Abstract
Objective
To characterize the results of surgery for GIST in the pre- and post-imatinib eras at a single-institution and identify current prognostic clinicopathologic factors.
Summary Background Data
Imatinib has radically changed the management of GIST, yet the magnitude of impact on outcome across the spectrum of GIST presentation and relevance of historical prognostic factors are not well defined.
Methods
We retrospectively analyzed 1,000 patients who underwent surgery for GIST at our institution from 1982–2016. Patients were stratified by presentation status as primary tumor only (PRIM), primary with synchronous metastasis (PRIM+MET), or metachronous recurrence/metastases (MET), as well as imatinib era (before and after it became available). Cox proportional hazard models and Kaplan Meier methods were used to model and estimate overall and recurrence-free survival (OS, RFS).
Results
OS was longer in the imatinib era compared to the pre-imatinib era in each presentation group, including in Miettinen high risk primary tumors. Among PRIM patients from the pre-imatinib era, tumor site, size, and mitotic rate were independently associated with OS and RFS on multivariate analysis. PRIM patients in the imatinib era who received imatinib (neoadjuvant and/or adjuvant) had higher risk tumors, but after adjusting for treatment, only size >10cm remained independently prognostic of RFS (HR 3.85, 95% CI 2.00–7.40, p<0.0001) and OS (HR 3.37, 95% CI 1.60–7.13, p=0.001).
Conclusions
Patients treated in the imatinib era had prolonged overall survival across all presentations. In the imatinib era, among site, size, and mitotic rate, high risk features were associated with treatment with the drug, but only size >10cm correlated with outcome. Imatinib should still be prescribed for patients with high risk features.
MINI-ABSTRACT
In the pre-imatinib era, primary tumor site, size, and mitotic rate predicted outcome as expected. In the modern era, survival was dramatically longer. While primary tumor high risk features were associated with imatinib treatment, only tumor size >10cm remained associated with outcome. Imatinib should be prescribed for high risk features.
INTRODUCTION
Since the discovery of a gain-of-function mutation in the KIT oncogene by Hirota in 19981, our understanding of GIST has evolved rapidly. We now know that GIST is the most common sarcoma.2 MicroGISTs (<1cm) occur in up to one-third of older patients3. GIST arises from the interstitial cells of Cajal (ICC) and depends on the transcription factor ETV14. KIT mutations are present in 75% of GISTs, while 10% instead have a PDGFRA mutation5. The remaining “wild-type” GISTs have a variety of other mutations and epimutations that may affect the SDH pathway6.
Imatinib is a small molecule tyrosine kinase inhibitor of KIT and PDGFR, originally used for chronic myelogenous leukemia5. Discovery of KIT mutations in GIST quickly led to the successful introduction of the drug in clinical trials7, in what became the modern paradigm for targeted therapy8. Despite a historical response rate of less than 10% to conventional systemic chemotherapy9, partial response or stable disease occurred in 80% of patients with advanced GIST treated with imatinib10. Median survival was 57 months11, compared to 19 months historically12.
Adjuvant imatinib was then tested in randomized clinical trials. After resection of primary GISTs ≥3cm, one year of adjuvant therapy prolonged recurrence-free survival (RFS) without affecting OS13, 14, with a similar result seen after two years of therapy in intermediate and high risk GISTs15, as defined by NIH consensus criteria10. Three years of adjuvant therapy proved superior to one year in modified NIH high risk patients, with prolonged RFS and a slight benefit in OS16, 17. While further study is ongoing to determine the optimal duration of therapy, these trials have resulted in adjuvant imatinib becoming the standard of care for intermediate and high risk GIST. Neoadjuvant imatinib has been evaluated in locally advanced primary GIST, typically for difficult anatomic locations such as the gastroesophageal junction, duodenum, or rectum, or for large tumors requiring multivisceral resection18–20. While these studies are mostly retrospective or single arm studies, neoadjuvant imatinib is safe and may facilitate R0 resection. Preoperative imatinib has been used before resection of metastatic GIST, with progression-free survival correlating to treatment response21, 22.
In 2000, we reported the recurrence patterns and prognostic factors for 200 GISTs (80 of which were PRIM) treated at Memorial Sloan Kettering Cancer Center12. While the randomized controlled trials have established the efficacy of imatinib in GIST in the advanced10, 23 and adjuvant13–17 settings, here we present the magnitude of imatinib’s effect across the spectrum of GIST presentation (PRIM, PRIM+MET, and MET) in the largest single-institution series of surgical management of GIST. Furthermore, we interrogate the factors associated with the PRIM+MET presentation, which are poorly characterized in the literature, and may require closer surveillance or alternate therapy to imatinib. Finally, the current relevance of traditional clinicopathologic prognostic factors in PRIM10, 12, 24–27 (i.e., site, size, and mitotic rate) is unclear.
METHODS
Patients and methods
From a prospective database, we identified patients who underwent surgery for GIST at our institution from July 1982 until April 2016. Diagnosis was confirmed using standard histology as well as immunohistochemistry for CD117 (KIT) and sometimes DOG-1. Patients were grouped by their presentation at our institution as primary tumor only (PRIM), primary with synchronous metastasis (PRIM+MET), or metachronous recurrence/metastasis (MET). Age, sex, characteristics of the primary tumor (size, site, and mitotic rate), histologic variant (spindle, epithelioid, or mixed), margins from first resection (R0/1/2), and significant dates (date of diagnosis, first surgery at MSKCC, recurrence, death, last follow-up), were queried. Fifty high-powered fields (HPF) were counted by the pathologist to determine mitotic rate28. Site of metastasis, mutation (starting in the mid-2000s), and second malignancies were recorded. Postoperative surveillance included history, physical exam, and imaging every 3–6 months. The database was locked in June 2016. Approval for all research was obtained from the Institutional Review Board and was Health Insurance Portability and Accountability Act compliant.
