removal). The same medical record reviews were also conducted. All remaining records were reviewed to select controls for the SCD-only group.
Cognitive measures
Processing Speed.
Processing speed was assessed using the Decision Speed subtest of the Woodcock-Johnson Tests of Cognitive Abilities, 3rd edition, which requires children to identify the two pictures from a row of stimuli that are most conceptually similar (McGrew & Woodcock, 2001). Age adjusted standardized scores were used for analyses.
Language Skills.
Three tests from the Test of Language Development-Primary: Third Edition (TOLD-P:3; Newcomer & Hammill, 1997) were administered. The TOLD-P:3 was selected due to its design for children in this age range, reliability at the subtest and domain level, supporting validity data, and lack of cultural bias for African-American children (Newcomer & Hammill, 1997). Oral Vocabulary requires the child to provide definitions for orally presented words. Grammatical Understanding requires that the child select the picture that best demonstrates the meaning of sentences that have increasingly complex syntax. Prior data with this task has suggested that in addition to measuring language skills, this test is likely sensitive to SCD-related deficits in verbal working memory (Sanchez et al., 2010). Word Discrimination requires the child to assess whether two similarly sounding words are the same word or different words (e.g., pig – pig versus big – pig). Age adjusted standardized scores were used for all tests.
Visual-Motor Skills.
Visual–motor skills were assessed using the Beery Developmental Test of Visual-Motor Integration (DVTMI), 5th edition (Beery, 2004). The DVTMI requires children to imitate and copy geometric forms of increasing complexity using pencil and paper. The age-adjusted standardized score was used for analyses.
Early Academic skills.
Academic/pre-academic skills were measured using the Letter-Word Identification and Applied Problems subtests of the Woodcock-Johnson Tests of Achievement, 3rd edition (McGrew & Woodcock, 2001). Letter-Word Identification is a measure of early reading skills and requires the child to identify letters and words without having to know the meaning of a word. Applied Problems serves as a measure of quantitative knowledge and reasoning and requires children to analyze and solve orally presented math problems. Age adjusted standardized scores were used for both subtests.
Data analysis
The Statistical Package for the Social Sciences, 25th Edition (SPSS) was used to conduct all statistical analyses. Chi squared tests and one-way analysis of variance (ANOVAs) tests were conducted to identify any group differences not controlled for by the matching process. Pearson bivariate correlations were run to identify any potential covariates of the relationship between chronic disease and cognitive functioning. One-way analysis of covariance (ANCOVAs) tests were run to evaluate any differences in cognitive functioning between the SCD+OSA, SCD only, and no CHC groups. Family income level was included as a covariate in these ANCOVA’s to reduce unexplained variance in the statistical model. For any significant one-way ANCOVAs, post hoc analyses were run using Tukey’s HSD. The alpha level was set as p < .05 for these analyses. Exploratory analyses were conducted using Pearson bivariate correlations to examine the relationship between measures of OSA severity (hypopnea index, mean O2 saturation, NADIR) and cognitive outcomes. The alpha level was set as p < .017 for these exploratory analyses (.05 / 3 OSA severity variables).
Results
Chi squared tests and one-way ANOVAs revealed no significant differences between the three groups on the matched variables of age, gender, and income distribution. Additionally, there were no significant differences in genotype severity between the SCD+OSA and SCD only groups (see Table 1).
Table 1.
