Abstract
Purpose.
A growing body of research suggests that inflammation plays a role in many chemotherapy-related toxicities such as fatigue, anxiety, and neuropathy. Regular exercise can change levels of individual cytokines (e.g., reducing IL-6, increasing IL-10); however, it is not known whether exercise during chemotherapy affects relationships between cytokines (i.e., whether cytokine concentrations change collectively vs. independently). This study assessed how 6 weeks of exercise during chemotherapy affected relationships between changes in concentrations of several cytokines.
Methods.
This is a secondary analysis of a randomized trial studying 6 weeks of moderate-intensity walking and resistance exercise during chemotherapy compared to chemotherapy alone. At pre- and post-intervention, patients provided blood to assess serum concentrations of cytokines IL-1β, IL-6, IL-8, IL-10, and IFN-γ, and receptor sTNFR1. We investigated relationships between cytokines using the correlations between changes in cytokine concentrations from pre- to post-intervention.
Results.
We obtained complete data from 293 patients (149 randomized to exercise). Exercise strengthened the correlation between concentration changes of IL-10 and IL-6 (r=0.44 in exercisers vs. 0.11 in controls; p=0.001). We observed the same pattern for IL-10:IL-1β and IL-10:sTNFR1. Exercise also induced an anti-inflammatory cytokine profile, per reductions in pro-inflammatory IFNγ (p=0.044) and perhaps IL-1β (p=0.099, trend-level significance).
Conclusions.
Our hypothesis-generating work suggests that regular exercise during 6 weeks of chemotherapy may cause certain cytokine concentrations to change collectively (not independently). This work enhances our understanding of relationships between cytokines and complements traditional analyses of cytokines in isolation. Future work should test for replication and relationships to patient outcomes.
Keywords: cytokine, cytokine network, cytokine matrix, cytokine correlation, exercise, chemotherapy
Introduction
The majority of patients receiving chemotherapy for cancer experience one or more severe side effects including pain, cognitive impairment, fatigue, and neuropathy [1]. Chemotherapy activates pro-inflammatory cytokines acutely [2] and chronically: even months after completion of treatment [3]. In healthy individuals, inflammation is regulated very effectively, such that acute changes in inflammatory cytokines toward a pro-inflammatory state are counteracted by anti-inflammatory cytokines to return the body to a healthy inflammatory state. During chemotherapy, however, inflammation is dysregulated so extensively that patients experience a chronic pro-inflammatory state. Many studies suggest that excessive levels of pro-inflammatory cytokines, particularly interleukin-6 (IL-6), contribute to the experience of pain [4], cognitive impairment [5], fatigue [6], and neuropathy [7] among chemotherapy patients.
Growing evidence suggests that exercise can reduce pain (e.g., [8]), cognitive impairment (e.g., [9]), fatigue [10], and symptoms of neuropathy (e.g., [11, 12]) in patients with cancer (for a review, see [13]), partially due to its potent anti-inflammatory effects [14]. During walking, resistance training, and other types of exercise, muscles secrete IL-6, which causes release of anti-inflammatory IL-10, which then reduces levels of IL-6 and inhibits pro-inflammatory IL-1β via release of IL-1 receptor agonist [14]. Thus, the strongest evidence for the effects of exercise on cytokines involves IL-6, IL-10, and IL-1β. Other cytokines have been implicated in the effect of exercise as well, such as IL-8, which has been shown to decrease in response to a 12-week exercise intervention in adults with metabolic syndrome [15]; tumor necrosis factor (TNF), which has been shown to decrease via exercise-induced release of cortisol [14]; and IFN-γ, which has been show to increase immediately after exercise, but then decrease after recovery from exercise (i.e., 24 hours post-exercise; [16]). It has been suggested that a regular exercise program will yield an overall reduction in inflammation at rest due to the anti-inflammatory environment induced by each exercise session combined with a reduction in visceral fat, which releases pro-inflammatory adipokines such as IL-6 and TNF [14]. Through these interactions, exercise helps individuals regulate inflammation through endogenous cytokine pathways. Thus, exercise may treat pain, cognitive impairment, fatigue, and neuropathy symptoms in patients with cancer by inhibiting and decreasing concentrations of pro-inflammatory cytokines, increasing concentrations of anti-inflammatory cytokines, and regulating the endogenous cytokine system.
Although many studies of patients with cancer have assessed individual markers of inflammation (e.g., in relation to pain [2], fatigue [17], quality of life [18]) and how they change in response to exercise (e.g., [19–21]), no intervention-based studies have examined the extent to which cytokine concentrations change collectively vs. independently, as has been done in immunological studies of cell cultures [22], non-interventional studies of cancer [23, 24], and research on other conditions (e.g., depression [25], HIV [26], subdural hematoma [27]). We define a collective change as a correlation in the changes in concentrations of two cytokines, which we speculate reflects related to regulation (or lack thereof) of the two cytokines. Indeed, focusing only on changes in individual cytokine concentrations—rather than inter-cytokine relationships—fails to assess processes related to how we know many of these cytokines function: collectively. We reasoned that by measuring the extent to which cytokine concentrations change together, we can provide complementary and unique insight into a patient’s inflammatory state and perhaps how their body regulates inflammation. Ultimately, clinical researchers might be able to optimize interventions to target elements of the inflammatory network that might cause or exacerbate symptoms.
