Abstract
Introduction:
As cancer trajectories change due to screening, earlier diagnoses, living longer with illnesses, and new successful treatments, cancer is increasingly a disease of older adults. While cancer diagnoses themselves are very stressful for patients and families, little is known about the health status, functional limitations, and social resources of older patients before they face a new cancer diagnosis.
Materials and Methods:
Using the National Health and Aging Trends Study (NHATS), a national survey of older Medicare beneficiaries linked to Medicare claims data, we examined the health characteristics, functional limitations and social and financial resources of older adults before a new diagnosis of lung, breast, prostate or colorectal cancer and how these factors vary by race/ethnicity.
Results:
We identified 274 community-dwelling older adults with incident cancer diagnoses: lung (30.6%), breast (20.3%), prostate (30.8%), and colorectal (18.3%) representing 1,202,920 older Medicare beneficiaries. The sample was 81% Non-Hispanic White, 10% Non-Hispanic Black, and 9% Hispanic/Other. Before diagnosis, patients had an average of three comorbidities and 29% of patients reported poor/fair health. Almost one-third were living alone, 13% received help with at least one activity of daily living (ADL), 11% had probable dementia and nearly one in ten already receive financial help from family members.
Discussion:
Before an older adult has ever been diagnosed with a major cancer, many face significant health and financial challenges and are dependent on others for care. These needs vary based on cancer type and race/ethnicity and must be considered as clinicians develop individualized care plans for patients alongside caregivers.
Introduction
Cancer is increasingly a disease of older adults, with persons over 65 years accounting for 60% of newly diagnosed malignancies and 70% of all cancer deaths.1,2 As the population of individuals in the United States age 65 years or older is projected to nearly double from 2000–2050,1 and with improved cancer survival, a substantial number of older adults will be living with cancer. Treatment of older adults with cancer is complex.3 The benefit of treatment to prolong survival must be weighed against potential treatment toxicity and reductions in quality of life (QoL).2 Yet minimal effort has been dedicated to developing guidelines for cancer treatment in older adults. Even though there is an increase in clinical trials evaluating patients above the age of 65 years, they are usually excluded and thus not well represented. Thus, new treatments are not effectively being tested in the segment of the population with the highest cancer incidence.4
In 2018 the American Society of Clinical Oncology (ASCO) released new guidelines recommending geriatric assessment (e.g. function, comorbidity, falls, depression, and cognition) for older adults5 with the goal of enabling providers to develop an integrated and individualized plan that informs cancer management. Because of their advanced age, many older adults receiving a new cancer diagnosis may already have significant functional and cognitive impairment, and suffer from one or multiple serious illnesses that require support from paid and family caregivers. In addition to physical and mental health conditions, older adults may face social isolation, reduced QoL, financial strain and significant unmet care needs that should be assessed in routine primary care. Such factors may complicate cancer decision-making and treatment adherence and outcomes.1,6 Patients and families must decide whether to even engage in cancer-directed treatment in the face of competing prognoses, and need to weigh costs versus benefits of treatment (e.g., considering the effect of treatment toxicity7 on a patient with reduced cognitive function.2,8) Family caregivers may be faced with increased responsibility for decision-making and associated stress as they are confronted with the intersection of cancer in the context of other health and social challenges.
New cancer diagnoses themselves are highly stressful, and are associated with a decrease in QoL and an increase in depression symptoms and financial strain.9–13 Yet surprisingly little is known about the health and well-being of older adults at the time of an incident cancer diagnosis. The intersection of these complex issues may necessitate additional support for older patients with cancer and their families. This may be especially true for racial/ethnic minorities who are increasingly burdened with multimorbidity, a more advanced stage at diagnosis and financial strain.14,15
This study sought to determine the health, well-being and existing challenges faced by patients immediately prior to the diagnosis of the four most common cancers in the United States (lung, breast, prostate, colorectal),16,17 using a longitudinal dataset consisting of a nationally representative sample of Medicare beneficiaries with detailed data on health status, functional limitations, and caregiving. The specific objectives are to: 1) identify Medicare beneficiaries with incident cancer diagnoses; 2) examine health, functional, financial, and caregiving status for these patients immediately prior to a cancer diagnosis; and 3) examine disparities in pre-diagnosis status based on race.
Methods
Sample
This study uses annual survey data collected from the National Health and Aging Trends Study (NHATS) linked to Medicare claims. NHATS, a longitudinal, population-based survey of late-life disability trends and trajectories, drew a random sample of individuals ages 65 years and older living in the contiguous U.S. from the Medicare enrollment file on September 30, 2010 with oversampling of those over age 90 years and non-Hispanic blacks18,19. The enrollment file represents 96% of all older adults in the United States. Study enrollment interviews were completed between May and November 2011. Annual follow up interviews were completed through 2016. In-person interviews involve the collection of detailed self-reported information on participants’ physical capacity, functional status, chronic health conditions, and socio-economic status. Physical and cognitive performance batteries are also conducted. For the current study, we used the NHATS cohort enrolled in 2011 (with the follow-up surveys collected from 2011 to 2016), and their corresponding Medicare claims from the Inpatient, Outpatient, and National Claims History (NCH) files from 2009–2016.
