Abstract
Background
The mass migrations experienced by the Western Balkans in the past decades have significantly changed the demographic structures and have probably altered the prevalence of transfusion-transmitted infections (TTIs) among blood donors. However, data on the prevalence of TTIs in the Western Balkans countries remain incomplete. This study reports the prevalence of TTIs among blood donors in Serbia in the period 2005–2017.
Materials and methods
Between January 2005 and December 2017, in the four largest Serbian transfusion centres, mandatory serology tests for screening HBV, HCV, HIV and syphilis infection were used for all blood donations.
Results
Of the total of 1,660,019 blood donations made, 3,377 (0.203%) were positive for one of the TTIs: 1,440 (0.087%), 1,055 (0.064%), 215 (0.013%), and 667 (0.040%) were positive for HBV, HCV, HIV and syphilis, respectively. Serbia showed a declining trend of prevalence of HBV and HCV infection, while prevalence of HIV and syphilis remained unchanged. Prevalence of TTIs varied between different transfusion centres and showed a north-to-south upward trend.
Discussion
The reported prevalence of TTIs among blood donors in Serbia was low and continued to follow a declining trend over the period of study.
Keywords: transfusion-transmitted infections, serological testing, blood donation testing, blood safety, epidemiology
Introduction
Serbia, in the Western Balkans, has slightly over 7 million inhabitants. Approximately 250,000 blood donations are collected each year (Blood Transfusion Institute of Serbia, unpublished data, 2018). To ensure a continuous blood supply, the Serbian blood transfusion system requires 40 blood donors per 1,000 inhabitants (4% of total population)1. The actual percentage of blood donors in the total population is 3%1, and to ensure a continuous supply, donated blood in Serbia comes from both voluntary and family/replacement blood donors2 who provide blood only when it is required by a member of their own family or community. It is estimated that up to 30% of donations are from family/replacement blood donors (Blood Transfusion Institute of Serbia, unpublished data, 2018).
The blood transfusion system of the Republic of Serbia is decentralised. It is organised on three levels: 3 Blood Transfusion Institutes (1 national and 2 regional, all within university centres); 44 blood transfusion centres within various health facilities (hospitals, healthcare centres, clinical centres); and 70 blood transfusion departments/laboratories within hospitals, clinics and institutes2. The Institute for Transfusiology and Haemobiology of the Military Medical Academy (MMA) is, unlike the others, under the auspices of the Ministry of Defence of the Republic of Serbia. The regulations proposed by Serbian National Regulations for safe blood donations in Serbia require that: 1) blood donors are aged from 18 to 65 years; 2) high infectious risk donors are identified and excluded from blood donation under procedures based on a self-exclusion questionnaire and medical-history evaluation; 3) mandatory serology tests on all blood donations are performed for HBV, HCV, HIV and syphilis infection3. Transfusion-transmitted infection (TTI)-positive or undetermined blood units are excluded from the blood supply. Regular testing is not carried out for other agents which could carry a risk for TTIs (hepatitis E virus, West Nile virus, human herpes viruses, parvovirus B19, influenza virus, Listeria monocytogenes, Plasmodium, Toxoplasma, Leishmania, Trypanosoma)4.
Although mandatory screening has significantly reduced the prevalence of TTIs worldwide, these infections still represent serious complications of blood transfusion leading to chronic and life-threatening disorders4. A varying degree of risk of TTI transmission still remains as a result of the failure to assess donor suitability for blood donation, the window period following infections when the testing assays applied cannot detect the virus, the sensitivity of tests applied, and/or releasing of infected donations in error (the highest proportion of errors are related to the processes of labelling, blood collection and issue of blood products). In Serbia, TTI-positive or undetermined blood units are excluded from the blood supply. However, since there is no national centralised electronic database, TTI-positive blood donors in Serbia cannot be permanently removed from the donor pool. Therefore, the risk continues because: i) there is a small but significant number (0.8–6.7%) of blood donors who are not sufficiently aware of the direct impact their donation can have on transfusion safety; ii) some blood donors only donate in order to be tested for HIV and other TTIs; and iii) some donors give false answers to questions relating to blood donation which would otherwise compromise their eligibility for blood donation5,6. Another important reason for the risk of TTI-associated transmission of infections is the fact that, in Serbia, mandatory screening of TTIs is based on serological testing, and nucleic acid amplification testing (NAT) for TTIs is still not compulsory. Mandatory serological testing in Serbia started in 1970 for HBV, in 1987 for HIV, and in 1994 for HCV1. Although Serbian transfusion centres apply the most up-to-date serology tests for TTIs, one HIV-positive donation (in 2017) and one HCV-positive donation (in 2016) were missed due to a donation made within the infectious window period and entered the blood supply. In addition, in 2002, one HIV-positive donation was released in error and this led to a transfusion-transmitted HIV infection (Ministry of Health of Republic of Serbia, data available upon official request).
The prevalence of these four TTIs could be significantly altered in a relatively short period of time7. Therefore, permanent recording of the prevalence of TTIs is necessary in order to implement more efficient public health measures and to improve blood transfusion safety. As pointed out by Byrne et al.8, blood donors are considered a sentinel population and recording the prevalence of TTIs among them provides valuable epidemiological information about a low-risk population. The study of prevalence of TTIs in the European Western Balkans is of particular importance. In the early 1990s, the large, multinational and multi-ethnic Socialist Federal Republic of Yugoslavia was broken up. The subsequent unresolved political issues led to armed conflicts and a civil war which laid the foundation for mass migrations of local populations. Such movements of people were followed by drastic changes in the demographic structure of the population and a fall in national incomes in most of the countries involved. Because of the large-scale displacement of people that befell the Western Balkans, Serbia and most of the neighbouring countries only have incomplete and often out-dated information available on the prevalence of TTIs; information which cannot be considered reliable. To the best of our knowledge, Croatia is the only Western Balkan country whose Institute of Transfusion Medicine provides up-to-date data on the prevalence of TTI9. Over the period 2000–2017, only one scientific paper reporting the prevalence of TTIs among blood donors in Serbia was published; the study concerned a survey of 155,479 blood donations/units collected throughout Serbia and analysed at the Military Medical Academy. Results showed the prevalence of 0.20%, 0.12%, 0.005% and 0.06%, for HBV, HCV, HIV and syphilis, respectively. These results also showed that there has been a declining trend for seroprevalence of HBV and HCV infection; the trend remained unchanged for HIV and syphilis infection during this period10.