Analysis and Statistics
Patients were stratified by treatment era, with the imatinib era defined as the earliest date when a patient in each group received the drug (8/2001, 3/2001, and 8/2000, respectively). Univariate associations used the Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical variables. OS and RFS were estimated from the time of the initial surgery at our institution using Kaplan Meier methods. For RFS, recurrence or death from any cause were counted as an event. Groups were compared using the log-rank test. Cox Proportional hazard models were used to model OS and RFS univariately and to build a multivariate model for each era. All statistical tests were two sided, and p-values less than 0.05 were considered statistically significant. Statistical analyses were performed in SAS 9.4 (SAS Institute, Inc, Cary, NC) and R version 3.4.0.
RESULTS
Clinicopathologic features
Among 11,323 patients with soft tissue sarcomas treated at our institution from 1982–2016, there were 1,000 (8.8%) who underwent surgery for GIST with a median follow-up of 4.6 years (Table 1). Overall, 66% were PRIM patients, 13% PRIM+MET, and 21% MET. The median time from disease presentation at an outside institution to surgery for MET at our institution was 4.1 years (interquartile range [IQR] 1.9–6.5). PRIM patients were older.
Table 1:
Characteristics of 1,000 GISTs by presentation status.
For this and all following tables, percentages reflect the proportion of those where variable is known, with unknowns excluded from statistical analysis.
|
PRIM n=660 |
PRIM+MET n=128 |
MET n=212 |
p-value | |||
|---|---|---|---|---|---|---|
| Age at first surgery (yrs) | Median (range) | 61.0 (8.1–94.7) | 64.5 (8.1–94.7) | 56.6 (22.6–85.3) | 54.7 (9.4–85.2) | <0.001 |
| Sex | F | 468 (46.8) | 326 (49.4) | 47 (36.7) | 95 (44.8) | 0.025 |
| M | 532 (53.2) | 334 (50.6) | 81 (63.3) | 117 (55.2) | ||
| Survivor follow-up (yrs) | Median (range) | 4.6 (0.0–29) | 4.6 (0.0–28) | 4.4 (0.20–22) | 5.3 (0.03–29) | |
| Primary tumor site | Stomach | 606 (60.6) | 467 (70.8) | 59 (46.1) | 80 (37.7) | <0.001 |
| Small Bowel | 264 (26.4) | 116 (17.6) | 55 (43) | 93 (43.9) | ||
| Rectum | 51 (5.1) | 44 (6.7) | 0 (0) | 7 (3.3) | ||
| Othera | 79 (7.9) | 33 (5) | 14 (10.9) | 32 (15.1) | ||
| Primary tumor Size (cm) | <5 | 377 (38.7) | 327 (49.5) | 17 (13.5) | 33 (17.5) | <0.001 |
| 5–10 | 314 (32.2) | 195 (29.5) | 40 (31.7) | 79 (41.8) | ||
| >10 | 284 (29.1) | 138 (20.9) | 69 (54.8) | 77 (40.7) | ||
| Unknown | 25 (N/A) | 0 (N/A) | 2 (N/A) | 23 (N/A) | ||
| Primary tumor mitotic rate per 50 HPF | ≤5 | 406 (54.8) | 370 (66) | 13 (21) | 23 (19.5) | <0.001 |
| >5 | 335 (45.2) | 191 (34) | 49 (79) | 95 (80.5) | ||
| Unknown | 259 (N/A) | 99 (N/A) | 66 (N/A) | 94 (N/A) | ||
| Histologic variant | Spindle | 485 (73.8) | 355 (76.8) | 59 (67) | 71 (66.4) | 0.047 |
| Epithelioid | 88 (13.4) | 55 (11.9) | 12 (13.6) | 21 (19.6) | ||
| Mixed | 84 (12.8) | 52 (11.3) | 17 (19.3) | 15 (14) | ||
| Unknown | 343 (N/A) | 198 (N/A) | 40 (N/A) | 105 (N/A) | ||
| Marginsb | R0 | 744 (75) | 597 (90.5) | 49 (38.6) | 98 (47.8) | <0.001 |
| R1 | 118 (11.9) | 47 (7.1) | 22 (17.3) | 49 (23.9) | ||
| R2 | 130 (13.1) | 16 (2.4) | 56 (44.1) | 58 (28.3) | ||
| Unknown | 8 (N/A) | 0 (N/A) | 1 (N/A) | 7 (N/A) | ||
| Site of metastasisc | ||||||
| any | No | 557 (55.7) | 557 (84.4) | 0 (0) | 0 (0) | c |
| Yes | 417 (42.8) | 82 (12.8) | 128 (100) | 207 (100) | ||
| any liver | No | 207 (49.6) | 40 (48.8) | 61 (47.7) | 106 (51.2) | |
| Yes | 210 (50.4) | 42 (51.2) | 67 (52.3) | 101 (48.8) | ||
| liver only | No | 293 (70.3) | 58 (70.7) | 85 (66.4) | 150 (72.5) | |
| Yes | 124 (29.7) | 24 (29.3) | 43 (33.6) | 57 (27.5) | ||
| any peritoneum | No | 151 (36.2) | 36 (43.9) | 53 (41.4) | 62 (30) | |
| Yes | 266 (63.8) | 46 (56.1) | 75 (58.6) | 145 (70) | ||
| peritoneum only | No | 230 (55.2) | 51 (62.2) | 73 (57) | 106 (51.2) | |
| Yes | 187 (44.8) | 31 (37.8) | 55 (43) | 101 (48.8) | ||
| liver and peritoneum | No | 339 (81.3) | 67 (81.7) | 108 (84.4) | 164 (79.2) | |
| Yes | 78 (18.7) | 15 (18.3) | 20 (15.6) | 43 (20.8) | ||
| any otherd | No | 398 (95.4) | 73 (89) | 122 (95.3) | 203 (98.1) | |
| Yes | 19 (4.6) | 9 (11) | 6 (4.7) | 4 (1.9) | ||
| Second malignancy | ||||||
| any | No | 782 (78.2) | 470 (71.2) | 116 (90.6) | 196 (92.5) | <0.001 |
| Yes | 218 (21.8) | 190 (28.8) | 12 (9.4) | 16 (7.5) | ||
| prior | No | 835 (83.5) | 521 (78.9) | 119 (93) | 195 (92) | <0.001 |
| Yes | 165 (16.5) | 139 (21.1) | 9 (7) | 17 (8) | ||
| synchronous | No | 938 (93.8) | 602 (91.2) | 125 (97.7) | 211 (99.5) | <0.001 |
| Yes | 62 (6.2) | 58 (8.8) | 3 (2.3) | 1 (0.5) | ||
| Mutationf | KIT exon 9 | 44 (7.6) | 13 (4.1) | 11 (10.9) | 20 (12.4) | f |
| KIT exon 11 deletion | 216 (37.1) | 128 (40) | 43 (42.6) | 45 (28) | ||
| KIT exon 11 other | 117 (20.1) | 76 (23.8) | 11 (10.9) | 30 (18.6) | ||
| KIT exon 13 | 9 (1.5) | 4 (1.3) | 3 (3) | 2 (1.2) | ||
| KIT exon 17 | 4 (0.7) | 1 (0.3) | 0 (0) | 3 (1.9) | ||
