Abstract
Objectives
At present, the modified inflation‐deflation method is accepted and widely used in the clinic, but the near‐infrared (NIR) fluorescence imaging with intravenous indocyanine green (ICG) method can also delineate the intersegmental demarcation. However, whether the two methods identify that the intersegmental plane is concordant with each other and match the real intersegmental demarcation is still unknown.
Methods
Between March 2019 to July 2019, 19 consecutive segmentectomies were performed, using both methods to delineate the intersegmental plane, in order to evaluate and verify whether the intersegmental plane results created by the two methods were concordant and matched the real intersegmental demarcation.
Results
Segmentectomies were carried out using uniportal video‐assisted thoracic surgery (UVATS) successfully with no intraoperative conversions or ICG‐related complications and only three cases (15.8%) with postoperative complications related to the operation. The intersegmental plane generated by the NIR fluorescence imaging with intravenous ICG method was found to be totally concordant with the modified inflation‐deflation method that was approaching the real intersegmental demarcation in all 19 cases.
Conclusions
Both methods revealed the intersegmental plane clearly, and the NIR fluorescence imaging with intravenous ICG method was found to be totally concordant with the modified inflation‐deflation method, which is highly concordant with the real intersegmental demarcation. NIR fluorescence imaging with intravenous ICG method may be more popular because of its safety, efficiency, and less complicated restrictions, especially in patients with pulmonary emphysema. Low doses of ICG do not affect the rate of identification of the intersegmental plane and is safer from drug toxicology.
Keywords: Intersegmental plane, segmentectomy, surgery
Introduction
With the development of current imaging technology, more and more early stage lung cancer is being detected. Therefore, precise resection of small pulmonary nodules has become an opportunity and challenge for contemporary thoracic surgeons.1 Segmentectomy is increasingly becoming a popular technique for the treatment of early stage lung cancer as it combines equivalent oncologic requirements and greater preservation of lung function. Multiple studies have shown that there is no difference between segmentectomy compared with lobectomy in the postoperative tumor recurrence rate and five‐year survival rate in the treatment of early stage lung cancer, with considerable oncological outcomes.2, 3, 4 Segmentectomy is an anatomical resection compared with wedge resection which maintains an adequate surgical margin, preserves the morphology of residual lungs and performs intrapulmonary lymph node sampling, which reduces local recurrence and metastasis of malignant tumors, and improves the accuracy rate of tumor staging.5, 6, 7
Although the feasibility of thoracoscopic segmentectomy has been widely demonstrated, there is still controversy regarding technical issues, such as the identification of the intersegmental plane. Although many studies have reported several methods for identifying the intersegmental demarcation, their essence is two types aiming to generate a demarcation: inflation‐deflation line and dyeing the plane.8, 9, 10, 11, 12, 13, 14, 15, 16 The former representative method is modified inflation‐deflation, and this method is precise, simple, easy to operate, and does not require additional auxiliary materials, widely used as a standard technology in clinical practice. However, it is not easy to visualize the demonstration of the intersegmental plane in pulmonary emphysema, and the inflated lung may obstruct the view of the target segment.10 The latter representative method is near‐infrared (NIR) fluorescence imaging with intravenous indocyanine green (ICG), which has been recently developed and several studies have reported the utility for the demonstration of the intersegmental plane.11, 12, 13 However, the concept of NIR fluorescence imaging with intravenous indocyanine green method for the demonstration of the intersegmental plane raises several controversial issues. The first is the issue of whether the two methods identify that the intersegmental plane is concordant with each other and match the real intersegmental demarcation. The second consideration relates to the cost of the imaging system modalities. Finally, there are safe challenges of ICG which may reflect on the applicable use of minimally invasive strategy. To the best of our knowledge, this report is the first to evaluate and verify whether the intersegmental plane results created by the two methods are concordant and attempt to resolve the uncertainty regarding the accuracy and effectiveness of the near‐infrared (NIR) fluorescence imaging with the intravenous indocyanine green (ICG) method.
Methods
This study was approved by Nanjing Chest Hospital Review Board and the Ethics Committee, and informed consent was obtained for all patients. We retrospectively reviewed the consecutive cases of patients who received uniportal video‐assisted segmentectomy at Nanjing Chest Hospital Affiliated to Southeast University between March 2019 to July 2019. Nineteen consecutive segmentectomies were enrolled in the retrospective study which combined both the modified inflation‐deflation and NIR fluorescence imaging with intravenous ICG method to visualize the distinct intersegmental plane by a team at Nanjing Chest Hospital. Indocyanine green (Eisai Liaoning Pharmaceutical, Liaoning, China; drug approval number H20045514, China Food and Drug Administration) is commonly used in clinical settings.