Here, we report the results for the period 2005–2017 of established serology tests for HBV, HCV, HIV and syphilis infection carried out in the four largest blood transfusion centres in Serbia. The following institutions from three different regions of Serbia participated in this study: 1) in Vojvodina, the northernmost region of Serbia, the Blood Transfusion Institute of Vojvodina in Novi Sad (Regional Institute); 2) in Belgrade, the Blood Transfusion Institute of Serbia (National Institute), and the Institute for Transfusiology and Haemobiology of the MMA; 3) in the Eastern and Southern regions of Serbia, the Blood Transfusion Institute of Clinical Centre Niš (Regional Institute). The Blood Transfusion Institute of Serbia and Institute for Transfusiology and Haemobiology of the MMA process and test the blood collected throughout the country. According to the Blood Transfusion Institute of Serbia (unpublished data), in the 2005–2017 period, 3,231,130 blood donations were collected and 51.4% of them were collected in the four largest transfusion centres.
Materials and methods
A total of 1,660,019 blood donations (98% whole blood units, and 2% plateletpheresis and plasma donations2) were collected from voluntary and family/replacement blood donors between January 2005 and December 2017. HBsAg, anti-HCV, HIV antigen/antibodies and anti-Treponema pallidum antibodies were tested by last-generation immunoenzyme assays. Repeatedly reactive samples were submitted to confirmatory testing. Confirmatory tests included HBsAg neutralisation test and anti-HBc testing, anti-HCV and anti-HIV1/2 immunoblot assays, and Treponema pallidum or syphilis immunoblot plus Veneral Disease Research Laboratory (VDRL) testing. A list of commercial test kits used for TTIs testing is given in the Online Supplementary Content, Table SI.
Prevalence, i.e. percent of total number of donations positive for TTIs (HBV, HCV, HIV, and syphilis) was calculated using the equation as follows:
The linear regression analysis (conducted in OriginPro 8, OriginLab Corporation, Northampton, MA, USA) was used to establish the time-course of prevalence of TTIs. p<0.05 was considered statistically significant. The χ2 test was used to assess the significance of the difference in seroprevalence between the transfusion centres as well as between the demographic characteristics of TTI-positive donors.
Results
A total of 1,660,019 blood donations were collected in four transfusion centres in Serbia during the period 2005–2017 (Table I). Overall, screening of 1,440 (0.087%; 87/100,000) blood donations showed positive test results for HBV infection, 1,055 (0.064%; 64/100,000) tested positive for HCV, 215 (0.013%; 13/100,000) for HIV infection, and 667 (0.040%; 40/100,000) tested positive for syphilis. The overall prevalence of TTIs was 0.203% (203/100,000).
Table I.
Blood donations positive for transfusion-transmissible infections in Serbia, 2005–2017.
| Transfusion centre | Donations | HBV | HCV | HIV | Syphilis | TTIs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N. | N. | (%) | N. | (%) | N. | (%) | No | (%) | N. | (%) | |
| Novi Sad | 388,014 | 131 | (0.034) | 85 | (0.022) | 10 | (0.003) | 52 | (0.013) | 278 | (0.072) |
| Belgrade BTI | 796,398 | 650 | (0.082) | 433 | (0.054) | 42 | (0.005) | 246 | (0.031) | 1,371 | (0.172) |
| Belgrade MMA | 225,226 | 387 | (0.172) | 216 | (0.096) | 8 | (0.004) | 128 | (0.057) | 739 | (0.328) |
| Niš | 250,381 | 272 | (0.109) | 321 | (0.128) | 155 | (0.062) | 214 | (0.096) | 983 | (0.395) |
| Serbia | 1,660,019 | 1,440 | (0.087) | 1,055 | (0.064) | 215 | (0.013) | 667 | (0.040) | 3,377 | (0.203) |
Data are presented as absolute numbers (N.) and percentage (%) of positive blood donations.
Significant difference between transfusion centres (χ2 test): HBV: p<0.001; HCV: p<0.001; HIV: p<0.001; syphilis: p<0.001.
HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; TTIs: total transfusion-transmissible infections; Novi Sad: Blood Transfusion Institute of Vojvodina; Belgrade BTI: Blood Transfusion Institute of Serbia; Belgrade MMA: Institute for Transfusiology and Haemobiology of Military Medical Academy, Belgrade; Niš: Blood Transfusion Institute, Clinical Centre Niš.
Most blood donations were collected in Belgrade. The Blood Transfusion Institute of Serbia is the largest blood supplier, and consequently the largest absolute number of positive test results for HBV and HCV was recorded here (650 and 433 positive donations, respectively). The highest absolute number of positive test results for syphilis was recorded in Belgrade, at the Blood Transfusion Institute of Serbia, and in Niš (246 and 214 positive donations, respectively). The highest absolute number of positive test results for HIV was recorded in Niš (155 positive donations). In the period 2005–2017, a north-to-south upward trend of prevalence was observed (Table I and Online Supplementary Content, Table SIII). The highest prevalence of HBV was detected in Belgrade, at the MMA (0.172%), and the highest prevalence for HCV, HIV and syphilis was recorded in Niš (0.128%, 0.062% and 0.096, respectively).
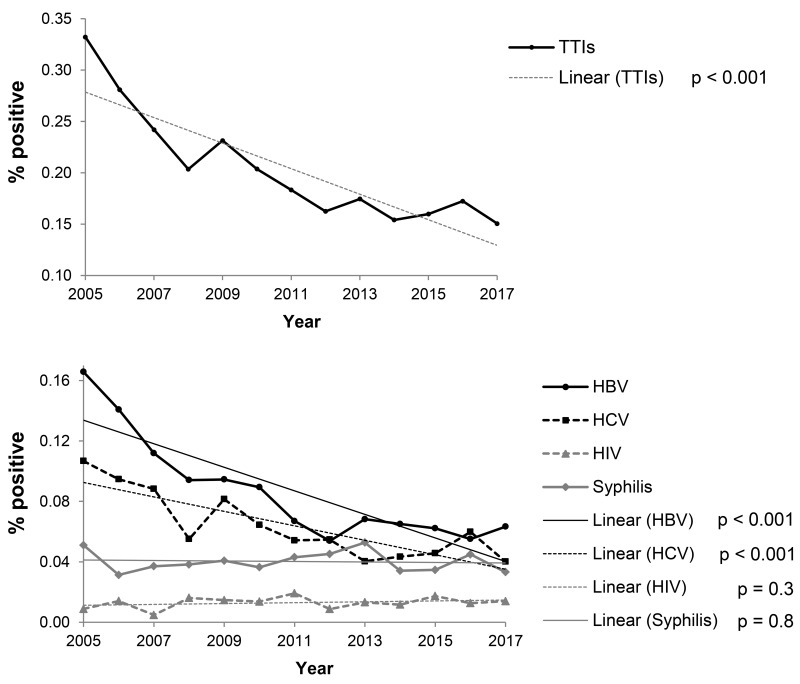
Over the period of study, a significant declining trend of TTI prevalence was recorded (from 0.332 to 0.150%) given the percentage decrease in HBV (from 0.166 to 0.063%) and HCV (from 0.107 to 0.040%) positive donations (Figure 1 and Online Supplementary Content, Table SII). Detailed analyses showed that trends of total TTIs, HBV and HCV prevalence fell sharply until 2011. After 2011, the prevalence continued its downward trend, although this was less pronounced (Online Supplementary Content, Figure S1), and the prevalence of HIV and syphilis infection in the period was registered as stable (Figure 1 and Online Supplementary Content, Table SII).
