Abstract
Anthropogenic nutrient enrichment and increased seawater temperatures are responsible for coral reef decline. In particular, they disrupt the relationship between corals and their dinoflagellate symbionts (bleaching). However, some coral species can afford either high temperatures or nutrient enrichment and their study can bring new insights into how corals acclimate or adapt to stressors. Here, we focused on the role of the nutrient history in influencing the response of the Mediterranean scleractinian coral Cladocora caespitosa to thermal stress. Colonies living naturally in nutrient-poor (<0.5 µM nitrogen, <0.2 µM phosphorus, LN) and nutrient-rich (ca. 10–20 µM nitrogen, 0.4 µM phosphorus, HN) locations were sampled, maintained under the right nutrient conditions, and exposed to a temperature increase from 17 °C to 24 °C and 29 °C. While both HN and LN colonies decreased their concentrations of symbionts and/or photosynthetic pigments, HN colonies were able to maintain significant higher rates of net and gross photosynthesis at 24 °C compared to LN colonies. In addition, while there was no change in protein concentration in HN corals during the experiment, proteins continuously decreased in LN corals with increased temperature. These results are important in that they show that nutrient history can influence the response of scleractinian corals to thermal stress. Further investigations of under-studied coral groups are thus required in the future to understand the processes leading to coral resistance to environmental perturbations.
Subject terms: Ecophysiology, Respiration, Animal physiology
Introduction
The health of scleractinian reef-building corals is rapidly declining, in particular due to heat wave events, which have been increasing in frequency and intensity due to global change. Elevated temperatures induce the loss of coral symbionts and/or photopigments, known as bleaching1. In recent years, shallow-water tropical reefs have already undergone massive bleaching events, followed by coral mortality2–5. Alongside deterioration in reef environment from global threats, local disturbances such as overfishing, nutrient runoff and pollution are likely to lower the resilience of corals to environmental change6–9. In particular, anthropogenic seawater nutrient enrichment, due to the use of chemical fertilizers or to discharges of human and animal wastes can cause shifts in trophic dynamics of coral reef ecosystems10, loss of coral cover and diversity11, increased coral diseases12 and susceptibility to bleaching13. It has also been associated to coral reef decline by disturbing the fine balance between the host and its symbiotic algae14. Seawater enrichment with nitrate seems to be more detrimental for corals than ammonium enrichment (reviewed by15), especially under imbalanced nitrogen to phosphorus ratio13, or in conditions, which are enriched with organic particulate matter16.
Despite the overall detrimental effect of nutrification on corals, it has been shown that some corals can acclimate or adapt to nutrient enriched environments17–20. For many Brazilian reefs, for example, there are no reports of diseases, and bleaching events have high recovery rates of corals21,22, despite the fact that they are both affected by high sedimentation levels23 and nutrient enrichment24. In some other cases, corals respond positively to nutrient addition, by increasing growth and metabolism25, especially under elevated pCO226–28, or thermal stress29,30. A reduced susceptibility to bleaching was also noticed29, in particular in regions with small-scale upwelling31. Overall, these antagonistic observations suggest that more research has to be done to better understand the adaptation or acclimation of corals to nutrification. Mediterranean corals, such as the scleractinian symbiotic coral Cladocora caespitosa, are among the few examples of corals that can be found both in nutrient-poor (Levantine basin, Cyprus32) or nutrient-rich environments20,33–35. They are thus the perfect model to study their responses and adaptations to the nutrient levels of their living environment. In addition, they are threatened by heat waves in summer, showing several episodes of tissue necrosis (tissue degradation, peeling) and subsequent mortality34,36–42, due to the significant increase in sea surface temperatures (SSTs) of the Mediterranean and the Levantine Sea over the past years43,44. Corals, as well as other sessile organisms such as gorgonians, are key species of the Mediterranean Sea, and their mortality can have significant consequences for the ecosystem functioning and the overall biodiversity of this Sea. It is therefore urgent to understand how temperature, but also nutrient conditions, can affect their physiology and their chance to survive both global and local changes.
In this study, we have investigated the thermal tolerance of the coral C. caespitosa acclimated to two different nutrient environments, in order to assess the effect of nutrient supply on the response of such coral species to thermal stress. For this purpose, colonies of C. caespitosa were sampled in two close environments of Cyprus island, with contrasting inorganic nutrient levels: a location with low levels in inorganic nutrients, hereafter called LN (Kryo-Nero, (<0.5 µM dissolved inorganic nitrogen and 0.2 µM phosphorus), as most of the waters of the Levantine basin32; a location with high levels in inorganic nutrients, hereafter called HN (Liopetri > 10 µM dissolved inorganic nitrogen, 0.4 µM phosphorus), situated in front of an on-land fish hatchery and agricultural area where a large community of C. caespitosa thrives. We hypothesize that the main physiological traits of the coral colonies will be different between nutrient-enriched and poor conditions and that the colonies will also present a different response to thermal stress.
Results
Normal growth temperature (17 °C): Nutrient effect on C. caespitosa physiology
The two-way ANOVA showed that both temperature and nutrients changed the physiology of C. caespitosa, with an interaction between the two parameters (Table 1). At 17 °C, nutrient enrichment significantly decreased the symbiont density per surface area (Fig. 1A; Table 1, Tukey’s test, p < 0.05), but did not significantly change the chlorophyll (a and c2) or protein content within the tissue of C. caespitosa (Fig. 1B–D; Table 1, Τukey’s test, p > 0.05). No significant changes were also observed concerning the rates of calcification (Fig. 2; Τukey’s test, p > 0.05), as well as the rates of respiration and net photosynthesis (Fig. 3A,B; Τukey’s test, p > 0.05). Gross photosynthesis was however lower in HN conditions (Fig. 3C; Τukey’s test, p < 0.01). TOC fluxes were also inversed (Fig. 4A; Τukey’s test, p < 0.01): while C. caespitosa released organic carbon (positive flux from the coral to the seawater) in the LN condition, it significantly took up organic carbon in HN treatment (negative flux from the coral to the seawater). This is explained by the fact that HN-corals presented lower rates of gross photosynthesis and also needed more carbon to compensate the higher levels of nitrogen input.
Table 1.
Results of the two-way ANOVAs (p value) testing the effect of temperature and nutrient condition on the physiological parameters of C. caespitosa. Net photosynthesis (Pn) and gross photosynthesis (Pg) normalized to surface area (cm−2) or symbiont cell (symbiont), chlorophyll a (Chl a) or c2 (Chl c2) concentration, total organic carbon (TOC) and nitrogen (NTN) fluxes. NS: non significant.
| Temperature | Nutrient | Interaction | |
|---|---|---|---|
| Symbiont density | <0.01 | <0.05 | <0.05 |
| Chl a (μg cm−2) | <0.001 | NS | NS |
| Chl c2 (μg cm−2) | <0.001 | NS | NS |
| Protein (μg cm−2) | NS | <0.05 | <0.001 |
| Calcification | <0.001 | 0.053 | <0.001 |
| Pn (cm−2) | <0.001 | NS | <0.05 |
| Pg (cm−2) | <0.001 | NS | <0.001 |
| Pg (100) (symbiont) | <0.001 | NS | <0.05 |
| Respiration | <0.001 | <0.05 | NS |
| TOC | <0.001 | NS | <0.05 |
| TN | NS | <0.001 | <0.01 |
Figure 1.