| KIT multiple exons | 48 (8.2) | 7 (2.2) | 16 (15.8) | 25 (15.5) | ||
| PDGFRA D842V/I | 23 (4) | 20 (6.3) | 0 (0) | 3 (1.9) | ||
| PDGFRA other | 27 (4.6) | 20 (6.3) | 3 (3) | 4 (2.5) | ||
| NF1 | 5 (0.9) | 4 (1.3) | 0 (0) | 1 (0.6) | ||
| SDH | 3 (0.5) | 0 (0) | 2 (2) | 1 (0.6) | ||
| Wild typee | 86 (14.8) | 47 (14.7) | 12 (11.9) | 27 (16.8) | ||
| Unknown | 418 (N/A) | 340 (N/A) | 27 (N/A) | 51 (N/A) | ||
Other primary sites included 12 (1.2%) colon, 9 (0.9%) esophagus, 2 (0.2%) pancreas, 1 (0.1%) appendix, and 1 (0.1%) perineum. The remainder of this group was comprised of tumors designed as omentum, mesentery, retroperitoneum or abdomen where a source organ could not be determined.
R2 margins include tumor rupture
Percentages in top category ‘any’ metastasis rows reflect percentage of total patients in that group. Percentages in the other rows (i.e. ‘any liver’) reflect percentages of those who developed metastases from that group. For PRIM, metastatic site denotes the site of metastasis in the 103 patients who recurred. For PRIM, MET, and PRIM+MET there were 21, 0, and 5 patients per group, respectively, for whom the site of metastasis was unknown, and these were not includes in the percentages listed. There were too many categories for meaningful statistical comparison.
Other metastatic sites occurred either in isolation or concomitantly with liver and or peritoneal metastasis and included 10 lymph node (2.3% of 443 total patients with metastases), 5 bone (1.1%), 3 lung (0.7%), and 1 each chest, chest wall, bronchus, perineum, paraspinal, and adrenal (0.7% each).
early mutational analysis included only KIT and PDGFRA, so some “wild type” likely carry other mutations/epimutations
There were too many categories (many with low numbers) for statistical comparison. Values listed here represent proportions of all mutations. Note that percentages in results section (where noted) reflect proportion of all KIT mutations.
Stomach was by far the most common site of origin in PRIM, whereas small bowel and stomach tumors were seen in similar proportions in the PRIM+MET and MET groups. Notably, there were no rectal tumors in the PRIM+MET group. PRIM patients had smaller tumors, with only 21% >10cm vs. 55 and 41% in the other groups. Primary tumor mitotic rate was available for 74% of all patients and was >5/50 HPF in 34% of PRIM tumors compared to 79 and 81% in the other groups.
Mutational analysis (done by Sanger sequencing prior to 201014 and MSK-IMPACT after29) was available in 58% of patients. KIT mutations were most common at 75% of all patients, followed by 9% PDGFRA (Table 1). KIT mutations, when analyzed separately, were most often exon 11 deletions (49% of KIT mutations) or other exon 11 mutations (including point mutations and insertions; 27%). Exon 9 mutations were less common (10% of KIT mutations), and exon 13 and exon 17 were rare (2.1 and 0.9%). Mutations in multiple KIT exons comprised 11% of KIT mutations, but were rare in PRIM (2.2% vs. 16% in the other groups), likely reflecting secondary mutations in patients on long-term imatinib. PDGFRA mutations were found mostly in the PRIM group and were D842V or D842I (known to be imatinib-resistant) in 46% of patients with PDGRFA mutations.
Second malignancy
GIST has previously been reported to have a high prevalence of second malignancy (preceding or synchronous)30, and since this would be expected to affect survival, we included it in our multivariate analysis. Overall, 22% of patients had a second malignancy (Table 1). PRIM patients had the highest rate (29 vs. 9 and 8%). In PRIM patients, among 190 second malignancies, 58 (31%) were synchronous, most commonly gastric, esophageal, colorectal, or pancreatobiliary carcinomas (Supplemental Table 1).
Recurrence and Survival
In patients with recurrence, the peritoneum was involved in 64%, liver in 50%, and both in 19% (Table 1). Rare sites included lymph nodes (2.3%), bone (1.1%), and lung (0.7%). The pattern of recurrence was similar by presentation group, except there was more peritoneal disease in the PRIM+MET group.
PRIM patients had longer median OS than PRIM+MET patients (13.6 vs. 5.3 years, Figure 1a). MET patients had a median OS of 4.0 years (Figure 1b)6. Patients in the MET group underwent surgery at our institution a median of 4.1 years after their initial outside surgery. Incomplete resection (R2) was rare in PRIM patients (2.4%) but common in the other groups (44 and 28%, Table 1). Remarkably, there was no difference between negative (R0) and microscopically positive (R1) margins in the 3 groups (Figures 1c–e).
Figure 1:
OS after surgery by group and margins.
OS after initial surgery is shown for PRIM and PRIM+MET (a) and MET (b). OS by margin status is shown for PRIM (c), PRIM+MET (d), and MET (e). The number of patients at risk at each time point is indicated on the x axis. The inset in each panel denotes the median survival, 95% confidence interval of the median, and p-value for a log rank test when multiple groups were compared.