Figure 1.
Trends in seroprevalence of total transfusion-transmitted infections and hepatitis B virus, hepatitis C virus, human immunodeficiency virus and syphilis-positive blood donations in the four largest transfusion centres in Serbia, 2005–2017.
HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; TTIs: total transfusion-transmissible infections. p: statistical significance of trends.
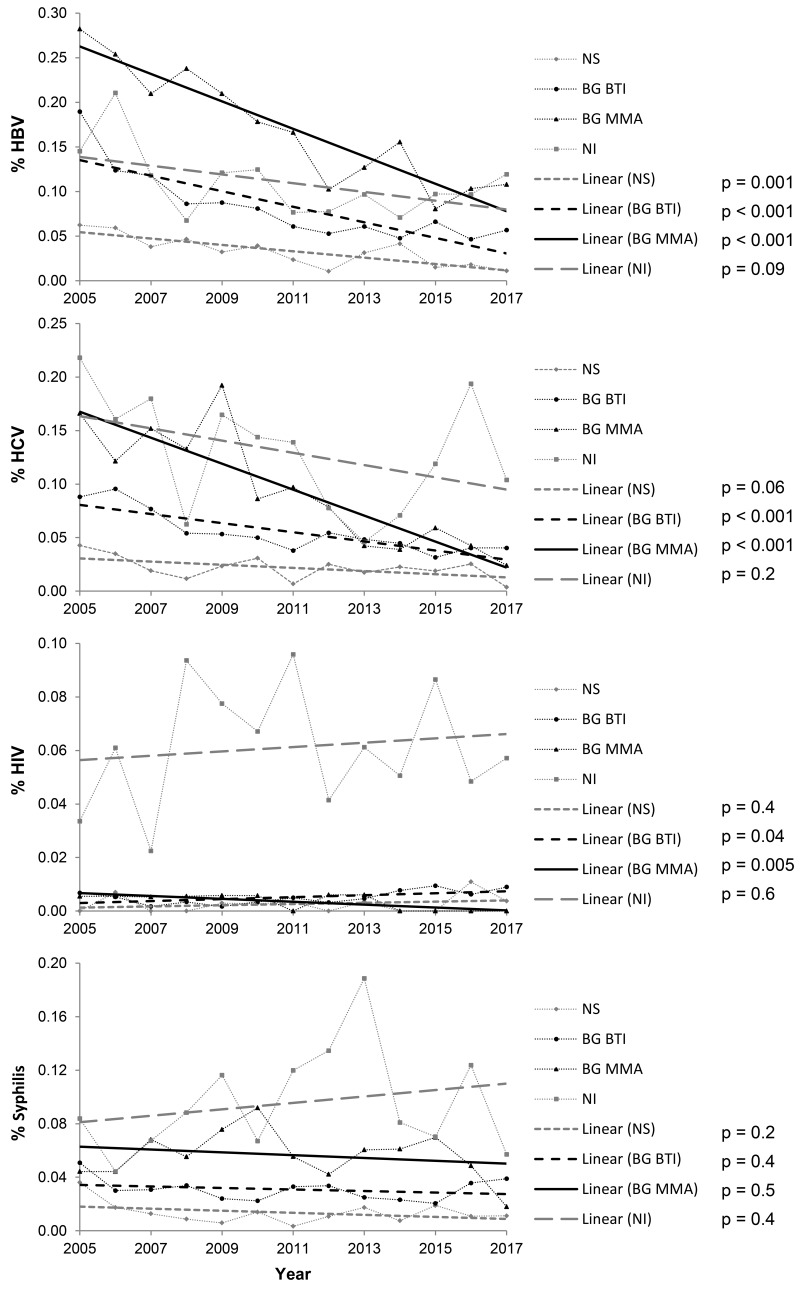
Trends in the prevalence of TTIs for the period 2005–2017 for each of the four largest Serbian centres are shown in Figure 2 and Online Supplementary Content, Table SIII. A descreasing trend of HBV and HCV prevalence was registered in three centres: at both centres in the Belgrade region (the Blood Transfusion Institute of Serbia and Transfusiology and Haemobiology of the MMA), and in Novi Sad. Prevalence of syphilis remained unchanged in all four centres. The prevalence of HIV, although low (0.013%), rose at the Blood Transfusion Institute of Serbia in Belgrade while it fell at the Institute for Transfusiology and Haemobiology of the MMA. Prevalence of HIV remained stable in Novi Sad and Niš.
Figure 2.
Trends in seroprevalence of hepatitis B virus, hepatitis C virus, human immunodeficiency virus and syphilis infections in each of the four largest transfusion centres in Serbia, 2005–2017.
p: statistical significance of trends; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; NS: Blood transfusion Institute of Vojvodina in Novi Sad; BG BTI: Blood Transfusion Institute of Serbia in Belgrade; BG MMA: Institute for Transfusiology and Haemobiology of the Military Medical Academy, Belgrade; NI: Blood Transfusion Institute, Clinical Centre Niš.
The total prevalence of TTIs varied between different transfusion centres: a downward trend was registered in three centres (two in Belgrade and one in Novi Sad); the total prevalence of TTIs remained unchanged in the fourth centre in Niš (Online Supplementary Content, Table SIII).
Demographic data (Tables II and III) showed that, in Serbia, blood donations positive for the main TTIs (HBV and HCV) were from males who had donated blood for the first time. HIV-positive donations were predominantly from male repeat donors. Syphilis-positive donations were predominantly from males, but were equally distributed among first-time and repeat donors. Donations positive for HBV and HCV were predominantly from the youngest donors (19–29 years old), whereas syphilis-positive donations were equally distributed among four donor age groups. In addition, different blood transfusion centres showed some variation in the prevalence of TTI-positive blood donations between repeat and first-time donors, as well as between donors of different age groups.
Table II.