The basic indications for segmentectomy were as follows: (i) benign nodules or undiagnosed nodules in the deep lung parenchyma; (ii) history of previous pulmonary surgery; (iii) it was impossible to diagnose whether the nodule was primary lung cancer or metastasis during surgery; (iv) multiple pulmonary nodules possibly requiring further surgical intervention in the future; and (v) according to NCCN guideline for NSCLC (Version 1.2016),17 as follows: (i) marginal cardiopulmonary reserve or multiple comorbidities meaning that the option of lobectomy was limited and (ii) CT scan indicating the presence of peripheral nodules, tumor diameter ≤2 cm, and with at least one of the following conditions: pathology confirmed as adenocarcinoma in situ; the ground‐glass component was ≥50%; the imaging examination indicated that the tumor doubling time was ≥400 days. The exclusion criteria for segmentectomy in this retrospective study included patients with a history of iodine or indocyanine green allergy and those with pulmonary emphysema.
3D‐CTBA reconstruction and surgical simulation
A preoperative evaluation by computed tomography angiography (CTA) scanning for patients who were suitable for segmentectomy was usually carried out. The CTA images were then reconstructed three‐dimensionally (3D) to identify the pulmonary bronchi and blood vessels of the target segment with reconstruction software IQQA system (intelligent/interactive qualitative and quantitative analysis: EDDA Technology, Princeton Junction, NJ, USA). Surgical simulation of segmentectomy was then performed. In patients with suspected lung cancer, the safe surgical margin should be greater than 2 cm or at least greater than the maximum diameter of the tumor. Centering on the nodules, bi‐ or trisegmentectomy was necessarily performed when they were close to intersegmental veins, and then the target segment to be identified with enough parenchymal margin. In order to minimize the anatomic resection of lung parenchyma and meet the requirements of oncology, all the structures belonging to the targeted segment were identified using 3D images, including the involved segmental bronchi, arterial branches, and intrasegmental veins, that need to be divided during operation. In addition, the intersegmental veins would be preserved when the targeted segmentectomy was performed.
Operative procedure
All patients underwent general anesthesia with double‐lumen endotracheal intubation and were placed in a lateral decubitus position. A uniportal thoracoscopic anatomical segmentectomy was performed with a 2.5–3.0 cm skin incision between the fourth or fifth intercostal space in the anterior axillary using single lung ventilation.
Under the guidance of the realtime 3D navigation and simulated navigation technique, we accurately identified and dissected targeted segmental arteries, bronchi, and intrasegmental veins. A modified inflation‐deflation method was used to identify the intersegmental plane.10 After the targeted segment structures were dissected, the collapsed lung was initiated to fully re‐expand with controlled airway pressure under 20 cm H2O, followed by single lung ventilation. After an interval of approximately 10 to 15 minutes, an irregularly curved demarcation was identified naturally between the deflated preserving segments and the inflated target segment, which was no longer changing over time. Simultaneously, ICG at 2 mL (25 mg dissolved in 10 mL saline, equivalent to 5 mg/bodyweight), was rapidly injected into the peripheral vein, and the demarcation was also visualized using NIR fluorescence thoracoscopy, approximately 10–15 seconds later, the surrounding segments were stained, but not the targeted segment so that a distinct boundary between the two areas was clearly identified.13 In the study, we used the following NIR thoracoscopy system: PINPOINT Endoscopic Fluorescence Imaging System (Novadaq, Ontario, Canada), and this was used throughout the entire surgical procedure from start to finish. Alternated use of fluorescence mode repeatedly, based on the NIR light, to evaluate and verify whether the intersegmental plane results created by the two methods were concordant and matched the real intersegmental demarcation. Finally, the intersegmental plane was tailored along the intersegmental pulmonary veins up to the outer third of the lung parenchyma using an electrocautery or ultrasonic scalpel, and the distal intersegmental parenchyma was dissected with stapling devices to reduce air leakage.
The resected segment was taken out in a specimen bag, and frozen section was then performed during the operation. According to the frozen section diagnosis, in malignant cases, N1 and N2 lymph nodes were sampled. If there was evidence of lymph node involvement, a lobectomy and systemic lymph node dissection was performed. In addition, fibrin glue or continuous sutures was applied on the surface of the intersegmental plane as appropriate and a chest tube placed through the single port. The pathologic diagnosis staging was made based on the eighth edition Union for International Cancer Control (UICC) Tumor Node Metastasis (TNM) Classification.