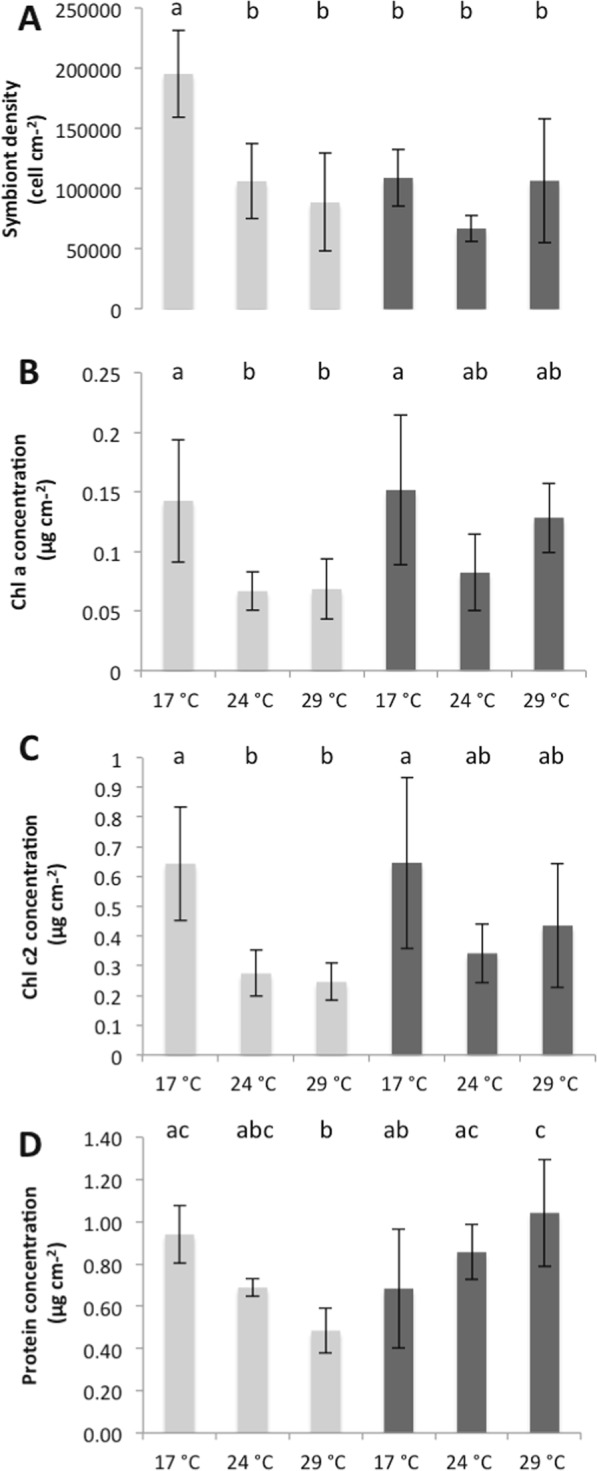
Symbiont density (A), concentrations in Chlorophyll-α (B), Chlorophyll-c2 (C) and protein (D) in nubbins maintained under low nutrient (LN, light grey) and high nutrient (HN, dark grey) conditions at different temperatures. Data represent mean ± standard deviation.
Figure 2.
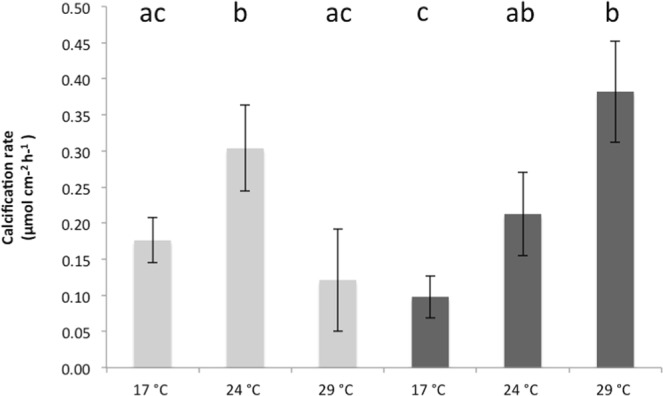
Average net photosynthesis (Pn) (A), respiration rates, gross photosynthesis (Pg) (B) and photosynthetic efficiency (Pg/symbiont) (C) of C. caespitosa under different temperatures, light intensities and nutrient levels (high (HN) and low (LN) nutrient). Data represent mean ± standard deviation.
Figure 3.
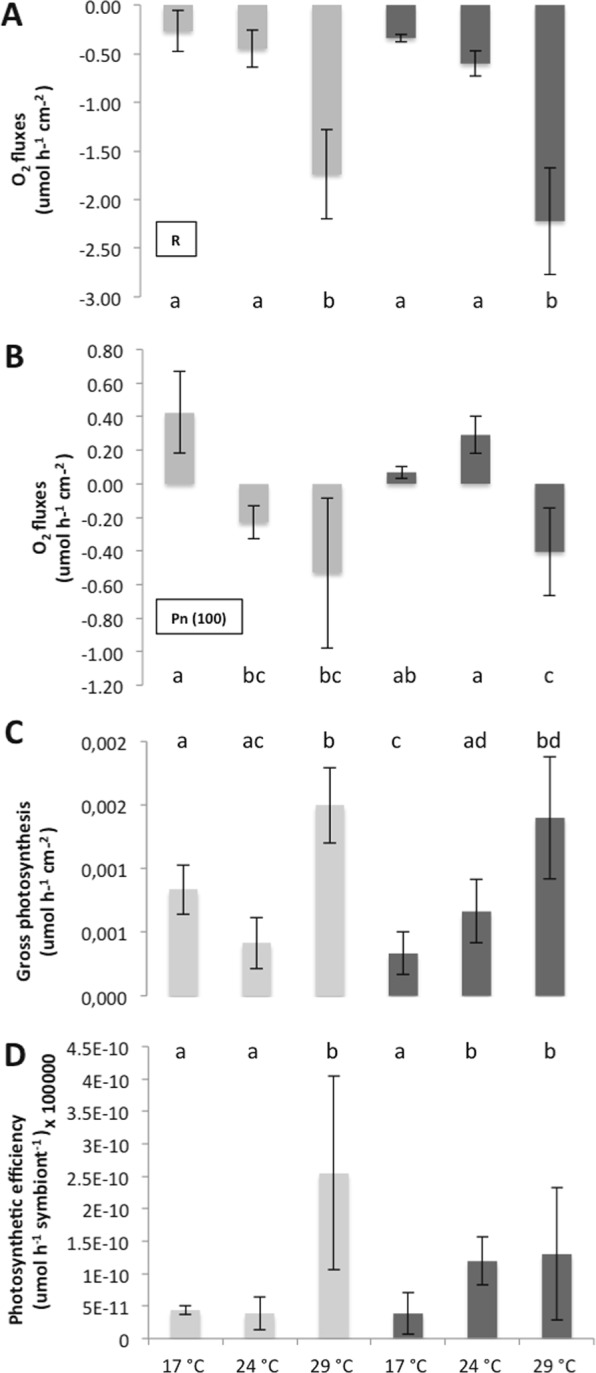
Calcification rate of C. caespitosa under low nutrient (light grey) and high nutrient (dark grey) levels at different seawater temperatures. Data represent mean ± standard deviation.
Figure 4.
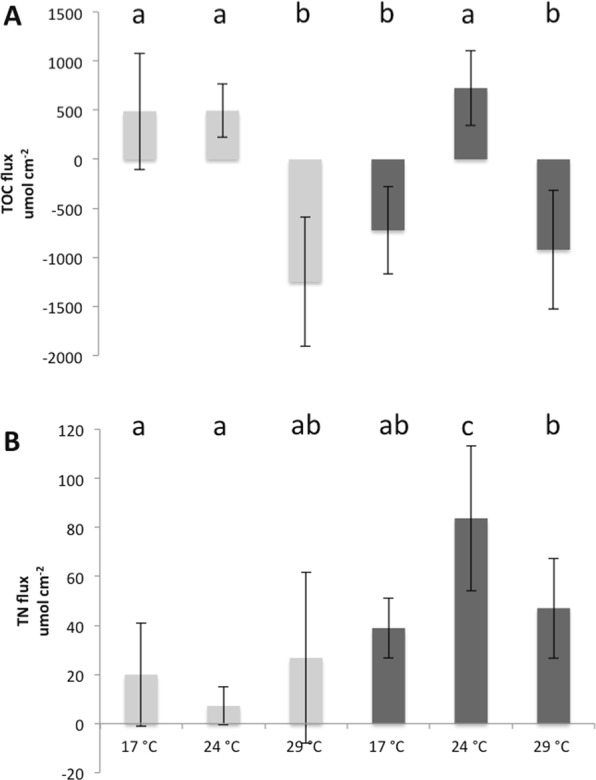
(A) Total organic carbon (TOC) and (B) total nitrogen (TN) fluxes under low nutrient (light grey) and high nutrient (dark grey) conditions, at different seawater temperatures. Data represent mean ± standard deviation.
