Abstract
Background
Growing evidence indicates that several inflammatory biomarkers may predict survival in patients with malignant tumors. The aim of this study was to evaluate the prognostic value of pretreatment biomarkers in patients with primary small‐cell carcinoma of the esophagus (PSCCE).
Methods
A total of 73 PSCCE patients enrolled between January 2009 and December 2017 at the Affiliated Cancer Hospital of Zhengzhou University. The total lymphocyte counts (TLC), neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) prior to anticancer therapy were collected as inflammation biomarkers. The cutoff value was determined by Receiver operating characteristic (ROC). The Kaplan‐Meier method was utilized to analyze overall survival (OS). Cox proportional hazards regression was used to identify univariate and multivariate prognostic factors.
Results
Univariate analysis showed that high NLR group (hazard ratio [HR] = 1.685; 95% CI: 1.001–2.838; P = 0.047) and high PLR group (hazard ratio [HR] = 1.716; 95% CI: 1.039–2.834; P = 0.033) were associated with poor OS, and TLC was not correlated with OS. On multivariate analysis, high PLR (hazard ratio [HR] = 1.751; 95% CI: 1.042–2.945; P = 0.035) was an independent prognostic factor of unfavorable OS.
Conclusions
Pretreatment PLR and NLR are correlated with OS. These biomarkers are easily accessible, cost effective, and can serve as a marker to identify high‐risk patients for further designing personalized treatment and predicting treatment outcomes.
Keywords: Inflammatory biomarker, neutrophil‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio, primary small‐cell carcinoma of the esophagus, prognosis, total lymphocyte counts
Introduction
Esophageal carcinoma is the sixth leading cause of cancer‐related deaths worldwide and the third most common cancer in China.1, 2 The common types of esophageal cancer are squamous cell carcinoma and adenocarcinoma and primary small cell carcinoma of the esophagus (PSCCE) which is a relatively rare histological subtype, accounting for only 0.5–2.8% of all esophageal malignant tumors.3 PSCCE is characterized by high aggression, early dissemination and poor prognosis.4, 5, 6, 7, 8, 9 Although the first case was noticed by McKeown in 1952, the lower incidence of PSCCE made it is difficult to establish a standard treatment.10 Currently, different treatments including surgery, chemotherapy and radiotherapy have been performed alone or in combined strategies, but the outcomes are inconsistent.6, 7 Therefore, it is critical to identify reliable biomarkers for predicting prognosis and distinguishing patients with negative prognoses. Taking into account individual variability, using the prognostic biomarker to select eligible patients and administration of specific treatments is a promising strategy in the era of precision medicine.
Previous studies have shown that systemic inflammatory response plays an important role in tumorigenesis, development, and metastasis.11, 12 In the tumor microenvironment, inflammatory cells involved in angiogenesis, viability, mobility, and invasion.13, 14 Numerous evidence demonstrates that inflammatory biomarkers are correlated with the survivals of distinct types of cancers such as nasopharyngeal carcinoma,15 liver cancer,16 cervical cancer,17 lung cancer,18 and esophageal cancer.16, 19 Patients outcomes can be effectively evaluated with pretreatment hematological biomarkers, including total lymphocyte count (TLC), neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR). In addition, the neutrophil, lymphocyte and platelet counts are easily available from the complete blood cell (CBC) counts in daily clinical practice and the cost of CBC is inexpensive. Nevertheless, there is little evidence of the relationship between these factors and the prognosis of PSCCE.
For the above reasons, we investigated whether the markers (TLC, NLR and PLR) have independent prognostic values in patients with PSCCE.
Methods
Patients
We performed a retrospective analysis on the hematologic and clinicopathological data of PSCCE patients from January 2009 to December 2017. The study was approved by the Ethical Board of the Affiliated Cancer Hospital of Zhengzhou University. Inclusion criteria were: (i) PSCCE proven by histopathology; (ii) blood samples prior to anticancer therapy were available; (iii) complete medical records. Exclusion criteria included: (i) non‐primary esophageal carcinoma; (ii) pathologically confirmed or combined with squamous cell carcinoma, adenocarcinoma and other neuroendocrine carcinoma; (iii) if patients had received any other treatment before blood samples were collected; (iv) incomplete medical records; and (v) any inflammatory infections.
