Abstract
African wolves (AWs) are sympatric with endangered Ethiopian wolves (EWs) in parts of their range. Scat analyses have suggested a dietary overlap between AWs and EWs, raising the potential for exploitative competition, and a possible conservation threat to EWs. However, in contrast to that of the well-studied EW, the foraging ecology of AWs remains poorly characterized. Accordingly, we studied the foraging ecology of radio-collared AWs (n = 11 individuals) at two localities with varying levels of anthropogenic disturbance in the Ethiopian Highlands, the Guassa-Menz Community Conservation Area (GMCCA) and Borena-Saynt National Park (BSNP), accumulating 845 h of focal observation across 2952 feeding events. We also monitored rodent abundance and rodent trapping activity by local farmers who experience conflict with AWs. The AW diet consisted largely of rodents (22.0%), insects (24.8%), and goats and sheep (24.3%). Of the total rodents captured by farmers using local traps during peak barley production (July to November) in GMCCA, averaging 24.7 ± 8.5 rodents/hectare/day, 81% (N = 3009) were scavenged by AWs. Further, of all the rodents consumed by AWs, most (74%) were carcasses. These results reveal complex interactions between AWs and local farmers, and highlight the scavenging niche occupied by AWs in anthropogenically altered landscapes in contrast to the active hunting exhibited by EWs in more intact habitats. While AWs cause economic damage to local farmers through livestock predation, they appear to play an important role in scavenging pest rodents among farmlands, a pattern of behaviour which likely mitigates direct and indirect competition with EWs. We suggest two routes to promote the coexistence of AWs and EWs in the Ethiopian highlands: local education efforts highlighting the complex role AWs play in highland ecosystems to reduce their persecution, and enforced protection of intact habitats to preserve habitat preferred by EWs.
Keywords: African wolf, ecosystem services, Ethiopian highlands, Ethiopian wolf, feeding ecology, pest rodents
1. Introduction
The midsize canids in northern Africa considered to be golden jackals (Canis aureus) were recently reclassified as African wolves (AWs), Canis lupaster, due to their close phylogenetic relationship to the grey wolf (C. lupus) [1,2]. AWs are found throughout the Ethiopian Highlands, often in sympatry with endangered Ethiopian wolves (EWs), Canis simensis [3,4], Africa's most threatened carnivore. At fewer than 500 individuals, the EW is the rarest canid in the world [5]. Restricted to several enclaves of Afro-alpine habitats, small EW populations are highly vulnerable to extinction, particularly because of habitat loss as well as rabies and canine distemper virus outbreaks stemming from interactions with local domestic animals [6,7].
Recent scat analyses revealed that the diet of AWs consists largely of rodents (48–57%) and varies by season [8,9]. Given that EWs depend on abundant rodent populations for their survival and reproduction [10–12], potential niche overlap and competition between these two species might have negative effects on EW populations. Based on intensive study at multiple sites, EWs are known to be active rodent hunters and only rarely kill livestock or scavenge [10,11]. However, because our knowledge of the diet of AWs in the Ethiopian Highlands is based primarily on scat analyses [8,9], we do not know the proportion of rodents acquired through hunting versus scavenging rodents killed by local farmers using traditional practices. To better understand the nature and extent of potential competition between EWs and AWs, it is necessary to learn more about the AW's diet. If AWs primarily scavenge rodents, direct exploitative competition between these species may be relatively minor. On the other hand, if AWs primarily engage in the active hunting of rodents, particularly where the two species overlap, the potential for competition may be significant. Recent work has indicated that sympatric AWs and EWs do actively defend their territories from each other via agonistic interactions [8].
A better characterization of AW foraging ecology will also permit inferences about the nature of human-wildlife conflict in the Ethiopian Highlands. AWs are presently considered one of the main livestock predators in the Ethiopian Highlands and are heavily persecuted [9]. However, they may also provide an ecological benefit to farmers if they feed upon pests such as rodents and insects, which cause significant damage to crops in small-holder farms in Ethiopia [13–16].
Accordingly, our goal is to evaluate the foraging ecology of AWs in greater detail than before and to assess the potential effects their dietary choices may have on EWs via competition for resources. We intensively studied the foraging ecology of the AW in the Ethiopian Highlands via direct observations of 11 radio-collared individuals at two sites, and compared our results with those from published studies of the diet and foraging behaviour of EWs. Specifically, we estimated (1) the proportion of rodents in the diet of the AW that derived from scavenging versus predation, (2) the extent to which AWs foraged in farmland versus intact habitat and (3) rodent abundance and level of trapping activity by local farmers who experience conflict with AWs due to sheep predation.