High temperatures (24 °C and 29 °C): Comparison of C. caespitosa physiology at low (LN) and high (HN) nutrient concentrations
There was no significant difference in the symbiont density, chl a and c2 content and rates of respiration between nutrient conditions at both 24 °C and 29 °C (Figs 1A–C, 3A; Τukey’s test, p > 0.05). However, at 24 °C, HN-corals presented higher rates of net and gross photosynthesis (Fig. 3B,C; Τukey’s test, p < 0.05), as well as higher rates of cell-specific photosynthesis (Fig. 3D; Τukey’s test, p < 0.05), and TN release (Fig. 4B; Τukey’s test, p < 0.001). At 29 °C, protein concentration as well as rates of calcification were also significantly higher in the HN condition compared to the LN condition (Figs 1D, 2; Τukey’s test, p < 0.001).
Changes observed in each nutrient condition between 17 °C and high temperatures
In the LN condition, at temperatures higher than 17 °C, we observed a significant decrease in symbiont density (Fig. 1A; Τukey’s test, p < 0.05), Chl-a and chl-c2 concentrations (Fig. 1B,C; Τukey’s test, p < 0.05 and p < 0.01 respectively), and net photosynthesis, Pn (Fig. 3; Τukey’s test, p < 0.05 for 24 °C and p < 0.01 for 29 °C). Protein concentration also significantly decreased from 17 °C to 29 °C (Fig. 1D; Τukey’s test, p < 0.01). Since the dark respiration significantly increased at 29 °C when compared to 24 °C and 17 °C (Fig. 3A; Τukey’s test, p < 0.001), gross photosynthesis rates per surface area and symbiont cell were significantly higher at 29 °C compared to 17 °C (Fig. 3C,D; Τukey’s test, p < 0.001 and < 0.01 respectively). Finally, calcification rates, measured through total alkalinity, significantly increased from 17 °C to 24 °C (Fig. 3; Τukey’s test, p < 0.05), before decreasing again to the initial value at 29 °C (Fig. 3; Tukey’s test, p < 0.001). While there were no significant changes in the TN fluxes with temperature conditions, TOC fluxes were inversed between 17 °C-24 °C and 29 °C (Fig. 4; Τukey’s test, p < 0.001): C. caespitosa released TOC at 17 °C and 24 °C while it took up TOC at 29 °C.
In the HN condition, there was no significant change in symbiont density (Fig. 1A; Τukey’s test, p > 0.05), and chl a and c2 levels (Fig. 1B,C; Τukey’s test, p > 0.05;) with increased seawater temperature. Some parameters significantly increased between 17 °C and 29 °C: protein concentration (Fig. 1D; Τukey’s test, p < 0.05), respiration rates (Fig. 2A; Τukey’s test, p < 0.001), Pg (Fig. 2C; Tukey’s test, p < 0.001), rates of photosynthesis per symbiont cell (Fig. 2D; Tukey’s test, p < 0.05) and rates of calcification (Fig. 3; Τukey’s test, p < 0.001). The two last parameters started to significantly increase at 24 °C (Figs 3, 4D; Τukey’s test, p < 0.05 and p < 0.05 respectively). On the contrary, Pn was significantly lower at 29 °C compared to the other temperatures (Fig. 2B; Tukey’s test, p < 0.05). Finally, TOC and TN fluxes were significantly higher at 24 °C when compared to 17 °C (Fig. 4A,B; Τukey’s test, p < 0.001) and 29 °C (Fig. 4A,B; Τukey’s test, p < 0.001).
Discussion
Although many coral species are vulnerable to increased sea surface temperature and/or nutrification45–47, some may acclimate or even adapt to these stressors, at both the physiological and molecular levels (i.e.48). For example, thermal history led to acclimation in several coral species49–53 (see5 for an alternative view) and some corals are able to grow in nutrified or eutrophic environments17,54. Although the above studies have highlighted the importance of understanding the flexibility of coral responses to environmental stressors, most of them have focused on the acclimation to high temperature conditions rather than nutrification. It is however important to understand the ability of different coral species to acclimate to high nutrient conditions, as this is going to affect many reefs in the future, due to the increasing urbanization of many coastal areas (e.g.8). The present study is thus one of the few that has focused on experimentally testing the effect of the long-term nutrient history on the bleaching susceptibility of a scleractinian coral species47,55. Mediterranean corals such as C. caespitosa are good examples of coral species able to thrive both in oligotrophic and nutrient-enriched, environments. In addition, they experience large temperature variations between summer and winter conditions56. The results of this study are important because they show that the nutrient history can influence the response of some scleractinian corals to thermal stress and therefore have implications for the understanding of the bleaching process and coral resilience57,58. We observed that colonies acclimated to very high levels of dissolved inorganic nutrients didn’t bleach more and even maintained higher rates of net and gross photosynthesis and higher protein content than non-enriched corals at elevated temperatures. Our results provide novel insights into the particular resilience of Mediterranen corals to nutrification, as also observed in some particular occasions with tropical corals17,54. They suggest that further investigation of under-studied coral groups are needed in the future to understand the processes leading to such coral resilience to environmental perturbations.
Colonies of C. caespitosa maintained under low inorganic nutrient concentrations, but fed twice a week with Artemia salina prey at repletion, presented a response to thermal stress similar to many tropical and temperate coral species37,46,59,60. Temperature increase induced a significant decrease in both symbiont density and areal chlorophyll content of C. caespitosa, followed by a decrease in the net photosynthesis measured at the in situ irradiance of 100 µmole photons m−2 s−1 (bleaching). As a consequence of the decreased autotrophic energy input and increased respiratory needs at high temperatures, the protein content of the coral tissue, which is a proxy of biomass, continuously decreased during the elevation in temperature. Such decrease occurred despite the fact that corals stopped releasing organic carbon and even started to take up the small amount of carbon available in seawater at 29 °C (Fig. 4). Bleaching also occurred while coral colonies were fed twice a week with Artemia salina nauplii. Feeding has been shown to decrease the bleaching susceptibility of tropical coral species61–63 but did not avoid bleaching in C. caespitosa, likely because it is an heterotrophic species with high energetic requirements. Mortality or bleaching of C. caespitosa has thereby been recorded in different locations of the Mediterranean Sea38,39,64,65 but also in Cyprus in 2012, concurring with temperature anomalies42. A similar effect of high temperature on C. caespitosa was observed in laboratory thermal stress experiments37. Despite significant bleaching, calcification rates were boosted under high temperature conditions. This is in agreement with in situ observations in the North-West Mediterranean Sea showing higher growth rates of C. caespitosa in summer, compared to almost no growth in winter, at temperatures of 12 °C66. The growth of C. caespitosa in a previous thermal stress experiment37 was also significantly enhanced during the first 3 weeks of temperature increase, contrary to another Mediterranean coral, Oculina patagonica, whose growth was rapidly impacted by thermal stress37. In tropical corals, the thermal optimum for calcification generally occurs between 26 °C and 28 °C, after which there is an inverse temperature dependency67,68. Calcification of C. caespitosa may follow the same trend, at least until the energetic reserves in coral tissue are able to sustain such high growth rate.