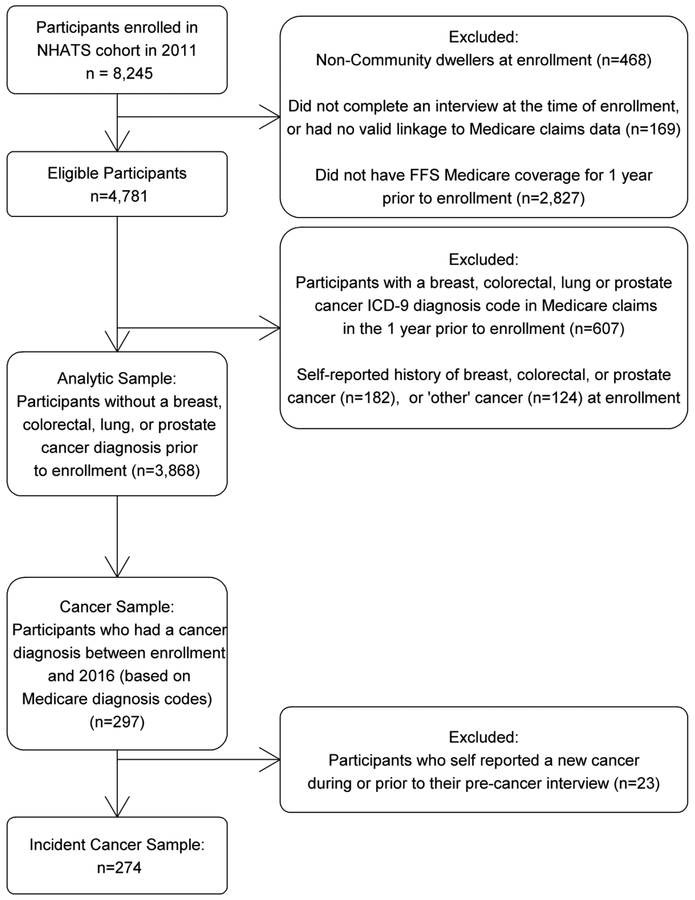
Of the 8,245 participants enrolled in NHATS in 2011 (71% response rate), those who were community dwelling, had complete enrollment interviews and complete continuous Part A and B Medicare coverage (i.e. with fee for service (FFS)), and no HMO coverage, for the 12 months prior to their enrollment in NHATS were included in the present analysis (n=4,781) (Figure 1). In order to define a cohort free of lung, breast, prostate or colorectal cancer, we restricted the sample to those with no ICD-9 diagnosis codes corresponding to breast, colorectal, lung, or prostate cancer in their Medicare claims within 1 year prior to their enrollment interview (See supplemental table 1 for list of ICD-9 codes) (n=4,174). We further excluded individuals who self-reported “breast”, “prostate” or “colon” cancer at the time of their enrollment interview, by answering “yes” to the question “Please tell me if a doctor ever told you that you had cancer?”, and “yes” to the subsequent question about specific cancer types as “breast”, “prostate” or “colon”. Since NHATS does not ask specifically about a history of lung cancer, participants who report a history of “other” cancer type were further excluded (n=306). Among 3,868 identified individuals, the linked Medicare FFS inpatient, outpatient and NCH files were queried for cancer diagnoses from the date of study enrollment through the end of 2016 (mean months of Medicare coverage =51.2). The NHATS interview that immediately preceded the incident cancer diagnosis will be referred to as the pre-cancer interview. Participants who self-reported that they had a new cancer during the previous year in any interview before the incidence cancer diagnosis from the claims were excluded.
Figure 1-.
Selection Criteria
Measures
Incident cancer diagnosis:
Individuals with at least one ICD-9 (through the end of September, 2015) or ICD-10 (starting October 1, 2015) diagnosis code for lung, breast, prostate, or colorectal cancer were identified as incident cancer cases (Supplemental Table 1). The date of the first appearance of a diagnosis code after the pre-cancer interview was used as the date of diagnosis. Cancer type was also based on the earliest diagnosis code.