2. Methods
2.1. Study area
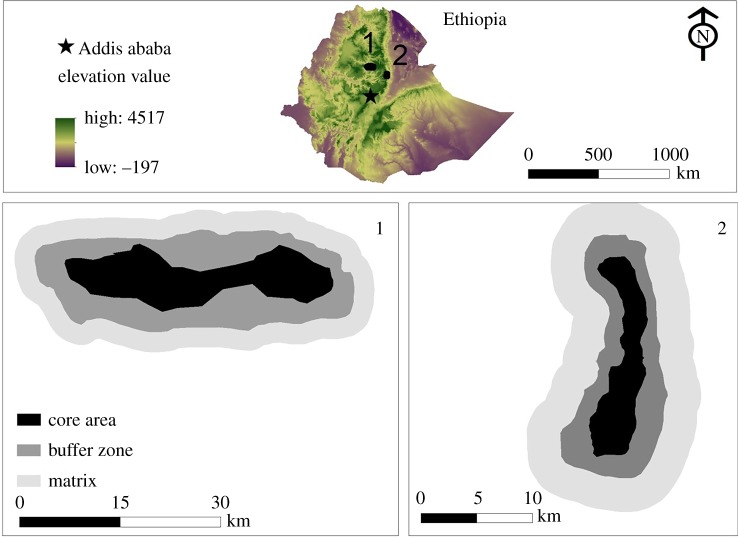
Our study was carried out in Guassa-Menz Community Conservation Area (GMCCA; 10°15′–10°27' N to 39°45'–39°49' E) and Borena-Saynt National Park (BSNP; 10°50'–10°53' N to 38°40'–38°54' E; figure 1), areas of Afro-alpine habitat located in the north-central highlands of Ethiopia. GMCCA spans 111 km2 with an elevational range of 3200–3600 m [17], while BSNP covers an area of 153 km2 with an elevational range of 1900–3700 m [18,19]. Both sites are also home to several mammal species endemic to the Ethiopian Highlands, including EWs, gelada monkeys (Theropithecus gelada) and Starck's hare (Lepus starcki) [11,18]. The Ethiopian wolf populations are estimated at approximately 21 individuals in GMCCA [11] and approximately 16 individuals in BSNP [5]. The local people in both areas are mostly agro-pastoralists who grow barley between June and November and keep a variety of livestock (mostly sheep but also goats, cattle and horses) [8,18]. The two study areas are 150 km apart, but their climates are broadly similar, with a wet season extending from June to November and a dry season from December to May [8,11,19]. Detailed climatic data are available only for GMCCA where rainfall averages 1650 ± 243 mm per year, average monthly temperature is 11.0 ± 1.2°C, and mean monthly low and high temperatures are 4.3 ± 0.5°C and 17.8 ± 0.3°C, respectively (n = 6 years) [17].
Figure 1.
Study localities, (1) Borena-Saynt National Park (BSNP) and (2) Guassa-Menz Community Conservation Area (GMCCA).
Livestock grazing is a common practice in most Ethiopian protected areas [20,21]. Based on the levels of anthropogenic disturbance, we divided each study area into three zones: core (the section of the protected areas where all human and livestock activities are prohibited), buffer (the section of the protected areas where controlled livestock grazing is permitted), and matrix (human-dominated areas adjacent to the protected area which consist mainly of farmland and settlements [8]). In GMCCA, EWs largely occupy the core while AWs mostly use the buffer zone [8].
2.2. Foraging behaviour
We captured 11 AWs using rubber-lined Soft Catch foothold traps (Woodstream Corporation, Lititz, Pennsylvania, USA) sizes 1.5 and 3 (for method details see [19]) and fitted them with VHF radio collars; two males and three females in GMCCA and three males and three females in BSNP (for more details see [19]). In the wet and dry seasons of 2016 and 2017, we followed collared individuals for 3–4 days per month during both day and night. Focal observations were carried out with binoculars from distances of 50–150 m after locating the focal individuals using a hand-held directional antenna. We recorded their activity, including successful and unsuccessful feeding attempts. A successful attempt was scored if the prey was killed and ingested. Accordingly, an unsuccessful attempt was scored when they failed to capture and kill the prey [22]. Scavenging was defined as feeding on a dead animal, typically taking dead rodents from traps set by farmers (difit, see below) ([10,11]; electronic supplementary material, table S1). We classified the food items consumed by AWs as hunted prey (including common molerats Tachyoryctes splendens, smaller rodents, and shrews), livestock carcasses (cattle, horses, sheep and goats), rodent carcasses (taken from difit traps) and arthropods (mainly grasshoppers, but also spiders and beetles). Whenever we observed AWs feeding or attempting to capture prey, we recorded the appropriate habitat type, classified as bushland (greater than 50% shrubs, predominantly Helichrysum and Erica spp.), grassland (greater than 50% open land; including rocky grassland, open grazing land dominated by Festuca spp.) or farmland (barley and other crops).