Comparison of clinical and demographic characteristics among children with SCD+OSA, SCD, and no CHC.
| Variable | Group | ||||
|---|---|---|---|---|---|
| SCD+OSA (n = 26) | SCD Only (n = 39) | No CHC (n = 59) | p-value | Test statistic | |
| Age in years (M (SD)) | 6.25 (1.02) | 6.35 (.96) | 6.50 (.93) | p = .479 | F(2, 123) = .74 |
| Gender (n (% Female)) | 14 (53.8%) | 20 (51.3%) | 32 (54.2%) | p = .857 | Χ2 = .087 |
| Household Income (n, %) | p = .970 | Χ2 = 2.31 | |||
| <$10,000 | 6 (23.1%) | 9 (23.1%) | 10 (16.9%) | ||
| $10,000–20,000 | 4 (15.4%) | 8 (20.5%) | 11 (18.6%) | ||
| $20,000–30,000 | 2 (7.7%) | 4 (10.3%) | 9 (15.3%) | ||
| $30,000–40,000 | 6 (23.1%) | 7 (17.9%) | 10 (16.9%) | ||
| >$40,000 | 8 (30.8%) | 11 (28.2%) | 19 (32.2.%) | ||
| SCD genotype (n) | p = .899 | Χ 2= .016 | |||
| (High risk total) | 21 | 31 | −− | ||
| HbSS | 17 | 30 | −− | ||
| HbSβ0 | 4 | 1 | −− | ||
| (Lower-risk total) | 5 | 8 | −− | ||
| HbSC | 4 | 5 | −− | ||
| HbSβ+ | 1 | 3 | −− | ||
| zBMI (M (SD)) | .31 (1.53) | .88 (1.40) | .94 (1.17) | p = .118 | F(2,123) = 2.17 |
| Obese Classification (n, %) | 5 (19.2%) | 12 (30.8%) | 36 (32.2%) | p = .459 | Χ2 = 1.56 |
| Hemoglobin (M (SD)) | 8.86 (1.55) | 9.19 (1.56) | −− | p = .417 | t(61) = .82 |
| WBC count (M (SD)) | 11.57 (3.64) | 11.35 (3.96) | −− | p = .821 | t(61) = .23 |
Note.
Hemoglobin is reported in grams per deciliter (g/dL) and WBC count is reported in thousands per cubic milliliter (K/uL). Genotype risk status was compared for high risk vs lower risk totals. BMI= Body mass index; no CHC= No chronic health condition; OSA=Obstructive sleep apnea; SCD= Sickle cell disease; WBC= White blood cells
Pearson bivariate correlations were run that included demographic and other descriptive variables as well as cognitive outcomes to identify potential covariates. Correlations revealed a significant relationship between family income and the WJ-III Letter Word Identification subtest, r = .285, p = .001. As such, family income was included as a covariate in later analyses (see Table 2).
Table 2.
Bivariate correlations of cognitive measures and descriptive variables.
| Cognitive measures | Family income | Preterm birth | BMI | Surgical treatment for OSAa |
|---|---|---|---|---|
| TOLD Oral Vocabulary | .07 | −.12 | .09 | .66** |
| TOLD Grammatical Understanding | .06 | .04 | .03 | .32 |
| TOLD Word Discrimination | .17 | −.05 | .01 | .08 |
| WJ-III Decision Speed | .07 | −.06 | .02 | .46* |
| WJ-III Letter Word Identification | .29** | −.10 | −.13 | .33 |
| WJ-III Applied Problems | .16 | .03 | −.01 | .34 |
| DVTMI | .16 | .00 | −.17 | .58** |
Note.
Values presented as Pearson correlations coefficients. DTVMI = Developmental Test of Visual-Motor Integration; TOLD = Test of Language Development Primary; WJ = Woodcock Johnson
These represent correlation between treatment history of adenoid removal and cognitive outcomes in the SCD+OSA group.