A total of 73 patients were screened in the analysis, and pathological diagnosis was confirmed PSCCE via endoscopic biopsy. Detailed physical and laboratory examination were performed after patients were admitted to the hospital. The tumor stage was classified according to the sixth edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual. Informed consent was obtained from all individuals prior to treatment.
Data collection
Clinical date including patient characteristics, laboratory outcomes, tumor location and stage, treatment, and pathological results were extracted from medical records. Blood samples were collected within 14 days prior to treatment in case the hematological parameters may have been influenced by antitumor treatments such as chemotherapy, radiotherapy, or nutritional support. The neutrophil, lymphocyte, and platelet counts were obtained from the pretreatment CBC. NLR was defined as the total neutrophil count divided by the total lymphocyte count. PLR was defined as the total platelet count divided by the total lymphocyte count. The optimal cutoff values of TLC, NLR, and PLR were calculated based on receiver operating curve. Patients were stratified according to the cutoff points. Other clinical characteristics were divided into different groups, including age (<60 or ≥60 years), gender (male or female), alcohol abuse (yes or no), tobacco abuse (yes or no), locations (upper, middle or lower), length of tumor lesion (≤6 or >6 cm), TNM stage (I, II, III, IV) and treatment modalities (surgery alone vs. chemoradiotherapy vs. surgery combined with chemoradiotherapy).
Statistical analysis
OS was served as the primary endpoint, and was calculated from the date of diagnosis by histopathology to the date of death from any cause, or the time of last follow‐up. The connections between TLC, NLR, PLR and clinicopathological factors were analyzed by Chi‐square test. The Kaplan‐Meier method was used to conduct univariate analysis of survival. The variable with P‐value less than 0.05 in univariate analysis were evaluated by multivariate logistic regression analysis. Cox proportional hazards regression was used to identify univariate and multivariate prognostic factors. All statistical analyses were conducted using SPSS version 22.0 (IBM Software Group, Chicago, USA). Differences were considered statistically significant at P < 0.05.
Results
Patient characteristics
The clinicopathological characteristics of PSCCE patients included in the study are illustrated in Table 1. There were 51 (69.9%) men and 22 (30.1%) women with a median age of 60 years, ranging from 37 to 77 years. The percentage of primary tumors located in the middle, upper and lower thoracic esophagus were 67.1% (n = 49), 2.7% (n = 2) and 30.1% (n = 22), respectively. The mean diameter of the tumor lesion was 5 cm (ranging from 1 to 11 cm). According to the sixth edition of the AJCC Cancer Staging Manual, six patients had stage I PSCCE (8.2%), 32 had stage II PSCCE (43.8%), 12 had stage III PSCCE (16.4%), and the remaining 23 patients had stage IV PSCCE (31.5%). Among the eligible individuals, 10 patients were treated by surgical resection (13.7%); 30 underwent surgery and chemoradiotherapy (41.1%); and 33 received chemoradiotherapy. The outcomes revealed that NLR and PLR were insignificantly associated with clinicopathological variables. In addition, significant correlations were observed between the TLC and alcohol and tobacco abuse, and there was no significant relationship between TLC and other features.
Table 1.