2.3. Traditional traps ‘difit’ as a source of rodents for AWs
In both GMCCA and BSNP, farmers use a traditional trapping method known as difit to protect their crops from rodents [9]. However, for this specific objective, we collected data only from GMCCA. Difit are made of a locally manufactured rope, a relatively heavy stone and some barley seeds as bait (electronic supplementary material, figure S1). We collected data on the use of difit in GMCCA, where 25 barley farm sites adjacent to GMCCA were investigated, recording the number of rodents trapped (per hectare per day) and the extent to which AWs exploited the traps by taking the dead rodents. Farmers usually set up their traps in the morning (07.00–9.00 h), visiting them at 1–4 h intervals and resetting them if a capture had taken place. Trapping concluded in the evening (17.00–18.00 h). Every morning, after the traps were set we checked them regularly at 2 h intervals. When we found rodents had been caught in the trap, we recorded their number (usually one per trap, but occasionally two) and the species to which they belonged. We then cleared and reset the trap. In addition, we recorded whenever carnivores and raptors were observed taking rodents from the traps.
2.4. Data analysis
We compared the proportions of food items consumed by the AWs in the GMCCA and BSNP by a mixed effect model using food items as response variable, localities as fixed effects and individual collared animals as random effects.
We estimated successful hunting by AWs on two food classes (rodents and sheep) in relation to seasons, using logistic regression by fitting a general linear model. The response variable was binomial (1/0), indicating successful or unsuccessful hunting attempts, respectively. The fixed effects were diet (at two levels: rodents and sheep) and season (at two levels: dry and wet). We combined the data collected from GMCCA and BSNP (proportion of food items consumed) after verifying no significant differences for the two sites using one-way ANOVA followed by Tukeys's HSD post hoc test.
We also compared the effect of habitat types (i.e. fixed effect factor at three levels: bushland, farmland and grassland) on the success probability (attempt to feed and outcome) of AWs capturing rodents (i.e. binomial response variable: 1/0 where 1 is successful) using logistic regression.
All analyses were done in R v. 3.3.1 (R Core Team 2016).
3. Results
3.1. Foraging ecology observations
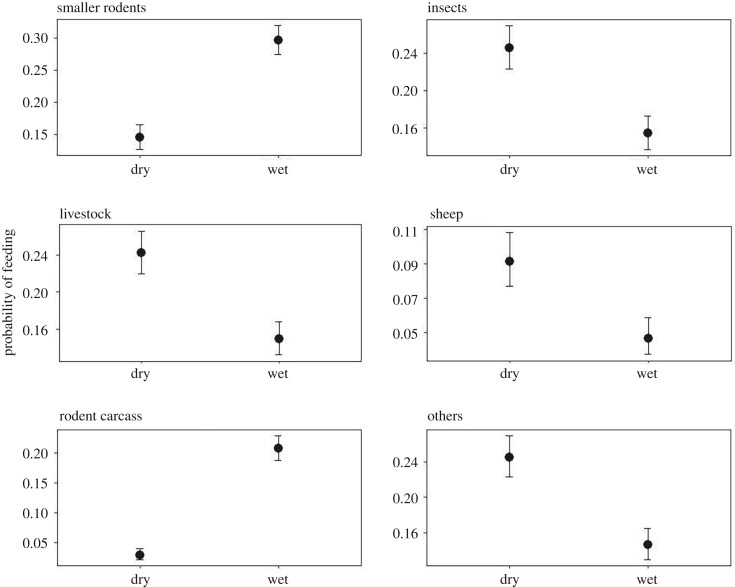
We observed radio-tracked AWs for 845 h across 16 months during 2016 and 2017 (392 h in GMCCA and 453 h in BSNP) resulting in a total of 2952 records of food items. Our focal observations revealed that the proportion of food items consumed by AWs in both study areas did not differ (electronic supplementary material, table S3). We found that AWs consumed primarily rodents during the wet season, and ate a more diverse diet, including more insects, livestock carcasses and sheep, during the dry season (figure 2 and table 1). Indeed, dietary composition differed significantly between the wet and dry seasons: rodents (z = 94.6, d.f. = 1, p < 0.001), sheep (z = 22.4, d.f. = 1, p < 0.001) and insects (z = 38.6, d.f. = 1, p < 0.001). The probability of AWs successfully hunting rodents (successful events as a proportion of total hunting attempts) also differed between seasons (z = 4.6, d.f. = 1, p < 0.001), but not on sheep (z = 1.5, d.f. = 1, p = 0.2; figure 3).