Demographic data of blood donations positive for transfusion-transmissible infections in Serbia, 2005–2017.
| HBV | HCV | HIV | Syphilis | Total TTIs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| N. | (%) | N. | (%) | N. | (%) | N. | (%) | N. | (%) | |
| Gender | ||||||||||
| Male | 1,211 | (84) | 831 | (79) | 181 | (84) | 530 | (79) | 2,753 | (82) |
|
|
||||||||||
| Female | 229 | (16) | 224 | (21) | 34 | (16) | 137 | (21) | 624 | (18) |
|
| ||||||||||
| Donor status | ||||||||||
| New | 1,163 | (81) | 733 | (69) | 83 | (39) | 370 | (55) | 2,349 | (70) |
|
|
||||||||||
| Repeat | 277 | (19) | 272 | (26) | 132 | (61) | 297 | (45) | 978 | (30) |
|
| ||||||||||
| Ages | ||||||||||
| 18–29 | 509 | (35) | 507 | (48) | 94 | (44) | 174 | (26) | 1284 | (38) |
|
|
||||||||||
| 30–39 | 326 | (23) | 245 | (23) | 50 | (23) | 153 | (23) | 774 | (25) |
|
|
||||||||||
| 40–49 | 342 | (24) | 182 | (17) | 41 | (19) | 146 | (22) | 711 | (21) |
|
|
||||||||||
| 50–65 | 263 | (18) | 121 | (11) | 30 | (14) | 194 | (29) | 606 | (18) |
Data are presented as absolute numbers (N.) and percentage (%) of positive blood donations. Significant difference between TTIs (χ2 test): gender: p=0.002; donor status: p<0.001; age: p<0.001. HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; TTIs: total transfusion-transmissible infections.
Table III.
Demographic data of blood donations positive for transfusion-transmissible infections in the four largest Serbian transfusion centres, 2005–2017 period.
| NS | BG BTI | BG MMA | NI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N. | (%) | N. | (%) | N. | (%) | N. | (%) | |||
| HBV+ | Gender | Male | 103 | (79) | 521 | (80) | 367 | (95) | 220 | (81) |
| Female | 28 | (21) | 129 | (20) | 20 | (5) | 52 | (19) | ||
| Donor status | New | 118 | (90) | 581 | (89) | 310 | (80) | 154 | (57) | |
| Repeat | 13 | (10) | 69 | (11) | 77 | (20) | 118 | (43) | ||
| Age (years) | 18–29 | 42 | (32) | 200 | (31) | 164 | (42) | 103 | (38) | |
| 30–39 | 23 | (18) | 174 | (27) | 61 | (16) | 68 | (25) | ||
| 40–49 | 35 | (27) | 163 | (25) | 84 | (22) | 60 | (22) | ||
| 50–65 | 31 | (24) | 113 | (17) | 78 | (20) | 41 | (15) | ||
| HCV+ | Gender | Male | 54 | (64) | 332 | (77) | 195 | (90) | 250 | (78) |
| Female | 31 | (36) | 101 | (23) | 21 | (10) | 71 | (22) | ||
| Donor status | New | 63 | (74) | 339 | (78) | 180 | (83) | 151 | (47) | |
| Repeat | 22 | (26) | 94 | (22) | 36 | (17) | 120 | (37) | ||
| Ages (years) | 18–29 | 31 | (36) | 167 | (39) | 139 | (64) | 170 | (53) | |
| 30–39 | 17 | (20) | 133 | (31) | 37 | (17) | 58 | (18) | ||
| 40–49 | 27 | (32) | 82 | (19) | 24 | (11) | 49 | (15) | ||
| 50–65 | 10 | (12) | 51 | (12) | 16 | (7) | 44 | (14) | ||
| HIV+ | Gender | Male | 8 | (80) | 37 | (88) | 7 | (88) | 129 | (83) |
| Female | 2 | (20) | 5 | (12) | 1 | (12) | 26 | (17) | ||
| Donor status | New | 3 | (30) | 24 | (57) | 6 | (75) | 50 | (32) | |
| Repeat | 7 | (70) | 18 | (43) | 2 | (25) | 105 | (68) | ||
| Ages (years) | 18–29 | 4 | (40) | 19 | (45) | 3 | (38) | 68 | (44) | |
| 30–39 | 3 | (30) | 14 | (33) | 1 | (12) | 32 | (21) | ||
| 40–49 | 1 | (10) | 6 | (14) | 4 | (50) | 30 | (19) | ||
| 50–65 | 2 | (20) | 3 | (7) | 0 | (0) | 25 | (16) | ||
| Syphilis | Gender | Male | 39 | (75) | 188 | (76) | 114 | (89) | 189 | (78) |
| Female | 13 | (25) | 58 | (24) | 14 | (11) | 52 | (22) | ||
| Donor status | New | 24 | (46) | 171 | (70) | 82 | (64) | 93 | (39) | |
| Repeat | 28 | (54) | 75 | (30) | 46 | (36) | 148 | (61) | ||
| Ages (years) | 18–29 | 6 | (12) | 53 | (22) | 26 | (20) | 89 | (37) | |
| 30–39 | 16 | (31) | 60 | (24) | 25 | (20) | 52 | (22) | ||
| 40–49 | 15 | (29) | 51 | (21) | 32 | (25) | 48 | (20) | ||
| 50–65 | 15 | (29) | 82 | (33) | 45 | (35) | 52 | (22) | ||
| TTIs | Gender | Male | 204 | (73) | 1,078 | (79) | 683 | (92) | 788 | (80) |
| Female | 74 | (27) | 293 | (21) | 56 | (8) | 201 | (20) | ||
| Donor status | New | 208 | (75) | 1,115 | (81) | 578 | (78) | 448 | (45) | |
| Repeat | 70 | (25) | 256 | (19) | 161 | (22) | 491 | (50) | ||
| Ages (years) | 18–29 | 83 | (30) | 439 | (32) | 332 | (45) | 430 | (43) | |
| 30–39 | 59 | (21) | 381 | (28) | 124 | (17) | 210 | (21) | ||
| 40–49 | 78 | (28) | 302 | (22) | 144 | (19) | 187 | (19) | ||
| 50–65 | 58 | (21) | 247 | (18) | 139 | (19) | 162 | (16) | ||
Data are presented as absolute numbers (N.) and percentage (%) of positive blood donations. Significant difference between transfusion centres (χ2 test):
- HBV+: gender: p<0.001; p<0.001; p<0.001;
- HCV+: gender: p<0.001; donor status: p<0.001; age: p<0.001;
- HIV+: gender: p=0.85; donor status: p=0.004; age: p=0;
- Syphilis+: gender: p=0.2; donor status: p<0.001; age: p<0.001;
- TTIs+: gender: p<0.001; donor status: p<0.001; age: p<0.001.