Patient characteristics
| NLR | PLR | TLC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 73) | NLR ≤ 2.37 n = 44, 60.3% | NLR > 2.37 n = 29, 39.7% | P‐value | PLR ≤ 136.5 n = 38, 52.1% | PLR > 136.5 n = 35, 47.9% | P‐value | TLC ≤1.8 *109/L n = 43, 58.9% | TLC ≤1.8 *109/L n = 30, 41.1% | P‐value |
| Age (years) | |||||||||
| <60 | 24 | 15 | 0.813 | 22 | 17 | 0.425 | 22 | 17 | 0.643 |
| ≥60 | 20 | 14 | 16 | 18 | 21 | 13 | |||
| Gender | |||||||||
| Male | 31 | 20 | 0.892 | 29 | 22 | 0.211 | 28 | 23 | 0.29 |
| Female | 13 | 9 | 9 | 13 | 15 | 7 | |||
| Alcohol abuse | |||||||||
| Yes | 10 | 12 | 0.089 | 12 | 10 | 0.78 | 9 | 13 | 0.04 |
| No | 34 | 17 | 26 | 25 | 34 | 17 | |||
| Tobacco abuse | |||||||||
| Yes | 23 | 18 | 0.409 | 23 | 18 | 0.434 | 20 | 21 | 0.047 |
| No | 21 | 11 | 15 | 17 | 23 | 9 | |||
| Location | |||||||||
| Upper | 1 | 1 | 0.458 | 0 | 2 | 0.206 | 2 | 0 | 0.128 |
| Middle | 32 | 17 | 27 | 22 | 31 | 18 | |||
| Lower | 11 | 11 | 11 | 11 | 10 | 12 | |||
| Length (cm) | |||||||||
| ≤6 | 30 | 17 | 0.404 | 28 | 19 | 0.084 | 27 | 20 | 0.734 |
| >6 | 14 | 12 | 10 | 16 | 16 | 10 | |||
| TNM stage | |||||||||
| I | 4 | 2 | 0.233 | 4 | 2 | 0.261 | 4 | 2 | 0.818 |
| II | 21 | 11 | 20 | 12 | 17 | 15 | |||
| III | 4 | 8 | 5 | 7 | 8 | 4 | |||
| IV | 15 | 8 | 9 | 14 | 14 | 9 | |||
| Treatment modalities | |||||||||
| S | 5 | 5 | 0.756 | 6 | 4 | 0.083 | 6 | 4 | 0.557 |
| S + CRT | 19 | 11 | 19 | 11 | 16 | 14 | |||
| CRT | 20 | 13 | 12 | 21 | 22 | 11 | |||
NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; TLC, total lymphocyte count; S, surgery alone; S + CRT, surgery combined with chemoradiotherapy; CRT, chemoradiotherapy.
Survival analyses
The median follow‐up time was 26.5 months, ranging from 1 to 116 months. At the end of follow‐up, 69 patients died (94.5%). The median survival time was 22.0 months. The one‐, three‐, and five‐year OS rates were 83.5%, 24.6%, and 6.8%, respectively. On univariate analysis, seven clinicopathologic features including tumor location, lesion length, TNM stage, treatment, pretreatment NLR and pretreatment PLR were found to be associated with OS (Table 2).
Table 2.
Univariate analysis of prognosis factors of overall survival
| Variable | Hazard ratio | 95% CI | P‐value |
|---|---|---|---|
| Age (year) | 1.105 | 0.682–1.789 | 0.685 |
| Gender | 1.081 | 0.639–1.826 | 0.772 |
| Alcohol abuse | 0.89 | 0.530–1.493 | 0.659 |
| Tobacco abuse | 1.134 | 0.701–1.835 | 0.609 |
| Location | 1.764 | 1.035–3.007 | 0.037 |
| Length (cm) | 1.672 | 1.004–2.785 | 0.048 |
| AJCC | 1.601 | 1.220–2.100 | 0.001 |
| Treatment modalities | 1.723 | 1.194–2.487 | 0.003 |
| NLR | 1.685 | 1.001–2.838 | 0.047 |
| PLR | 1.716 | 1.039–2.834 | 0.033 |
| TLC | 0.798 | 0.488–1.305 | 0.113 |
Relationships between inflammation biomarkers and OS
In the study, we determined cutoff points for TLC, NLR, and PLR to be 1.8, 2.37 and 145, respectively. According to the cutoff points,patients were divided into two separate groups (TLC ≥ 1.8 × 109 as high TLC group,TLC < 1.8 × 109 as low TLC group;NLR ≥ 2.37 as high NLR group, NLR < 2.37 as low NLR group;PLR ≥1 45 as high PLR group, PLR < 145 as low PLR group). Patients in the high NLR group had significantly poorer OS than those in the low NLR group (hazard ratio (HR) = 1.685; 95% CI: 1.001–2.838; P = 0.047, Fig 1). The patients in the high PLR group had significantly worse OS than those in the low PLR group (hazard ratio (HR) = 1.716; 95% CI: 1.039–2.834; P = 0.033, Fig 2). Meanwhile, no statistical difference was observed in patients with different TLC (Fig 3). Furthermore, the multivariate analysis showed that low pretreatment PLR (hazard ratio (HR) = 1.751; 95% CI: 1.042–2.945; P = 0.035) was an independent predictor of superior survival in PSCCE. Treatment strategies (hazard ratio (HR) = 1.563; 95% CI: 1.081–2.262; P = 0.018) and tumor location (hazard ratio (HR) = 1.788; 95% CI: 1.037–3.083; P = 0.036) were significantly correlated with survival. There was no significant relationship between low pretreatment NLR and OS (Table 3).