The goal of this exploratory secondary analysis was to investigate correlations between changes in cytokine concentrations over time. We tested three hypotheses: (1) exercise during chemotherapy strengthens correlations between changes in concentrations of IL-6 and IL-10 because exercise increases both IL-6 and IL-10 [14]. Specifically, we predicted that exercisers would have a more positive correlation of change in IL-6 and change in IL-10 because we reasoned that regular exercise could entrain or sensitize the biochemical pathways linking increases in IL-6 to increases in IL-10, such as increases in local concentrations of IL-6 receptors and other mediators or inducers of IL-10 expression including epigenetic changes [28]. (2) Exercise during chemotherapy strengthens correlations between changes in concentrations of other cytokines as well (IFNγ, IL-8, IL-1β, IL-6, IL-10, and receptor sTNFR1). Specifically, we predicted exercisers would have more positive correlations between (i) changes in IL-10 and IL-1β, and (ii) changes in IL-6 and IL-1β, based on their known responses to exercise [14]. We did not have strong predictions for the remaining cytokine-cytokine correlations. (3) Exercise during chemotherapy produces a more favorable inflammatory state (decreased concentrations of pro-inflammatory cytokines such as IFNγ, IL-8, and IL-1β, and increased concentrations of anti-inflammatory cytokines such as IL-10 and sTNFR1). We used data from our phase III nationwide randomized controlled trial (RCT) designed to study fatigue in response to six weeks of either exercise during chemotherapy or chemotherapy alone in mixed type, early/mid-stage, chemotherapy-naïve patients with cancer. Our analysis focused on the 293 patients who provided blood both pre- and post-intervention.
Methods
Study design.
This work is based on an RCT (ClinicalTrials.gov ) designed to assess the effects of exercise on fatigue. Briefly, the trial was conducted and analyzed through the University of Rochester Cancer Center (URCC) National Cancer Institute (NCI) Community Oncology Research Program (NCORP) Research Base across 20 community oncology practices in the United States from 2009–2016. Participants were randomly assigned to receive six weeks of either (1) standard chemotherapy or (2) standard chemotherapy plus exercise in a 1:1 allocation ratio with randomization block size 4 or 6. Allocation was concealed from coordinators until after participant registration and concealed from participants until baseline assessments were complete. Each institutional review board approved the study before participants were enrolled. All participants provided written informed consent. As part of the pre- and post-intervention assessments, participants completed questionnaires and daily diaries and wore a pedometer (Walk 4 Life Classic; Oswego, IL). After beginning the study, we applied for and obtained additional funding to assess serum markers of inflammation, and thus all patients including and after number 303 were asked to provide blood samples at pre- and post-intervention; 343 (89%) of the patients agreed to do so.
Study participants.
To be eligible for the parent RCT, patients must have (1) been ≥21 years old, (2) had a primary diagnosis of cancer other than leukemia, without distant metastasis, (3) been chemotherapy naïve, (4) started chemotherapy after enrollment and been scheduled for at least six weeks of chemotherapy with treatment cycles of either two, three, or four weeks; (5) had a Karnofsky Performance Status ≥70, (6) been able to read English, (7) not received concurrent radiation therapy, (8) not had physical limitations that contraindicate participation in a low-to-moderate-intensity home-based walking and progressive resistance program, and (9) not been identified as in the active or maintenance stage of exercise behavior as assessed by the Exercise Stages of Change [29], wherein we defined exercise as planned physical activity performed to increase physical fitness and performed at least 3–5 times per week for 20–60 min per session at an intensity that increases breathing rate and induces sweating. To put this into context, before randomization all patients in the study reported exercising less than 60 min/week, which is much less than the American College of Sports Medicine recommendations for cancer survivors of 150 min/week [30]. This exploratory secondary analysis only included patients who provided blood samples and pre- and post-intervention.
Exercise intervention.
The exercise intervention began on the first day of chemotherapy for all patients and continued for the next six weeks. Exercise for Cancer Patients (EXCAP©®) is a low-moderate intensity individualized at-home walking and resistance exercise program involving walking a prescribed number of daily steps, which increases 5–20% per week, and completing a prescribed number of sets and repetitions (1–4 sets of 8–15 repetitions) of resistance band exercises that increase over time [31]. For details, see our prior publication from this RCT [11].
Standard care control condition.
Control participants completed all assessments and were followed in the same manner as the exercise participants. Control participants were offered the exercise intervention after all assessments were complete.
Measures.
Clinical and demographic information were collected from patients and medical records. Exercise adherence was reported daily using (1) steps from a pedometer, (2) minutes of resistance exercise, and (3) a rating of perceived exertion of the resistance exercise on the American College of Sports Medicine revised rating scale [32], where 1=no exertion and 10=maximal exertion. For measures of inflammation, participants were instructed to fast before providing approximately 50 mL of blood. Blood samples were processed and cytokine concentrations were assessed using a multiplex assay.
Assessing inflammation.
For serum samples, the tubes (20 mL of blood in red-top tubes) were inverted 3–4 times, allowed to clot for 30 minutes at room temperature, and centrifuged for 10 min at 1500 g. The serum (supernatant) was aliquoted into 6–10 microfuge tubes (between 0.5 to 1 mL per tube) and frozen at −80°C. After completing data acquisition for the study, samples were thawed and analyzed using a multiplex kit (EMD Millipore; Billerica, MA) following the kit’s instructions. One kit (HSCYTMAG-60SK) was used to analyze IL-1β, IL-6, IL-8, IL-10, and IFNγ all at once. A second kit (HSCRMAG-32K) was used to analyze sTNFR1; this second kit was used to prevent overlap in the resulting fluorescence spectrum. Samples were loaded onto a 96-well plate and concentrations were assessed using a Luminex MagPix (Austin, TX).