Since imatinib has dramatically altered management of GIST5, we sought to dissect the impact of the drug. In order to avoid treatment bias in our retrospective analysis, we stratified each group by when the first patients in that group received imatinib (i.e., when imatinib became available or was employed for that indication). In each of the 3 groups, survival in the imatinib era was longer. Five-year OS was 87, 63, and 51% in the imatinib era compared to 57, 19, and 35% in the pre-imatinib era, respectively (Figure 2a–c). Since the PRIM group was the largest and most homogenous because the patients had been followed at our institution from their initial diagnosis, we selected these patients for further analysis. Age, sex, and histologic variant were similar between the pre-imatinib and imatinib eras (Table 2). Although statistical comparison of mutation was not possible, the distributions were similar. There were more stomach tumors, and less small bowel, rectal, and other tumors in the imatinib era. Tumors were smaller in the imatinib era as only 16% were >10cm compared to 32%. R1 margins were more common in the pre-imatinib era. Overall, however, better prognostic features in the imatinib era did not explain the survival differences, since similar survival differences were also noted when only Miettinen high risk25, 31 patients were analyzed (Figure 2d).
Figure 2:
OS after surgery by group, stratified by imatinib era.
OS after initial surgery is shown for PRIM (a), PRIM+MET (b) MET (c), and Miettinen high risk patients from the PRIM group (d) with imatinib era stratified by the date of the first patient in each group to receive the drug. The number of patients at risk at each time point is indicated on the x axis. The inset in each panel denotes the median survival, 95% confidence interval of the median, and p-value for a log rank test comparison between groups.
Table 2:
Characteristics of completely resecteda primary GISTs (PRIM) by imatinib era.
| Pre-imatinib era (n=137) | Imatinib era (n=507) | p-value | ||
|---|---|---|---|---|
| Age at first Surgery (yrs) | Median (range) | 66.6 (15.8–94.7) | 64.0 (8.1–90.1) | 0.657 |
| Sex | F | 57 (41.6) | 260 (51.3) | 0.054 |
| M | 80 (58.4) | 247 (48.7) | ||
| Primary tumor site | Stomach | 83 (60.6) | 380 (75) | 0.003 |
| Small Bowel | 29 (21.2) | 83 (16.4) | ||
| Rectum | 14 (10.2) | 28 (5.5) | ||
| Other | 11 (8) | 16 (3.2) | ||
| Primary tumor Size (cm) | <5 | 46 (33.6) | 281 (55.4) | <0.001 |
| 5–10 | 47 (34.3) | 144 (28.4) | ||
| >10 | 44 (32.1) | 82 (16.2) | ||
| Primary tumor mitotic rate per 50 HPF | ≤5 | 61 (54) | 306 (70) | 0.002 |
| >5 | 52 (46) | 131 (30) | ||
| Unknown | 24 (N/A) | 70 (N/A) | ||
| Histologic variant | Unknown | 117 (N/A) | 72 (N/A) | 0.268 |
| Epithelioid | 3 (15) | 52 (12) | ||
| Mixed | 0 (0) | 52 (12) | ||
| Spindle | 17 (85) | 331 (76.1) | ||
| Marginsa | R0 | 121 (88.3) | 476 (93.9) | 0.040 |
| R1 | 16 (11.7) | 31 (6.1) | ||
| Second malignancyb | No | 108 (78.8) | 348 (68.6) | 0.020 |
| Yes | 29 (21.2) | 159 (31.4) | ||
| Mutation | KIT exon 9 | 3 (2.9) | 10 (4.8) | c |
| KIT exon 11 deletion | 44 (42.7) | 78 (37.1) | ||
| KIT exon 11 other | 21 (20.4) | 55 (26.2) | ||
| KIT exon 13 | 0 (0) | 4 (1.9) | ||
| KIT exon 17 only | 1 (1) | 0 (0) | ||
| KIT mult exons | 1 (1) | 6 (2.9) | ||
| PDGFRA D842V/I | 3 (2.9) | 17 (8.1) | ||
| PDGFRA other | 4 (3.9) | 16 (7.6) | ||
| NF1 | 0 (0) | 4 (1.9) | ||
| SDH | 0 (0) | 0 (0) | ||
| Wild type (WT) | 26 (25.2) | 20 (9.5) | ||
| Unknown | 34 (N/A) | 297 (N/A) | ||
R2 margins were excluded from this analysis of disease-free survival.
Any preceding or synchronous second malignancy
There were too many categories (many with low numbers) for statistical comparison.
Prognostic factors by era
Among 507 completely resected PRIM patients in the imatinib era, 162 patients received imatinib, 42 as neoadjuvant only, 86 as adjuvant only, and 34 as both (Supplemental Table 2). Patients who received imatinib were younger and were more likely to have a non-gastric site, large tumor size, high mitotic rate, and R1 margin. Tumor site, size, and mitotic rate are well-known traditional prognostic factors of recurrence after resection of primary GIST10, 12, 24–27. In order to determine whether these factors remained important in the era of imatinib, we analyzed RFS separately in the pre-imatinib and imatinib (Table 3) eras using Cox proportional hazards models. In the pre-imatinib era, as expected, site (small bowel or rectal), large size, and high mitotic rate were independently associated with worse RFS on the multivariate analysis. However, in the imatinib era, despite adjusting for imatinib treatment and using a large cohort of patients, of these variables, only size >10cm remained statistically significant on multivariate analysis (HR 3.85, 95% CI 2.00–7.40, p<0.0001). Additionally, mixed histologic variant (HR 2.1, 95% CI 1.19–3.79, p=0.01) and second malignancy (HR 2.37, 95% CI 1.49–3.76, p=0.0003) emerged as negative predictors. Since patients who develop recurrence are often salvaged with further treatment including imatinib, we performed a similar analysis of OS with similar results, this time including the 16 patients who had R2 margins (Supplemental Table 3).