Health and functional status:
Participants were asked whether they received help with any of the following activities of daily living (ADL): eating, getting cleaned up, using the toilet, dressing, walking around inside, and getting out of bed and Instrumental activities of daily living (IADL): laundry, shopping for groceries, making hot meals, handling bills and banking, and handling of prescribed medicines. We used measures of overall self-reported health and a count of the following chronic conditions: heart disease, hypertension, arthritis, osteoporosis, diabetes, lung disease, stroke, cancer, hip fracture, depression, and anxiety based on self-reports of whether a doctor had ever told study participants they had that health condition. Depressive symptoms and anxiety were measured using the four-item brief screening scale Patient Health Questionnaire-4. The recommended cutoff of three or greater for each subscale,20,21 allowed for the categorization of participants as having probable anxiety and/or probable depression. As we have done previously,22,23 we used criteria defined by the NHATS investigators to identify participants with probable dementia. This algorithm has been validated for use in community-based epidemiologic surveys to identify individuals likely to have dementia24 and closely mirrors a clinical diagnostic assessment. We ascertained the presence of frailty phenotype as the presence of three or more of the following: unintentional weight loss, exhaustion, weakness (decreased grip strength), slow walking speed, and decreased physical activity.25 Participants also self-reported falls in the last month, hospitalizations and whether they were bothered by pain.
Social resources:
Receipt of help refers to older adults’ reports of assistance in the last month with personal care, mobility, household activities, or medical activities (keeping track of medications, attending medical visits, decisions about medical insurance). Older adults reported whether anyone helped with these activities, whether help was paid, hours of care received, and each helper’s relationship to the respondent. We identified those who provided the most hours of help as the primary caregiver. If a respondent indicates that either someone helps them with a specific household, mobility or self-care task, or that they have difficulty doing it on their own, they are asked about adverse consequences because no one was there to help them, or because it was too difficult to do on their own (e.g. if in the last month, they ever went without eating because no one was there to help them). If an adverse consequence is endorsed, this is defined as an unmet need. Consistent with previous research, unmet needs are categorized as occurring across three domains: self-care tasks (eating, bathing, using toilet, getting dressed), household activities (doing laundry, shopping, meal preparation, keeping track of medications), and mobility tasks (going outside home, getting around inside home, getting out of bed).26 We also examined social network size, characterized by the number of people they “talk to about important things”.19 We used criteria previously defined27 to identify social isolation if participants indicated ≥4 of the following: Not currently married or with a partner, do not talk to family about important things, do not talk to friends about important things, have not visited (in-person) with friends or family in the last month, have not attended religious services in the past month, have not participated in any organized activities in the past month.
Financial resources:
Total annual household income is assessed biannually. NHATS allows individuals to estimate amounts in bracketed ranges if exact figures are unknown and all estimates are converted to $2016. Receipt of government assistance was defined if participants reported receipt of help with food, utilities, or housing.
Other covariates:
Older adults’ demographic characteristics included age, gender, race, education, marital status, Medicaid status and whether or not they lived in a residential or assisted living community.
Analysis
Descriptive statistics (percentages for categorical variables; means and standard errors for continuous variables) were performed overall, and by cancer type to examine the challenges (including comorbid conditions, functional limitations, social and financial resources and caregiver needs) present for older adults before a cancer diagnosis, using the responses from the pre-cancer interview. Pre-cancer characteristics were also compared across race/ethnicity, using χ2 tests for categorical variables, and univariate linear regression for continuous variables accounting for the NHATS complex survey design and weights. All analyses were performed using the survey procedures in SAS software, v9.4 (SAS Institute, Cary NC), and weighted statistics are reported for all percentages, means, and standard errors.
All NHATS participants gave informed consent and ethical approval for the study was given by the IRB of the JHSPH.
Results
Cancer incidence:
From 2011–2016 we identified 274 newly diagnosed cancer cases (82 lung, 57 breast, 86 prostate, 49 colorectal cases), representing 1,202,920 older Medicare beneficiaries nationally with an incident lung, breast, prostate, or colorectal cancer diagnosis (30.6% lung, 20.3% breast, 30.8% prostate, 18.3% colorectal cases).
Overall characteristics:
On average, the pre-cancer interview was conducted twelve months prior to the new cancer diagnosis (range: 0.1–62 months), when participants’ mean age was 77.2 years. They were majority male (55%), non-Hispanic white (81%), married (56%), with no more than a high school education (52%). A quarter of participants were socially isolated, with almost a third living alone. Participants had significant health issues at the pre-cancer interview: 29% reported fair or poor overall health, 11% had probable dementia, 50% had pain, and over 10% had probable depression. Almost a quarter of participants reported a hospital admission within the last year and 9% reported a fall in the last month. Participants also reported needing significant help with ADLs (13%) and IADLs (21%). Approximately 15% of participants were receiving government assistance, while 9% percent said they received financial help from family members. Thirteen percent endorsed an unmet need, most commonly around mobility (8.8%) (Table 1). The majority (84%) of the sample reported receiving help from a paid or unpaid caregiver. Those receiving help received a mean of 29 hours per week of help and the vast majority (94%) were receiving help from family members (Table 2).