p < .01
p <.05
One-way ANCOVAs were run with group as the independent variable (SCD+OSA, SCD only, no CHC), cognitive outcomes as the dependent variable, and family income as a covariate (see Table 3). There were significant differences on the WJ-III Decision Speed test between the three groups, F(2, 120) = 9.18, p <.001, eta squared =.134. Follow up tests using Tukey’s HSD revealed differences between the SCD+OSA and no CHC group, p = .001, and the SCD only and no CHC group, p = .002. In addition, there were significant differences in group performance on the WJ-III Letter-Word Identification test, (F(2,120) = 11.74, p = < .001, eta squared = .164); Tukey’s HSD revealed significant differences between the SCD+OSA and no CHC group (p = .024) and the SCD only and no CHC group (p < .001). There were also significant differences on the WJ-III Applied problems test, F(2,120) = 4.02, p = .02, eta squared = .063, and the DVTMI, F(2,120) = 5.39, p = .006, eta squared = .083); Tukey’s HSD revealed significant differences between the SCD only and no CHC group for both subtests, p = .025, p = .003, respectively. There were no significant differences in performance between groups on the TOLD Oral Vocabulary, F(2, 120) = .53, p = .592, eta squared = .009, Grammatical Understanding, F(2, 120) = 1.11, p = .334, eta squared = .018, and Word Discrimination tests, F(2, 120) = 1.41, p = .248, eta squared = .023. In summary, although significant group differences were found, none of the a priori hypotheses were supported.
Table 3.
Group scores on cognitive tests
| Cognitive Test | Group | |||
|---|---|---|---|---|
| SCD+OSA (n = 26) | SCD Only (n = 39) | No CHC (n = 59) | p-value | |
| WJ-III Decision Speed | 89.31 (15.27)a | 91.49 (11.96)a | 102.67 (17.67)b | p < .001 |
| TOLD Oral Vocabulary | 9.12 ( 2.27)a | 9.15 ( 2.63)a | 9.61 (2.46)a | p = .592 |
| TOLD Grammatical Understanding | 8.92 ( 3.15) a | 8.51 (2.85) a | 9.39 (2.62) a | p = .334 |
| TOLD Word Discrimination | 7.85 (3.26) a | 8.23 (3.02) a | 9.07 (3.50) a | p = .248 |
| DTVMI | 91.73 (13.99) a,b | 85.82 (13.13) a | 94.76 (11.93) b | p = .006 |
| WJ-III Letter-Word Identification | 100.92 (12.38) a | 96.44 (11.86) a | 108.93 (13.56) b | p < .001 |
| WJ-III Applied Problems | 96.08 (16.93) a,b | 95.18 (9.70) a | 102.53 (13.85) b | p = .02 |
Note.
Values are presented as mean score (SD). Standardized WJ-III and DVTMI scores are based on a scale with a mean of 100 and standard deviation of 15. Standard scores for the TOLD are based on a scale with a mean of 10 and standard deviation of 3. DTVMI = Developmental Test of Visual-Motor Integration; TOLD = Test of Language Development Primary; WJ = Woodcock Johnson; Cells in each row with different superscripts represent statistically significant differences in group means (p < .05).
Subsequent data analyses indicated our choice of covariates impacted the study results. Pearson bivariate correlations revealed no significant associations between OSA surgical treatment and cognitive outcomes in the overall sample, however, follow up analyses did identify significant associations between OSA surgical treatment and cognitive outcomes for the subgroup of participants in the SCD+OSA group. Specifically, surgical treatment was related to outcomes on the TOLD Oral Vocabulary, WJ-III Decision Speed, and DVTMI (see Table 2). As such, an alternative data analytic plan was used with OSA treatment included as a covariate (in addition to family income) for the a priori data analyses described above (see Supplemental Table 1). This alternate approach changed the statistical significance for two of the cognitive measures: TOLD Oral Vocabulary, F(2,119) = 4.48, p =.013, eta squared = .070, and WJ-III Decision Speed tests, F(2,119) = 11.67, p < .001, eta squared = .165. Tukey’s HSD revealed significant differences between the SCD+OSA and SCD only groups on both the TOLD Oral Vocabulary (p = .015) and WJ-III Decision Speed tests (p = .034), with the SCD+OSA group evidencing greater impairment than the SCD only group.