Altogether, we assessed six cytokines/receptors: three that we considered pro-inflammatory (IFNγ, IL-8, and IL-1β), two that we considered anti-inflammatory (IL-10 and sTNFR1), and one that we did not categorize (IL-6) [33].
Adverse events.
Adverse events were monitored by the URCC Data Safety Monitoring Committee. All unexpected, serious, life-threatening, and fatal adverse events were reported.
Statistical analyses.
All patients were analyzed according to their randomization assignment (i.e., intent to treat analysis). All outcomes were examined with two-tailed tests using α=0.05, with a trend-level significance considered as p<0.1, and we did not adjust for multiple tests due to the exploratory nature of this project [34]. For demographics and clinical characteristics (Table 1), to test for differences in baseline characteristics between study arms, we used t-tests and χ2 tests for continuous and categorical characteristics, respectively. For hypotheses 1 and 2, to calculate coordination in changes of cytokine concentrations, we computed Pearson’s correlations between changes (post-intervention minus pre-intervention) in log10 concentrations of each cytokine and receptor. We compared correlation coefficients using z-tests. For hypothesis 3, to test for changes in cytokine concentrations from pre- to post-intervention, we used linear regression to predict post-intervention log10 concentration while controlling for pre-intervention log10 concentration and testing for an effect of study arm (exercise vs. control). Analyses were performed using JMP v.13 (SAS Institute Inc., Cary, NC) and MATLAB (Mathworks, Natick, MA).
Table 1.
Participant demographics, characteristics, and clinical measures at baseline.
| Characteristic | Control | Exercise | Total | Control vs. Exercise p-valuea |
|---|---|---|---|---|
| Total participants | 170 | 173 | 343 | |
| Female sex | 161 (95%) | 159 (92%) | 320 (93%) | 0.298 |
| Age, years (mean ± std. dev) | 55.6 ± 11.8 | 56.3 ± 12.9 | 55.9 ± 12.4 | 0.655 |
| Body mass index, kg/m2 (mean ± std. dev.) | 29.9 ± 6.5 | 30.6 ± 7.0 | 30.3 ± 6.7 | 0.090 |
| Obese (body mass index > 30 kg/m2) | 68 (40%) | 85 (49%) | 153 (45%) | 0.103 |
| Race | 0.712 | |||
| White | 143 (84%) | 148 (86%) | 291 (85%) | |
| Non-White | 27 (16%) | 25 (14%) | 52 (15%) | |
| Employment | 0.555 | |||
| Employed outside the house | 105 (62%) | 98 (57%) | 203 (59%) | |
| Self-employed / homemaker | 18 (11%) | 18 (10%) | 36 (10%) | |
| Unemployed | 47 (28%) | 57 (33%) | 104 (30%) | |
| Marital status | 0.107 | |||
| Married or long-term committed relationship | 108 (64%) | 124 (72%) | 232 (68%) | |
| Divorced, separated, single, widowed | 62 (36%) | 49 (28%) | 111 (32%) | |
| Education | 0.623 | |||
| At least some college | 117 (69%) | 118 (68%) | 235 (69%) | |
| High school/GED degree | 44 (26%) | 49 (28%) | 93 (27%) | |
| No high school or GED degree | 8 (5%) | 5 (3%) | 13 (4%) | |
| Cancer typeb | 0.272 | |||
| Breast | 136 (80%) | 131 (76%) | 267 (78%) | |
| Lymphoma | 3 (2%) | 8 (5%) | 11 (3%) | |
| Colon | 7 (4%) | 14 (8%) | 21 (6%) | |
| Lung | 2 (1%) | 1 (1%) | 3 (1%) | |
| Other | 9 (5%) | 8 (5%) | 17 (5%) | |
| Cancer stage | 0.843 | |||
| Stage I | 44 (26%) | 44 (25%) | 88 (26%) | |
| Stage II | 78 (46%) | 75 (43%) | 153 (45%) | |
| Stage III | 44 (26%) | 47 (27%) | 91 (27%) | |
| Stage IV | 2 (1%) | 4 (2 %) | 6 (2%) | |
| Not reported | 2 (1%) | 3 (2%) | 5 (1%) | |
| Chemotherapy type | 0.565 | |||
| Cyclophosphamide + doxorubicin | 66 (39%) | 62 (36%) | 128 (37%) | |
| Cyclophosphamide + docetaxel/paclitaxel | 60 (35%) | 50 (29%) | 110 (32%) | |
| Carboplatin + docetaxel/paclitaxel | 20 (12%) | 22 (13%) | 42 (12%) | |
| FOLFOX (leucovorin, 5-fluorouracil, oxaliplatin) | 7 (4%) | 7 (4%) | 14 (4%) | |
| Otherc | 4 (2%) | 10 (6%) | 14 (4%) | |
| CMF (cyclophosphamide, methotrexate, fluorouracil) | 4 (2%) | 8 (5%) | 12 (3%) | |
| Carboplatin/cisplatin/oxaliplatin | 5 (3%) | 6 (3%) | 11 (3%) | |
| R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) | 2 (1%) | 5 (3%) | 7 (2%) | |
| Unspecified | 2 (1%) | 3 (2%) | 5 (1%) | |
| Previous treatment | 0.464 | |||
| Previous surgery | 148 (87%) | 155 (90%) | 303 (88%) | |
| Previous radiation therapy | 1 (1%) | 5 (3%) | 6 (2%) | |
| Previous hormone therapy | 6 (4%) | 9 (5%) | 15 (4%) | |
| Time since previous treatment | ||||
| Weeks since end of first surgery for cancer (mean ± std. dev) | 4.5 ± 4.3 | 5.0 ± 6.4 | ± | 0.426 |
| Weeks since end of first radiation for cancer (mean ± std. dev) | 3 ± NA | 5 ± 470.6 | NA | |
| Weeks since end of first hormone therapy for cancer (mean ± std. dev) | 11 ± 19.1 | 71.8 ± 169.7 | 0.318 | |
| Karnofsky performance status (mean ± std. dev) | 94.8 ± 6.7 | 94.5 ± 7.0 | 94.6 ± 6.9 | 0.616 |
Statistical tests includes t-test or χ2 test
Other cancer types include endometrial, ovary, testes, uterine, brain, cervical, fallopian tube, head or neck, kidney, pancreas, and peritoneum.