Table 1:
Demographic, Health and Social Characteristics of Community Dwelling Older Adults Before a New Diagnosis of Breast, Colorectal, Lung, or Prostate Cancer
| Variable | Overall Weighted n 1,202,920 |
Breast Weighted n 244,771 (20.3%) |
Colorectal Weighted n 220,000 (18.3 %) |
Lung Weighted n 367,965 (30.6%) |
Prostate Weighted n 370,184 (30.8%) |
|---|---|---|---|---|---|
| Weighted % | Weighted % | Weighted % | Weighted % | Weighted % | |
| Demographics | |||||
| Age at Diagnosis (years) [Mean (SE)] | 78.7 (0.4) | 78.2 (1.0) | 79.5 (1.1) | 78.7 (0.7) | 78.5 (0.6) |
| Female | 45.2 | 100.0 | 59.1 | 45.9 | -- |
| Race/Ethnicity | |||||
| Non-Hispanic White | 81.3 | 80.3 | 83.9 | 78.1 | 83.6 |
| Non-Hispanic Black | 9.8 | 10.6 | 8.1 | 9.4 | 10.8 |
| Hispanic/Other | 8.9 | 9.1 | 8.0 | 12.5 | 5.6 |
| Marital Status | |||||
| Married/Living with partner | 56.1 | 42.5 | 46.9 | 50.0 | 76.6 |
| Education | |||||
| < HS Education | 25.4 | 18.6 | 19.6 | 36.5 | 22.5 |
| HS/GED | 26.4 | 10.7 | 48.4 | 25.0 | 25.1 |
| Some College | 21.9 | 40.8 | 17.8 | 15.0 | 18.7 |
| ≥Bachelors | 26.2 | 29.8 | 14.2 | 23.5 | 33.7 |
| Lives in Residential Care | 5.6 | 1.5 | 17.1 | 2.4 | 4.7 |
| Financial resources | |||||
| Household income quartiles. (2016 $) | |||||
| <$15,321 | 23.4 | 23.5 | 31.9 | 29.2 | 12.5 |
| $15,321–$28,807 | 18.9 | 14.3 | 24.0 | 24.6 | 13.2 |
| $28,808–$53,349 | 25.1 | 30.0 | 28.9 | 20.9 | 23.9 |
| ≥$53,349 | 32.6 | 32.2 | 15.2 | 25.3 | 50.5 |
| Has Medicaid | 19.6 | 12.9 | 20.6 | 29.3 | 14.0 |
| Receives Government Assistance | 14.8 | 17.3 | 18.4 | 18.3 | 7.6 |
| Financial Help From Family | 8.5 | 16.9 | 7.6 | 8.9 | 3.1 |
| Credit or Medical Debt | 18.8 | 17.0 | 16.8 | 27.6 | 12.6 |
| Social resources | |||||
| Number in social network [Mean (SE)] | 1.9 (0.1) | 2.7 (0.2) | 1.5 (0.2) | 1.8 (0.2) | 1.8 (0.1) |
| Socially Isolated | 25.2 | 20.8 | 34.9 | 29.1 | 18.5 |
| Lives Alone | 30.6 | 38.9 | 43.3 | 30.6 | 17.7 |
| Health characteristics | |||||
| Self Reported Health Fair/Poor | 29.4 | 20.2 | 34.8 | 39.6 | 22.2 |
| Probable Dementia | 11.3 | 8.4 | 17.3 | 14.4 | 6.7 |
| Count of Medical Conditions [Mean(SE)] | 2.9 (0.2) | 3.1 (0.3) | 2.9 (0.3) | 3.1 (0.2) | 2.5 (0.2) |
| Bothered by pain | 49.5 | 58.7 | 50.2 | 43.6 | 49.0 |
| Probable Depression | 11.7 | 7.1 | 13.7 | 14.3 | 11.1 |
| Probable Anxiety | 10.9 | 12.6 | 14.1 | 11.9 | 6.9 |
| Frail | 22.7 | 25.6 | 18.6 | 33.9 | 12.1 |
| Hospital Admission in last year | 24.4 | 12.3 | 24.0 | 36.0 | 20.9 |
| Fall in last month | 8.7 | 9.6 | 4.4 | 7.0 | 12.2 |
| Functional Limitations | |||||
| Help with ≥1 ADL | 12.9 | 17.5 | 17.5 | 9.6 | 10.5 |
| Help with ≥1 IADL | 20.8 | 30.5 | 19.8 | 23.1 | 12.7 |
| Receives any help | 83.8 | 83.1 | 79.6 | 83.1 | 87.5 |
| Unmet Needs | |||||
| Any | 13.1 | 14.6 | 14.7 | 16.7 | 7.6 |
| Self-care tasks | 6.5 | 3.0 | 14.7 | 6.4 | 4.2 |
| Household tasks | 6.5 | 10.1 | 5.4 | 6.0 | 5.3 |
| Mobility tasks | 8.8 | 12.2 | 9.5 | 10.0 | 4.9 |
All percentages are among non-missing values. For all variables, the missing accounted for ≤4.6% of the sample
Table 2-.