NS: Blood Transfusion Institute of Vojvodina, Novi Sad; BG BTI: Blood Transfusion Institute of Serbia, Belgrade; BG MMA: Institute for Transfusiology and Haemobiology of the Military Medical Academy, Belgrade; NI: Blood Transfusion Institute, Clinical Centre Niš; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; TTIs: total transfusion-transmissible infections.
Demographic data for total and TTI-positive blood donors over the period 2005–2014 were available in electronic form only at the Blood Transfusion Institute of Serbia. According to their data, the number of donations per donor is 1.27. In reference to the total number of blood donations, our data showed that most donors were male and that they were repeat blood donors. The proportion of total new vs total repeat donors was 0.31. Donors who tested positive for TTIs were also predominantly males and were first-time blood donors (Table IV).
Table IV.
Demographic data on total and transfusion-transmissible infection-positive blood donations at the Institute for Blood Transfusion of Serbia, Belgrade, 2005–2014.
| Gender | Donor status | ||||
|---|---|---|---|---|---|
| Male | Female | New | Repeat | ||
| Donations | N. | 443,798 | 159,592 | 143,103 | 459,038 |
| (%) | (74) | (26) | (24) | (76) | |
| TTIs | N. | 861 | 249 | 925 | 185 |
| (%) | (78) | (22) | (83) | (17) | |
| HBV | N. | 439 | 101 | 488 | 52 |
| (%) | (81) | (19) | (90) | (10) | |
| HCV | N. | 268 | 92 | 286 | 74 |
| (%) | (74) | (26) | (79) | (21) | |
| HIV | N. | 23 | 3 | 16 | 10 |
| (%) | (88) | (12) | (62) | (38) | |
| Syphilis | N. | 131 | 53 | 135 | 49 |
| (%) | (71) | (29) | (73) | (27) | |
Data are reported as absolute numbers (N.) and percentages (%) of positive blood donations.
TTIs: total transfusion-transmissible infections; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus.
Discussion
Based on the data collected for the period 2005–2017 from the four largest Serbian blood transfusion centres, here we report the prevalence of TTIs for four mandatory blood tests: HBV, HCV, HIV, and syphilis. The centres collect 51.4% of blood donations across Serbia: 12% at the Blood Transfusion Institute of Vojvodina, Novi Sad; 24.6% at the Blood Transfusion Institute of Serbia, Belgrade; 7.0% at the Institute for Transfusiology and Haemobiology of the MMA; and 7.8% at the Blood Transfusion Institute of Clinical Centre Niš (Serbian Blood Transfusion Institute, unpublished data, 2018). We have noticed that recorded TTI prevalence varies among the blood transfusion centres which participated in this study. According to Serbian laws regulating the blood service, there is no centralised procedure for the acquisition of either equipment or key medical items (blood bags, test kits) used to detect TTIs. Instead, essential items are purchased on an individual basis by the management of blood transfusion centres or the management of hospital-based transfusion centres. However, the safety policy, the blood testing algorithm following standard protocols and the use of medical items of the same generation, have been standardised across all Serbian transfusion centres3, thus allowing a comparison to be made between the results in this survey.
The prevalence of HBV, HCV, HIV and syphilis infection in the period 2005–2017 was, according to this survey, 0.087%, 0.064%, 0.013%, and 0.040%, respectively. For the period 2005–2013, prevalence rates of HBV, HCV and syphilis were higher (0.20%, 0.12%, and 0.06%, respectively) while lower rates of prevalence of HIV (0.005%) were found among blood donors at the Institute for Transfusiology and Haemobiology of the MMA in Belgrade10. Based on the published results10 and those observed in the present study, the prevalence of TTIs among Serbian blood donors is low and corresponds to the prevalence of TTIs in upper/middle-income countries reported by the World Health Organization11. Thirty percent of Serbian blood donors are family/replacement blood donors. Paid donations are not allowed according to national legislation, and, as far as we know, there is no record of paid donors that serve as “surrogate” family/replacement donors. Although it is generally accepted that there is higher prevalence of TTIs among true family/replacement blood donors12, some reports show that these donors do not represent an increased risk of TTI transmission13. Since there is no general database in Serbian transfusion centres, the prevalence of TTIs among family/replacement blood donors could not be calculated, and so their influence on TTI prevalence among Serbian blood donors is currently not known.
The percentage of new cases of HBV, HCV, HIV and syphilis infections in the general population in Serbia for the period 2005–2017 was 0.0063%, 0.0079%, 0.0007% and 0.0013%, respectively14. We believe that the low percentage of new cases of these diseases in Serbia reflects the success of the implementation of public health measures: permanent education about TTI transmission and preventive strategies (in schools, through public media), HBV vaccination (which became mandatory for new-born children in 2002), free testing for HIV and other TTIs (anonymous testing in a number of health centres across the country), the mandatory testing of child-bearing women for HBV; there remains no routine testing for HIV.
The data collected from the four major blood transfusion centres in Serbia from 2005–2017 presented in this study show that, among Serbian blood donors, there has been a decline in the prevalence of HBV and HCV infection, while the prevalence of HIV and syphilis remained unchanged. Over this period, the number of new HBV and HCV cases in the general population also declined14. The trend of prevalence of HIV among blood donors in this study, and the number of new HIV cases in the general population, remained stable throughout this period14. Although the number of new cases of syphilis in the general population is low, a sharp upward trend was recorded over the period 2010–2017 (from 0.0012 to 0.0023)14. Reynolds et al.15 have stated that the decline in HCV-positive blood donors may reflect: 1) an increase in donor compliance; 2) a decrease in HCV positivity in the general population; 3) a reduction in the proportion of undiagnosed infection as a result of the increase in testing in the general population; 4) a change in donor profile; or 5) a combination of these elements. We believe that the declining trend of HBV and HCV infection is the consequence of the successful implementation of public health measures and the decrease in the diseases in the general Serbian population. In our previous study10, we showed that the reorganisation of the Serbian army started in 2005 and the abolishment of conscription in 2009, followed by the need to manage staff redundancies and a generational change, together with changes in the educational system, correlated with a reduced prevalence of mandatory TTI testing among military personnel. We showed (Clinical Centre Kososvska Mitrovica, unpublished data, 2018) that mass movements of population in the southernmost Serbian region of Kosovo and Metohija resulted in a significant reduction in prevalence of TTIs in the transfusion centre in Kosovska Mitrovica, which operates within municipalities with a Serbian majority. In the period 2005–2014, the prevalence of TTIs among 14,374 blood donations declined from 2.61% to 0.21%, due to a decline in prevalence of HBV from 1.99% to 0.21%, and of HCV from 1.37% to 0.07%. The 10-year (2005–2014) prevalence of HBV and HCV, recorded in Kosovska Mitrovica, was 0.59 and 0.54 for HBV and HCV, respectively, and the prevalence recorded in the Sector for Public Health in the Municipality of Prishtina (operating within municipalities with an Albanian majority) was 4.2% and 0.3% for HBV and HCV, respectively16.