Figure 1.
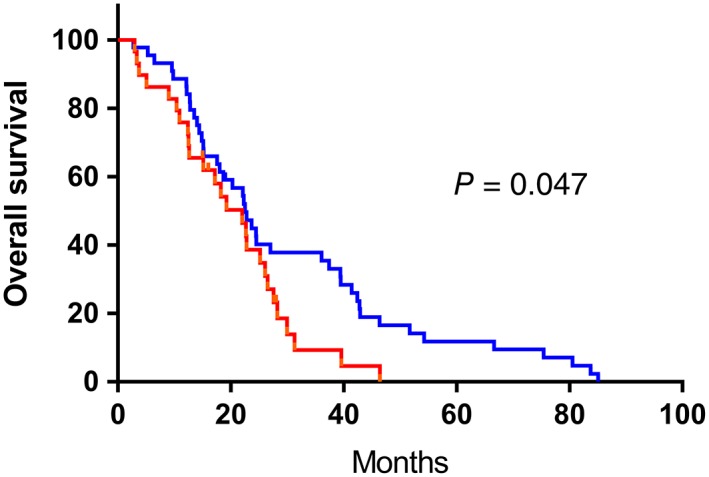
Kaplan‐Meier analysis of NLR for overall survival in patents with PSCCE. ( ) low NLR group, (
) low NLR group, ( ) high NLR group, (
) high NLR group, ( ) low NLR group‐censored, and (
) low NLR group‐censored, and ( ) high NLR group‐censored.
) high NLR group‐censored.
Figure 2.
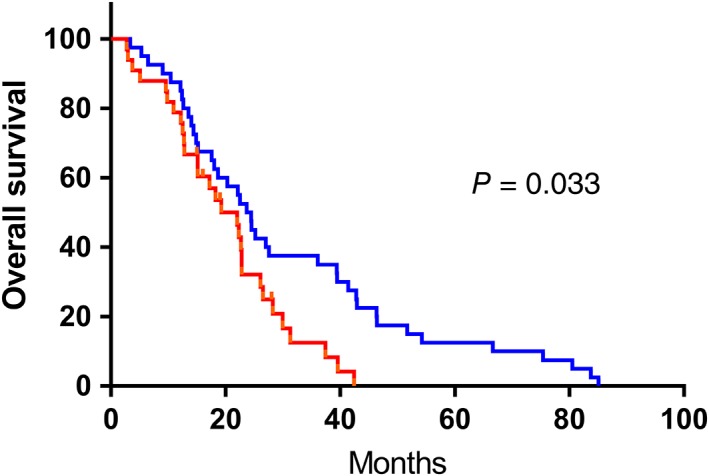
Kaplan‐Meier analysis of PLR for overall survival in patents with PSCCE. ( ) low PLR group, (
) low PLR group, ( ) high PLR group, (
) high PLR group, ( ) low PLR group‐censored, and (
) low PLR group‐censored, and ( ) high PLR group‐censored.
) high PLR group‐censored.
Figure 3.