Table 3:
Univariate and multivariate analysis of RFS in the pre-imatinib era (top) and imatinib era (bottom).
| PRE-IMATINIB ERAa | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| Class value | Reference | HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age at first surgery (yrs) | N/A | N/A | 1.01 (0.998–1.029) | 0.088 | 1.02 (1.002–1.037) | 0.030 | |
| Sex | M | F | 1.52 (0.99–2.33) | 0.053 | - | - | |
| Primary tumor site | Small Bowel | Stomach | 1.35 (0.81–2.25) | 0.250 | 2.13 (1.67–3.90) | 0.014 | |
| Rectum | 2.63 (1.41–4.91) | 0.002 | 2.25 (1.08–4.68) | 0.030 | |||
| Other | 1.47 (0.73–2.95) | 0.280 | 1.36 (0.65–2.83) | 0.409 | |||
| Primary tumor size (cm) | 5–10 | <5 | 1.21 (0.73–2.00) | 0.466 | 1.72 (0.96–3.09) | 0.071 | |
| >10 | 1.82 (1.10–3.01) | 0.020 | 2.63 (1.44–4.8) | 0.002 | |||
| Primary tumor mitotic rate per 50 HPF | >5 | ≤5 | 2.56 (1.65–3.99) | <0.001 | 2.80 (1.71–4.61) | <0.001 | |
| Marginsb | R1 | R0 | 1.10 (0.58–2.07) | 0.769 | - | - | |
| Second Malignancyc | Yes | No | 0.79 (0.47–1.32) | 0.370 | - | - | |
| IMATINIB ERA | Univariate Analysis | Multivariate Analysisd | |||||
|---|---|---|---|---|---|---|---|
| Class value | Reference | HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age at first surgery (yrs) | N/A | N/A | 1.02 (1.00–1.04) | 0.014 | - | - | |
| Sex | M | F | 1.24 (0.85–1.81) | 0.274 | - | - | |
| Primary tumor site | Small Bowel | Stomach | 1.29 (0.78–2.11) | 0.318 | - | - | |
| Rectum | 0.93 (0.38–2.30) | 0.873 | - | - | |||
| Other | 2.06 (0.95–4.47) | 0.068 | - | - | |||
| Primary tumor size (cm) | 5–10 | <5 | 1.43 (0.90–2.25) | 0.138 | 1.66 (0.96–2.87) | 0.070 | |
| >10 | 2.72 (1.71–4.32) | <0.001 | 3.85 (2.00–7.40) | <0.001 | |||
| Primary tumor mitotic rate per 50 HPF | >5 | ≤5 | 1.91 (1.21–3.00) | 0.005 | - | - | |
| Histologic variant | Epithelioid | Spindle | 1.25 (0.63–2.47) | 0.526 | 1.09 (0.55–2.16) | 0.811 | |
| Mixed | 2.64 (1.50–4.66) | <.001 | 2.1 (1.19–3.79) | 0.011 | |||
| Marginsb | R1 | R0 | 1.29 (0.63–2.65) | 0.492 | - | - | |
| Imatinib treatment | Any | None | 1.78 (1.21–2.63) | 0.004 | 1.11 (0.65–1.91) | 0.693 | |
| Second Malignancyc | Yes | No | 1.90 (1.30–2.77) | <0.001 | 2.37 (1.49–3.76) | 0.0003 | |
Histologic variant was not routinely recorded in the pre-imatinib era.
R2 margins were excluded from this analysis of recurrence-free survival.
Any preceding or synchronous second malignancy
All statistically significant values from the univariate analysis were included in the initial multivariate model. The least significant values (p>0.05) were removed stepwise to provide the final strongest model which is shown. Although age and mitotic rate were significant univariately in the imatinib era, they were never significant on multivariate analysis. Imatinib treatment was left in the final model to adjust for any undetected treatment bias.
5-year RFS was longer in each size category (<5, 5–10, and >10cm) at 84, 78, and 61% in the imatinib era compared to 62, 49, and 36% in the pre-imatinib era (Figure 3a–c; p=0.008, 0.004, 0.016 respectively). Since the <5cm group was the largest (n=327), and there is debate about the size threshold to resect a small GIST, we examined it more closely. While there were 75 RFS events in this group (Figure 3a), there were only 11 recurrences of GIST and 5 deaths due to GIST. The other events were deaths due to other causes, usually due to second malignancies which were found in 40% of the patients with GISTs <5cm (Supplemental Table 4). In the <3cm subgroup (n=150), there were no recurrences or deaths due to GIST. In the 3–5cm subgroup (n=177), 10 of 11 recurrences were seen with non-gastric tumors and/or high mitotic rates. Only one of these patients had received imatinib.
Figure 3:
RFS after surgery in the PRIM group size categories, stratified by imatinib era.
RFS after initial surgery is shown for PRIM patients with tumors <5cm (a), 5–10cm (b) and >10 (c), stratified by imatinib era. Along the x axis of each panel, the number of patients at risk at each time point is indicated. The inset in each panel denotes the median survival, 95% confidence interval of the median, and p-value for a log rank test comparison between groups.