Figure 2.
Probability of African wolves feeding on different diets in the dry and wet seasons.
Table 1.
Composition of African wolf diet. Recorded as successful hunting attempts from focal animal observations of 11 individuals in GMCCA and BSNP.
| BSNP |
GMCCA |
||||||
|---|---|---|---|---|---|---|---|
| food items | total n = 2952 |
dry n = 902 |
wet n = 753 |
total n = 1655 |
dry n = 450 |
wet n = 847 |
total n = 1297 |
| small rodents | 22.76 | 16.30 | 31.08 | 23.02 | 11.56 | 28.22 | 22.44 |
| arthropods | 19.00 | 26.72 | 17.00 | 22.30 | 18.44 | 12.87 | 14.80 |
| livestock caracasses | 18.56 | 20.51 | 14.48 | 17.76 | 29.78 | 14.17 | 19.58 |
| unidentified | 18.53 | 23.28 | 16.73 | 20.30 | 25.11 | 11.57 | 16.27 |
| rodent carcasses | 12.13 | 2.22 | 14.74 | 7.92 | 4.00 | 24.68 | 17.50 |
| sheep | 6.50 | 9.20 | 2.92 | 6.34 | 8.22 | 5.90 | 6.71 |
| grass | 1.32 | 0.78 | 1.86 | 1.27 | 1.11 | 1.53 | 1.39 |
| potatoes | 0.54 | 0.22 | 0.93 | 0.54 | 0.22 | 0.71 | 0.54 |
| wild birds | 0.20 | 0.33 | 0.13 | 0.24 | 0.44 | 0.00 | 0.15 |
| duikers | 0.17 | 0.33 | 0.00 | 0.18 | 0.22 | 0.12 | 0.15 |
| chickens | 0.14 | 0.11 | 0.00 | 0.06 | 0.44 | 0.12 | 0.23 |
| hares | 0.14 | 0.00 | 0.13 | 0.06 | 0.44 | 0.12 | 0.23 |
Figure 3.
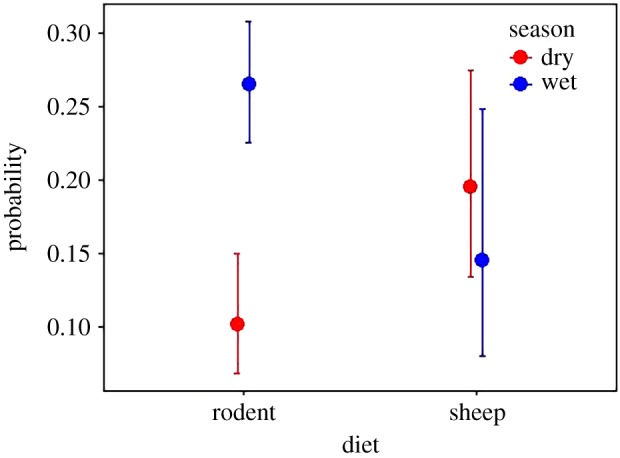
Probability of African wolves successfully capturing (successful events per total hunting attempts) rodents and sheep during hunting attempts in the wet and dry seasons.
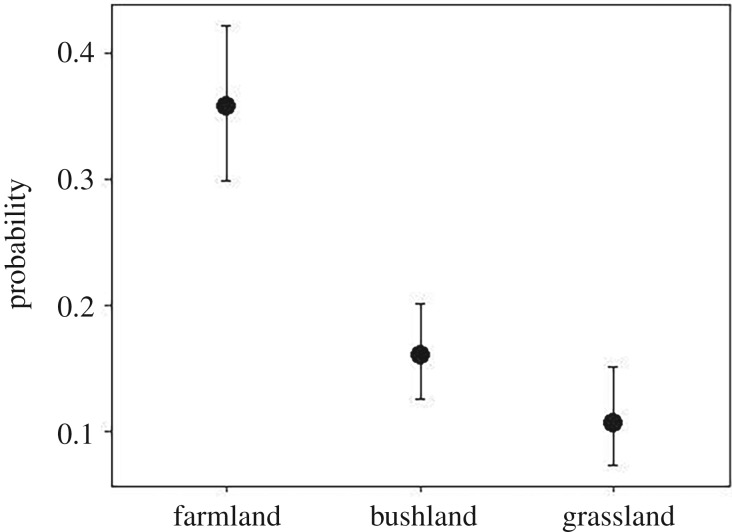
Among the 491 rodents consumed by AWs, 28% were hunted and 72% were scavenged from traps. AWs exhibited a higher proportion of successful feeding attempts in farmland (36%, n = 229) than in bushland (17.3%, n = 324) or grassland (10.7%, n = 244) (figure 4). The proportion of successful feeding attempts did not differ between bushland and grassland (table 2).