Exploratory analyses were run examining the relationship between measures of OSA severity and all seven cognitive outcomes. Measures of OSA severity for the OSA+SCD group included AHI-RDI (M = 11.40, SD = 9.35), NADIR (M = 85.08, SD = 12.52), and mean oxygen saturation levels (M = 94.85, SD = 2.99). There were no missing OSA severity data for the 26 participants in the SCD+OSA group. Pearson correlations revealed significant associations between NADIR scores and scores on the TOLD Word Discrimination test, with higher NADIR scores correlated with better cognitive performance, r = .430, p = .028. There was also a significant relationship between apnea indices (AHI or RDI) and scores on the WJ-III Decision Speed, r = −.533, p = .006, and Applied Problems tests, r = −.482, p = .015, with higher apnea indices correlated with decreased performance. However, when the scatterplot was examined for the NADIR correlation, it was identified that the effect was due to a single, extreme outlier score for NADIR. The correlation was no longer significant following removal of the outlier, identifying it as an influential observation. Scatterplots showing the association of apnea indices with cognitive outcomes are shown in Figures 1 & 2. Although Figure 1 shows a significant association between apnea indices and the WJ-III Applied Problems test, we note that the relationship was tested for a linear effect but the data appears as if it could be curvilinear. There were no significant correlations between mean oxygen saturation levels and cognitive outcomes.
Figure 1.

Scatterplot showing the correlation between AHI-RDI scores and performance on the WJ-III Applied Problems task for the SCD+OSA group.
Figure 2.
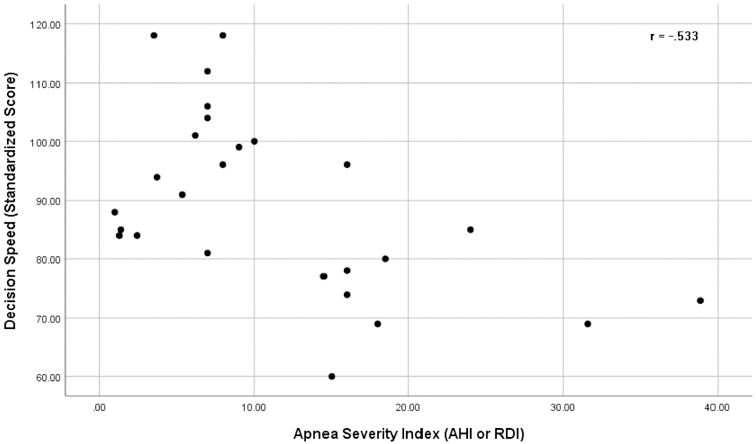
Scatterplot showing the correlation between AHI-RDI scores and performance on the WJ-III Decision Speed task for the SCD+OSA group.
Discussion
OSA and SCD are common co-morbid diagnoses that are independently associated with insufficient oxygen delivery to the brain and neurocognitive deficits; however, the overall paucity of research on the combined effects of SCD and OSA on cognitive functioning highlights the need for additional studies to better elucidate risks to children with both conditions. The current study compared neurocognitive functioning in children with both SCD and OSA, children with SCD only, and children without a chronic health condition (no CHC) to examine the additive effects of SCD and OSA. Overall, the results did not indicate the combination of OSA and SCD imparted a greater risk to cognitive functioning than SCD alone. Significant differences in quantitative reasoning and visual-motor skills were observed between the SCD only group and the no CHC group. In addition, there were significant differences in processing speed and reading skills between the no CHC comparison group and both SCD groups. These findings are in line with well-documented research on the negative impact of pediatric SCD on neurocognitive outcomes (Hijmans Channa T. et al., 2010; Schatz, Finke, Kellett, & Kramer, 2002; Schatz, Puffer, Sanchez, Stancil, & Roberts, 2009b).