GED, general educational development
Other chemotherapy types include combinations of cyclophosphamide, epirubicin, bendamustine, rituximab, bleomycin, doxorubicin, vinblastine, vincristine, irinotecan, 5-fluorouracil, and lenalidomide
Handling missing cytokine data.
Cytokine data were censored if they were below the detection limit of the instrument: IL-1β and IFNγ data were censored the most (49–62% of observations), and all other cytokines/receptor data were censored minimally (<3% of observations). For assessing cytokine correlations, we handled missing data using two methods: (1) case-wise deleting data points below the detection limit and (2) multiple imputation, as suggested by Uh et al. 2008 [35]. Specifically, we used the Multiple Imputation using Chained Equations (MICE) library in R [36] to impute each patient’s change in log10 cytokine concentration using only the available (i.e., not missing) changes in cytokine concentrations across all patients. We obtained identical conclusions between these two methods and thus we present results in which data points below the detection limit were deleted.
Results
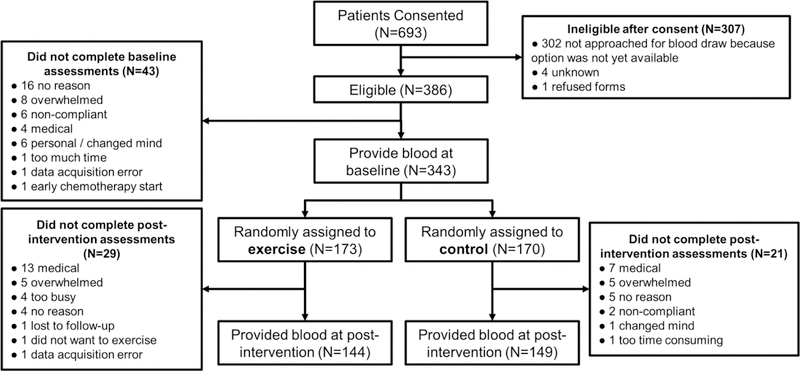
Participant flow (Figure 1).
Figure 1.
CONSORT diagram of study participants.
Of the 343 patients who provided blood at baseline, 293 (85%; i.e., 15% attrition) also provided blood at post-intervention (144 exercisers, 149 controls). The most common reasons for incomplete data were medical issues (20 participants), feeling overwhelmed (10 participants), or not reported (9 participants). Withdrawal from the study after providing blood at baseline exhibited trend-level predictions by increased patient-reported fatigue (rated 0–10; OR=1.213; 95% CI=0.979, 1.502; p=0.077) and age (OR=1.031; CI=0.996, 1.072; p=0.084), but was not significantly predicted by study arm, other patient-reported symptoms (pain, distress, quality of life; all rated using 0–10 scales), education, gender, BMI, race, marital status, cancer site, cancer stage, or Karnofsky Performance Status (all p>0.2). Furthermore, although there were more dropouts due to medical reasons in the exercise group than in the control group: 13 (7.5%) vs. 7 (4.1%; Figure 1), this difference was not statistically significant (p=0.167).
Baseline characteristics (Table 1).
Study participants were primarily middle-aged, married women with early-stage breast cancer, at least some college education, and employment outside the home. The three most common chemotherapy regimen types were cyclophosphamide + doxorubicin, cyclophosphamide + docetaxel/paclitaxel, and carboplatin + docetaxel/paclitaxel. There were no significant baseline differences between patients in the two study arms.
Intervention adherence.
At baseline, there were no significant differences between exercise and control conditions in terms of daily steps (exercisers 4,257 steps/day, controls 4,444 steps/day; p=0.615) or minutes of resistance band exercise (both groups reported zero). After the intervention, exercisers increased daily steps by 651 and walked more steps than control participants, who decreased daily steps by 120 (4,908 vs. 4,324, respectively; p=0.061, trend-level effect). For resistance band exercise, 94% of exercise participants reported performing at least some resistance exercise during the study. These sessions were on average 26.9 min long with a perceived exertion of 3.8 and were performed 3.7 days/week. Exercise contamination was minimal in the control participants. Only 8% of controls reported any resistance exercise during the study, and, on average, these participants exercised fewer than two times during the six-week study. Thus, exercisers performed significantly more resistance exercises than controls (average of 3.7 days/week vs. 0.3; p<0.001).
Hypothesis 1: Exercise during chemotherapy strengthens correlations between changes in concentrations of IL-6 and IL-10 (Figure 2).
Figure 2.
Regular exercise during six weeks of chemotherapy increases the correlation between change in IL-6 concentration and change in IL-10 concentration. Each data point shows one participant, with 143 participants randomized to chemotherapy alone (left) and 142 participants randomized to chemotherapy plus exercise (right). Each figure shows the Pearson’s correlation r and the corresponding p-value from a two-tailed test.