One of the major observations of this study is the particular resistance of C. caespitosa to long-term nutrification. The same can be observed in other parts of the Mediterranean Sea, such as close to the city of La Spezia (North West Mediterranean Sea), where many colonies also thrive next to a river mouth in a nutrient rich environment66. At the in situ temperature of 17 °C, high nitrogen supply did not increase the symbiont density, which is in contrast with many tropical corals68,69. In these later corals, increased symbiont density may even lead to a decrease in rates of photosynthesis and calcification70–72. It has to be noticed that C. caespitosa, under natural conditions, can afford relatively high symbiont densities, particularly in the North-West Mediterranean Sea, where it can host more than 2 and up to 6 × 10−6 zooxanthellae cm−2 37,73. The lack of nitrogen enhancement of symbiont growth can be partly due to the fact that symbionts are already nutrient-repleted, due to the particular heterotrophic nature of C. caespitosa, which mainly feed on planktonic prey throughout the year74. We also observed at 17 °C, under high-nutrient condition, an adjustment with lower symbiont density, but higher cell-specific photosynthetic pigments compared to corals maintained under low-nutrient condition. All together, the areal photosynthetic pigment concentration, as well as the rates of net photosynthesis did not change between nutrient-enriched and poor conditions, which did not affect the rates of calcification. Nutrification also promoted the uptake of (dissolved and particulate) organic carbon contained in seawater by C. caespitosa, suggesting that the corals had to counterbalance the high nitrogen input by acquiring more carbon from seawater.
In the tropics, chronic enrichment in dissolved inorganic nutrients, especially nitrogen, has direct but also indirect effects on corals (reviewed in14). It enhances the prevalence and severity of coral disease12,55, leads to imbalanced N:P ratios within the coral tissue13,75, and increases coral bleaching susceptibility, especially under a combined enrichment in nitrate and particulate organic matter16. At the ecosystem level, it mainly increases the density and productivity of macroalgae, which can overgrow and replace corals76, alter the coral microbial communities and interfere with recruitment of planulae by allelopathic interactions77,78. The success of C. caespitosa in shallow eutrophic areas of the Mediterranean Sea can thus partly rely on the lack of competition with algae, due to water turbidity or algal grazing by sea urchin35. Although C. caespitosa banks can be observed in Spain in the middle of a high algal coverage of Dictyopteris polypodioides, Halimeda tuna, Cystoseira sauvageauana and Cystoseira compressa35, the algae were indeed never observed overgrowing coral colonies. Finally, the heterotrophic nature of C. caespitosa can explain its presence in eutrophic environments74. The same observation was made in tropical areas, where the increased productivity of nutrient enriched waters has benefited corals with a high heterotrophic capacity11,79,80.
Another major observation of this study is that nutrification did not induce enhanced bleaching of C. caespitosa under high temperatures compared to control corals and even maintained higher rates of photosynthesis at 24 °C, as well as a higher protein content at 29 °C. Although moderate inorganic nitrogen supply (ca. 1–3 µM) has been shown to promote coral growth and metabolism17,81, in particular under elevated pCO225–27 or thermal stress30, other studies on tropical corals have also suggested that elevated inorganic nitrogen levels may impact corals by decreasing their thermal thresholds for bleaching. Nitrogen addition indeed tends to enhance symbiont growth inside the coral host tissue and increase oxidative stress13,55,82–84. To reconcile these two opposite observations, Wiedenmann et al.13, as well as some other studies75,85 demonstrated that an imbalance N:P ratio was the key factor explaining coral bleaching. A condition where phosphorus is in limited amount while nitrate is fully available indeed promotes coral bleaching13,75,85. In this study, while the seawater N:P ratio was high and should have induced bleaching in C. caespitosa, the contrary was observed. A plausible explanation is that the internal N:P ratio of the coral tissue was not imbalanced, due to the provision of heterotrophic food to the coral colonies, which may have delivered large amounts of organic phosphorus to the coral74. Heterotrophy may have also avoided carbon limitation of the symbionts under high nutrient supply13,63. Such carbon limitation has often been reported in coral-dinoflagellate symbiosis86,87, enhancing bleaching under thermal stress63,87,88. Since the physiological traits of the coral host are partly shaped by the dominant symbiont type present within its tissues89, we also suggest that the symbionts of C. caespitosa have particular adaptation to nutrient enrichment and can provide ecological advantages to C. caespitosa in nutrient-rich conditions. Symbionts in C. caespitosa belong to formerly clade B (now Breviolum sp.), which is common in the Mediterranean temperate and subtropical regions90–92. In the light of these observations, more studies are needed to fully understand the interactions between organic and inorganic nutrients on the resistance of corals to thermal stress, in particular by taking into account how external nutrients modify the internal C:N:P ratio of coral tissue. In addition, the response of corals to environmental changes may be light dependent, as shown in recent studies93,94. This experiment was performed in late autumn/winter on samples that were acclimated to relatively low light levels (100 µmol photons m−2 s−1). The experiment should therefore be repeated during the summer season, when irradiance can be 3 times higher.
Cladocora caespitosa is an emblematic coral of the Mediterranean Sea, and its conservation is an important concern now that its bioconstructions are endangered by the climate change effects35. A better knowledge of its response to environmental stressors is thus needed to further understand how this species can be preserved. This study conclusively demonstrates that the long time scale acclimation to high nutrient levels can reduce the bleaching susceptibility of C. caespitosa and has not necessarily adverse effects on its growth. This is maybe due to the high heterotrophic capacities of the coral host, which can maintain a balanced C:N:P ratio within the tissues and counterbalance the nutrient-enhancement of symbiont growth. However, this coral model need more in depth studies to fully understand the different acclimation or adaptation ways to eutrophication.
Materials and Methods
Study sites and sample collection
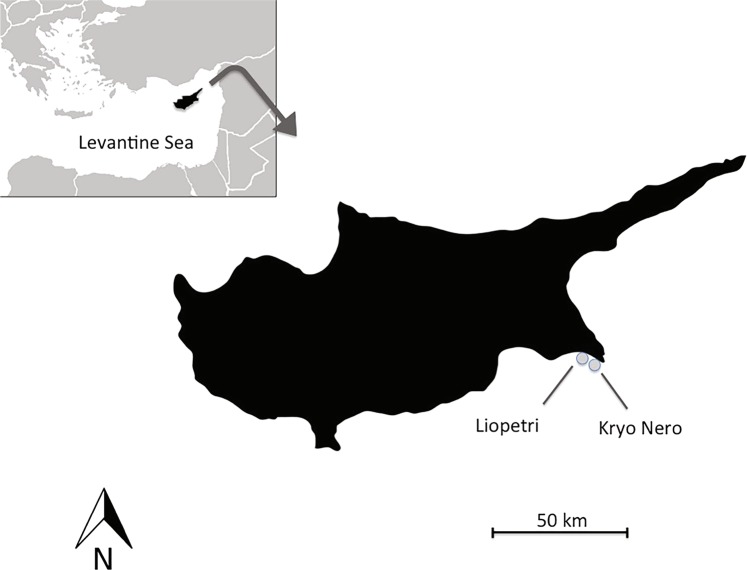
Coral colonies originated from two close areas in Cyprus, both holding > 100 colonies of C. caespitosa at very shallow depths (<4 m). ‘Kryo Nero’ site (i.e. nutrient-poor site, LN), is found on the coast of Ayia Napa village in the South-east of Cyprus (34°58.949′N, 34°1.014′E). ‘Liopetri’ site (nutrient-enriched site, HN) lies approximately 10 km west of ‘Kryo Nero’ right in front of a small on-land fish hatchery and very close to a large agricultural area (34°57.537′N, 33°53.755′E) (Fig. 5).
Figure 5.
Location of field-sites in SE Cyprus.