Alternative analyses identified greater impairments in processing speed and semantic processing in the comorbid SCD+OSA group in comparison to the SCD only group when controlling for history of OSA surgical treatment. These findings suggest that surgical treatments for OSA may play a role in ameliorating the detrimental impacts of OSA on cognitive functioning. It is notable that children with more severe OSA are more likely to receive surgical treatment and yet surgical treatment was associated with better cognitive scores on three of the cognitive outcomes. This pattern of correlations suggests benefits from the surgery rather than a selection effect for who receives surgery. As such, future research should be careful in considering treatment factors in assessing the cognitive effects of OSA. Replication of these analyses in a different sample are important given that these analyses were not part of our a priori analytic plan.
Contrary to our hypothesis, there were no significant differences in performance between children with co-morbid OSA and SCD and those with SCD only; however, additional exploratory analyses indicated a significant link between OSA severity and cognitive functioning in the co-morbid OSA and SCD group. Specifically, youth with more severe OSA evidenced poorer performance on measures of processing speed and quantitative reasoning. These results are similar to those reported by Hollocks et al. (2012), who found that higher apnea indices were associated with lower performance on tests of fluid reasoning. Therefore, it may be that additional risk for cognitive impairment is conferred by increased OSA disease severity, while in cases of milder OSA the neurocognitive risk factors associated with SCD could outweigh the contributions of OSA. For example, studies have identified significant nighttime hypoxemia occurs in individuals with SCD (Little et al., 2014; Whitesell et al., 2016). These small-scale studies have suggested only about half of the cases with significant nighttime hypoxemia also demonstrated OSA. Thus, nighttime oxygen deprivation, along with other factors that contribute to silent cerebral infarction in SCD, may occur frequently enough that the impact of mild OSA is not detectable in a group comparison study as we conducted. However, studies with larger sample sizes are needed that directly assess differences in cognitive functioning between children with SCD with milder OSA and those with severe OSA. In addition, longitudinal studies of within-person effects might be more sensitive to detect the impact of mild OSA.
There were a number of limitations associated with the current study. First, the sample size of the three groups is comparable to many studies of SCD but is small in absolute terms and this limits statistical power. However, the observed absolute differences between the groups of children with SCD suggested the equivalent of approximately a two standard score point decrement for children with OSA, at most, on selected tests. Even with a much larger sample, such differences may not be clinically meaningful. Another possible limitation is the presence of differences between the three groups on factors we were not able to assess. For example, clinical neuroimaging was not available to determine if there were group differences in the rate of silent cerebral infarction across groups. However, the groups were well matched on available measures of demographics and disease severity. A third limitation concerns the determination of the onset of OSA. OSA can often go undiagnosed in children, with diagnosis only occurring when symptoms have reached a higher threshold (Perkin & Young, 2000; Pijpers et al., 2004). As such, these challenges make it difficult to control for the degree of exposure to OSA symptomatology in the current sample. Finally, the lack of an OSA only control group makes it difficult to establish the extent to which the cognitive measures and timing of the administration of measures provides a sensitive indicator of OSA-related deficits. The current test battery was developed to be sensitive to screening for SCD-related neurocognitive effects, but its degree of sensitivity to OSA is not established.
In summary, this study adds to limited preexisting research on the relationship between OSA and neurocognitive deficits in children with SCD by suggesting that increased OSA severity, rather than a diagnosis of OSA in general, may be associated with deficits in cognitive functioning. Although further studies are needed to confirm and expand on these findings, this more nuanced understanding of risk for children with comorbid SCD and OSA has the potential to aid in the early identification of cognitive deficits. For instance, in clinical settings with limited resources, cognitive testing could be prioritized for children with SCD with more severe OSA symptomatology. As such, the current findings highlight the need for further investigation of the impact of OSA on neurocognitive functioning in pediatric SCD to aid in the development of more nuanced treatment approaches for this particularly vulnerable population.
Supplementary Material
Acknowledgements
Funding
This publication was made possible in part by Grant Number T32-GM081740 from NIHNIGMS. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS or NIH.