Complete data on the prevalence of TTIs between 2005 and 2017 in the Balkan countries are not available except for Croatia. According to the available data9, we calculated that the prevalence of TTIs in Croatia was 0.013, 0.007, 0.001 and 0.007 for HBV, HCV, HIV and syphilis, respectively, and that the prevalence of HBV, HCV, and syphilis showed declining trends. Data on the prevalence of TTIs among blood donations in neighbouring countries (Croatia, Hungary, Bulgaria, Romania and the Former Yugoslav Republic [FYR]of Macedonia9,17,18) showed that the prevalence of HBV, HCV, HIV and syphilis is lowest in Croatia. The prevalence of HBV and HCV was highest (at almost equal levels of 0.7% for HBV and 0.1% for HCV) in Bulgaria and Romania. The highest prevalence of HIV (0.013%) was in Serbia, and the highest prevalence of syphilis (0.148%) was in the FYR of Macedonia. In the Balkan countries, the highest value of HBV prevalence was found in Albania (7.5–8.1%)19.
A critical analysis of our results would require a direct comparison of the differences between the different countries under study, but this is problematic. It is known that the risk of acquiring a TTI (HIV) infection is not uniformly distributed; it varies according to geographical region in Europe and reflects the effects of different national legislation on donor selection20. Differences are also known to depend on the equipment available, commercially available assays, quality of testing, processing, and staff education and training2.
In the countries and centres where nucleic acid test (NAT) is adopted as a molecular technique for screening blood donations and combined with serology tests, it has made a measurable contribution to blood safety, i.e. a reduction in the risk of TTI transmission6,9,21–26. After implementation of mandatory Individual Donation NAT testing (ID-NAT) in Croatia in 2013, one HIV infectious window period and 50 occult HBV infections were detected26. Calculated NAT incidence rates (i.e. risk of transmission of infection to the transfused recipient) for HBV in Croatia and Slovenia are 1:10,900 and 1:15,60026. Residual risk for TTI transmission in other former Yugoslav countries and the Balkans is not known. In EU countries where NAT has been carried out for over 15 years, the main residual risk is lower compared to Croatia (implemented in 2013) and Slovenia (implemented for HCV in 2000, and for HIV and HBV in 2007). Thus, calculated residual risk for transmitted HBV infections ranges from 10 per million donations in Spain to 1.6 per million donations in France and Germany27, 57.8 per million donations in Italy28, and 1.37 per million donations in England and Wales29. Since NAT implementation, the residual risk for HCV transmission has ranged between 0.1 (France) to 2.33 (Spain) per million donations, and for HIV, from 0.18 (Germany) to 1.1 (Italy) per million donations27. In England, calculated residual risk for HCV is 1 in 8 million donations and 1 in 30 million donations for HCV30. In 2008, Velati et al. reported the risk of 2.5 HCV and 1.8 HIV infectious units per million donations entering the blood supply28. The risk has declined over time, and the same length of study in 2018 reported a residual risk of one in 12,979,949 donations for HCV and of one in 1,917,250 for HIV31.
In Serbia, NAT was implemented only at the Institute for Transfusiology and Haemobiology of the Military Medical Academy in Belgrade in 2007. With NAT (mini pool NAT of 24 samples), 3 HCV-positive and one HBV-positive positive blood units were detected. Among these, one HCV positive was in an infectious window period and could not be identified with the serology tests used6, and two HCV-positive and one HBV-positive donations stayed undetermined after serological testing; the infections were confirmed with NAT (Institute for Transfusiology and Haemobiology, MMA, unpublished data, 2018). Due to the absence of mandatory NAT testing, one HIV-positive and one HCV-positive donation in an infectious window period entered the blood supply in Serbia. TTIs infections were confirmed, and information about this was publicly announced. A planned reorganisation of Serbian Blood Transfusion System, and centralised NAT screening implementation, has been envisaged for 2019.
Besides NAT, other approaches and techniques have been introduced to improve blood safety. The introduction of a new reverse syphilis algorithm in 2015 in Greece resulted in higher seroprevalence compared to traditional screening by using a non-treponemal rapid plasma reagin (RPR) test32. In the FYR of Macedonia, a slight increase in the prevalence of TTIs was recorded after the implementation of a new transfusion algorithm for blood screening33.
In the Balkan countries, a north-to-south upward trend of TTI-positive blood donations has also been noted; a conclusion drawn from published papers in the literature8,17,19,24–26,32–37. Our results showed that there is a north-to-south upward trend of prevalence of TTI in Serbia. The lowest prevalence of TTI for all four mandatory tests for TTIs was recorded in Novi Sad, located in Vojvodina, the northernmost region of Serbia. These results are in accordance with the general European rising north-to-south trend of prevalence of TTI20,31,38–40 and are a result of the moderately restricted geographic area of TTIs in Mediterranean countries20,41,42.
In the absence of a generalised database in Serbian transfusion centres, a detailed analysis of the influence of demographic characteristics on TTI-positive blood donors could not be made. However, gender differences in blood donation are significant; only 17% of positive blood donations were from female donors. A study from 201543 reported that 22% of Serbian HCV-positive first-time donors are women. These results are to be expected because women are under-represented among blood donors; data from the Serbian blood Transfusion Institute show that 74% of blood donations were from men and 26% from women. According to the Institute of Public Health of Serbia, the incidence of HIV infection in the general population is 3.4 times higher in men than in women14. To the best of our knowledge, there have been no data published on the influence of gender on the incidence of other TTIs in Serbia. In general, as far as the gender of blood donors is concerned, Serbia follows the trend seen in the Balkans26,44,45. Regarding donor status among blood donors in Serbia for the period 2005–2017, more blood donors are repeat donors: at the Blood Transfusion Institute of Serbia, Belgrade, 24% of blood donors are first-time donors while 76% are repeat donors (Blood Transfusion Institute of Serbia, unpublished data, 2018). Even though there are more repeat blood donors than first-time donors, the prevalence of HBV and HCV was higher among first-time donations, because occasional donors are less aware of the risk of infection and they tend not to be selected. In fact, the 2015 results of Mitrovic et al. clearly show that first-time donors in Serbia are less aware of the risk43. According to this study, the main risk factors for HCV infection among first-time blood donors are drug use, tattooing, and previous blood transfusion (before the 1980s); also, non-use of condoms and multiple sex partners are identified as risk factors. The data clearly show that more than 85% of HCV-positive first-time donors reported at least one of these risk behaviours and 20% of the donors reported presumed that they knew when the infections had occurred. The most common way for HIV transmission in the general population is unprotected sex (45%) and sharing needles among drug injectors (35%)14.