Prior to the experiments, water samples were collected from both locations (35 times between 2012–2015 from Liopetri; 12 times between 2014–2015 from Kryo Nero) and analyzed to determine inorganic nutrient concentrations using standard spectrophotometric methods95. Inorganic nutrient analyses showed significantly higher concentrations at Liopetri than in Kryo Nero. Mean nutrient concentrations at Liopetri were 1232 μg L−1 or 19.87 µM for nitrate (NO3−), 92 μg L−1 or 5 µM for ammonium (NH4+) and 24 μg L−1 or 0.24 µM for phosphate (PO43−). At Kryo Nero, mean concentrations equaled 74 μg L−1 or 1.2 µM NO3−, 13 μg L−1 or 0.72 µM NH4+, 12 μg L−1 or 0.12 µM PO43− (Supplementary Fig. S1). The particulate organic carbon (POC) and nitrogen (PON) content of the water was also analyzed using an elemental analyzer (Shimadzu). POC concentrations were equal to 27.6 ± 5.7 µM and 25.5 ± 2.5 in Kryo Nero and Liopetri respectively. PON concentrations ranged from 1.4 ± 0.4 µM in Kryo Nero to 1.8 ± 0.14 µM in Liopetri. Both levels were not significantly different between locations and in agreement with previous measurements for the Mediterranean Sea96.
Coral fragments (of 6–8 polyps) were collected from 36 large colonies at Liopetri and Kryo Nero, end of November 2015. They were identified, kept in separated bags containing the original seawater and rapidly transported to the aquarium system of the Centre Scientifique de Monaco (CITES no CY/exp/005/2015). Here, each fragment was divided in two smaller fragments of 3–4 polyps, making a total of 72 fragments, which were distributed into 12 tanks, so that each tank contained 6 different original colonies. All tanks were maintained at the seawater temperature at the time of collection (17 °C). Six tanks were maintained under low nutrient condition (ca. 0.5 µM NO3−, 0.1 µM NH4+ and 0.2 µM PO43−) whereas the other six received high nitrogen levels (6–7 μM NO3− and 5–6 μM NH4+). These concentrations were lower than the mean in situ concentrations, but where applied continuously to the corals for the 6 weeks experiment. Nutrient enrichment was thus performed using a peristaltic pump, which continuously supplied the experimental tanks with a solution of NO3− and NH4+ at a rate of 15 ml h−1, together with a 12 L h−1 seawater flow-through. Nutrient concentrations were monitored twice a week with an auto-analyzer (Alliance Instrument, France), according to Tréguer and Le Corre (1975)97. Light (100 ± 10 μmol photons m−2 s−1, with a 12:12 h photoperiod) was provided by HQI lamps and set up to the mean daily irradiance received by the corals at the time of collection (daily photon flux density of 4 mol m−2). It was measured using a spherical quantum sensor (LiCor LI-193, Lincoln, NE, USA). As C. caespitosa is a mixotrophic/heterotrophic species, colonies were fed twice a week with nauplii of Artemia salina. This ensured to have the same level of heterotrophic feeding but a different autotrophic level linked to the two inorganic nutrient conditions.
Experimental setup
Corals were kept three weeks under the two nutrient conditions and at 17 °C (control). Two aquaria per nutrient condition were kept as control while seawater temperature was slowly increased (0.5 °C per day) in two other aquaria to 24 °C and the last two aquaria to 29 °C. Once the last two aquaria reached 29 °C, corals were all maintained for 10 days before the physiological measurements described below were performed. While 17 °C corresponds to the temperature at the time of collection, 24 °C and 29 °C represent respectively the mean annual temperature in Cyprus and the mean maximal temperature recorded in summer times using a Star-Oddi starmon mini temperature logger.
Measurements
Calcification and release of organic carbon and nitrogen
Calcification rates were assessed using the alkalinity anomalous technique/principle, according to Smith and Kinsey (1978)98. Six nubbins from each condition (3 per tank) were placed in separate sealed containers with 350 mL of 0.22 μm-filtered seawater (FSW). An extra container with only FSW was also incubated to serve as control. All containers were placed in a water bath at the right temperature (17 °C, 24 °C, 29 °C) and light and incubated for 6 hours. Stirring was applied by magnetic stir bars. At the beginning and end of the incubation period, three seawater samples (50 mL) were collected from each container and transferred in borosilicated vials. The TA was immediately measured in duplicate by automatic titration using a Metrohm Titrando 888 following Dickson et al.99.
The same coral nubbins were used to estimate the total organic carbon (TOC) and nitrogen fluxes (TN) with the use of Shimadzu TOC-L analyser, according to the established beaker incubation technique (e.g.100). Briefly, corals were transferred without aerial exposure into acid-washed and seawater-rinsed 250 ml glass beakers filled with 0.2 µm filtered seawater. Three control beakers containing only seawater were also prepared. All beakers were placed in a water bath and incubated for 6 h as described above. After 6 h, corals were removed from the incubation beakers and kept for surface determination. Before and after incubations, seawater subsamples were drawn by sterile syringe from the thoroughly homogenised incubation media to quantify TOC and TON concentrations. Subsamples were transferred into pre-combusted (450 °C, 5 h) glass vials, acidified with phosphoric acid (20%, 250 μl) to pH < 2 and kept frozen (−20 °C) until analysis.
Photosynthesis/respiration
Rates of net photosynthesis (Pn) and respiration (R) were measured using six nubbins per condition (three per tank). Each nubbin was placed in a temperature-controlled airtight chamber filled with ~50 ml of 0.45 μm-FSW, equipped with optodes (OCY-4 micro, PreSens, Germany), and continuously stirred using magnetic stirrers. The optodes were calibrated before each treatment using nitrogen gas (N2) and air saturated water for 0% and 100% oxygen saturation values respectively. Measurements were performed during 15 minutes initially at 100 μmol photons m−2 s−1, and then 20 minutes in total darkness. Rates of gross photosynthesis (Pg) were calculated by adding R to Pn. Rates of cell photosynthesis (Pg/zoox) were calculated by normalizing Pg to symbiont density. Each rate was expressed per polyp surface area (µmol O2 h−1 cm−2) or per symbiont cell (µmol O2 h−1 symbiont cell−1) according to Rodolfo-Metalpa et al.37. Samples were frozen for later determination of tissue parameters (symbiont, chlorophyll concentration, and protein concentration).
Tissue parameters were determined according to Hoogenboom et al.73. Coral tissue was removed from the skeleton with an airbrush, using 0.45 μm filtered seawater and homogenized with a potter tissue grinder. A 1 mL sub-sample was used to determine symbiont density with a Beckman coulter counter (France). Protein content was assessed in another 1 mL sample according to Smith et al.101 by the use of a BCAssay Protein Quantification Kit (Uptima, Interchim) and a Xenius® spectrofluorometer (SAFAS, Monaco). In order to measure Chlorophyll-a concentration, the remaining 5 mL sub-sample was centrifuged at 8000 g for 10 min at 4 °C. After removing the supernatant, symbionts were resuspended into 5 mL acetone and placed at 4 °C overnight. Chlorophyll a and c2 concentrations were determined following the method of Jeffrey and Humphrey (1975)102 by the use of a spectrophotometer (Safas, Monaco). Data were normalized to the surface area (cm2). The main Symbiodiniacae genotype hosted by C. caespitosa in each location was checked according to the protocol of Santos et al.103. Symbionts from both sampling sites belong to clade B.
Statistical analyses
Two-way analysis of variance (ANOVA) was used to compare TA, TOC, TN, Pn, Pg, Pg/zoox, symbiont density, chlorophyll-a/chlorophyll-c2 and protein concentrations between nutrient conditions and temperatures. When significant interaction effects were detected, Tukey’s HSD multiple comparison tests were conducted to examine the differences. All data were checked prior to analyses for normal distribution and were log-transformed when required. All analyses were computed using PAST statistical package104. Comparisons with p < 0.05 were considered significant.
Supplementary information
Acknowledgements
We would like to thank Aristos laboratories for providing assistance with the nutrient analyses and Loizos Hadjioannou, Julia Hartingerova, AP Marine and Telia Hatchery for assistance in the field. Many thanks to Leila Ezzat for laboratory assistance and Thetis Christoforou for technical help. We are also grateful to Elena Erotokritou from the Department of Environment in Cyprus for providing CITES permits. Three anonymous reviewers largely improved the manuscript. This project was partially supported by the CSM, ENALIA, The University of Cyprus and The Cyprus Institute (EU-FP7 288710 MERMAID project).