Consistent with our hypothesis, the exercise group exhibited a modest positive correlation between changes in IL-6 and IL-10 concentrations (r=0.45, p<0.001) that was greater (z=3.06, p=0.001) than the positive correlation observed in the chemotherapy alone group (r=0.11, p=0.194; Figure 2). Next, we performed the same analysis across all 15 pairs of the six cytokines/receptors (i.e., not just IL-6 and IL-10).
Hypothesis 2: Exercise during chemotherapy strengthens correlations between changes in concentrations of other cytokines (Figure 3, Table 2).
Figure 3.
Regular exercise during six weeks of chemotherapy induces greater correlations among changes in cytokine and receptor concentrations. The network diagram shows the correlation strength between changes in each cytokine or receptor concentration (like the one shown in Figure 2, which involves IL-6 and IL-10). Stronger correlations are shown with thicker lines that vary continuously; positive correlations are red, and negative correlations are blue. Each asterisk (*) indicates that the correlation coefficient differs from zero (p<0.05, two-tailed). Each dagger (†) indicates that the correlation coefficient differs by study arm (p<0.05, two-tailed). These data are also shown in Table 2.
Table 2.
Correlation matrix of changes in cytokine or receptor concentrations.
| Study Arm: Chemotherapy Alone | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔIL- 1β | ΔIL-6 | ΔIL‒8 | ΔIL‒10 | ΔsTNFR1 | ΔIFNγ | Mean | Standard error | |||
| ΔIL-1β | 0.10 | 0.13 | 0.15† | ‒0.20 | 0.42* | 0.12 | 0.10 | |||
| ΔIL-6 | 0.10 | 0.17* | 0.11† | 0.14 | 0.28* | 0.16*† | 0.03 | |||
| ΔIL-8 | 0.13 | 0.17* | 0.14 | ‒0.03 | 0.24 | 0.13* | 0.04 | |||
| ΔIL-10 | 0.15† | 0.11† | 0.14 | 0.33*† | 0.22 | 0.19* | 0.04 | |||
| ΔsTNFR1 | ‒0.20 | 0.14 | ‒0.03 | 0.33*† | 0.02 | 0.05 | 0.09 | |||
| ΔIFNγ | 0.42* | 0.28* | 0.24 | 0.22 | 0.02 | 0.24* | 0.06 | |||
| Study Arm: Chemotherapy + Exercise | ||||||||||
| ΔIL-1β | ΔIL-6 | ΔIL‒8 | ΔIL-10 | ΔsTNFR1 | ΔIFNγ | Mean | Standard error | |||
| ΔIL-1β | 0.27 | 0.15 | 0.48*† | ‒0.03 | 0.52* | 0.28 | 0.10 | |||
| ΔIL-6 | 0.27 | 0.30* | 0.44*† | 0.23* | 0.26 | 0.30*† | 0.04 | |||
| ΔIL-8 | 0.15 | 0.30* | 0.31* | 0.00 | 0.00 | 0.15* | 0.07 | |||
| ΔIL-10 | 0.48*† | 0.44*† | 0.31* | 0.11† | 0.48* | 0.36* | 0.07 | |||
| ΔsTNFR1 | ‒0.03 | 0.23* | 0.00 | 0.11† | ‒0.11 | 0.04 | 0.06 | |||
| ΔIFNγ | 0.52* | 0.26 | 0.00 | 0.48* | ‒0.11 | 0.23 | 0.13 | |||
Note. Each cell shows the correlation coefficient (Pearson’s r) of the change in the cytokine or receptor concentration listed in the row with the change in cytokine or receptor concentration listed in the column. These data are also shown visually in Figure 3.
The correlation coefficient differs from zero (p < 0.05, two-tailed).
The correlation coefficient differs by study arm (p < 0.05, two-tailed).
Consistent with our hypothesis, the exercise group exhibited stronger correlations among the changes in cytokine/receptor concentrations compared to the control group (thicker lines in Figure 3, greater correlation values in Table 2). Out of the 15 paths in this correlation matrix, 3 of the correlation values differed between exercise and control groups: IL-10:IL-6, IL-10:IL-1β, and IL-10:sTNFR1 (all p<0.05). Moreover, Table 2 (right two columns) shows the mean correlation coefficients for each cytokine/receptor by averaging across the other five cytokines/receptors; we found greater mean correlations for IL-6 in exercise compared to control (p=0.024).
Hypothesis 3: Exercise during chemotherapy produces a more favorable inflammatory state (Figure 4).
Figure 4.
Regular exercise during six weeks of chemotherapy induces an anti-inflammatory state, as evidenced by reduction in IFNγ (p=0.044) and perhaps IL1β (p=0.099, trend-level effect). Sample sizes are provided at the bottom of each figure. Each asterisk (*) indicates that the change in cytokine concentration differs from zero within study arm (p<0.05, two-tailed). Each dagger (†) indicates that the change in cytokine concentration differs by study arm (p<0.05, two-tailed). Error bars are standard errors and units are log10(pg/mL).