Footnotes
Disclosure statement
No potential conflict of interest
References
- Baldeweg T, Hogan AM, Saunders DE, Telfer P, Gadian DG, Vargha-Khadem F, & Kirkham FJ (2006). Detecting white matter injury in sickle cell disease using voxel-based morphometry. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 59(4), 662–672. 10.1002/ana.20790 [DOI] [PubMed] [Google Scholar]
- Beebe DW, Wells CT, Jeffries J, Chini B, Kalra M, & Amin R (2004). Neuropsychological effects of pediatric obstructive sleep apnea. Journal of the International Neuropsychological Society, 10(7), 962–975. 10.1017/S135561770410708X [DOI] [PubMed] [Google Scholar]
- Berkelhammer LD, Williamson AL, Sanford SD, Dirksen CL, Sharp WG, Margulies AS, & Prengler RA (2007). Neurocognitive sequelae of pediatric sickle cell disease: a review of the literature. Child Neuropsychology, 13(2), 120–131. 10.1080/09297040600800956 [DOI] [PubMed] [Google Scholar]
- Friedman BC, Hendeles-Amitai A, Kozminsky E, Leiberman A, Friger M, Tarasiuk A, & Tal A (2003). Adenotonsillectomy improves neurocognitive function in children with obstructive sleep apnea syndrome. Sleep, 26(8), 999–1005. 10.1093/sleep/26.8.999 [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Chase C, Vezina RM, Heeren TC, Corwin MJ, Auerbach SH, … Lesko SM (2004). Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. The Journal of Pediatrics, 145(4), 458–464. 10.1016/j.jpeds.2004.05.039 [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Vezina RM, Chase C, Lesko SM, Heeren TC, Weese-Mayer DE, … Corwin MJ (2003). Symptoms of sleep-disordered breathing in 5-year-old children are associated with sleepiness and problem behaviors. Pediatrics, 112(4), 870–877. 10.1542/peds.112.4.870 [DOI] [PubMed] [Google Scholar]
- Gozal D, Kheirandish-Gozal L, Bhattacharjee R, & Spruyt K (2010). Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics, 126(5). 10.1542/peds.2010-0688 [DOI] [PubMed] [Google Scholar]
- Gozal D, & Pope DW (2001). Snoring during early childhood and academic performance at ages thirteen to fourteen years. Pediatrics, 107(6), 1394–1399. 10.1542/peds.107.6.1394 [DOI] [PubMed] [Google Scholar]
- Hijmans CT, Fijnvandraat K, Grootenhuis MA, van Geloven N, Heijboer H, Peters M & Oosterlaan J (2010). Neurocognitive deficits in children with sickle cell disease: a comprehensive profile. Pediatric Blood & Cancer, 56(5), 783–788. 10.1002/pbc.22879 [DOI] [PubMed] [Google Scholar]
- Hollocks MJ, Kok TB, Kirkham FJ, Gavlak J, Inusa BP, DeBaun MR, & de Haan M (2012). Nocturnal oxygen desaturation and disordered sleep as a potential factor in executive dysfunction in sickle cell anemia. Journal of the International Neuropsychological Society, 18(1), 168–173. 10.1017/S1355617711001469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz T (2015). The prevalence rate and neurocognitive morbidity associated with obstructive sleep apnea in children with sickle cell disease ProQuest Information & Learning; Retrieved from https://login.pallas2.tcl.sc.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2015-99140-158&site=ehost-live [Google Scholar]
- Katz T, Schatz J, & Roberts CW (2018). Comorbid obstructive sleep apnea and increased risk for sickle cell disease morbidity. Sleep and Breathing, 22(3), 797–804. 10.1007/s11325-018-1630-x [DOI] [PubMed] [Google Scholar]