Author Contributions
L.H., C.F.P., S.S. and C.J. conceived and designed the experiment. L.H. and C.R. performed the experiments. All authors wrote the manuscript and participated to the scientific discussion.
Data Availability
All material, data and associated protocols are available from the authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-50716-w.
References
- 1.Hoegh-Guldberg O, Poloczanska ES, Skirving W, Dove S. Coral Reef Ecosystems under Climate Change and Ocean Acidification. Front. Mar. Sci. 2017;4:158. [Google Scholar]
- 2.Precht WF, Gintert BE, Robbart ML, Fura R, van Woesik R. Unprecedented Disease-Related Coral Mortality in Southeastern Florida. Sci. Rep. 2016;6:31374. doi: 10.1038/srep31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heron SF, Maynard JA, van Hooidonk R, Eakin CM. Warming Trends and Bleaching Stress of the World’s Coral Reefs 1985–2012. Sci. Rep. 2016;6:38402. doi: 10.1038/srep38402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes TP, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–377. doi: 10.1038/nature21707. [DOI] [PubMed] [Google Scholar]
- 5.Hughes TP, et al. Global warming transforms coral reef assemblages. Nature. 2018;556:492–496. doi: 10.1038/s41586-018-0041-2. [DOI] [PubMed] [Google Scholar]
- 6.Burke, L., Reytar. K., Spalding, M. D. & Perry, A. Reefs at Risk Revisited. World Resources Institute, Washington, DC, USA (2011).
- 7.Kennedy, E. V. et al. Avoiding Coral Reef Functional Collapse Requires Local and Global Action. Curr. Biol. 1–7 (2013). [DOI] [PubMed]
- 8.Duprey NN, Yasuhara M, Baker DM. Reefs of tomorrow: eutrophication reduces coral biodiversity in an urbanized seascape. Glob. Chang. Biol. 2016;22(11):3550–3565. doi: 10.1111/gcb.13432. [DOI] [PubMed] [Google Scholar]
- 9.Zaneveld JR, et al. Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat. Commun. 2016;7:11833. doi: 10.1038/ncomms11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szmant AM. Nutrient enrichment on coral reefs: Is it a major cause of coral reef decline? Estuaries. 2002;25:743–766. [Google Scholar]
- 11.Fabricius KE. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bull. 2005;50:125–146. doi: 10.1016/j.marpolbul.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 12.Thurber RV, Payet JP, Thurber AR, Correa AM. Virus-host interactions and their roles in coral reef health and disease. Nat. Rev. Microbiol. 2017;15(4):205–216. doi: 10.1038/nrmicro.2016.176. [DOI] [PubMed] [Google Scholar]
- 13.Wiedenmann J, et al. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Chang. 2013;2:1–5. [Google Scholar]
- 14.D’Angelo C, Wiedenmann J. Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr. Opin. Environ. Sustain. 2014;7:82–93. [Google Scholar]
- 15.Shantz AA, Burkepile DE. Context-dependent effects of nutrient loading on the coral-algal mutualism. Ecology. 2014;95(7):1995–2005. doi: 10.1890/13-1407.1. [DOI] [PubMed] [Google Scholar]
- 16.Ezzat L, Towle E, Irisson JO, Langdon C, Ferrier-Pagès C. The relationship between heterotrophic feeding and inorganic nutrient availability in the scleractinian coral T. reniformis under a short-term temperature increase. Limnol. Oceanogr. 2016;61:89–102. [Google Scholar]
- 17.Sawall Y, et al. Nutritional status and metabolism of the coral Stylophora subseriata along a eutrophication gradient in Spermonde Archipelago (Indonesia) Coral Reefs. 2011;30:841–853. [Google Scholar]
- 18.Sawall Y, et al. Coral Communities, in Contrast to Fish Communities, Maintain a High Assembly Similarity along the Large Latitudinal Gradient along the Saudi Red Sea Coast. J. Ecosys. Ecograph. 2014;S4:003. [Google Scholar]
- 19.Bongiorni L, Shafir S, Angel D, Rinkevich B. Survival, growth and gonad development of two hermatypic corals subjected to in situ fish-farm nutrient enrichment. Mar. Ecol. Prog. Ser. 2003;253:137–144. [Google Scholar]
- 20.Peirano A, Morri C, Bianchi CN. Skeleton growth and density pattern of the temperate, zooxanthellate scleractinian Cladocora caespitosa from the Ligurian Sea (NW Mediterranean) Mar. Ecol. Prog. Ser. 1999;185:195–201. [Google Scholar]
- 21.Migotto, A. E. Anthozoan bleaching on the southeastern coast of Brazil in the summer of 1994. In Proc 6th Int Conf Coelenterate Biol, vol 1: National Museum of Natural History, Leiden, pp 329–335 (1997).
- 22.Castro CB, Pires DO. A bleaching event in a Brazilian Reef. Revta bras. Oceanogr. 1999;47(1):87–90. [Google Scholar]
- 23.Addad J, Martins-Neto MA. Deforestation and coastal erosion: a case from East Brazil. J. Coastal. Res. 2000;16:423–431. [Google Scholar]
- 24.Costa OS, Jr., Leão ZMAN, Nimmo M, Attrill M. Nutrification impacts on coral reefs from northern Bahia, Brazil. Hydrobiologia. 2000;440:307–315. [Google Scholar]
- 25.Meyer JL, Schultz ET. Tissue condition and growth rate of corals associated with schooling fish. Limnol. Oceanogr. 1985;30:157–166. [Google Scholar]
- 26.Langdon, C., Atkinson, M. J. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J. Geophys. Res. Oceans. 110, 10.1029/2004JC002576 (2005).