At baseline, there were no significant differences between groups in any cytokine or cytokine receptor concentration (all p>0.357). All three pro-inflammatory markers decreased significantly in exercise from pre- to post-intervention (IFNγ p=0.030, IL-8 p=0.005, and IL-1β p<0.0001) whereas only one pro-inflammatory marker (IL-8) decreased significantly in controls (IFNγ p=0.813, IL-8 p=0.005, IL1β p=0.073). For the anti-inflammatory markers, all three increased significantly in the exercise group (IL-6 p=0.020, IL-10 p=0.0004, and sTNFR1 p<0.0001), whereas only two (IL-10 and sTNFR1) increased significantly in controls (IL-6 p=0.395, IL-10 p<0.0001, and sTNFR1 p<0.0001). When directly comparing the study arms from pre- to post-intervention, exercise significantly reduced pro-inflammatory IFNγ compared to control (p=0.044), and exercise exhibited a trend-level reduction in pro-inflammatory IL-1β compared to control (p=0.099); there were no other demonstrated changes between the two study arms (all p>0.296).
Adverse events.
There were two adverse events (grade >3) during the study; both were neutropenia (one grade 3 and one grade 4). All adverse events were unrelated to participation in the study.
Discussion
Our results suggest that six weeks of exercise during chemotherapy coordinates changes in cytokine concentrations, particularly IL-6 and IL-10. We speculate that this coordinated change is related to inflammatory regulation. Both IL-6 and IL-10 are dysregulated by chemotherapy [23] and are influenced by exercise in healthy individuals [14]. Our results are encouraging because we observed significant effects on cytokine coordination and cytokine concentrations using a modest dose of low-to-moderate intensity exercise (i.e., an additional 651 steps/day and 3.7 sessions/week of resistance exercise at an RPE of 3.8 out of 10), consistent with studies showing that moderate intensity exercise affects inflammation in healthy individuals [37]. This is a realistic amount of exercise for patients to achieve during chemotherapy, and it can be performed unsupervised at home, thus enhancing feasibility.
Our work extends prior studies of inflammation in cancer and in general, with studies suggesting that lower inter-cytokine correlations reflect physiological stress or an unhealthy state. These studies have shown significant reductions in correlation between IL-6 and IL-10 in 60 patients with lymphoma (compared to 20 healthy controls) [24] and in response to chemotherapy in 54 patients with multiple myeloma (compared to 24 healthy controls) [23]. Our study builds on this work by suggesting an intervention—exercise—that may increase correlations between changes in cytokine concentrations, which we are speculating may be related to improved inflammatory regulation. Our results also suggest that exercise affects other inter-cytokine correlations besides IL-6 and IL-10, namely the relations between IL-10 and IL-1β, and IL-10 and sTNFR1 (Hypothesis 2, Figure 3). Our observed effects of exercise on inter-relations between IL-6, IL-10, and IL-1β is wholly consistent with the established effects of exercise on IL-6, IL-10, and IL-1β [14]. Our findings that exercise reduced serum levels of IFNγ and perhaps IL-1β (Hypothesis 3, Figure 4) are consistent with prior findings that exercise reduces serum IFNγ in healthy adults (e.g., [16]), although prior studies in patients receiving cancer chemotherapy showed no changes in IFNγ (e.g., [38]) and IL-1β (e.g., [39]) perhaps due to their small sample size (both N < 31).
Regarding the clinical significance of our observed exercise-induced reduction in IFNγ (an approximately 2-fold reduction from 61 to 26 pg/mL) and reduction in IL-1β (almost a 2-fold reduction from 1.2 to 0.7 pg/mL), a recent review concluded that there is little research on identifying clinical cutoffs or significance of levels of various cytokines [40]. However, serum IFNγ has been associated with other disease states such as surgical site infection with cutoffs of 34 pg/mL and 115 pg/mL across two studies, and IL-1β with a cutoff of 8.26 pg/mL in another study. And, in general, very low levels of IFNγ are expected in the absence of infection, with one study reporting approximately 10 pg/mL in healthy adults [41]. Therefore, it is tempting to speculate that our observed reduction in IFNγ might be clinically meaningful in relation to infection or other conditions that patients experience during chemotherapy, but clearly further research is needed to study changes in cytokine levels and changes in cytokine-cytokine correlations in relation to signs and symptom from patients receiving chemotherapy for cancer.
This study has several major strengths. First, it is a randomized study, thus suggesting a causal role for the beneficial effects of exercise on correlations between changes in cytokines. We also used a large sample size of nearly 300 patients drawn from multiple locations across the United States, increasing the precision and generalizability of our results compared to a single-site study. Patients adhered to the exercise intervention, with significantly more resistance exercise performed by exercise subjects than by control subjects, and a modest increase in steps/day in exercise subjects than in control subjects. In our view, this amount of exercise is quite impressive for patients during chemotherapy, who were dealing with an array of side effects and who were previously not exercising.
This study also has limitations. First, we only assessed six molecular markers of inflammation, whereas inflammatory regulation actually involves hundreds of inter-molecular interactions [42]. However, we captured key exercise-related cytokines (IL-6, IL-10, and IL-1β) [14] and we sampled both pro- and anti-inflammatory cytokines. Missing data (i.e., dropouts) and undetectable cytokine concentrations could have biased our results, but our attrition of 15% is less than that of typical clinical trials in supportive oncology, which average 26% attrition [43], and our key results involving IL-6 and IL-10 had minimal undetectable cytokine values (<3%). Furthermore, there we no significant differences in dropout across the exercise and control groups, so we do not expect dropout to confound our observed differences between the exercise and control groups. Our sample was mostly white, well-educated, female breast cancer patients, thus limiting generalizability to patients with other tumor types and demographic characteristics. Finally, the probability of Type-I error was inflated due to the exploratory nature of our secondary analysis [34].