Results
Clinical characteristics of the patients
The baseline characteristics of the 19 patients (six men and 13 women, mean age 50.58 ± 13.71, range: 28–72 years) in this study are described in Table 1. Targeted segments included 11 simple and eight complex types. The mean nodule size was 9.58 ± 2.28 mm. Pathological diagnoses exhibited invasive adenocarcinoma (five cases), minimally invasive adenocarcinoma (eight cases), adenocarcinoma in situ (three cases), atypical adenomatous hyperplasia (one case), and benign (two cases). Of the 16 cases of lung cancer, pathological staging included Tis (three cases), T1mi (eight cases), IA1 (T1aN0M0, two cases), and IA2 (T1bN0M0, three cases).
Table 1.
Patient characteristics and target segments†
| Patient | Age (years) | Sex | Emphysema | FEV1 (L) | Targeted segment | Tumor size (mm) | Pathologic diagnosis | TNM stage |
|---|---|---|---|---|---|---|---|---|
| 1 | 48 | F | None | 2.24 | LS1 + 2 + 3 | 8 | MIA | T1mi |
| 2 | 52 | M | None | 2.57 | RS2 | 10 | MIA | T1mi |
| 3 | 62 | F | None | 1.98 | LS1 + 2 | 9 | MIA | T1mi |
| 4 | 65 | F | None | 2.12 | LS6 | 7 | AIS | 0 (Tis) |
| 5 | 36 | F | None | 2.44 | RS1 | 8 | AIS | 0 (Tis) |
| 6 | 59 | M | None | 2.96 | LS1 + 2 + 3 | 15 | IAC | IA2 (T 1bN0M0) |
| 7 | 53 | F | None | 2.14 | LS1 + 2 | 11 | MIA | T1mi |
| 8 | 64 | F | None | 1.75 | LS6 | 14 | IAC | IA2 (T 1bN0M0) |
| 9 | 59 | F | None | 1.87 | RS3 | 6 | AAH | ‐ |
| 10 | 33 | M | None | 4.12 | LS4 + 5 | 9 | IAC | IA1 (T 1aN0M0) |
| 11 | 51 | M | None | 2.78 | RS2 | 9 | IAC | IA1 (T 1aN0M0) |
| 12 | 38 | F | None | 2.45 | LS1 + 2 + 3 | 11 | Benign | ‐ |
| 13 | 28 | F | None | 3.12 | LS1 + 2 + 3 | 8 | MIA | T1mi |
| 14 | 58 | F | None | 2.02 | RS6 | 9 | MIA | T1mi |
| 15 | 72 | M | None | 1.64 | RS3 | 12 | IAC | IA2 (T 1bN0M0) |
| 16 | 43 | F | None | 2.41 | LR1 | 8 | MIA | T1mi |
| 17 | 37 | F | None | 2.86 | LS6 | 7 | AIS | 0 (Tis) |
| 18 | 71 | F | None | 1.43 | LS1 + 2 | 13 | MIA | T1mi |
| 19 | 32 | M | None | 3.42 | RS2 | 8 | Benign | ‐ |
| Mean | 50.58 ± 13.71 | 2.44 ± 0.66 | 9.58 ± 2.28 |
Five males, 14 females. Right: S1, apical segment; S2, posterior segment; S3, anterior segment; S6, superior segment; Left: S1 + 2, apico‐posterior segment; S3, anterior segment; S4, superior lingular segment; S5, inferior lingular segment; S6, superior segment; AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; FEV1, forced expiratory volume in one second; IAC, invasive adenocarcinoma; MIA, minimally invasive adenocarcinoma; TNM, tumor node metastasis; TNM, tumor node metastasis.
Surgical outcomes
As shown in Table 2, all segmentectomies were carried out using UAVTS successfully with no intraoperative conversions or ICG‐related complications. The surgical outcomes were as follows: the surgical margin width of each targeted segment met the margin requirements (≥2 cm or ≥ the maximum diameter of the tumor); operating time, median of 101 minutes (range: 60–150 minutes); blood loss, median of 43 mL (range: 10–120 mL); duration of chest tube drainage, median of three days (range, 1–9 days); duration of hospital stay after surgery, median of five days (range: 2–10 days); the number of dissected lymph nodes, median of six (range: 0–22); and postoperative complications related to operation occurred in three cases (15.8%): prolonged air leak (>7 days) in two cases, pulmonary infection in one case, and all patients recovered after conservative management.
Table 2.