Figure 4.
Probability of African wolves successfully capturing rodents in different habitat types.
Table 2.
Comparison of African wolves' success in capturing rodents (active hunting) in different habitat types using Tukey multiple comparisons test.
| habitat | different | lower | upper | p adj |
|---|---|---|---|---|
| farmland–bushland | 0.197 | 0.121 | 0.274 | 0.0000 |
| grassland–bushland | −0.054 | −0.129 | 0.021 | 0.212 |
| grassland–farmland | −0.251 | −0.335 | −0.168 | 0.0000 |
During the study period, we observed AWs killing 192 sheep, of which 163 (85%) were killed by solitary AWs, 21 (11%) by pairs and four (2%) by groups of three AWs.
3.2. AW foraging on dead rodents from traps
During the period of barley production (July–November) in 2016 and 2017, 3009 rodents were trapped in difits at an average rate of 24.7 ± 8.5 rodents ha−1 d−1 (electronic supplementary material, table S2). The Natal multimammate mouse (Mastomys natalensis) was the most frequently captured species in difits, accounting for 72.6% of the total. The other four captured species were the Ethiopian white-footed mouse (Stenocephalemys albipes, 17.3%), grey-tailed narrow-headed rat (S. griseicauda, 5.1%), Abyssinian grass rat (Arvicanthis abyssinicus, 4.3%) and Lophuromys spp. (0.8%). Eleven rodent species were caught in Sherman live traps in the study. The Natal multimammate mouse, Ethiopian white-footed mouse and grey-tailed narrow-headed rat were all caught only in farmland (matrix). The remaining species were either captured in the buffer and core zones or in all three zones (table 3).
Table 3.
Frequency (%) of rodents and shrews (n = 420) captured in the three zones of GMCCA using Sherman live traps and percentage of rodent species captured in farmland using difit. For comparison, frequency (%) of occurrence of rodents per scat (348 scat samples) of EWs in the same study site (data from [11]).
| matrix |
buffer |
core |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| species | dry | wet | dry | wet | dry | wet | total | captured in difit | EW |
| Arvicanthis abyssinicus | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 4.3 | 59.5 |
| Dendromus lovati | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.2 | 0 | |
| Lophuromys brevicaudus | 0.0 | 1.0 | 4.5 | 14.5 | 12.4 | 12.9 | 45.2 | 0.8 | |
| Lophuromys flavopunctatus | 0.0 | 0.0 | 1.4 | 1.4 | 0.0 | 4.0 | 6.9 | ||
| Mastomys natalensis | 3.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.1 | 72.6 | |
| Otomys typus | 0.0 | 0.5 | 0.2 | 1.7 | 0.0 | 0.7 | 4.0 | 0 | 25.6 |
| Stenocephalemys albipes | 6.0 | 6.0 | 0.0 | 0.0 | 0.0 | 0.0 | 6.0 | 17.3 | |
| Stenocephalemys albocaudata | 0.0 | 0.0 | 0.2 | 6.0 | 3.3 | 3.3 | 17.1 | 0 | |
| Stenocephalemys griseicauda | 2.9 | 2.9 | 4.5 | 2.1 | 0.0 | 1.7 | 11.4 | 5.1 | |
| Tachyoryctes splendens | 30.5 | ||||||||
| Crocidura baileyi | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.5 | 4.3 | 0 | |
| Crocidura macmillani | 0.0 | 0.0 | 0.5 | 3.1 | 0.2 | 0.0 | 1.4 | 0 | |
| grand total | 12.1 | 2.14 | 11.4 | 29.5 | 17.4 | 27.4 | 100 | 0 | |
AWs took most of the rodents from difit (81% of events), followed by raptors (Milvus migrans, Buteo augur: 12%), caracals (Caracal caracal: 2.4%), domestic dogs (2.2%) and domestic cats (1.4%). EWs were observed taking rats in only 1% of difit scavenging events.