Imatinib treatment
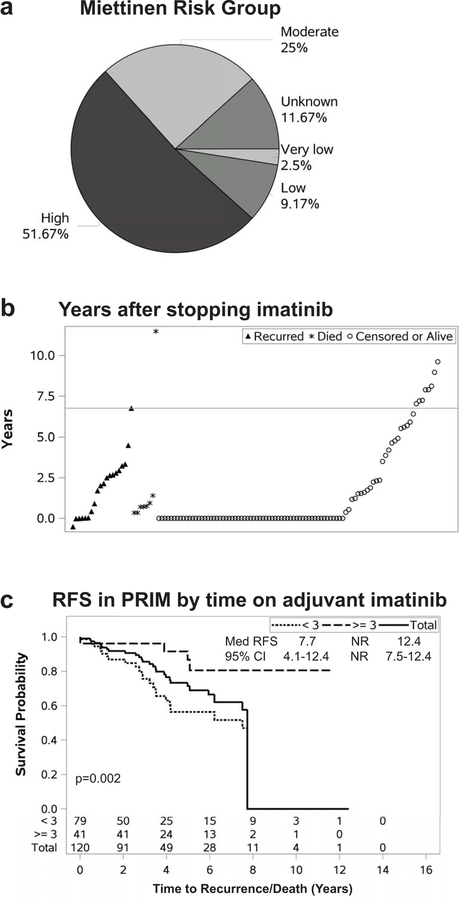
There were 76 PRIM patients treated with neoadjuvant treatment for a median of 6.5 months (IQR 3.3–8.5), with RECIST partial response, stable disease, and progressive disease in 32, 56 and 12%, respectively (not shown). There were 120 patients who received adjuvant imatinib for a median duration of 1.8 years (IQR 0.8–3.5). Most patients were Miettinen moderate and high risk (Figure 4a), although 12% of patients were low or very low risk. These were usually patients who had received the drug in clinical trials before more stringent criteria were adopted. Another 12% had unknown risk stratification due to lack of pre-treatment mitotic rate before neoadjuvant therapy – likely these were moderate or high risk patients. There were 20 recurrences among patients who received adjuvant therapy, of whom 7 died of disease, and another 8 died due to unknown (3) or other (4) causes (Figure 4b). Notably, 14 of these 20 patients developed recurrence with an IQR of 0.9–1.4 years after stopping imatinib. The other six recurred on the drug, with 4 of these recurring early in their course of therapy (0.5–1.6 years on imatinib) – these were later found to have resistant or less responsive mutations (KIT exon 9, KIT L576P, PDGFRA, and wild type). The other two recurred on long-term imatinib at 5.0 and 5.9 years, one with a known sensitive mutation in the primary (KIT exon 11 deletion/insertion) and the other unknown due to necrosis after neoadjuvant imatinib. Overall, patients who stayed on imatinib for more than 3 years (n=41; similar Miettinen criteria proportions as Figure 4a, data not shown) had a longer 5-year RFS (92 vs. 60%) than the 79 patients treated less than 3 years (Figure 4c).
Figure 4:
Adjuvant imatinib therapy in primary GIST.
Miettinen risk category (a) is listed for 120 patients who received adjuvant imatinib. Patients listed as unknown could not be classified due to lack of available mitotic rate to complete classification – these patients had undergone neoadjuvant imatinib therapy without pre-treatment core biopsy. (b) Time to recurrence or death is plotted relative to discontinuation of imatinib. Open circles represent patients who were alive at last follow-up or censored. Closed triangles represent GIST recurrence, while asterisks depict deaths due to unknown or other causes. The Y axis shows time from discontinuing the drug to the depicted event. The horizontal line represents the median time to recurrence or death from stopping the drug by the Kaplan-Meier method. Patients at 0 remained on the drug, and a negative number in one patient indicates staying on the drug briefly after recurrence was detected. (c) RFS is shown stratified by time on imatinib. The solid line represents all PRIM patients who received adjuvant imatinib. The upper dashed line represents chronic (>3 years) imatinib, while the lower dashed line shows short term (≤3 years) treatment. The number of patients at risk at each time point is indicated on the x axis. The inset denotes the median survival, 95% confidence interval of the median, and p-value for a log rank test comparison between the <3 and ≥3 year groups.
DISCUSSION
We present here the largest single-institution comprehensive clinical series on the management of GIST. While large pre-imatinib pathologic series on GIST have been published by the Armed Forces Institute of Pathology25, 31–33 and others, those were based on specimens submitted for review. Our series is unique not only in number, but in its long time period, spanning the introduction of imatinib. There were clearly different outcomes among the three groups, with a median OS of 13.6 years in PRIM versus 5.3 in PRIM+MET, while MET had 4.0 years following a median additional 4.1 years from initial presentation elsewhere.
Not surprisingly, PRIM+MET and MET had higher risk clinicopathologic variables and mutation profiles than PRIM. For most of these factors, these two groups were indistinguishable, with more small bowel tumors and more high mitotic rate tumors than PRIM, at almost identical proportions. Genomically, PRIM+MET and MET had higher proportions of KIT exon 9 mutations, which carry a worse prognosis11, 34. These groups also had more mutations in multiple KIT exons, which usually result from secondary mutations due to imatinib use35–37. Despite these similarities between PRIM+MET and MET, several factors were associated with the more aggressive presentation in PRIM+MET. Peritoneal metastasis was more common in PRIM+MET. This group had the largest tumors, with 55% >10cm. R2 resection was associated with poor prognosis in all groups, but was most common in PRIM+MET, with almost half of these patients undergoing incomplete resection. In some cases, R2 resection in this group may reflect a staged resection, or intentionally leaving imatinib-responsive disease in situ while resecting progressing disease or a symptomatic primary tumor. Likewise, mixed histologic variant has previously been shown to be a poor prognostic factor38, and we saw the highest proportion of this subtype in PRIM+MET. Remarkably, there were no rectal tumors in PRIM+MET. Overall, we believe that better understanding of PRIM+MET, the presentation associated with the worst outcome, may lead to closer postoperative radiologic surveillance, and also help identify patients at risk of imatinib failure who may be candidates for more potent KIT inhibitors than imatinib, such as cabozantinib39 or avapritinib40.
While R2 margins were associated with poor prognosis in all groups, there was no difference between R0 and R1 margins. In PRIM, this is consistent with ACOSOG Z9001 data in which patients with R0 versus R1 margins had similar outcomes regardless of receiving imatinib41. We now extend this finding to the PRIM+MET and MET groups. Overall, our institutional approach to GIST of any presentation status is to attempt resection if a complete (R0/R1) resection is possible. In some cases, particularly for rectal GIST, it is reasonable to plan for a close or R1 margin and treat with adjuvant imatinib, if doing so allows for an organ-sparing approach. For example, in the imatinib era, 97% of patients with rectal GIST underwent organ-sparing surgery (local excision or low anterior resection), compared to only 41% in the pre-imatinib era42. Despite less radical surgery after resection of rectal GIST in the imatinib era, and approximately 1/3 positive margins in each era, there were no local recurrences in the imatinib era. Analogous considerations occur in the duodenum or gastroesophageal junction, and apply to debulking multiple peritoneal nodules, which we consider as an R1 resection.