Details of the segmentectomy
| Patient | Operative time (minutes) | Blood loss (mL) | Duration of chest tube drainage (days) | Duration of hospital stay after surgery (days) | Number of lymph nodes dissected (n) | Dose of ICG (mg) | ICG‐related complications | Complications related to operation | Consistency of the two methods |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 120 | 115 | 4 | 5 | 6 | 5 | None | None | Yes |
| 2 | 85 | 80 | 6 | 7 | 5 | 5 | None | None | Yes |
| 3 | 125 | 100 | 3 | 4 | 6 | 5 | None | None | Yes |
| 4 | 85 | 20 | 4 | 5 | 0 | 5 | None | Pulmonary infection | Yes |
| 5 | 65 | 50 | 2 | 3 | 2 | 5 | None | None | Yes |
| 6 | 100 | 60 | 3 | 4 | 18 | 5 | None | None | Yes |
| 7 | 105 | 10 | 1 | 3 | 4 | 5 | None | None | Yes |
| 8 | 120 | 40 | 3 | 4 | 14 | 5 | None | None | Yes |
| 9 | 110 | 10 | 4 | 5 | 0 | 5 | None | None | Yes |
| 10 | 140 | 30 | 2 | 3 | 22 | 5 | None | None | Yes |
| 11 | 150 | 120 | 3 | 4 | 17 | 5 | None | None | Yes |
| 12 | 80 | 10 | 2 | 4 | 0 | 5 | None | None | Yes |
| 13 | 70 | 20 | 2 | 4 | 3 | 5 | None | None | Yes |
| 14 | 105 | 50 | 3 | 5 | 5 | 5 | None | None | Yes |
| 15 | 130 | 60 | 9 | 10 | 11 | 5 | None | Prolonged air leak | Yes |
| 16 | 60 | 10 | 1 | 2 | 6 | 5 | None | None | Yes |
| 17 | 110 | 20 | 4 | 6 | 0 | 5 | None | None | Yes |
| 18 | 90 | 10 | 8 | 9 | 3 | 5 | None | Prolonged air leak | Yes |
| 19 | 75 | 10 | 3 | 4 | 0 | 5 | None | None | Yes |
| Mean | 101.32 ± 25.76 | 43.42 ± 37.05 | 3.53 ± 2.12 | 4.79 ± 2.02 | 6.42 ± 6.78 |
ICG, indocyanine green; Consistency: the intersegmental plane results created by the near‐infrared fluorescence imaging with intravenous indocyanine green method and the modified inflation‐deflation method.
Consistency of both methods
Both methods clearly revealed the intersegmental plane and the intersegmental plane generated by the NIR fluorescence imaging with intravenous ICG method was found to be totally concordant with the modified inflation‐deflation method in all 19 cases. The operative findings of each method are shown in Figs 1 and 2. Using the fluorescence mode alternately, we could see that the intersegmental plane produced by the two methods were completely concordant, even detail such as the irregular convex surface was completely matched (Fig 3), approaching to the real intersegmental demarcation.
Figure 1.
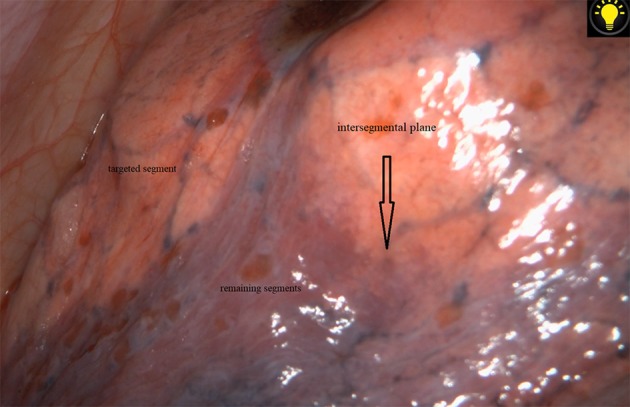
The intersegmental plane was clearly identified via the modified inflation‐deflation method.
Figure 2.
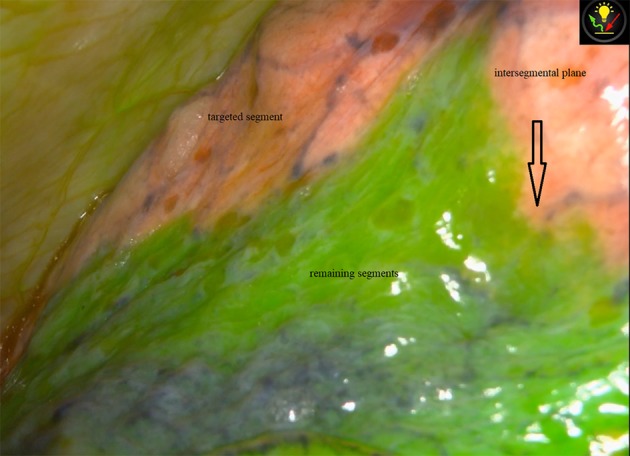
The distinct intersegmental plane between the targeted and remaining segments via NIR fluorescence imaging with ICG.