- Kawadler JM, Clayden JD, Kirkham FJ, Cox TC, Saunders DE, & Clark CA (2013). Subcortical and cerebellar volumetric deficits in paediatric sickle cell anaemia. British Journal of Haematology, 163(3), 373–376. 10.1111/bjh.12496 [DOI] [PubMed] [Google Scholar]
- Kheirandish L, & Gozal D (2006). Neurocognitive dysfunction in children with sleep disorders. Developmental Science, 9(4), 388–399. 10.1111/j.1467-7687.2006.00504.x [DOI] [PubMed] [Google Scholar]
- Kirkham F, Hewes D, Prengler M, Wade A, Lane R, & Evans J (2001). Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. The Lancet, 357(9269), 1656–1659. 10.1016/S0140-6736(00)04821-2 [DOI] [PubMed] [Google Scholar]
- Kuhle S, Urschitz MS, Eitner S, & Poets CF (2009). Interventions for obstructive sleep apnea in children: a systematic review. Sleep Medicine Reviews, 13(2), 123–131. 10.1016/j.smrv.2008.07.006 [DOI] [PubMed] [Google Scholar]
- Little JA, Rotz S, Kim C, O’Riordan M, Langer N, & Lance C (2014). Nocturnal hypoxemia (not sleep apnea) may drive reticulocytosis in sickle cell disease. Sleep Medicine, 22, 47–49. 10.1016/j.sleep.2016.05.006 [DOI] [Google Scholar]
- McClellan CB, Schatz J, Sanchez C, & Roberts CW (2008). Validity of the pediatric quality of life inventory for youth with sickle cell disease. Journal of Pediatric Psychology, 33(10), 1153–1162. 10.1093/jpepsy/jsn036 [DOI] [PubMed] [Google Scholar]
- McGrew KS, & Woodcock R (2001). Technical manual. Woodcock-Johnson III Itasca, IL: Riverside Publishing. [Google Scholar]
- Montgomery-Downs H, Crabtree V, & Gozal D (2005). Cognition, sleep and respiration in at-risk children treated for obstructive sleep apnoea. European Respiratory Journal, 25(2), 336–342. 10.1183/09031936.05.00082904 [DOI] [PubMed] [Google Scholar]
- Naismith S, Winter V, Gotsopoulos H, Hickie I, & Cistulli P (2004). Neurobehavioral functioning in obstructive sleep apnea: differential effects of sleep quality, hypoxemia and subjective sleepiness. Journal of Clinical and Experimental Neuropsychology, 26(1), 43–54. 10.1076/jcen.26.1.43.23929 [DOI] [PubMed] [Google Scholar]
- Panepinto JA, O’mahar KM, DeBaun MR, Loberiza FR, & Scott J (2005). Health-related quality of life in children with sickle cell disease: Child and parent perception. British Journal of Haematology, 130(3), 437–444. 10.1111/j.1365-2141.2005.05622.x [DOI] [PubMed] [Google Scholar]
- Perkin R, & Young T (2000). Obstructive sleep apnea in children. Reversing the trend of underdiagnosis. Advance for Nurse Practitioners, 8(10), 57. [PubMed] [Google Scholar]
- Pijpers M, Poels PJ, Vaandrager JM, de Hoog M, van den Berg S, Hoeve HJ, & Joosten KF (2004). Undiagnosed obstructive sleep apnea syndrome in children with syndromal craniofacial synostosis. Journal of Craniofacial Surgery, 15(4), 670–674. [DOI] [PubMed] [Google Scholar]
- Powars DR, Conti PS, Wong W-Y, Groncy P, Hyman C, Smith E, … Harold Y (1999). Cerebral vasculopathy in sickle cell anemia: diagnostic contribution of positron emission tomography. Blood, 93(1), 71–79. [PubMed] [Google Scholar]
- Reed W, Jagust W, Al-Mateen M, & Vichinsky E (1999). Role of positron emission tomography in determining the extent of CNS ischemia in patients with sickle cell disease. American Journal of Hematology, 60(4), 268–272. [DOI] [PubMed] [Google Scholar]
- Robertson PL, Aldrich MS, Hanash SM, & Goldstein GW (1988). Stroke associated with obstructive sleep apnea in a child with sickle cell anemia. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 23(6), 614–616. [DOI] [PubMed] [Google Scholar]