The issue of donor selection leads to an increased prevalence among first-time blood donors in Serbia, and this might be due to several reasons. Firstly, among blood donors in Serbia, there is a small but significant number of people who are not aware of the direct impact their health behaviours have on safe transfusion therapy, who donate blood only to get tested for HIV and other TTIs, and who give false answers to questions related to blood donation which could otherwise affect their eligibility for blood donation5,6. Secondly, in the absence of a centralised electronic database, they cannot be removed from the donor pool and they can donate blood in any other transfusion centre, where they are treated as first-time donors (Blood Transfusion Institute of Serbia and Institute for Transfusiology and Haemobiology, MMA, unpublished data, 2018). One way to eliminate such irresponsible donors (and donors in an infectious window period) could be to apply predonation and donation screening for newly registered blood donors17. Higher prevalence of HIV and syphilis among repeat blood donors could indicate that infections are acquired through sexual or other blood contacts between adults. However, an occurrence of TTI positivity among repeat blood donors could be a result of improved sensitivity of serodiagnostic tests over time.
Conclusion
The reported prevalence of TTIs among blood donors in Serbia was low and it continued to follow a declining trend over the period under study. Even though we are still facing challenges regarding financing, resources, and a comprehensive regulatory framework to help co-ordinate the Serbian blood transfusion system, our results do reflect an adequate implementation of criteria for blood donor selection and testing. Estimates for the prevalence of TTIs that are based on the results of screening tests presented in this study are a valuable resource for both transfusion services and to enable an epidemiological evaluation of TTIs in Serbia and the Western Balkans.
Online supplementary content
Acknowledgements
This work was supported by the Ministry of Defence of the Republic of Serbia (Grant VMA/06-10B.20) and by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant n. 175,062). We would like to thank the colleagues from Serbian transfusion centres who helped us in the preparation of this manuscript: Nevenka Bujandrić (Novi Sad), Anastazija Mrdja (Subotica), Selena Grković (Sombor), Mirjana Guteša (Vrbas), Srbislava Drndarski (Kikinda), Dragana Popov (Zrenjanin), Jelena Pešić (Pančevo), Snežana Martinović (Sremska Mitrovica), Ivana Rodić (IBT, Belgrade), Svetlana Stojković (KBC Dr Dragiša Mišović, Beograd), Milutin Mihajlović (Valjevo), Radmila Popović (Loznica), Vera Divac (Užice), Zvezdana Lojpur (Kragujevac), Marija Simonović (Kragujevac),Vitković Marijana (Kraljevo), Nataša Drljača (Smederevo), Snežana Milutinović (Bor), Biljana Todorović-Kazimirović (Negotin), Marina Džunić (Pirot), Zoran Andjelković (Niš), Vesna Milentijević (Kosovska Mitrovica).
Footnotes
Authorship contributions
DV and VI designed the research; DV, MJ, SB, AA, ZS made serological analysis and analysed data; GF analysed data and analysed scientific literature; DV, IM and VI analysed and interpreted data, analysed scientific literature, and prepared the manuscript.
The Authors declare no conflicts of interest.
References
- 1.Blood Transfusion Institute of Serbia. Voluntary blood donor. [Accessed 5/12/2018]. Available at: http://www.nbti.org.rs/NBTI/1102/Voluntary-blood-donor.shtml.
- 2.Hafner V. Blood services in south-eastern Europe: Current status and challenges. Copenhagen: WHO, Regional Office for Europe; 2007. [Google Scholar]
- 3.Ministry of Health of the Republic of Serbia. 16th edition. [Guide to the preparation, use and quality assurance of blood components]. 2011. [In Serbian.] [Google Scholar]
- 4.Barbara JAJ, Eglin R. Introduction: transfusion-transmitted infections, then and now. In: Barbara JAJ, Regan F, Contreras M, editors. Transfusion Microbiology. New York: Cambridge University Press; 2008. pp. 1–7. [Google Scholar]
- 5.Bogdanović S, Đurić P, Jovanović R, Bogdanović J. Blood donors awareness and attitudes towards blood transfusion safety in the Autonomous Province of Vojvodina, Serbia. Transfus Med. 2017;27:303–6. doi: 10.1111/tme.12398. [DOI] [PubMed] [Google Scholar]
- 6.Balint B, Vučetić D, Todorović-Balint M, et al. Safety improving by complementary serological and molecular testing combined with pathogen reduction of the donated blood in window period. Transfus Apher Sci. 2013;49:103–4. doi: 10.1016/j.transci.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48:148–62. doi: 10.1016/j.jhep.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Byrne L, Reynolds C, Brailsford S, et al. Fifteen years of testing the nation: the role of blood donor infection surveillance in informing the safe supply of blood. J Epidemiol Community Health. 2011;65:A62–3. [Google Scholar]
- 9.Croatian Institute for Transfusion Medicine Zagreb, Croatia. [Accessed on 15/08/2018]. Available at: http://www.hztm.hr/glasilo/ [In Croatian.]
- 10.Vučetić D, Kecman G, Ilić V, et al. Blood donors’ positivity for transfusion-transmissible infections: the Serbian Military Medical Academy experience. Blood Transfus. 2015;13:569–75. doi: 10.2450/2015.0314-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Blood safety and availability. 2017. [Accessed on 11/01/2019]. Available at: https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability.
- 12.World Health Organization, Department of Blood Safety and Clinical Technologies. Blood transfusion safety. Information Sheet for National Health Authorities. 2016. [Accessed on 11/01/2019]. Available at: http://digicollection.org/hss/documents/s15395e/s15395e.pdf.
- 13.Allain JP, Sibinga CT. Family donors are critical and legitimate in developing countries. Asian J Transfus Sci. 2016;10:5–11. doi: 10.4103/0973-6247.164270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Public Health of Serbia. Dr Milan Jovanovic Batut. Health Statistical Yearbooks of Republic of Serbia 2005–2012. [Accessed on 05/12/2018]. Available at: http://www.batut.org.rs/download/publikacije.