Figure 3.
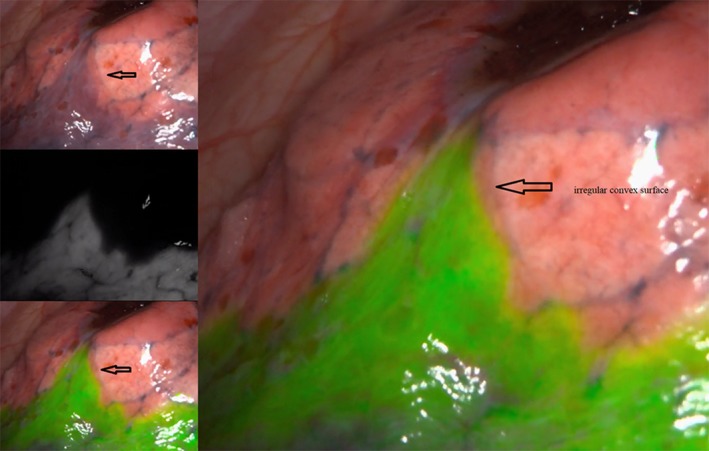
The intersegmental plane produced by the two methods were completely concordant, even in some details, such as the irregular convex surface was completely matched, approaching to the real intersegmental demarcation.
Discussion
Thoracoscopic segmentectomy may be used as a mini‐invasive procedure that represents significant advancement in thoracic surgery technology, and is increasingly being utilized as a more prominent option for the treatment of early‐stage lung cancer,1, 18 owing to the small surgical incision and resection range. However, the most commonly performed segmentectomies are lingular, left upper lobectomy, superior and basilar segmentectomy, that are simple segments. Most other types of segmentectomies are technically sophisticated, due to the complicated anatomical variations of segmental structures. Therefore, the technical difficulties of thoracoscopic segmentectomy include the ability to accurately identify and dissect targeted segmental arteries, bronchi, and intrasegmental veins, preserve the intersegmental veins, identify and tailor the intersegmental plane, and in patients with lung cancer, to ensure there is a sufficient surgical margin.
As the prerequisite for identifying a good intersegmental plane between the targeted and preserving segments, accurate identification and dissection of the targeted segmental arteries, bronchi and intrasegmental veins, and preservation of the intersegmental veins should be guaranteed by combining the guidance of 3D navigation. In our center, a preoperative evaluation by computed tomography angiography scanning for patients who were suitable for segmentectomy was made, using the reconstruction software IQQA system, then surgical simulation of segmentectomy was performed. In addition, the conditions of the lung during intraoperative discernment and intraoperatively were slightly different, thus experience was required for accurate identification of the targeted segmental structures.
Nevertheless, there is no obvious anatomic demarcation between the targeted and preserving segments and identifying the intersegmental planes remains controversial with regard to the technical aspects of precise segmentectomy, in addition to the two methods previously mentioned in this study, some surgeons have explored many other methods, such as, Okada et al.8 who used a fiber bronchoscope to insert the targeted segment of tracheal continuous high‐frequency ventilation (40 Hz, 2 kg/cm2), so that the target segment of the lung segment was inflated to show the intersegmental plane. Although the method was rapid with little visual field effect, the requirements for the anesthesiologist were highly professional, and the positioning of the segmental bronchus was quite difficult when the patient was in the lateral decubitus position during the operation; Oh et al.14 reported that after isolating targeted segment bronchus, ICG (25 mg dissolved in 50 mL saline) was injected at the distal bronchus to determine the intersegmental plane. Although this method was simple, the injection pressure was not well controlled and if the ICG was injected too quickly, diffusion through the Kohn pores resulted, unsuccessfully obtaining the demarcation. Zhang et al.15 injected methylene blue 0.1% (20 mL) into the bronchus of the targeted segments using an intravenous needle; however, the demarcation result was closely related to the extremely precise dose of methylene blue. Oizumi et al.16 reported a modified Roeder knot technique for bronchial ligation, generating the intersegmental plane that was a similar technique to the modified inflation‐deflation; however, the latter optimized the surgical procedure by dissecting the bronchi, which saved the step of making additional knots and tying them to dissect the targeted segmental bronchi after the whole lung was re‐expanded.