Although formal statistical analysis of mutational subgroups was not possible due to so many categories, several observations are noteworthy. The minority (15%) of tumors were categorized as wild type, yet many of these were early cases in which only KIT and PDGFRA were tested and likely carried other undetected mutations or epimutations. Our institutional approach to genomic analysis has evolved; most GISTs are now interrogated with MSK-IMPACT29, an institutional next-generation sequencing panel of all introns and exons of over 400 cancer-related genes including SDH, NF1, and others that have been found altered in GIST6. True wild-type GISTs are rare.6 Initially, we used imatinib empirically, but now generally perform mutational analysis first.
There have been several reports of the high incidence of second malignancies seen with GIST43–45, including one from our institution30. Here we present the largest single-institution analysis of second malignancy in GIST, demonstrating that among PRIM, one-third had a second malignancy, with one third of these synchronous and two-thirds preceding. This is a far higher incidence of cancer than that estimated by the American Cancer Society for the 60–69 year old age group (the median age of PRIM was 65), in which 14% of males and 10% of females would be expected to have cancer46. The high incidence is likely related to our status as a tertiary referral center. Synchronous lesions were often incidental during resection or pathologic analysis for other cancers. Preceding lesions were often a patient’s index cancer, and GIST was identified subsequently during surveillance. Second malignancies were most commonly coincident with small low risk GISTs, with second malignancies seen in 55% of <3cm tumors, compared to 28% of 3–5cm tumors. Among small tumors, recurrence of GIST was uncommon; in fact, we saw no recurrences or deaths due to GIST among 150 primary tumors <3cm, although most of those tumors were gastric. The few recurrences in the 3–5cm group occurred in primary tumors with a high mitotic rate or non-gastric origin. Only one of those 11 patients had received imatinib, underscoring the importance of consideration of adjuvant imatinib in small tumors if other high risk features are present.
In the two pivotal trials of imatinib in advanced GIST10, 23, the analyses were not distinguished by whether the patients had an unresectable primary GIST, a primary GIST with metastases, or metastases alone. Here we show the magnitude of imatinib’s effect in PRIM+MET and MET patients undergoing surgery. We compared all patients selected for surgery at a single institution stratified based on the availability of the drug to that cohort at the time of their initial surgery, which may provide the most direct evidence. Directly proving an OS benefit of adjuvant imatinib, as opposed to just increasing RFS, has been difficult because recurrence was treated with crossover to imatinib or other tyrosine kinase inhibitors, and metastasectomy.13–17 OS was greater in PRIM patients in the imatinib era; although patients in the pre-imatinib era overall had higher risk primary tumors, the difference remained in the subset of high risk patients. Of note, we believe that the modern cohort includes more small, low risk gastric GISTs due to more frequent incidental detection from increased use endoscopy and cross-sectional imaging, as well as the NCCN recommendation that GISTs 2cm or greater should be resected. We did not assess the contribution of the individual components of imatinib therapy (neoadjuvant, adjuvant, or treatment for recurrent/metastatic disease) except we did find that PRIM patients who received chronic adjuvant imatinib ≥3 years had longer RFS than those who received <3 years despite similar risk group proportions. Although this subanalysis is underpowered, it is in agreement with data for the PERSIST5 trial, a single arm study of 5 years of adjuvant imatinib47. Like PERSIST5, in our study, most recurrences in patients treated with adjuvant imatinib occurred after stopping the drug, and the few patients who recurred on long-term imatinib had less responsive or resistant mutations.
Primary tumor site, size, and mitotic rate are currently used clinically to estimate the risk of recurrence after resection, and to guide the need for adjuvant therapy10, 12, 24–27. The pre-imatinib cohort recapitulated their prognostic importance. In contrast, only size >10cm remained independently predictive of RFS or OS in the imatinib era. This does not make site and mitotic rate irrelevant in the imatinib era, since high risk features were associated with imatinib treatment. Rather, these data imply that adjuvant imatinib was appropriately applied to high risk patients, and after treatment, tumor site and mitotic rate did not further estimate outcome. In the ACOSOG Z9001, all three factors were prognostic of recurrence on multivariate analysis after 1 year of adjuvant imatinib or placebo14. Our PRIM group received longer imatinib treatment (median 1.8 years, IQR 0.8–3.5), with neoadjuvant therapy given to 76 patients with locally advanced tumors, many of whom had rectal or duodenal (included as small bowel) tumors. Our cohort had a higher proportion of rectal tumors at 6.7% compared to 1.4% in the ACOSOG study. Mixed histologic variant was also independently predictive of outcome in the imatinib era. We believe that imatinib should still be prescribed to patients with high risk features, and once appropriately applied, further prognostication of outcome is limited to tumor size and presence of the mixed histologic subtype.
This study is limited by its retrospective nature. While we do report some baseline differences between cohorts, for example larger tumors in the earlier era, there may be additional undetected differences contributing to the associations. In comparing the outcomes of the pre-imatinib era to the imatinib era, we used an “intention to treat” type analysis. In other words, patients from the pre-imatinib era who survived into the imatinib era and received drug when indicated (33 of 246 patients, 13%) were still analyzed with the pre-imatinib era group. The net effect of these patients would have been to dampen the magnitude of the drug in the imatinib era, however, the effect remained striking. Meanwhile, 113 patients either died or had their last follow-up in the imatinib era. Only 9 of these patients had an indication to receive imatinib in the imatinib era, but were never treated. We believe this “intention to treat” analysis was less biased than grouping based on treatment alone, which would carry substantial treatment bias, with higher risk patients receiving the drug.
Thus, we show longer OS regardless of presentation group in the imatinib era. PRIM patients had lower risk primary tumor size, site, and mitotic rate and fewer KIT exon 9 and multiple exon mutations. The most aggressive presentation status (PRIM+MET) was associated with incomplete resection and mixed histologic subtype. Among PRIM patients in the imatinib era, high risk features were associated with imatinib treatment, but after accounting for treatment, only large tumor size remained independently prognostic.