4. Discussion
This study provides the first detailed observational data on the foraging behaviour of AWs and provides inferences into the extent of dietary overlap with EWs. Earlier scat analyses indicated that rodents comprise a high proportion (47–57%) of the diet of the AW [8,9]. Here, we show that a large proportion (72%; electronic supplementary material, table S1) of the rodents consumed by AWs are obtained via scavenging from traditional traps (difits) rather than by hunting. Unlike the rodent specialist EWs [10,12], AWs feed on a greater diversity of food items, including insects, livestock carcasses and live sheep. Surprisingly, arthropods comprised the second most frequently consumed food items by AWs at 19.0%. Given that other sympatric mammals like EWs and gelada monkeys consume insects much less often [11,17], AWs may be unique among large mammals in the Ethiopian Highlands in exploiting a dietary niche in which insects play a major role.
AWs appear to be generally less efficient in capturing live rodents in Afro-alpine habitats (less than 17% success rate) than EWs, which exhibit capture efficiencies between 25 and 66% at Guassa [22] and 45% in the Bale mountains [10]. Whereas active hunting accounted for only 6% of the AW diet, EWs are almost exclusively (greater than 90%, [10]) rodent hunters and seldom scavenge (electronic supplementary material table S1). Thus, AWs exhibit a more omnivorous diet with a prominent scavenging component, whereas EWs are more strict rodent hunting specialists. This difference may be due to EWs preferring intact grassland habitat, and thus not encountering live rodents as frequently as carcasses. Further, the proclivity of AWs for scavenging rodents may reduce the extent of direct exploitative competition between AWs and EWs.
These results highlight the flexible nature of AW foraging behaviour. The foraging behaviour of AWs is highly seasonal and appears to track rodent abundance [8]. Consistent with previous research [8,9], we found that AWs forage on rodents more in the wet season and exploit livestock (sheep) more during the dry season. Indeed, while rodents comprise a large proportion of AW foraging efforts and capture frequency, active hunting and scavenging of livestock was probably a major, if not the main, component of the diet in terms of biomass. AWs are also more proficient at rodent capture in farmlands compared to more intact habitats (bushland, grassland and woodland) where EWs thrive. The success of AWs in such disturbed habitats may be attributed to the higher visibility of farmland habitats, which evince little above-ground biomass, and/or the nature of rodent abundance and species composition in farmlands [23,24].
The reliance by AWs on rodents and insects implies that they play a role in pest control that may be beneficial to local farmers during certain times of the year [25]. Further, their scavenging of carcasses may have a hygiene benefit around human habitation [26]. Mesocarnivores seem to be on the increase in farm communities worldwide [27,28]. They benefit humans by feeding on crop vermin, and by removing garbage and carcasses, thus reducing health risks [26–28].
The present study points to two conservation recommendations that would facilitate the coexistence of EWs and AWs. First, given the adaptable nature of the foraging ecology of AWs in comparison to EWs, it is crucial that future EW conservation efforts focus on preserving intact habitats that are inherently preferred by EWs. Second, given the extent of persecution of AWs, local farmers should be informed about the potential benefits that AWs have for their farms.
5. Conclusion
This study shows that a large proportion of the rodents whose remains have been found in the scats of AWs were from dead animals caught in traditional traps, rather than obtained through predation, distinguishing them in their foraging habitats from EWs. As a consequence, we may conclude that exploitative food competition between the AW and EW is probably limited. This study also highlights the importance of AWs in rodent control (with their greater efficiency at capturing live rodents in farmland habitats) and waste management (through their removal of rodent and livestock carcasses near farms) in the Ethiopian Highlands. Lastly, it is important to underline that human agricultural expansion into EW habitats is likely attracting AWs, thereby adversely affecting EWs though interference competition [8].
Supplementary Material
Acknowledgement
We thank EWCA for research permission and the Ethiopian Wolf Conservation Programme for capture equipment and assistance capturing AWs.
Ethics
Ethiopian Wildlife Conservation Authority (EWCA): Policy for management of wildlife resources guidelines have been followed.
Data accessibility
Data available at the Dryad Digital Repository: https://doi.org/10.5061/dryad.p9g41sd [29].
Authors' contributions
G.T.M., A.A., A.B., C.S.Z. and N.C.S. conceived the study; G.T.M. and M.K. performed the fieldwork; G.T.M, A.A. and D.T. analysed the data and interpreted the results; G.T.M, A.A., V.V.V., P.J.F., D.T. and N.C.S. drafted the manuscript; and C.S.Z., J.M.R., V.V.V., M.K., A.B. and P.J.F. commented on the manuscript and contributed to its final version.