Currently, the commonly used technology to identify the intersegmental plane is a modified inflation‐deflation method, which is considered as a prominent method superior to other methods without additional auxiliary materials. The specific procedure of the method is that under the condition of single‐lung ventilation in the contralateral side, after accurately dissecting the targeted segmental bronchi, arteries and intrasegmental veins, under the guidance of 3D navigation, the anesthesiologist confirms the whole lung has been re‐expanded, and it is suggested that the airway pressure should not exceed 20 cm H2O. When the targeted segment is inflated, restoring the opposite side lung of the single‐lung ventilation, so that the remaining segments of the lung is completely dark red, the targeted segment remains inflated and then a clear demarcation is formed between the targeted segment and the adjacent retained lung segments, and this does not change over time. The theory of this method is still unclear, and one of the most widely accepted explanations is that without the exchange of gas and blood in the targeted segments, while blocking the targeted segment bronchus, gaseous exchange between the targeted segment and the atmosphere cannot take place. At the same time, the Kohn pores nonopening led to the continuous expansion of the targeted segment.19, 20
However, this method still has some limitations: (i) The inflated lung parenchyma may obstruct the visualization of the targeted segment particularly in the context of VATS; (ii) it is difficult to form a distinct intersegmental plane in emphysematous lung patients or without the involved intrasegmental veins accurate dissection; (iii) sufficient waiting time for a clear intersegmental plane is varied from case to case, for example, if a distinct plane at the first inflation‐deflation process does not develop as the preoperative 3D navigation misleading actual anatomy, so the targeted segment structures need re‐identify and remove accurately, thus prolonging the duration of the operation; (iv) this method requires additionally engagement of the anesthesiologist. In some cases, the anesthesiologist with less experience cannot control the pressure to re‐expand the whole lungs including the bronchus‐dissected segment, that maybe lead to pressure trauma and interfere with the accuracy of the intersegment plane; and (v) the inflated targeted segment is not conducive to removal from the small incision, easily broken, leading difficulty to the touch the lesions, and the expansion of the incision may increase the postoperative pain.
The technique for the formation of the intersegmental plane based on NIR fluorescence imaging with intravenous administration of ICG has recently advanced considerably due to its simplicity, rapidity, and less complicated restrictions, and its application has been reported in several studies.11, 12, 13, 21, 22, 23 The mechanism is based on the fact that the intravenous ICG can rapidly combine with plasma, and in plasma the emission spectrum of ICG peaked at 780‐805nm fluorescence. The infrared thoracoscope irradiates and detectes 700‐900 nm fluorescence, reflecting on the monitor, providing an image that is visible to the human eye. There is no ICG in the blood flowing to the targeted segment, when the targeted segment arteries are cutting off, however, the remaining lung parenchyma receives cardiac output undergoing the ICG blood flow; thus, the ICG defect area remaines unchanged and the ICG distributed area appeares as a blue colour on the monitor, thereby exhibiting a distinct intersegmental plane.24 Although the NIR fluorescence imaging with intravenous ICG method may overcome some disadvantages of the previous methods, including the modified inflation‐deflation, there are still a number of difficulties as to the issue of whether the two methods identify that the intersegmental plane are concordant with each other and match the real intersegmental demarcation, the cost of the imaging system modalities and the safety of ICG.
In previous studies, many colleagues have confirmed the safety of ICG which are widely used in thoracic surgery, such as sentinel lymph node localization,25 lung lesions localization,26 thoracic duct imaging,27 as well as intersegmental plane identification. Notwithstanding the evidence, the incidence of adverse events due to ICG has been reported to be 0.05%.28 Furthermore, anaphylaxis to ICG may be mediated by a dose‐dependent pseudo‐allergic mechanism,29 and the dose of ICG used for segmentectomy ranged from 0.25 to 5 mg/kg, with even a lower dose of 5 mg/bodyweight, and no complications attributed to ICG.11, 12, 13, 21, 22, 23 The dose of ICG routinely used in our central segmentectomy was 2 mL (25 mg dissolved in 10 mL saline, equivalent to 5 mg/body), which had accumulated to more than 280 cases, and no ICG‐related complications had been found. In addition, low‐dose ICG might achieve a clear intersegmental plane while avoiding anaphylactic shock and pharmacological toxicity, so ICG may be generally considered safe.