- Rosen CL, Debaun MR, Strunk RC, Redline S, Seicean S, Craven DI, … Roberts I (2014). Obstructive sleep apnea and sickle cell anemia. Pediatrics, 134(2), 273–281. 10.1542/peds.2013-4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz J, Brown R, Pascual J, Hsu L, & DeBaun M (2001). Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology, 56(8), 1109–1111. 10.1212/WNL.56.8.1109 [DOI] [PubMed] [Google Scholar]
- Schatz J, & Buzan R (2006). Decreased corpus callosum size in sickle cell disease: relationship with cerebral infarcts and cognitive functioning. Journal of the International Neuropsychological Society, 12(1), 24–33. 10.1017/S1355617706060085 [DOI] [PubMed] [Google Scholar]
- Schatz J, Finke RL, Kellett JM, & Kramer JH (2002). Cognitive functioning in children with sickle cell disease: a meta-analysis. Journal of Pediatric Psychology, 27(8), 739–748. 10.1093/jpepsy/27.8.739 [DOI] [PubMed] [Google Scholar]
- Schatz J, & McClellan CB (2006). Sickle cell disease as a neurodevelopmental disorder. Mental Retardation and Developmental Disabilities Research Reviews, 12(3), 200–207. 10.1002/mrdd.20115 [DOI] [PubMed] [Google Scholar]
- Schatz J, Puffer ES, Sanchez C, Stancil M, & Roberts CW (2009). Language processing deficits in sickle cell disease in young school-age children. Developmental Neuropsychology, 34(1), 122–136. 10.1080/87565640802499191 [DOI] [PubMed] [Google Scholar]
- Sforza E (2012). Sleep apnea syndrome and cognition. Frontiers in Neurology, 3, 87 10.3389/fneur.2012.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RG, Fineberg-Buchner C, Hankins G, Weiss L, Prifitera A, & Mulhern RK (2005). Cognitive deficits in children with sickle cell disease. Journal of Child Neurology, 20(2), 102–107. 10.1177/08830738050200020301 [DOI] [PubMed] [Google Scholar]
- Tauman R, & Gozal D (2011). Obstructive sleep apnea syndrome in children. Expert Review of Respiratory Medicine, 5(3), 425–440. 10.1586/ers.11.7 [DOI] [PubMed] [Google Scholar]
- Whitesell P, Owoyemi O, Oneal P, Nouraie M, Klings E, Rock A, … Taylor R (2016). Sleep-disordered breathing and nocturnal hypoxemia in young adults with sickle cell disease. Sleep Medicine, 22, 47–49. 10.1016/j.sleep.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery KE (2004). The Beery-Buktenica developmental test of visual-motor integration: Beery VMI, with supplemental developmental tests of visual perception and motor coordination, and stepping stones age norms from birth to age six Minneapolis, MN: NCS Pearson. [Google Scholar]
- Hassell KL (2010). Population estimates of sickle cell disease in the US. American Journal of Preventive Medicine, 38(4), S512–S521. [DOI] [PubMed] [Google Scholar]
- Puffer ES, Schatz JC & Roberts CW (2009). Relationships between somatic growth and cognitive functioning in young children with sickle cell disease. Journal of Pediatric Psychology, 35(8), 892–904. [DOI] [PubMed] [Google Scholar]
- Newcomer PL, & Hammill DD (1988). Test of language development-primary Austin, TX: Pro-ed. [Google Scholar]
- Sanchez C, Schatz J, & Roberts C (2010). Cerebral blood flow velocity and language functioning in pediatric sickle cell disease. Journal of the International Neuropsychological Society,16(2), 326–334. 10.1017/S1355617709991366 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