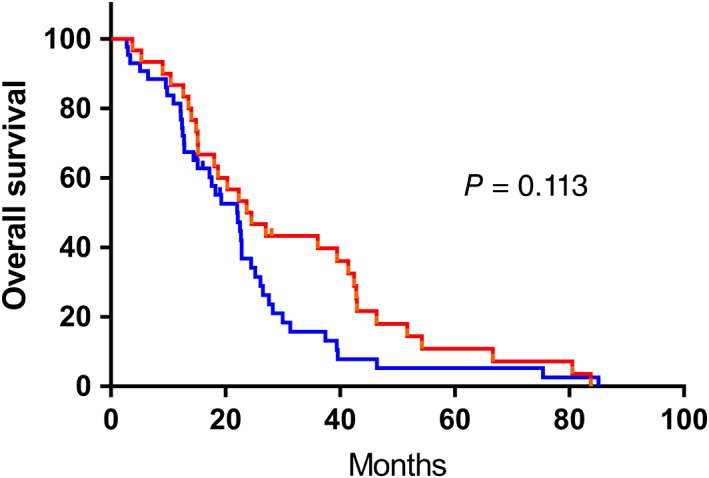
Kaplan‐Meier analysis of TLC for overall survival in patents with PSCCE. ( ) low TLC group, (
) low TLC group, ( ) high TLC group, (
) high TLC group, ( ) low TLC group‐censored, and (
) low TLC group‐censored, and ( ) high TLC group‐censored.
) high TLC group‐censored.
Table 3.
Multivariate analysis for potential prognostic factors of overall survival
| Variable | Hazard ratio | 95% CI | P‐value |
|---|---|---|---|
| Treatment modalities | 1.563 | 1.081–2.261 | 0.018 |
| PLR | 1.751 | 1.042–2.945 | 0.035 |
| Location | 1.788 | 1.037–3.083 | 0.036 |
| Length (cm) | 1.604 | 0.935–2.750 | 0.086 |
Discussion
The present study demonstrated that pretreatment PLR is an independent prognostic factor for OS. Moreover, patients diagnosed as PSCCE with low PLR may have superior OS than those with the high PLR. NLR was also correlated with OS and TLC, NLR, as well as PLR were uncorrelated with other clinicopathologic factors. As far as we know, this is the first study to analysis the pretreatment TLC, NLR and PLR in the prediction of OS in patients with PSCCE.
Systemic inflammation involved in the process of tumorigenesis has been previously reported.20 Chronic inflammation triggers molecular cascades in tumor cells, which promote tumor invasion and immune cell evasion.21 The cancer‐related inflammation recruiting T lymphocytes and activating chemokines, forming an immunosuppressive microenvironment, results in inhibited antitumor immunity which promotes tumor growth and metastasis.20, 22 Theoretically, after inflammatory cytokines have been released, the blood cells including neutrophils, lymphocytes, platelets and so on proliferate and instantly differentiate.23 It is well known that neutrophils produce angiogenic cytokines and induce angiogenesis in tumor cells. Neutrophilia is frequently found in cancer patients and is associated with a poor prognosis.24 In antitumor immune reactions, lymphocytes induce tumor cell apoptosis and suppress tumor cell proliferation and metastasis.25 Platelets also contribute considerably to tumor growth, infiltration and dissemination.26 The activation of platelets can lead to the release of angiogenic growth factors. Also, their adherence to tumor microvessels may enhance vascular permeability.27 Many studies have reported that cancer produces interleukin‐1, and interleukin‐6, granulocyte colony‐stimulating factor, as well as tumor necrosis factor‐alpha, which may cause neutrophilia. Neutrophilia and thrombocytosis always symbolize a nonspecific response to the cancer‐related inflammation.22, 28 Above all, systemic inflammatory biomarkers such as TLC, NLR, and PLR are expected to predict tumor prognosis. Systemic chemotherapy, radiotherapy or postoperative stress response will inevitably influence the CBC. Thus, this study assessed the potential prognostic value of TLC, NLR and PLR in patients with PSCCE who were newly diagnosed.