- 15.Reynolds CA, Davison KL, Andrews N, et al. Dissecting the decline of hepatitis C in first-time donors in England and Wales. Vox Sang. 2018;113:329–38. doi: 10.1111/vox.12638. [DOI] [PubMed] [Google Scholar]
- 16.Fejza H, Telaku S. Prevalence of HBV and HCV among blood donors in Kosovo. Virol J. 2009;6:21. doi: 10.1186/1743-422X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieshout-Krikke RW, Domanovic D, De Kort W, et al. Selection strategies for newly registered blood donors in European countries. Blood Transfus. 2017;15:495–501. doi: 10.2450/2016.0107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makarovska Bojadzieva T, Nikolova J, Velkova E, et al. Comparative study on transfusion transmissible infections in blood donors. Bilt Transfusion. 2018;63:93. [Google Scholar]
- 19.Durro V, Qyra S. Trends in prevalence of hepatitis B virus infection among Albanian blood donors, 1999–2009. Virol J. 2011;8:96. doi: 10.1186/1743-422X-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suligoi B, Raimondo M, Regine V, et al. Epidemiology of human immunodeficiency virus infection in blood donations in Europe and Italy. Blood Transfus. 2010;8:178–85. doi: 10.2450/2009.0126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsoulidou A, Paraskevis D, Magiorkinis E, et al. Molecular characterization of occult hepatitis B cases in Greek blood donors. J Med Virol. 2009;81:815–25. doi: 10.1002/jmv.21499. [DOI] [PubMed] [Google Scholar]
- 22.Zervou EZ, Kourgia FK, Vini MV. The effect of nucleic acid testing (NAT) in the safety of blood transfusions in NW Greece. Vox Sang. 2015;109(Suppl 1):217. [Google Scholar]
- 23.Saygan MB, Beker CM, Demirel K, et al. The implementation of nucleic acid amplification testing for screening blood donors in Turkey, preliminary data. Vox Sang. 2015;109(Suppl 1):205. [Google Scholar]
- 24.Bingulać-Popović J, Babić I, Maslović M, et al. NAT testing of blood donors in Croatia: results of two years testing in Croatian institute of transfusion medicine. Vox Sang. 2015;109(Suppl 1):210. [Google Scholar]
- 25.Juraković-Loncar N, Safić H, Babić I, et al. Hepatitis B window period infection in blood donor detected by ID-NAT testing: case report. Vox Sang. 2016;111(Suppl 1):170. [Google Scholar]
- 26.Safić Stanić H, Babić I, Maslović M, et al. Three-year experience in NAT screening of blood donors for transfusion transmitted viruses in Croatia. Transfus Med Hemother. 2017;44:415–20. doi: 10.1159/000457965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laperche S. Blood safety and nucleic acid testing in Europe. Euro Surveill. 2005;10:3–4. [PubMed] [Google Scholar]
- 28.Velati C, Romanò L, Fomiatti L, et al. Impact of nucleic acid testing for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus on the safety of blood supply in Italy: a 6-year survey. Transfusion. 2008;48:2205–13. doi: 10.1111/j.1537-2995.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 29.Brant LJ, Reynolds C, Byrne L, et al. Hepatitis B and residual risk of infection in English and Welsh blood donors, 1996 through 2008. Transfusion. 2011;51:1493–502. doi: 10.1111/j.1537-2995.2011.03108.x. [DOI] [PubMed] [Google Scholar]
- 30.Soldan K, Barbara JA, Ramsay ME, Hall AJ. Estimation of the risk of hepatitis B virus, hepatitis C virus and human immunodeficiency virus infectious donations entering the blood supply in England, 1993–2001. Vox Sang. 2003;84:274–86. doi: 10.1046/j.1423-0410.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 31.Velati C, Romanò L, Piccinini V, et al. Prevalence, incidence and residual risk of transfusion-transmitted hepatitis C virus and human immunodeficiency virus after the implementation of nucleic acid testing in Italy: a 7-year (2009–2015) survey. Blood Transfus. 2018;16:422–32. doi: 10.2450/2018.0069-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pape M, Konstantinidou A, Bakaloudi V, et al. Experience from the implementation of the reverse syphilis screening in greek blood donors. Vox Sang. 2016;111(Suppl 1):82. [Google Scholar]
- 33.Nikolova JN, Makarovska-Bojadzieva TM, Velkova VE, et al. Prevalence of transfusion transmitted infectious diseases markers in Macedonian blood donors-A comparison study. Vox Sang. 2015;109(Suppl 1):217. [Google Scholar]
- 34.Kochovska E. TTI marker prevalence of the donors in the regional center Tetovo. Vox Sang. 2017;112(Suppl 1):168. [Google Scholar]
- 35.Öner S, Yapici G, Şaşmaz CT, et al. Hepatitis B, hepatitis C, HIV, and VDRL seroprevalence of blood donors in Mersin, Turkey. Turk J Med Sci. 2011;41:35–41. [Google Scholar]
- 36.Yilmaz SY, Cetinkaya RAC, Savasci US, et al. Analysis of four years seroprevalence data of blood donors based on the donor type. Vox Sang. 2015;109(Suppl 1):209. [Google Scholar]
- 37.Veresa V, Kamchev N, Shorova M, et al. Incidence of HBsAg, anti HCV, anti-HIV and Treponema pallidum antibodies in blood donors of the eastern part of the Republic of Macedonia in the past 10 years. Bilt Transfuziol. 2012;58:139. [Google Scholar]
- 38.de Kort W, Mayr W, Jungbauer C, et al. Blood donor selection in European Union directives: room for improvement. Blood Transfus. 2016;14:101–8. doi: 10.2450/2015.0148-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.González R, Torres P, Castro E, et al. Efficacy of hepatitis B virus (HBV) DNA screening and characterization of acute and occult HBV infections among blood donors from Madrid, Spain. Transfusion. 2010;50:221–30. doi: 10.1111/j.1537-2995.2009.02343.x. [DOI] [PubMed] [Google Scholar]
- 40.Romanò L, Velati C, Cambiè G, et al. SIMTI study group for HBV infection among first-time blood donors. Hepatitis B virus infection among first-time blood donors in Italy: prevalence and correlates between serological patterns and occult infection. Blood Transfus. 2013;11:281–8. doi: 10.2450/2012.0160-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–55. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 42.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–42. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 43.Mitrović N, Delić D, Marković-Denić L, et al. Seroprevalence and risk factors for hepatitis C virus infection among blood donors in Serbia: A multicentre study. Dig Liver Dis. 2015;47:572–6. doi: 10.1016/j.dld.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Makarovska Bojadzjieva T, Nikolova J, Velkova E, et al. Demographic characteristics of blood donors with positive TTI markers. Vox Sang. 2016;111(Suppl 1):179. [Google Scholar]
- 45.Yildiz SM, Candevir A, Kibar F, et al. Hepatitis B, hepatitis C, human immunodeficiency virus and syphilis frequency among blood donors: a single center study. Transfus Apher Sci. 2015;53:308–14. doi: 10.1016/j.transci.2015.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.