Future studies should continue to test the validity, generalizability, and utility of these inter-cytokine correlation-based markers of inflammation. Specifically, we need to establish normal ranges for these markers by assessing healthy individuals and by exploring their relationships to patient-reported symptoms. More sophisticated analytical approaches (e.g., machine learning) to assessing inter-cytokine relationships are also warranted, as they have proven useful in studies of cognitive impairment of breast cancer survivors [44]. More granular data would also likely improve power and predictive ability, e.g., by collecting blood every week or both pre- and post-exercise instead of six weeks apart. Indeed, the first six weeks of chemotherapy are very stressful physiologically and psychologically and often toxicities accumulate week-by-week with each infusion (e.g., as seen in neuropathy [45]). If we measured cytokines earlier or more often (e.g., every week), we might be able to see an accumulating effect on inter-cytokine correlations or levels as well, and we would likely have improved sensitivity using repeated-measures analyses across more time points. With a longer intervention (beyond six weeks), we might have seen more or larger differences due to the accumulating effects of the exercise intervention. In the far future, inter-cytokine markers of inflammation may help predict and track cancer treatment-related toxicities before they become too severe, thereby informing the course of cancer treatments and recommendations for interventions such as exercise. These markers could perhaps help identify how to dose the exercise (type, intensity, frequency, duration) to achieve a certain level of improvement while not suggesting too much exercise, which can be harmful [30]. The markers could also help exercise promotion and adherence by increasing the patient’s self-efficacy if they saw improvements in their blood-based markers resulting from their exercise during chemotherapy. These measures might also provide mechanistic insight and might help inform development of therapeutics to treat or prevent cancer treatment-related toxicities.
Conclusion
Our exploratory work suggests that exercise during chemotherapy improves the inflammatory profile while also inducing coherent changes in concentrations of individual cytokines. Our work highlights the importance of interactions between cytokines, and it complements traditional inflammatory biomarkers used to study exercise, chemotherapy, and chemotherapy toxicities. Thus, this approach may similarly enhance research on other inflammation-mediated phenomena.
Acknowledgements:
The authors would like to thank Drs. Susan Rosenthal and Amber Kleckner for reviewing this manuscript. We extend special thanks to the cancer patients, as well as the research staff of the University of Rochester Cancer Center NCORP Research Base at each of the NCORP affiliates who recruited and followed participants in this study. We also thank the staff of the PEAK Human Performance Laboratory and the Cancer Control Psychoneuroimmunology Laboratory, especially Ms. Ann Colasurdo for conducting the cytokine assays. We thank the National Cancer Institute for their funding support of this project. Funding was provided by the National Cancer Institute, including K07CA221931 to I.R.K. and funds from NCORP (formerly CCOP) parent grant U10 CA037420, NCORP (formerly CCOP) supplement U10 CA037420, and R25 CA102618.
Trial Registration: Clinical Trials.gov, # , http://www.clinicaltrials.gov.
Footnotes
Conflict of interest statement
The authors have no conflict of interest to declare as the research was voluntarily conducted. The authors have full control of all primary data and agree to allow the journal to review the data if requested.
References
- 1.Mustian KM, Sprod LK, Janelsins M, et al. Exercise Recommendations for Cancer-Related Fatigue, Cognitive Impairment, Sleep Problems, Depression, Pain, Anxiety, and Physical Dysfunction—A Review. Oncology & Hematology Review (US) 2012;8(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pusztai L, Mendoza TR, Reuben JM, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 2004;25(3):94–102. [DOI] [PubMed] [Google Scholar]
- 3.Collado-Hidalgo A, Bower JE, Ganz PA, et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 2006;12(9):2759–66. [DOI] [PubMed] [Google Scholar]
- 4.Zhou YQ, Liu Z, Liu ZH, et al. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation 2016;13(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janelsins MC, Kesler SR, Ahles TA, et al. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry 2014;26(1):102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun 2013;30 Suppl:S48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang XM, Lehky TJ, Brell JM, et al. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine 2012;59(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNeely ML, Parliament MB, Seikaly H, et al. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: a randomized controlled trial. Cancer 2008;113(1):214–22. [DOI] [PubMed] [Google Scholar]
- 9.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 2003;21(9):1653–9. [DOI] [PubMed] [Google Scholar]
- 10.Mustian KM, Alfano CM, Heckler C, et al. A Comparative Meta-Analysis of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue. JAMA Oncology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleckner IR, Kamen C, Gewandter JS, et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support Care Cancer 2018;26(4):1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleckner IR, Kamen C, Gewandter JS, et al. Response to Crevenna and Ashbury, Vallance and Bolam, and Crevenna and Keilani regarding the effects of exercise on chemotherapy-induced peripheral neuropathy. Support Care Cancer 2019;27(1):7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleckner IR, Dunne RF, Asare M, et al. Exercise for Toxicity Management in Cancer-A Narrative Review. Oncol Hematol Rev 2018;14(1):28–37. [PMC free article] [PubMed] [Google Scholar]