In previous studies, the utility of inflammation biomarkers as a prognostic factor was investigated in various types of solid tumors. Chen et al. demonstrated high NLR was an independent poor prognostic marker in colorectal cancer.29 Suzuki et al. identified low TLC and high NLR was associated with inferior survival in the extensive‐stage small‐cell lung cancer.30 Luo et al. indicated high PLR was an independent prognostic indicator of short OS in patients of early stage non‐small cell lung cancer who received SABR.31 Ye et al. reported that both high NLR and PLR were correlated with poor survival in patients of nasopharyngeal carcinoma.32 A meta‐analysis by Yodying et al. showed elevated pretreatment NLR and PLR were remarkably associated with unfavorable OS of esophageal cancer.33 In patients undergoing surgery for esophageal squamous cell cancer, PLR was revealed as an independent prognostic factor; moreover, a significantly different survival rate was found between patients in the high NLR group and low NLR group.34 These results were similar to our analysis. Likewise, Feng et al. suggested that PLR should be superior to NLR as a predictive factor in esophageal squamous cell cancer.35 In addition, others reported that NLR was regarded as an independent prognostic factor for patients with PSCCE.36 This is mainly because of different inclusion criteria and a various cutoff value of NLR. In the current study, the patients who underwent surgery preceded by neoadjuvant therapy or only accepted chemoradiotherapy and patients diagnosed with distant metastasis were included in the analysis. Wang and Liu suggested the cutoff value to be 2.97 by the ROC analysis and the area under the curve was 0.702. With the same methods, the cutoff value of this study was calculated as 2.37 and the area under the curve was 0.713. To date, there is no standardized optimal cutoff point of inflammatory biomarkers and further research is therefore required.
The limitations of our retrospective study, include a small sample size and data from a single institution. In addition, other biomarkers of the systemic inflammatory response, for example C‐reactive protein, fibrinogens, albumin were not included in the analysis. Therefore, large, prospective, multi‐center and randomized controlled trials are required to confirm the results of this study.
Conclusion
In conclusion, our study suggested that pretreatment inflammatory biomarkers containing NLR and PLR are related to the survival of patients with PSCCE. The PLR could be deemed as a valuable independent prognostic factor of PSCCE. PLR can be considered as a supplement in distinguishing higher risk group of PSCCE, predicting treatment outcomes and tailoring treatment based on risk stratification. Future multi‐center and large clinical trials should be carried out to determine optimal cutoff values of inflammatory biomarkers, after which the further exploration of the independent prognostic value of these inflammatory biomarkers should be considered.
Disclosure
The authors declare they have no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant No. 81372436, 81773230) and the Technology Open and Cooperation Program of Henan (grant No. 182106000062). All procedures performed in studies involving human participants were in accordance with the ethical standards of the Affiliate Cancer Hospital of Zhengzhou University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Contributor Information
Nan Wang, Email: blancyk@hotmail.com.
Hong Ge, Email: progh@gs.zzu.edu.cn, Email: gehong616@126.com.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (6): 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66 (2): 115–32. [DOI] [PubMed] [Google Scholar]
- 3. Medgyesy CD, Wolff RA, Putnam JB Jr, Ajani JA. Small cell carcinoma of the esophagus: The University of Texas M. D. Anderson cancer Center experience and literature review. Cancer 2000; 88 (2): 262–7. [PubMed] [Google Scholar]
- 4. Ku GY, Minsky BD, Rusch VW, Bains M, Kelsen DP, Ilson DH. Small‐cell carcinoma of the esophagus and gastroesophageal junction: Review of the memorial Sloan‐Kettering experience. Ann Oncol 2008; 19 (3): 533–7. [DOI] [PubMed] [Google Scholar]
- 5. Xu L, Li Y, Liu X et al Treatment strategies and prognostic factors of limited‐stage primary small cell carcinoma of the Esophagus. J Thorac Oncol 2017; 12 (12): 1834–44. [DOI] [PubMed] [Google Scholar]
- 6. Zhu Y, Qiu B, Liu H et al Primary small cell carcinoma of the esophagus: Review of 64 cases from a single institution. Dis Esophagus 2014; 27 (2): 152–8. [DOI] [PubMed] [Google Scholar]
- 7. Zou B, Li T, Zhou Q et al Adjuvant therapeutic modalities in primary small cell carcinoma of Esophagus patients: A retrospective cohort study of Multicenter clinical outcomes. Medicine 2016; 95 (17): e3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu Z, Ma JY, Yang JJ, Zhao YF, Zhang SF. Primary small cell carcinoma of esophagus: Report of 9 cases and review of literature. World J Gastroenterol 2004; 10 (24): 3680–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen WW, Wang F, Zhang DS et al Primary small cell carcinoma of the esophagus: Clinicopathological study of 44 cases. BMC Cancer 2014; 14: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McKeown F. Oat‐cell carcinoma of the oesophagus. J Pathol Bacteriol 1952; 64 (4): 889–91. [DOI] [PubMed] [Google Scholar]