Supplementary Material
Acknowledgments
Funding: NIH RO1-CA102613 (RPD); Kristen Ann Carr Surgical Oncology Fellowship (MJC); and NIH/NCI P30 CA008748
Footnotes
Conflicts of interest: The authors disclose no conflicts of interest.
REFERENCES
- 1.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998; 279(5350):577–80. [DOI] [PubMed] [Google Scholar]
- 2.Ducimetiere F, Lurkin A, Ranchere-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One 2011; 6(8):e20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol 2006; 37(12):1527–35. [DOI] [PubMed] [Google Scholar]
- 4.Chi P, Chen Y, Zhang L, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature 2010; 467(7317):849–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med 2012; 63:247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkhuziem M, Burgoyne AM, Fanta PT, et al. The Call of “The Wild”-Type GIST: It’s Time for Domestication. J Natl Compr Canc Netw 2017; 15(5):551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 2001; 358(9291):1421–3. [DOI] [PubMed] [Google Scholar]
- 8.Antonescu CR. The GIST paradigm: lessons for other kinase-driven cancers. J Pathol 2011; 223(2):251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dematteo RP, Heinrich MC, El-Rifai WM, et al. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol 2002; 33(5):466–77. [DOI] [PubMed] [Google Scholar]
- 10.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002; 347(7):472–80. [DOI] [PubMed] [Google Scholar]
- 11.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008; 26(4):620–5. [DOI] [PubMed] [Google Scholar]
- 12.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000; 231(1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009; 373(9669):1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol 2014; 32(15):1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casali PG, Le Cesne A, Poveda Velasco A, et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol 2015; 33(36):4276–83. [DOI] [PubMed] [Google Scholar]
- 16.Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012; 307(12):1265–72. [DOI] [PubMed] [Google Scholar]
- 17.Joensuu H, Eriksson M, Sundby Hall K, et al. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol 2016; 34(3):244–50. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 2009; 99(1):42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 2013; 20(9):2937–43. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol 2012; 19(4):1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeMatteo RP, Maki RG, Singer S, et al. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg 2007; 245(3):347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fairweather M, Balachandran VP, Li GZ, et al. Cytoreductive Surgery for Metastatic Gastrointestinal Stromal Tumors Treated With Tyrosine Kinase Inhibitors: A 2-institutional Analysis. Ann Surg 2017. [DOI] [PMC free article] [PubMed]
- 23.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004; 364(9440):1127–34. [DOI] [PubMed] [Google Scholar]
- 24.Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008; 112(3):608–15. [DOI] [PubMed] [Google Scholar]
- 25.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006; 23(2):70–83. [DOI] [PubMed] [Google Scholar]
- 26.Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol 2009; 10(11):1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012; 13(3):265–74. [DOI] [PubMed] [Google Scholar]
- 28.Miettinen M, El-Rifai W, L HLS, et al. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol 2002; 33(5):478–83. [DOI] [PubMed] [Google Scholar]
- 29.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn 2015; 17(3):251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hechtman JF, DeMatteo R, Nafa K, et al. Additional Primary Malignancies in Patients with Gastrointestinal Stromal Tumor (GIST): A Clinicopathologic Study of 260 Patients with Molecular Analysis and Review of the Literature. Ann Surg Oncol 2015; 22(8):2633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006; 130(10):1466–78. [DOI] [PubMed] [Google Scholar]
- 32.Miettinen M, Makhlouf H, Sobin LH, et al. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 2006; 30(4):477–89. [DOI] [PubMed] [Google Scholar]
- 33.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005; 29(1):52–68. [DOI] [PubMed] [Google Scholar]
- 34.Gastrointestinal Stromal Tumor Meta-Analysis G. Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 2010; 28(7):1247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 2005; 11(11):4182–90. [DOI] [PubMed] [Google Scholar]
- 36.Heinrich MC, Corless CL, Blanke CD, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol 2006; 24(29):4764–74. [DOI] [PubMed] [Google Scholar]
- 37.Wardelmann E, Merkelbach-Bruse S, Pauls K, et al. Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin Cancer Res 2006; 12(6):1743–9. [DOI] [PubMed] [Google Scholar]
- 38.Singer S, Rubin BP, Lux ML, et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 2002; 20(18):3898–905. [DOI] [PubMed] [Google Scholar]
- 39.Cohen NA, Zeng S, Seifert AM, et al. Pharmacological Inhibition of KIT Activates MET Signaling in Gastrointestinal Stromal Tumors. Cancer Res 2015; 75(10):2061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans EK, Gardino AK, Kim JL, et al. A precision therapy against cancers driven by KIT/PDGFRA mutations. Sci Transl Med 2017; 9(414). [DOI] [PubMed] [Google Scholar]
- 41.McCarter MD, Antonescu CR, Ballman KV, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg 2012; 215(1):53–9; discussion 59–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cavnar MJ, Wang L, Balachandran VP, et al. Rectal Gastrointestinal Stromal Tumor (GIST) in the Era of Imatinib: Organ Preservation and Improved Oncologic Outcome. Ann Surg Oncol 2017; 24(13):3972–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agaimy A, Wunsch PH, Sobin LH, et al. Occurrence of other malignancies in patients with gastrointestinal stromal tumors. Semin Diagn Pathol 2006; 23(2):120–9. [DOI] [PubMed] [Google Scholar]
- 44.Smith MJ, Smith HG, Mahar AL, et al. The impact of additional malignancies in patients diagnosed with gastrointestinal stromal tumors. Int J Cancer 2016; 139(8):1744–51. [DOI] [PubMed] [Google Scholar]
- 45.Murphy JD, Ma GL, Baumgartner JM, et al. Increased risk of additional cancers among patients with gastrointestinal stromal tumors: A population-based study. Cancer 2015; 121(17):2960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Society AC. Cancer Facts & Figures 2017 2017. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed August 17th, 2017.
- 47.Raut CP, Espat NJ, Maki RG, et al. Efficacy and Tolerability of 5-Year Adjuvant Imatinib Treatment for Patients With Resected Intermediate- or High-Risk Primary Gastrointestinal Stromal Tumor: The PERSIST-5 Clinical Trial. JAMA Oncol 2018:e184060. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.