- 14.Gleeson M, Bishop NC, Stensel DJ, et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011;11(9):607–15. [DOI] [PubMed] [Google Scholar]
- 15.Troseid M, Lappegard KT, Claudi T, et al. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur Heart J 2004;25(4):349–55. [DOI] [PubMed] [Google Scholar]
- 16.Baum M, Muller-Steinhardt M, Liesen H, et al. Moderate and exhaustive endurance exercise influences the interferon-gamma levels in whole-blood culture supernatants. Eur J Appl Physiol Occup Physiol 1997;76(2):165–9. [DOI] [PubMed] [Google Scholar]
- 17.Mills PJ, Parker B, Dimsdale JE, et al. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol Psychol 2005;69(1):85–96. [DOI] [PubMed] [Google Scholar]
- 18.Mommersteeg PM, Kupper N, Schoormans D, et al. Health-related quality of life is related to cytokine levels at 12 months in patients with chronic heart failure. Brain Behav Immun 2010;24(4):615–22. [DOI] [PubMed] [Google Scholar]
- 19.Zimmer P, Baumann FT, Bloch W, et al. Impact of exercise on pro inflammatory cytokine levels and epigenetic modulations of tumor-competitive lymphocytes in Non-Hodgkin-Lymphoma patients-randomized controlled trial. Eur J Haematol 2014;93(6):527–32. [DOI] [PubMed] [Google Scholar]
- 20.Rogers LQ, Vicari S, Trammell R, et al. Biobehavioral factors mediate exercise effects on fatigue in breast cancer survivors. Med Sci Sports Exerc 2014;46(6):1077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez AM, Martinez C, Fiuza-Luces C, et al. Exercise training and cytokines in breast cancer survivors. Int J Sports Med 2011;32(6):461–7. [DOI] [PubMed] [Google Scholar]
- 22.Schindler R, Mancilla J, Endres S, et al. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 1990;75(1):40–7. [PubMed] [Google Scholar]
- 23.Alexandrakis MG, Roussou P, Pappa CA, et al. Relationship between circulating BAFF serum levels with proliferating markers in patients with multiple myeloma. Biomed Res Int 2013;2013:389579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labidi SI, Menetrier-Caux C, Chabaud S, et al. Serum cytokines in follicular lymphoma. Correlation of TGF-beta and VEGF with survival. Ann Hematol 2010;89(1):25–33. [DOI] [PubMed] [Google Scholar]
- 25.Dhabhar FS, Burke HM, Epel ES, et al. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J Psychiatr Res 2009;43(11):962–9. [DOI] [PubMed] [Google Scholar]
- 26.Berg A, Patel S, Gonca M, et al. Cytokine network in adults with falciparum Malaria and HIV-1: increased IL-8 and IP-10 levels are associated with disease severity. PLoS One 2014;9(12):e114480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pripp AH, Stanisic M. The correlation between pro- and anti-inflammatory cytokines in chronic subdural hematoma patients assessed with factor analysis. PLoS One 2014;9(2):e90149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horsburgh S, Robson-Ansley P, Adams R, et al. Exercise and inflammation-related epigenetic modifications: focus on DNA methylation. Exerc Immunol Rev 2015;21:26–41. [PubMed] [Google Scholar]
- 29.Marcus BH, Selby VC, Niaura RS, et al. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport 1992;63(1):60–6. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42(7):1409–26. [DOI] [PubMed] [Google Scholar]
- 31.Mustian K, Janelsins M, Peppone L, et al. EXCAP exercise effects on cognitive impairment and inflammation: A URCC NCORP RCT in 479 cancer patients. In: American Society for Clinical Oncology Annual Meeting Chicago, IL, 2015. [Google Scholar]
- 32.American College of Sports Medicine. ACSM’s Guidelines for exercise testing and prescription Baltimore, MD: Lippincott, Williams, & Wilkins; 2010. [Google Scholar]
- 33.Cavaillon JM. Pro- versus anti-inflammatory cytokines: myth or reality. Cell Mol Biol (Noisy-le-grand) 2001;47(4):695–702. [PubMed] [Google Scholar]
- 34.Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol 2001;54(4):343–9. [DOI] [PubMed] [Google Scholar]
- 35.Uh HW, Hartgers FC, Yazdanbakhsh M, et al. Evaluation of regression methods when immunological measurements are constrained by detection limits. BMC Immunol 2008;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate imputation by chained equations in R. Journal of Statistical Software 2011;45(3). [Google Scholar]
- 37.Leggate M, Nowell MA, Jones SA, et al. The response of interleukin-6 and soluble interleukin-6 receptor isoforms following intermittent high intensity and continuous moderate intensity cycling. Cell Stress Chaperones 2010;15(6):827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen JF, Tolver A, Andersen JL, et al. Resistance training does not protect against increases in plasma cytokine levels among germ cell cancer patients during and after chemotherapy. J Clin Endocrinol Metab 2014;99(8):2967–76. [DOI] [PubMed] [Google Scholar]
- 39.Allgayer H, Nicolaus S, Schreiber S. Decreased interleukin-1 receptor antagonist response following moderate exercise in patients with colorectal carcinoma after primary treatment. Cancer Detect Prev 2004;28(3):208–13. [DOI] [PubMed] [Google Scholar]
- 40.Monastero RN, Pentyala S. Cytokines as Biomarkers and Their Respective Clinical Cutoff Levels. Int J Inflam 2017;2017:4309485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koksal D, Unsal E, Poyraz B, et al. The value of serum interferon-gamma level in the differential diagnosis of active and inactive pulmonary tuberculosis. Tuberk Toraks 2006;54(1):17–21. [PubMed] [Google Scholar]
- 42.Dinarello CA. Historical insights into cytokines. Eur J Immunol 2007;37 Suppl 1:S34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui D, Glitza I, Chisholm G, et al. Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer 2013;119(5):1098–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henneghan AM, Palesh O, Harrison M, et al. Identifying cytokine predictors of cognitive functioning in breast cancer survivors up to 10years post chemotherapy using machine learning. J Neuroimmunol 2018;320:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loprinzi CL, Reeves BN, Dakhil SR, et al. Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. J Clin Oncol 2011;29(11):1472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]