- 27.Holcomb M, McCorkle DC, Cohen AL. Long-term effects of nutrient and CO2 enrichment on the temperate coral Astrangia poculata (Ellis and Solander, 1786) J. Exp. Mar. Biol. Ecol. 2010;386:27–33. [Google Scholar]
- 28.Chauvin A, Denis V, Cuet P. Is the response of coral calcification to seawater acidification related to nutrient loading? Coral Reefs. 2011;30:911–923. [Google Scholar]
- 29.McClanahan TR, et al. Interaction between nutrients and herbivory in controlling algal communities and coral condition on Glover’s Reef, Belize. Mar. Ecol. Prog. Ser. 2003;261:135–147. [Google Scholar]
- 30.Beraud E, Gevaert F, Rottier C, Ferrier-Pagès C. The response of the scleractinian coral Turbinaria reniformis to thermal stress depends on the nitrogen status of the coral holobiont. J. Exp. Biol. 2013;216:2665–2674. doi: 10.1242/jeb.085183. [DOI] [PubMed] [Google Scholar]
- 31.Riegl B, Piller WE. Possible refugia for reefs in times of environmental stress. Int. J. Earth. Sci. 2003;92:520–531. [Google Scholar]
- 32.Krom M. The oceanography of the eastern Mediterranean Sea. Ocean Challenge. 1995;5:22–28. [Google Scholar]
- 33.Schiller C. Ecology of the symbiotic coral Cladocora caespitosa (L.) (Faviidae, Scleractinia) in the Bay of Piran (Adriatic Sea): I. Distribution and biometry. Mar. Ecol. 1993;14:205–219. [Google Scholar]
- 34.Kružić P, Benkovic L. Bioconstructional features of the coral Cladocora caespitosa (Anthozoa, Scleractinia) in the Adriatic Sea (Croatia) Mar. Ecol. 2008;29:125–139. [Google Scholar]
- 35.Kersting DK, Linares C. Cladocora caespitosa bioconstructions in the Columbretes Islands Marine Reserve (Spain, NW Mediterranean): distribution, size structure and growth. Mar. Ecol. 2012;33:427–436. [Google Scholar]
- 36.Rodolfo-Metalpa R, Bianchi CN, Peirano A, Morri C. Tissue necrosis and mortality of the temperate coral Cladocora caespitosa. Ital. J. Zool. 2005;72(4):271–276. [Google Scholar]
- 37.Rodolfo-Metalpa R, et al. Response of zooxanthellae in symbiosis with the Mediterranean corals Cladocora caespitosa and Oculina patagonica to elevated temperatures. Mar. Biol. 2006;150:45–55. doi: 10.1242/jeb.02550. [DOI] [PubMed] [Google Scholar]
- 38.Kružić P, Lipej L, Mavric B, Rodic P. Impact of bleaching on the coral Cladocora caespitosa in the eastern Adriatic Sea. Mar. Ecol. Prog. Ser. 2014;509:193–202. [Google Scholar]
- 39.Kersting DK, Bensoussan N, Linares C. Long-Term Responses of the Endemic Reef-Builder Cladocora caespitosa to Mediterranean Warming. PLoS ONE. 2013;8(8):e70820. doi: 10.1371/journal.pone.0070820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kersting DK, Teixido N, Linares C. Recruitment and mortality of the temperate coral Cladocora caespitosa: implications for the recovery of endangered populations. Coral Reefs. 2014;33:403. [Google Scholar]
- 41.Kersting DK, et al. Experimental evidence of the synergistic effects of warming and invasive algae on a temperate reef-builder coral. Sci. Rep. 2015;5:18635. doi: 10.1038/srep18635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiménez C, et al. Mortality of the scleractinian coral Cladocora caespitosa during a warming event in the Levantine Sea (Cyprus). Reg. Environ. Change. 2016;16(7):1963–1973. [Google Scholar]
- 43.Perez T, Garrabou J, Sartoretto S, Harmelin JG, Francour P. Mass mortality of marine invertebrates: an unprecedented event in the Northwestern Mediterranean. CR. Acad. Sci. Paris III. 2000;323:853–865. doi: 10.1016/s0764-4469(00)01237-3. [DOI] [PubMed] [Google Scholar]
- 44.Samuel-Rhoads, Y., Zodiates, G., Hayes, D., Konnaris, G. & Georgiou, G. Climate change impacts on Sea Surface Temperature in the Eastern Mediterranean, Levantine Sea. Rapp. Comm. int. Mer Médit. 40 (2013).
- 45.Fabricius KE, Cséke S, Humphrey C, De’ath G. Does trophic status enhance or reduce the thermal tolerance of scleractinian corals? A review, experiment and conceptual framework. PloS one. 2013;8(1):e54399. doi: 10.1371/journal.pone.0054399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoegh-Guldbergh O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshwater. Res. 1999;50:839–66. [Google Scholar]
- 47.Hall ER, et al. Eutrophication may compromise the resilience of the Red Sea coral Stylophora pistillata to global change. Mar. Pollut. Bull. 2018;131:701–711. doi: 10.1016/j.marpolbul.2018.04.067. [DOI] [PubMed] [Google Scholar]
- 48.Middlebrook R, Hoegh-Guldberg O, Leggat W. The effect of thermal history on the susceptibility of reef-building corals to thermal stress. J. Exp. Biol. 2008;211(7):1050–1056. doi: 10.1242/jeb.013284. [DOI] [PubMed] [Google Scholar]
- 49.Brown B, Downs DC, Dunne RP, Gibb SW. Exploring the bases of thermotolerance in the reef coral Goniastrea aspera. Mar. Ecol. Prog. Ser. 2002;242:119–129. [Google Scholar]
- 50.Ulstrup KE, Ralph PJ, Larkum AWD, Kühl M. Intra-colonial variability in light acclimation of zooxanthellae in coral tissues of Pocillopora damicornis. Mar. Biol. 2006;149:1325–1335. [Google Scholar]
- 51.Howells EJ, et al. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Chang. 2012;2(2):116. [Google Scholar]
- 52.Oliver TA, Palumbi SR. Many corals host thermally resistant symbionts in high-temperature habitat. Coral Reefs. 2011;30(1):241–250. [Google Scholar]
- 53.Krueger T, et al. Common reef-building coral in the Northern Red Sea resistant to elevated temperature and acidification. R. Soc. Open. Sci. 2017;4(5):170038. doi: 10.1098/rsos.170038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moura RL, et al. An extensive reef system at the Amazon River mouth. Sci. Adv. 2016;2(4):e1501252. doi: 10.1126/sciadv.1501252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vega Thurber RL, et al. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob. Chang. Biol. 2013;20(2):544–54. doi: 10.1111/gcb.12450. [DOI] [PubMed] [Google Scholar]
- 56.Shaltout M, Omstedt A. Recent sea surface temperature trends and future scenarios for the Mediterranean Sea. Oceanologia. 2014;56(3):411–443. [Google Scholar]
- 57.Pawlik JR, Burkepile DE, Vega Thurber R. A Vicious Circle? Altered Carbon and Nutrient Cycling May Explain the Low Resilience of Caribbean Coral Reefs. BioScience. 2016;66(6):470–476. [Google Scholar]
- 58.Mumby PJ, Steneck RS. Paradigm Lost: Dynamic Nutrients and Missing Detritus on Coral Reefs. BioScience. 2018;68(7):487–495. [Google Scholar]
- 59.Hoegh-Guldberg Ove. Coral Reefs: An Ecosystem in Transition. Dordrecht: Springer Netherlands; 2010. The Impact of Climate Change on Coral Reef Ecosystems; pp. 391–403. [Google Scholar]
- 60.Kružić P, Popijač A. Mass mortality events of the coral Balanophyllia europaea (Scleractinia, Dendrophylliidae) in the Mljet National Park (eastern Adriatic Sea) caused by sea temperature anomalies. Coral Reefs. 2015;34:109. [Google Scholar]
- 61.Ferrier-Pagès C, Rottier C, Beraud E, Levy O. Experimental assessment of the feeding effort of three scleractinian coral species during a thermal stress: Effect on the rates of photosynthesis. J. Exp. Mar. Bio. Ecol. 2010;390(2):118–124. [Google Scholar]
- 62.Borell EM, Bischof K. Feeding sustains photosynthetic quantum yield of a scleractinian coral during thermal stress. Oecologia. 2008;157(4):593. doi: 10.1007/s00442-008-1102-2. [DOI] [PubMed] [Google Scholar]
- 63.Tremblay P, Gori A, Maguer JF, Hoogenboom M, Ferrier-Pagès C. Heterotrophy promotes the re-establishment of photosynthate translocation in a symbiotic coral after heat stress. Sci. Rep. 2016;6:38112. doi: 10.1038/srep38112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Metalpa RR, Bianchi CN, Peirano A, Morri C. Coral mortality in NW Mediterranean. Coral Reefs. 2000;19(1):24. [Google Scholar]
- 65.Kružić P, Sršen P, Benković L. The impact of seawater temperature on coral growth parameters of the colonial coral Cladocora caespitosa (Anthozoa, Scleractinia) in the eastern Adriatic Sea. Facies. 2012;58(4):477–491. [Google Scholar]
- 66.Peirano A, et al. Monthly variations in calyx growth, polyp tissue, and density banding of the Mediterranean scleractinian Cladocora caespitosa (L.) Coral Reefs. 2005;24:404–409. [Google Scholar]