Competing interests
We declare we have no competing interests.
Funding
Rufford Small Grants Foundation, Mohamed Bin Zayed Species Conservation Fund, Jimma University and U.S.-Norway Fulbright Foundation.
References
- 1.Rueness EK, Asmyhr MG, Sillero-Zubiri C, Macdonald DW, Bekele A, Atickem A, Stenseth NC. 2011. The cryptic African wolf: Canis aureus lupaster is not a golden jackal and is not endemic to Egypt. PLoS ONE 6, e16385 ( 10.1371/journal.pone.0016385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koepfli KP, Pollinger J, Godinho R, Robinson J, Lea A, Hendricks S, Yurchenko AA. 2015. Genome-wide evidence reveals that African and Eurasian golden jackals are distinct species. Curr. Biol. 25, 2158–2165. ( 10.1016/j.cub.2015.06.060) [DOI] [PubMed] [Google Scholar]
- 3.Marino J. 2003. Threatened Ethiopian wolves persist in small isolated Afroalpine enclaves. Oryx 37, 62–71. ( 10.1017/S0030605303000139) [DOI] [Google Scholar]
- 4.Viranta S, Atickem A, Lars A, Stenseth NC. 2017. Rediscovering a forgotten canid species. BMC Zool. 2, 6 ( 10.1186/s40850-017-0015-0) [DOI] [Google Scholar]
- 5.Marino J, Sillero-Zubiri C. 2011. Canis simensis. The IUCN Red List of Threatened Species 2011: e.T3748A10051312 ( 10.2305/IUCN) [DOI]
- 6.Eshete G, Tesfay G, Bauer H, Ashenafi ZT, de Iongh H, Marino J. 2015. Community resource uses and Ethiopian wolf conservation in Mount Abune Yosef. Environ. Manage. 56, 684–694. ( 10.1007/s00267-015-0529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marino J, Sillero-Zubiri C, Deressa A, Bedin E, Bitewa A, Lema F, Rskay G, Banyard A, Fooks AR. 2017. Rabies and distemper outbreaks in smallest Ethiopian wolf population. Emerg. Infect. Dis. 23, 2102 ( 10.3201/eid2312.170893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutema TM, et al. 2018. Competition between sympatric wolf taxa: an example involving African and Ethiopian wolves. R. Soc. open sci. 5, 172207 ( 10.1098/rsos.172207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atickem A, Simeneh G, Bekele A, Mekonnen T, Sillero-Zubiri C, Hill RA, Stenseth NC. 2017. African wolf diet, predation on livestock and conflict in the Guassa Mountains of Ethiopia. Afr. J. Ecol. 55, 632–639. ( 10.1111/aje.12456) [DOI] [Google Scholar]
- 10.Sillero-Zubiri C, Gottelli D. 1995. Diet and feeding behavior of Ethiopian wolves (Canis simensis). J. Mammal. 76, 531–541. ( 10.2307/138236) [DOI] [Google Scholar]
- 11.Ashenafi ZT, Coulson T, Sillero-Zubiri C, Leader-Williams N. 2005. Behaviour and ecology of the Ethiopian wolf (Canis simensis) in a human-dominated landscape outside protected areas. Anim. Conserv. 8, 113–121. ( 10.1017/S1367943005001952) [DOI] [Google Scholar]
- 12.Marino J, Mitchell R, Johnson PJ. 2010. Dietary specialization and climatic-linked variations in extant populations of Ethiopian wolves. Afr. J. Ecol. 48, 517–525. ( 10.1007/s00267-015-0529-6) [DOI] [Google Scholar]
- 13.Takele S, Bekele A, Belay G, Balakrishnan M. 2008. Pest status of rodents in Wonji sugarcane plantation, Ethiopia. Int. J. Ecol. Environ. Sci. 34, 157–163. ( 10.3390/agriculture7120099) [DOI] [Google Scholar]
- 14.Meheretu Y, Cížková D, Těšíková J, Welegerima K, Tomas Z, Kidane D, Bryjová A, Goüy de Bellocq J. 2012. High diversity of RNA viruses in rodents. Ethiop. Emerg. Infect. Dis. 18, 2047–2050. ( 10.3201/eid1812.120596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stenseth NC, et al. 2003. Mice, rats, and people: the bio-economics of agricultural rodent pests. Front. Ecol. Environ. 1, 367–375. ( 10.1890/1540-9295(2003)001[0367:mraptb]2.0.co;2) [DOI] [Google Scholar]