There is no doubt about the accuracy of the intersegmental plane generated by the modified inflation‐deflation method. In this study, all 19 cases were concordant without suspense, the intersegmental plane generated by the NIR fluorescence imaging with intravenous ICG method was found to be totally concordant, and even the convex details were absolutely matched with the real intersegmental plane. To the best of our knowledge, we have for the first time proved this appearance. The two methods compared the pleural surface boundaries, not the lung parenchyma, by visual evaluation of surgical imaging data. All intersegmental planes of 19 patients in this study were evaluated by comparing the details on the intraoperative photographs with the naked eye which were consistently successfully visualized. Despite the lack of quantitative analysis of ICG imaging results, our preliminary results assessed by the naked eye were reliable as we repeatedly alternated use of fluorescence mode based on the NIR light to evaluate the intersegmental plane results created by the two methods in surgery, which is objective and repeatable. This method of NIR fluorescence imaging with intravenous ICG can identify the intersegmental plane without lung expansion, thus minimizing the effects of surgical visions obstruction and emphysema, and avoid the waiting time required for the inflated lung to deflate allowing the intersegmental plane to reveal, thereby reducing the duration of the operation, which could be time‐saving and more in line with the enhanced recovery after surgery. Another important feature of this method is that it can avoid procedure‐related complications such as pressure trauma by ignoring the overexpansion of the target segment. An additional consideration is that the PINPOINT endoscopic fluorescence imaging system used in this study is relatively expensive, costing in the region of $290 000 in US currency. However, it provided not only fluorescence imaging with a sufficient duration of fluorescence to perform labeling between the intersegmental plane, but also normal light imaging, which has almost the same specifications as a conventional thoracoscopic system. Therefore, the application of VATS in conventional thoracic surgery can be also performed by the endoscopic fluorescence imaging system, thus saving the extra cost of repurchasing other imaging system modalities.
The limitations of this study include a small number sample size, and a lack of statistical data analysis with NIR fluorescence imaging with intravenous ICG method, such as the specific duration of fluorescence, as well as the improved imaging time window. Besides, the consistency of the two methods was evaluated by comparing the details on the image with the naked eye, which was lacking an exhaustive and quantifiable standard. In addition, as a result of the currently limited evidence, large‐scale randomized controlled studies are required to confirm NIR fluorescence imaging with intravenous ICG and the concrete physiological mechanisms of these two coincidences remain to be further studied.
In conclusion, both methods clearly revealed the intersegmental plane and the intersegmental plane generated by the NIR fluorescence imaging with intravenous ICG method was found to be totally concordant with the modified inflation‐deflation method, that is highly concordant with the real intersegmental demarcation. NIR fluorescence imaging with intravenous ICG method may be more popular with clinicians because of its safety, efficiency, and less complicated restrictions, especially in patient with pulmonary emphysema. Low doses of ICG do not affect the rate of identification of intersegmental plane and are safer from drug toxicology. Further, it will be a promising imaging system modality with an acceptable cost.
Disclosure
The authors declare that they have no conflicts of interest.
Acknowledgments
We thank Dr Wei Wang and Dr Fei Zhao for their constructive suggestions and comments.
Contributor Information
Yungang Sun, Email: suryig@163.com.
Feng Shao, Email: doctorshao1982@sina.com.
Rusong Yang, Email: njyrs_md@188.com.
References
- 1. Nakazawa S, Shimizu K, Mogi A, Kuwano H. VATS segmentectomy: Past, present, and future. Gen Thorac Cardiovasc Surg 2018; 66: 81–90. [DOI] [PubMed] [Google Scholar]
- 2. Landreneau RJ, Normolle DP, Christie NA et al Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non‐small‐cell lung cancer: A propensity‐matched analysis. J Clin Oncol Off J Am Soc Clin Oncol 2014; 32: 2449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamel MK, Rahouma M, Lee B et al Segmentectomy is equivalent to lobectomy in hypermetabolic clinical stage IALung adenocarcinomas. Ann Thorac Surg 2019; 107: 217–23. [DOI] [PubMed] [Google Scholar]
- 4. Nomori H, Shiraishi A, Cong Y, Sugimura H, Mishima S. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg 2018; 53: 640–7. [DOI] [PubMed] [Google Scholar]