- 67.Reynaud-Vaganay S, Gattuso JP, Cuif JP, Jaubert J, Juillet-Leclerc A. A novel culture technique for scleractinian corals: application to investigate changes in skeletal δ18O as a function of temperature. Mar. Ecol. Prog. Ser. 1999;180:121–130. [Google Scholar]
- 68.Edmunds PJ. The effect of sub-lethal increases in temperature on the growth and population trajectories of three scleractinian corals on the southern Great Barrier Reef. Oecologia. 2005;146:350–364. doi: 10.1007/s00442-005-0210-5. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka Y, Inoue M, Nakamura T, Suzuki A, Sakai K. Loss of zooxanthellae in coral under high seawater temperature and nutrient enrichment. J. Exp. Mar. Bio. Ecol. 2014;457:220–225. [Google Scholar]
- 70.Marubini F, Davies PS. Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Mar. Biol. 1996;127:319–328. [Google Scholar]
- 71.Marubini F, Atkinson MJ. Effects of lowered pH and elevated nitrate on coral calcification. Mar. Ecol. Prog. Ser. 1999;188:117–121. [Google Scholar]
- 72.Wooldridge SA. Excess seawater nutrients, enlarged algal symbiont densities and bleaching sensitive reef locations: 1. Identifying thresholds of concern for the Great Barrier Reef. Mar. Pollut. Bull. 2016 doi: 10.1016/j.marpolbul.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 73.Hoogenboom MO, Beraud E, Ferrier-Pagès C. Relationship between symbiont density and photosynthetic carbon acquisition in the temperate coral Cladocora caespitosa. Coral Reefs. 2010;29:21–29. [Google Scholar]
- 74.Ferrier-Pagès C, et al. Summer autotrophy and winter heterotrophy in the temperate symbiotic coral Cladocora caespitosa. Limnol. Oceanogr. 2011;56(4):1429–1438. [Google Scholar]
- 75.Rosset S, Wiedenmann J, Reed AJ, D’Angelo C. Phosphate deficiency promotes coral bleaching and is reflected by the ultrastructure of symbiotic dinoflagellates. Mar. Pollut. Bull. 2017;118(1-2):180–187. doi: 10.1016/j.marpolbul.2017.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCook L, Jompa J, Diaz-Pulido G. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral reefs. 2001;19:400–417. [Google Scholar]
- 77.Morrow KM, Bromhall K, Motti CA, Munn CB, Bourne DG. Allelochemicals produced by brown macroalgae of the Lobophora genus are active against coral larvae and associated bacteria, supporting pathogenic shifts to Vibrio dominance. Appl. Environ. Microbiol. 2017;83:e02391–16. doi: 10.1128/AEM.02391-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beatty DS, Clements CS, Stewart FJ, Hay ME. Intergenerational effects of macroalgae on a reef coral: major declines in larval survival but subtle changes in microbiomes. Mar. Ecol. Prog. Ser. 2018;589:97–114. doi: 10.3354/meps12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoogenboom MO, Campbell DA, Beraud E, DeZeeuw K, Ferrier-Pagès C. Effects of light, food availability and temperature stress on the function of photosystem II and photosystem I of coral symbionts. PLoS ONE. 2012;7:e30167. doi: 10.1371/journal.pone.0030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anthony KRN. Coral suspension feeding on fine particulate matter. J. Exp. Mar. Biol. Ecol. 1999;232:85–106. [Google Scholar]
- 81.Tanaka Y, MIiyajama T, Koike I, Hayashibara T, Ogawa H. Imbalanced coral growth between organic tissue and carbonate skeleton caused by nutrient enrichment. Limnol. Oceanogr. 2007;52:1139–1146. [Google Scholar]
- 82.Nordemar I, Nystroem M, Dizon R. Effects of elevated seawater temperature and 759 nitrate enrichment on the branching coral Porites cylindrica in the absence of 760 particulate food. Mar. Biol. 2003;142:669–677. [Google Scholar]
- 83.Wooldridge SA. Water quality and coral bleaching thresholds: Formalising the linkage for the inshore reefs of the Great Barrier Reef. Australia. Mar. Poll. Bull. 2009;58:745–751. doi: 10.1016/j.marpolbul.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 84.Wooldridge SA, Done TJ. Improved water quality can ameliorate effects of climate change on corals. Ecol. App. 2009;19:1492–1499. doi: 10.1890/08-0963.1. [DOI] [PubMed] [Google Scholar]
- 85.Ezzat L, Maguer JF, Grover R, Ferrier-Pagès C. Limited phosphorus availability is the Achilles heel of tropical reef corals in a warming ocean. Sci. Rep. 2016;6:31768. doi: 10.1038/srep31768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marubini F, Ferrier-Pages C, Furla P, Allemand D. Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism. Coral reefs. 2008;27(3):491–499. [Google Scholar]
- 87.Krueger T, et al. Temperature and feeding induce tissue level changes in autotrophic and heterotrophic nutrient allocation in the coral symbiosis–A NanoSIMS study. Sci. Rep. 2018;8(1):12710. doi: 10.1038/s41598-018-31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Slavov C, et al. “Super-quenching” state protects Symbiodinium from thermal stress—implications for coral bleaching. BBA-Bioenergetics. 2016;1857(6):840–847. doi: 10.1016/j.bbabio.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 89.Little A, van Oppen MJH, Willis BL. Flexibility in algal endosymbioses shapes growth in reef corals. Science. 2004;302:1492–1494. doi: 10.1126/science.1095733. [DOI] [PubMed] [Google Scholar]
- 90.Finney JC, et al. The relative significance of host-habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microb Ecol. 2010;6:250–263. doi: 10.1007/s00248-010-9681-y. [DOI] [PubMed] [Google Scholar]
- 91.Rodriguez-Lanetty M, Loh W, Carter D, Hoegh-Guldberg O. Latitudinal variability in symbiont specificity within the widespread scleractinian coral Plesiastrea versipora. Mar. Biol. 2001;138:1175–1181. [Google Scholar]
- 92.LaJeunesse TC, Parkinson J, Reimer JD. A genetic-based description of Symbiodinium minutum sp. nov. and S. psygmophilum sp. nov. (Dinophyceae), two dinoflagellates symbiotic with cnidarian. J. Phycol. 2012;48:1380–1391. doi: 10.1111/j.1529-8817.2012.01217.x. [DOI] [PubMed] [Google Scholar]
- 93.Rodolfo-Metalpa R, Martin S, Ferrier-Pagès C, Gattuso JP. Response of the temperate coral Cladocora caespitosa to mid-and long-term exposure to pCO2 and temperature levels projected for the year 2100 AD. Biogeosciences. 2010;7(1):289–300. [Google Scholar]
- 94.Scheufen T, Krämer WE, Iglesias-Prieto R, Enríquez S. Seasonal variation modulates coral sensibility to heat-stress and explains annual changes in coral productivity. Sci. Rep. 2017;7(1):4937. doi: 10.1038/s41598-017-04927-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strickland JD, Parsons TR. A practical handbook of seawater analysis. J. Fish. Res. Board. Can. 1968;167:1–311. [Google Scholar]
- 96.Tuğrul, S., Yücel, N., & Akcay, I. Chemical oceanography of north eastern Mediterranean. The Turkish Part of the Mediterranean Sea, 15–29 (2016).
- 97.Tréguer, P. & Le Corre, P. Manuel d’analyse des sels nutrifs dans l’eau de mer: utilisation de l’Autoanalyzer II Technicon (R). Université de Bretagne occidentale (1975).
- 98.Smith, S.V., Kinsey, D.W. Calcification and organic carbon metabolism as indicated by carbon dioxide, In Stoddart, D., Johannes, R. (Eds) Coral Reefs: Research Methods. Monographs on Oceanographic Methodology, UNESCO, Paris, pp. 469–484 (1978).
- 99.Dickson AG, Sabine CL. Guide to best practice for ocean CO2 measurements. PICES Special Publication. 2007;3:191. [Google Scholar]
- 100.Naumann MS, et al. Organic matter release by dominant hermatypic corals of the Northern Red Sea. Coral Reefs. 2010;29:649–659. [Google Scholar]
- 101.Smith PK, et al. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 102.Jeffrey SW, Humphrey GF. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975;167:191–194. [Google Scholar]
- 103.Santos SR, et al. Molecular 635 phylogeny of symbiotic dinoflagellates inferred from partial chloroplast large subunit (23S)-636 rDNA sequences. Mol. Phylogenet. Evol. 2002;23:97–111. doi: 10.1016/S1055-7903(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 104.Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4(1):9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All material, data and associated protocols are available from the authors.