- 11. Thibodeau J, Bourgeois‐Daigneault MC, Lapointe R. Targeting the MHC class II antigen presentation pathway in cancer immunotherapy. Oncoimmunology 2012; 1 (6): 908–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140 (6): 883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rice TW, Rusch VW, Ishwaran H, Blackstone EH. Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: Data‐driven staging for the seventh edition of the American joint committee on cancer/International Union against Cancer cancer staging manuals. Cancer 2010; 116 (16): 3763–73. [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Jia Y, Wang N et al The clinical significance of tumor‐infiltrating neutrophils and neutrophil‐to‐CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med 2014; 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu A, Li H, Zheng Y et al Prognostic significance of neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio, and platelet to lymphocyte ratio in patients with nasopharyngeal carcinoma. Biomed Res Int 2017; 2017: 3047802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aleksandrova K, Boeing H, Nothlings U et al Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology 2014; 60 (3): 858–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu ES, Oduyebo T, Cobb LP et al Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol 2016; 140 (1): 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang C, Liao Z, Gomez D et al Lymphopenia association with gross tumor volume and lung V5 and its effects on non‐small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys 2014; 89 (5): 1084–91. [DOI] [PubMed] [Google Scholar]
- 19. Grossman SA, Ellsworth S, Campian J et al Survival in patients with severe Lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw 2015; 13 (10): 1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature 2008; 454 (7203): 436–44. [DOI] [PubMed] [Google Scholar]
- 21. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011; 144 (5): 646–74. [DOI] [PubMed] [Google Scholar]
- 22. Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet 2001; 357 (9255): 539–45. [DOI] [PubMed] [Google Scholar]
- 23. Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer‐related inflammation. Cytokine 2008; 43 (3): 374–9. [DOI] [PubMed] [Google Scholar]
- 24. Fuchs TA, Abed U, Goosmann C et al Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007; 176 (2): 231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fridman WH, Pages F, Sautes‐Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer 2012; 12 (4): 298–306. [DOI] [PubMed] [Google Scholar]
- 26. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost 2011; 9 (2): 237–49. [DOI] [PubMed] [Google Scholar]
- 27. Kono SA, Heasley LE, Doebele RC, Camidge DR. Adding to the mix: Fibroblast growth factor and platelet‐derived growth factor receptor pathways as targets in non‐small cell lung cancer. Curr Cancer Drug Targets 2012; 12 (2): 107–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 2003; 6 (4): 283–7. [DOI] [PubMed] [Google Scholar]
- 29. Chen ZY, Raghav K, Lieu CH et al Cytokine profile and prognostic significance of high neutrophil‐lymphocyte ratio in colorectal cancer. Br J Cancer 2015; 112 (6): 1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suzuki R, Lin SH, Wei X et al Prognostic significance of pretreatment total lymphocyte count and neutrophil‐to‐lymphocyte ratio in extensive‐stage small‐cell lung cancer. Radiother Oncol 2018; 126 (3): 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luo H, Ge H, Cui Y et al Systemic inflammation biomarkers predict survival in patients of early stage non‐small cell lung cancer treated with stereotactic ablative radiotherapy ‐ a single center experience. J Cancer 2018; 9 (1): 182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ye L, Oei RW, Kong F et al Prognostic values of hematological biomarkers in nasopharyngeal carcinoma patients treated with intensity‐modulated radiotherapy. Eur Arch Otorhinolaryngol 2018; 275 (5): 1309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yodying H, Matsuda A, Miyashita M et al Prognostic significance of neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio in oncologic outcomes of Esophageal cancer: A systematic review and meta‐analysis. Ann Surg Oncol 2016; 23 (2): 646–54. [DOI] [PubMed] [Google Scholar]
- 34. Xie X, Luo KJ, Hu Y, Wang JY, Chen J. Prognostic value of preoperative platelet‐lymphocyte and neutrophil‐lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer. Dis Esophagus 2016; 29 (1): 79–85. [DOI] [PubMed] [Google Scholar]
- 35. Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol 2014; 12: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y, Liu JF. A retrospective study on the prognostic value of preoperative neutrophil/lymphocyte ratio in patients with primary small‐cell carcinoma of the esophagus. Onco Targets Ther 2017; 10: 2453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