- 5. Tsutani Y, Miyata Y, Nakayama H et al Appropriate sublobar resection choice for ground glass opacity‐dominant clinical stage IA lung adenocarcinoma: Wedge resection or segmentectomy. Chest 2014; 145: 66–71. [DOI] [PubMed] [Google Scholar]
- 6. Dziedzic R, Rzyman W. Lobectomy versus segmentectomy and wedge resection in the treatment of stage I non‐small cell lung cancer. J Thorac Dis 2018; 10: E234–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hou B, Deng XF, Zhou D, Liu QX, Dai JG. Segmentectomy versus wedge resection for the treatment of high‐risk operable patients with stage I non‐small cell lung cancer: A meta‐analysis. Ther Adv Respir Dis 2016; 10: 435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okada M, Mimura T, Ikegaki J, Katoh H, Itoh H, Tsubota N. A novel video‐assisted anatomic segmentectomy technique: Selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007; 133: 753–8. [DOI] [PubMed] [Google Scholar]
- 9. Sekine Y, Ko E, Oishi H, Miwa M. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012; 143: 1330–5. [DOI] [PubMed] [Google Scholar]
- 10. Wang J, Xu X, Wen W, Wu W, Zhu Q, Chen L. Modified method for distinguishing the intersegmental border for lung segmentectomy. Thorac Cancer 2018; 9: 330–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iizuka S, Kuroda H, Yoshimura K et al Predictors of indocyanine green visualization during fluorescence imaging for segmental plane formation in thoracoscopic anatomical segmentectomy. J Thorac Dis 2016; 8: 985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mun M, Okumura S, Nakao M, Matsuura Y, Nakagawa K. Indocyanine green fuorescence‐navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017; 3: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Motono N, Iwai S, Funasaki A, Sekimura A, Usuda K, Uramoto H. Low‐dose indocyanine green fluorescence‐navigated segmentectomy: Prospective analysis of 20 cases and review of previous reports. J Thorac Dis 2019; 11: 702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oh S, Suzuki K, Miyasaka Y, Matsunaga T, Tsushima Y, Takamochi K. New technique for lung segmentectomy using indocyanine green injection. Ann Thorac Surg 2013; 95: 2188–90. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Z, Liao Y, Ai B, Liu C. Methylene blue staining: A new technique for identifying intersegmental planes in anatomic segmentectomy. Ann Thorac Surg 2015; 99: 238–42. [DOI] [PubMed] [Google Scholar]
- 16. Oizumi H, Kato H, Endoh M, Inoue T, Watarai H, Sadahiro M. Slip knot bronchial ligation method for thoracoscopic lung segmentectomy. Ann Thorac Surg 2014; 97: 1456–8. [DOI] [PubMed] [Google Scholar]
- 17. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) . Non‐Small Cell Lung Cancer. Version 2. 2016. Available from URL: http://www.nccn.org/patients.
- 18. Oizumi H, Kanauchi N, Kato H et al Anatomic thoracoscopic pulmonary segmentectomy under 3‐dimensional multidetector computed tomography simulation: A report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011; 141: 678–82. [DOI] [PubMed] [Google Scholar]
- 19. Van Allen CM, Lindskog GE, Richter HG. Collateral respiration transfer of air collaterally between pulmonary lobules. J Clin Invest 1931; 10: 559–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cordingley JL. Pores of Kohn. Thorax 1972; 27: 433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Misaki N, Chang SS, Igai H, Tarumi S, Gotoh M, Yokomise H. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010; 140: 752–6. [DOI] [PubMed] [Google Scholar]
- 22. Kasai Y, Tarumi S, Chang SS et al Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: Comparative analysis of 2‐ and 1‐wavelength methods. Eur J Cardiothorac Surg 2013; 44: 1103–7. [DOI] [PubMed] [Google Scholar]
- 23. Tarumi S, Misaki N, Kasai Y, Chang SS, Go T, Yokomise H. Clinical trial of videoassisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014; 46: 112–5. [DOI] [PubMed] [Google Scholar]
- 24. Frangioni JV. New technologies for human cancer imaging. J Clin Oncol 2008; 26: 4012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nomori H, Cong Y, Sugimura H. Utility and pitfalls of sentinel node identifcation using indocyanine green during segmentectomy for cT1N0M0 non‐small cell lung cancer. Surg Today 2016; 46: 908–13. [DOI] [PubMed] [Google Scholar]
- 26. Anayama T, Qiu J, Chan H et al Localization of pulmonary nodules using navigation bronchoscope and a near‐infrared fluorescence thoracoscope. Ann Thorac Surg 2015; 99: 224–30. [DOI] [PubMed] [Google Scholar]
- 27. Chang TI, Chen YS, Huang SC. Intraoperative indocyanine green fluorescence lymphography to detect chylous leakage sites after congenital heart surgery. J Thorac Cardiovasc Surg 2014; 148: 739–40. [DOI] [PubMed] [Google Scholar]
- 28. Hope‐Ross M, Yannuzzi LA, Gragoudas ES et al Adverse reactions due to indocyanine green. Ophthalmology 1994; 101: 529–33. [DOI] [PubMed] [Google Scholar]
- 29. Speich R, Saesseli B, Hoffmann U, Neftel KA, Reichen J. Anaphylactoid reactions after indocyanine‐green administration. Ann Intern Med 1988; 109: 345–6. [DOI] [PubMed] [Google Scholar]