Help received by older adults before a new diagnosis of breast, colorectal, lung, and prostate cancer *
| Variable | Overall Weighted n 1,008,328 |
Breast Weighted n 203,457 (20.2%) |
Colorectal Weighted n 175,176 (17.4%) |
Lung Weighted n 305,799 (30.3%) |
Prostate Weighted n 323,895 (32.1%) |
|---|---|---|---|---|---|
| Weighted % | Weighted % | Weighted % | Weighted % | Weighted % | |
| Number of Helpers [Mean (SE)] | 1.6 (0.05) | 1.7 (0.1) | 1.7 (0.1) | 1.5 (0.1) | 1.5 (0.1) |
| Total hours/week of help [Mean (SE)] | 28.6 (4.2) | 18.9 (7.2) | 27.8 (11.6) | 29.2 (4.3) | 34.6 (6.9) |
| Paid help | |||||
| No | 94.1 | 88.2 | 94.5 | 95.5 | 96.2 |
| Yes | 5.9 | 11.8 | 5.5 | 4.5 | 3.8 |
| Family help | |||||
| No | 5.6 | 9.4 | 5.7 | 6.4 | 2.5 |
| Yes | 94.4 | 90.6 | 94.2 | 93.6 | 97.6 |
| Category of Primary Caregiver | |||||
| Spouse | 56.2 | 38.4 | 45.3 | 52.3 | 77.0 |
| Other relative | 32.7 | 48.2 | 37.7 | 34.1 | 19.1 |
| Non-relative | 11.0 | 13.4 | 17.0 | 13.6 | 3.9 |
Among those who indicated that they received help
Characteristics by cancer type:
As expected, participant characteristics before incident cancer diagnosis varied by cancer type. For example, socioeconomic status was lower among those in the colorectal and lung groups, with 68% and 62% of participants having a high school or lower education, and only 15% and 25% in the highest income quartile, respectively. Those in the colorectal group were most likely to be socially isolated (34.9%). Over a third of participants in the lung cancer group reported a hospital stay in the previous year and met criteria for frailty. Anxiety was highest before a breast cancer (13%) or colorectal cancer diagnosis (14.1%). Receipt of help with ADLs varied with only 10% among those who developed lung cancer to 18% among those in the colorectal and breast groups. Those who developed lung cancer reported the most unmet needs (17%) compared to less than 8% among those in prostate cancer group. Among those who reported receiving help before a cancer diagnosis (84% of sample), those diagnosed with prostate cancer received the most hours per week of help (mean=34.6) at the pre-cancer interview. Those with breast cancer were most likely to receive paid caregiver help (12%) and to report not receiving any help from family (9%) (Table 2). While spouses were primary caregivers for those with prostate cancer (77%), they were less involved in the care of individuals with colorectal cancer (45%) and breast cancer (38%).
Characteristics by race/ethnicity (Table 3):
Table 3:
Selected demographic, health and social characteristics of community dwelling older adults before a new diagnosis of breast, colorectal, lung, or prostate cancer, according to race
| Variable | Non-Hispanic White Weighted n 972,707 (81.3%) |
Non-White Weighted n 223,687 (18.7%) |
|
|---|---|---|---|
| Weighted % | Weighted % | P-value* | |
| Demographics | |||
| Cancer Type | 0.632 | ||
| Lung | 29.2 | 35.7% | |
| Breast | 20.1 | 21.4% | |
| Prostate | 31.7% | 27.1% | |
| Colorectal | 19.0% | 15.8% | |
| Age at Diagnosis (years) [Mean (SE)] | 78.9 (0.5) | 77.6 (0.8) | 0.087 |
| Marital Status | |||
| Married/Living with a Partner | 58.4% | 45.5% | 0.079 |
| Education | 0.001 | ||
| < HS Education | 20.5% | 47.0% | |
| HS/GED | 26.9% | 25.0% | |
| Some College | 22.4% | 18.9% | |
| ≥Bachelors | 30.3% | 9.1% | |
| Financial resources | |||
| Household income quartiles. (2016 $) | 0.008 | ||
| <$ 15,321 | 20.0% | 38.4% | |
| $15,321–$28,807 | 17.6% | 25.1% | |
| $28,808–$ 53,349 | 25.6% | 23.5% | |
| ≥$53,349 | 36.9% | 13.0% | |
| Has Medicaid | 12.9% | 48.4% | <0.001 |
| Receives Government Assistance | 12.3% | 26.1% | 0.016 |
| Financial Help from Family | 6.3% | 18.3% | 0.066 |
| Credit or Medical Debt | 13.8% | 39.1% | 0.001 |
| Social resources | |||
| Number in social network [Mean (SE)] | 2.0 (0.1) | 1.8 (0.2) | 0.418 |
| Socially Isolated | 23.9% | 31.2% | 0.297 |
| Lives Alone | 30.0% | 34.4% | 0.564 |
| Health Characteristics | |||
| Self Reported Health Fair/Poor | 28.7% | 33.6% | 0.492 |
| Probable Dementia | 11.0% | 13.0% | 0.626 |
| Number of Medical Conditions [Mean (SE)] | 3.0 (0.2) | 2.7 (0.2) | 0.207 |
| Probable Depression | 11.3% | 14.2% | 0.521 |
| Probable Anxiety | 12.0% | 6.5% | 0.167 |
| Functional Limitations | |||
| Help with ≥1 ADL | 10.8% | 22.9% | 0.014 |
| Help with ≥1 IADL | 19.5% | 26.1% | 0.221 |
| Unmet Needs | |||
| Any | 12.4% | 16.5% | 0.394 |
| Self-care tasks | 5.8% | 10.2% | 0.272 |
| Household tasks | 5.9% | 9.4% | 0.461 |
| Mobility tasks | 7.7% | 13.8% | 0.189 |
χ2 for categorical variables, univariate linear regression for continuous, among non-missing values