- 16.Swanepoel LH, Swanepoel CM, Brown PR, Eiseb SJ, Goodman SM, Keith M, Malebane P. 2017. A systematic review of rodent pest research in Afro-Malagasy small-holder farming systems: are we asking the right questions? PLoS ONE 12, e0174554 ( 10.1371/journal.pone.0174554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fashing PJ, Nguyen N, Venkataraman VV, Kerby JT. 2014. Gelada feeding ecology in an intact ecosystem at Guassa, Ethiopia: variability over time and implications for theropith and hominin dietary evolution. Am. J. Phys. Anthropol. 155, 1–16. ( 10.1002/ajpa.22559) [DOI] [PubMed] [Google Scholar]
- 18.Eshetu AA. 2014. Development of community based ecotourism in Borena-Saynt National Park, North central Ethiopia: opportunities and challenges. J. Hospital. Manag. Tourism 5, 1–12. ( 10.5897/JHMT2013.0103) [DOI] [Google Scholar]
- 19.Gutema TM, Atickem A, Lemma A, Bekele A, Sillero-Zubiri C, Zinner D, Stenseth NC. 2018. Capture and immobilization of African wolves (Canis lupaster) in the Ethiopian Highlands. J. Wildl Dis. 54, 175–179. ( 10.7589/2017-03-063) [DOI] [PubMed] [Google Scholar]
- 20.Stephens PA, D'Sa CA, Sillero-Zubiri C, Leader-Williams N. 2001. Impact of livestock and settlement on the large mammalian wildlife of Bale Mountains National Park, southern Ethiopia. Biol. Conserv. 100, 307–322. ( 10.1016/S0006-3207(01)00035-0) [DOI] [Google Scholar]
- 21.Abebe FB, Bekele SE. 2018. Challenges to National Park Conservation and Management in Ethiopia. J. Agric. Sci. 10, 52 ( 10.5539/jas.v10n5p52) [DOI] [Google Scholar]
- 22.Venkataraman VV, Kerby JT, Nguyen JN, Ashenafi ZT, Fashing PJ. 2015. Solitary Ethiopian wolves increase predation success on rodents when among grazing gelada monkey herds. J. Mammal. 96, 129–137. ( 10.1093/jmammal/gyu013) [DOI] [Google Scholar]
- 23.Hileman KS, Brodie JED. 1994. Survival strategies of the salamander Desmognathus ochrophaeus: interaction of predator-avoidance and anti-predator mechanisms. Anim. Behav. 47, 1–6. ( 10.7717/peerj.665) [DOI] [Google Scholar]
- 24.Klecka J, Boukal DS. 2014. The effect of habitat structure on prey mortality depends on predator and prey microhabitat use. Oecologia 176, 183–191. ( 10.1007/s00442-014-3007-6) [DOI] [PubMed] [Google Scholar]
- 25.Humphries BD, Ramesh T, Downs CT. 2016. Diet of black-backed jackals (Canis mesomelas) on farmlands in the KwaZulu-Natal Midlands, South Africa. Mammalia 80, 405–412. ( 10.1515/mammalia-2014-0103) [DOI] [Google Scholar]
- 26.Giannatos G, Marinos Y, Maragou P, Catsadorakis G. 2005. The status of the golden jackal (Canis aureus L.) in Greece. Belgian J. Zool. 135, 145–149. ( 10.11609/JoTT.o3954.6927-33) [DOI] [Google Scholar]
- 27.Beinart W. 1998. The night of the jackal: sheep, pastures and predators in the Cape. Past and Present 158, 172–206. ( 10.1093/past/158.1.172) [DOI] [Google Scholar]
- 28.Ćirović D, Penezić A, Krofel M. 2016. Jackals as cleaners: ecosystem services provided by a mesocarnivore in human-dominated landscapes. Biol. Conserv. 199, 51–55. ( 10.1016/j.biocon.2016.04.027) [DOI] [Google Scholar]
- 29.Gutema TM, et al. 2019. Data from: Foraging ecology of African wolves (Canis lupaster) and its implications for the conservation of Ethiopian wolves (Canis simensis) Dryad Digital Repository. ( 10.5061/dryad.p9g41sd) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gutema TM, et al. 2019. Data from: Foraging ecology of African wolves (Canis lupaster) and its implications for the conservation of Ethiopian wolves (Canis simensis) Dryad Digital Repository. ( 10.5061/dryad.p9g41sd) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available at the Dryad Digital Repository: https://doi.org/10.5061/dryad.p9g41sd [29].