Non-Hispanic White (NHW) participants were older at the time of their diagnosis than participants of other races/ethnicities (non-White) (78.9 years vs. 77.6 years, p=0.09), and more often married (58% vs. 46%, p=0.08). Non-White participants had significantly lower levels of education (p=0.001) and income (p=0.008), and were more likely to be on Medicaid (p=0.0004). Non-White participants were significantly more likely to receive government assistance (p=0.0159), to report credit or medical debt (p=0.001) and to need help with ADLs (p=0.01) than NHW. Non-White participants trended towards more social isolation, poorer overall self-reported health, more depression, more need for help IADLs and more often reported unmet needs. (Table 3) Non-white participants also tended to have a significantly larger network of helpers than NHW (1.9 vs. 1.5, p=0.02), including more paid help (13% vs 5%, p=0.09).
Discussion
Using a national sample of older adults and a prospective design, we describe the health and social challenges faced by older adults before a new cancer diagnosis. Consistent with previous clinical assessments,3 we find that before diagnosis with a major cancer, older adults commonly face significant challenges, including living with functional impairments that require caregiver assistance. A large proportion of participants have pain, and many already have symptoms of depression and anxiety. About one in ten have dementia, and are already relying on family caregivers to assist with activities and to handle complex treatment decision-making. Deficits in function, cognition and care resources are important factors for cancer care and outcomes as they may affect treatment tolerance and outcomes3 health-related QoL.28
Financial challenges
We also find that many older adults already have limited financial resources before a cancer diagnosis, situation that may be exacerbated by the diagnostic and treatment costs associated with cancer. Financial toxicity related to cancer treatment is associated with poor survival.29 It is possible that this observed phenomenon is exacerbated among the older and the frail. Patients with dementia are already experiencing healthcare spending and escalated economic burden for these families,30 in addition to the high costs of cancer care.31
Vulnerable populations
Many challenges pre-cancer diagnosis may be more severe for non-White older adults, as our results showed that, compared to their white counterparts, they tended to have poorer overall health, more depression, greater unmet care needs, and fewer financial resources. Furthermore, other studies have reported that black race is associated with lower QoL.28 Our results emphasize the need for dedicated resources among these more vulnerable populations and we may want to focus intervention efforts on these populations.
Improving support for older adults and their caregivers in cancer care
Our findings around complex needs among older adults with cancer is consistent with the significant efforts that have been made to include geriatric assessments that better capture the unique health situations of older patients with cancer for more than a decade.32 Although geriatric assessment can successfully identify patients at risk of poorer overall survival and treatment toxicity,33 it is not widely implemented in part because it can be highly burdensome for clinicians.34 The need for increased focus on this work is reflected by 2018 ASCO guidelines recommending geriatric assessment (e.g. function, comorbidity, falls, depression, and cognition) for older adults5 in order to allow providers to develop an integrated and individualized plan that informs cancer management. Future research examining how to best identify and treat functional limitations and comorbid conditions is necessary to provide the critical supportive services needed to improve the care and outcomes of older adults with cancer.
Additionally, our work highlights the huge reliance on family caregivers among older adults prior to a cancer diagnosis highlighting a need to increase accesses to supportive service for caregivers who are facing additional caregiver strain related to a cancer diagnosis. Cancer caregivers consistently state that they want more supportive services, yet they are underutilized35 suggesting an opportunity for intervention. It is critical that screening processes are integrated into the routine clinical care of patients and sensitive enough to identify caregivers most in need of formal support. Interventions should be developed that target increased support service utilization by family caregivers, especially for those who are distressed and underprepared. Recommendations to promote family caregiver assessment,36 the systematic evaluation of a caregiving situation, determining caregiver ability and willingness to assist a care recipient, and identifying areas of need, are critical first steps to elucidate family structure, resources and potential challenges. While caregiver assessment is not commonly integrated in health delivery settings, it is particularly important in settings where seriously ill older adults frequently receive care, such as oncology practices. In addition, the Caregiver Advise, Record, Enable (CARE) Act,37 which has been passed in 36 states, requires hospitals to identify and engage caregivers post-discharge and is a timely opportunity to expand caregiver assessment and support.
The increasing need to identify and address the complex needs of older adults including their pain management and reduced quality of life and the need to support their family caregivers is in keeping with palliative care models of care delivery. These models call for upstream support of individuals with serious illness and their caregivers regardless of disease stage.38,39 Older adults with new cancer diagnoses will likely benefit from a referral to palliative support teams that are increasingly available in hospital and community-based settings. Cancer providers must become increasingly aware of the challenges faced by patients prior to the initiation of a complex cancer treatment regimen.
Limitations and next steps
There are a number of limitations to this study. Importantly, while we identify new cancers using self-report data and Medicare claims, cancer cases are not confirmed via registry and lack staging data. In sensitivity analyses, we examined the validity of the claims-based case identification by examining confirmation of a new cancer diagnosis in the post-cancer interview: “Since the time of your last interview has a doctor told you that you had cancer?” We also restricted the cohort to those who had at least two cancer diagnosis codes in their Medicare claims and a confirmed self-report of a new cancer. Key findings remained similar using these more stringent criteria for new cancer identification. Furthermore, while we present differences across cancer subtypes in this descriptive study, our limited sample size precludes more detailed analysis of the needs of individuals with different types of cancer. Our work thus far highlights very different care patterns among those with breast cancer (less family involvement) and prostate cancer (more likely to have a spouse) which warrants further study. Future research should further examine pre-cancer limitations by subtype in order to optimize assessment and targeting of intervention strategies in cancer setting. Additionally, while we examined racial differences in pre-cancer characteristics, a small sample size limited our abilities to detect more nuanced issues. Despite these limitations, because of its unique combination of measures of QoL, health conditions, and functional status, NHATS is a valuable dataset to assess the physical, emotional and financial status of these participants before a new cancer diagnosis.
Finally, this is a descriptive study in nature and is only able to characterize the challenges faced by older adults with cancer. By highlighting the scope of the challenges faced by older adults prior to a new cancer diagnosis in a national sample, our work sets the stage for new research including larger studies that examine social and health characteristics by race and cancer type and stage and qualitative and quantitative research that examines how these challenges impact treatment decision-making for patients and families. Our work also critically highlights the frailty of older adults prior to a cancer diagnosis, and how this may affect the additional infrastructural and social needs these patients face after a cancer diagnosis. Financial challenges faced by older adults before a cancer diagnosis is an area that will require considerable research incorporating data on insurance status and family structure.
Implications
In conclusion, our results have several implications for clinicians and future research. This national survey of older adults with incident cancer found that older adults face multiple existing health, financial and social challenges before a cancer diagnosis, including a reliance on family caregivers. Non-white adults may be especially challenged before a new cancer diagnosis. Early assessment and intervention by clinicians is critical to improving cancer treatment, outcomes, QoL and to aid in decision-making for patients and their families. Additionally, research surrounding the emotional and financial strain on older patients with cancer and their families is warranted. The coming decades will see marked increases in the number of older persons living with serious cancers in the community.40,41 It will be imperative that early assessment of care needs and support be provided to older adults and families facing cancer diagnoses.
Supplementary Material
Funding
The National Health and Aging Trends Study (NHATS) is sponsored by the NIA (U01AG032947) through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health. Dr. Ornstein is supported by the NIA (K01AG047923)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts/Disclosures
Dr. Smith received an honorarium from Teva Pharmaceuticals. No others disclosures
Bibliography
- 1.Berger NA et al. Cancer in the Elderly. Trans. Am. Clin. Climatol. Assoc 117, 147–156 (2006). [PMC free article] [PubMed] [Google Scholar]
- 2.Li D, de Glas NA & Hurria A Cancer and Aging: General Principles, Biology, and Geriatric Assessment. Clin. Geriatr. Med 32, 1–15 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Jolly TA et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. The Oncologist 20, 379–385 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marosi C & Köller M Challenge of cancer in the elderly. ESMO Open 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohile SG et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 36, 2326–2347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ording AG et al. Comorbidity and survival of Danish breast cancer patients from 2000–2011: a population-based cohort study. Clin. Epidemiol 5, 39–46 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muss HB et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 25, 3699–3704 (2007). [DOI] [PubMed] [Google Scholar]
- 8.McWilliams L et al. Cancer-related information needs and treatment decision-making experiences of people with dementia in England: a multiple perspective qualitative study. BMJ Open 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SMITH HR Depression in cancer patients: Pathogenesis, implications and treatment (Review). Oncol. Lett 9, 1509–1514 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishel MH, Hostetter T, King B & Graham V Predictors of psychosocial adjustment in patients newly diagnosed with gynecological cancer. Cancer Nurs. 7, 291–299 (1984). [PubMed] [Google Scholar]
- 11.Linden W, Vodermaier A, Mackenzie R & Greig D Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J. Affect. Disord 141, 343–351 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Brintzenhofe-Szoc KM, Levin TT, Li Y, Kissane DW & Zabora JR Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type. Psychosomatics 50, 383–391 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Okamura M, Yamawaki S, Akechi T, Taniguchi K & Uchitomi Y Psychiatric disorders following first breast cancer recurrence: prevalence, associated factors and relationship to quality of life. Jpn. J. Clin. Oncol 35, 302–309 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Koroukian SM et al. Social determinants, multimorbidity, and patterns of end-of-life care in older adults dying from cancer. J. Geriatr. Oncol 8, 117–124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrera PM, Kantarjian HM & Blinder VS The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA. Cancer J. Clin 68, 153–165 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen J Common cancers in the elderly. Drugs Aging 13, 467–478 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Common Cancer Types. National Cancer Institute (2015). Available at: https://www.cancer.gov/types/common-cancers. (Accessed: 18th September 2018)
- 18.Montaquila J, Freedman VA, Edwards B & Kasper JD National Health and Aging Trends Study Round 1 Sample Design and Selection. NHATS Technical Paper #1. (2012). [Google Scholar]
- 19.Kasper JD & Freedman VA. National Health and Aging Trends Study User Guide: Rounds 1, 2, 3, 4 & 5 Beta Release. (2016).
- 20.Kroenke K, Spitzer RL, Williams JBW & Löwe B An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics 50, 613–621 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Löwe B et al. A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J. Affect. Disord 122, 86–95 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Soones T, Federman A, Leff B, Siu AL & Ornstein K Two-Year Mortality in Homebound Older Adults: An Analysis of the National Health and Aging Trends Study. J. Am. Geriatr. Soc 65, 123–129 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ornstein KA, Kelley AS, Bollens-Lund E & Wolff JL A national profile of end-of-life caregiving in the United States. Health Aff. Proj. Hope 36, 1184–1192 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasper JD, Freedman VA & Spillman BC Classification of Persons by Dementia Status in the National Health and Aging Trends Study. 14 (2013). [Google Scholar]
- 25.Fried LP et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A. Biol. Sci. Med. Sci 56, M146–156 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Allen SM, Piette ER & Mor V The adverse consequences of unmet need among older persons living in the community: dual-eligible versus Medicare-only beneficiaries. J. Gerontol. B. Psychol. Sci. Soc. Sci 69 Suppl 1, S51–58 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pohl JS, Cochrane BB, Schepp KG & Woods NF Measuring Social Isolation in the National Health and Aging Trends Study. Res. Gerontol. Nurs 10, 277–287 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Pergolotti M et al. Activities, function, and health-related quality of life (HRQOL) of older adults with cancer. J. Geriatr. Oncol 8, 249–254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrone F et al. The association of financial difficulties with clinical outcomes in cancer patients: secondary analysis of 16 academic prospective clinical trials conducted in Italy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol 27, 2224–2229 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Kelley AS, McGarry K, Gorges R & Skinner JS The Burden of Health Care Costs in the Last 5 Years of Life. Ann. Intern. Med 163, 729–736 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narang AK & Nicholas LH Out-of-Pocket Spending and Financial Burden Among Medicare Beneficiaries With Cancer. JAMA Oncol. 3, 757–765 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Extermann M & Hurria A Comprehensive geriatric assessment for older patients with cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 25, 1824–1831 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Li D, Soto-Perez-de-Celis E & Hurria A Geriatric Assessment and Tools for Predicting Treatment Toxicity in Older Adults With Cancer. Cancer J. Sudbury Mass 23, 206–210 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Puts MTE et al. Use of Geriatric Assessment for Older Adults in the Oncology Setting: A Systematic Review. JNCI J. Natl. Cancer Inst. 104, 1134–1164 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dionne-Odom JN et al. Participation and interest in support services among family caregivers of older adults with cancer. Psychooncology. 27, 969–976 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carman KL et al. Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Aff. Proj. Hope 32, 223–231 (2013). [DOI] [PubMed] [Google Scholar]
- 37.AARP. New State Law to Help Family Caregivers. (2017).
- 38.Meier DE et al. A National Strategy For Palliative Care. Health Aff. Proj. Hope 36, 1265–1273 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Kelley AS & Morrison RS Palliative Care for the Seriously Ill. N. Engl. J. Med 373, 747–755 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kent EE et al. Revisiting the Surveillance Epidemiology and End Results Cancer Registry and Medicare Health Outcomes Survey (SEER-MHOS) Linked Data Resource for Patient-Reported Outcomes Research in Older Adults with Cancer. J. Am. Geriatr. Soc 64, 186–192 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Miller KD et al. Cancer treatment and survivorship statistics, 2016. CA. Cancer J. Clin 66, 271–289 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.