OBJECTIVES:
Obesity is an established risk factor of erosive esophagitis, and metabolic unhealthiness has been implicated in the pathogenesis of erosive esophagitis. Yet, the risk of erosive esophagitis among obese individuals without obesity-related metabolic unhealthiness, a condition referred to as “metabolically healthy obese (MHO)”, remains unclear. We examined the association between body mass index (BMI) categories and the development of erosive esophagitis in a cohort of metabolically healthy individuals.
METHODS:
We conducted a cohort study of 14,725 asymptomatic adults free of erosive esophagitis and metabolic abnormalities, who underwent repeated health checkups including screening endoscopy. A metabolically healthy state was defined as having no metabolic syndrome components and a homeostasis model assessment of insulin resistance <2.5. The presence of erosive esophagitis was determined using endoscopy.
RESULTS:
During 81,385.2 person-years of follow-up, 1,865 participants developed erosive esophagitis (incidence rate, 22.9 per 1,000 person-years). The multivariable adjusted hazard ratios (95% confidence intervals) for incident erosive esophagitis comparing overweight (BMI 23.0–24.9) and obese (≥25) with normal-weight participants (18.5–22.9) were 1.12 (1.00–1.25) and 1.29 (1.14–1.47), respectively. In dose-response analyses, increasing BMI also showed positive association with overall and LA-B grade or higher. The association persisted in MHO individuals without central obesity. The association between waist circumference categories and the development of erosive esophagitis was also evident.
DISCUSSION:
In a large cohort of strictly defined metabolically healthy men and women, the MHO phenotype was associated with an increased incidence of erosive esophagitis, providing evidence that the MHO phenotype is not protective from gastroesophageal reflux disease.
INTRODUCTION
Gastroesophageal reflux disease is a widespread gastrointestinal disorder that frequently occurs in primary care settings, imposing considerable burdens on global health and economics (1). The disease has prevalence of 18.1%–27.8% in North America, 8.8%–25.9% in Europe, and 2.5%–7.8% in East Asia; the prevalence continues to increase (2). Among its various risk factors, obesity is considered a significant contributing factor for a spectrum of reflux-related esophageal disorders ranging from erosive esophagitis to Barrett's esophagus to esophageal adenocarcinoma (3–6). Although the exact mechanisms have not been fully identified, several studies have demonstrated that the pattern of body fat distribution may be more important than the general adiposity for increasing the risk of erosive esophagitis (7,8). In addition to the increased intra-abdominal pressure caused by visceral adiposity, metabolically active visceral adipose tissue creates a proinflammatory and insulin-resistant condition (9–12). Thus, the reflux-independent effect of adiposity on erosive esophagitis may contribute to the association, which cannot be solely explained by the mechanical effect of obesity. Indeed, obesity-related hypertension, hyperglycemia, dyslipidemia, and other metabolic abnormalities are significant risk factors for reflux esophagitis (13).
Recent interest has focused on a unique subgroup of obese individuals who do not have metabolic abnormalities, referred to as “metabolically healthy obese (MHO)”, despite their increased adiposity (14,15). Although the role of obesity-induced metabolic abnormalities in erosive esophagitis is previously described, MHO individuals seem to have a favorable profile with no metabolic abnormalities (13,16,17). The association between MHO and erosive esophagitis is largely unknown. The only study available found positive association (18), but the comparison between MHO and nonobese participants could be biased because the reference group included overweight participants, and metabolically unhealthy participants were defined as those with 2 or more metabolic components. Therefore, we examined the association between categories of body mass index (BMI) and the risk of erosive esophagitis in a large sample of metabolically healthy adults.
METHODS
Study population
We conducted a cohort study of healthy participants, who underwent a routine health checkup that included endoscopy. The participants underwent their health checkup at the Center for Health Promotion, Samsung Medical Center, Seoul, South Korea, between August 2006 and December 2011. In South Korea, regular health screenings are very common owing to the Korean Industrial Safety and Health Law. The Korean National Cancer Screening Program recommends biennial gastric cancer screening using endoscopy for people aged 40 years or older.
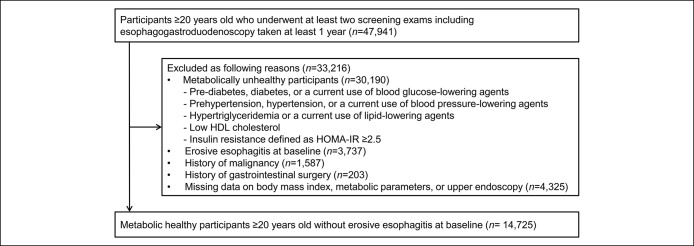
The study population consisted of healthy adults ≥20 years old who underwent at least 2 screening examinations including esophagogastroduodenoscopy (EGD) taken at least 1 year during the study period (n = 47,941). We selected metabolically healthy participants by excluding participant who had any of the following metabolic abnormalities at baseline (n = 33,216) (14): (i) fasting blood glucose (FBG) ≥100 mg/dL or drug treatment for previously diagnosed diabetes; (ii) blood pressure (BP) ≥130/85 mm Hg or drug treatment for previously diagnosed hypertension; (iii) triglyceride levels ≥150 mg/dL or drug treatment for this lipid abnormality; (iv) high-density lipoprotein cholesterol (HDL-C) <40 mg/dL in men or <50 mg/dL in women (19); or (v) insulin resistance defined as homeostasis model assessment of insulin resistance (HOMA-IR) ≥ 2.5 (14). We excluded participants meeting any of the following exclusion criteria: erosive esophagitis at baseline endoscopy (n = 3,737), history of malignancy (n = 1,587), or previous gastrointestinal surgery (n = 203). We also excluded participants with missing data on important covariates: upper endoscopy, BMI, or metabolic parameters (n = 4,325). Finally, 14,725 metabolically healthy adults without erosive esophagitis at baseline were included in this study (Figure 1). This study was approved by the Samsung Medical Center Institutional Review Board and was conducted in accordance with the Declaration of Helsinki and current legal regulations in Korea. Institutional Review Board approval was obtained, but did not require specific informed consent because the study used only de-identified data collected for clinical purposes as part of the health screening. However, informed consent was obtained from all subjects for their examinations during the health checkup.
Figure 1.
Flow diagram of the study participants. HDL, high-density lipoprotein.
Data collection
The comprehensive health-screening program included anthropometric measurements, endoscopy, serum biochemical measurements, and completion of an epidemiological questionnaire regarding smoking habits, alcohol consumption, physical activity, personal medical history, and medication history (including current use) (20). Personal medical history included hypertension, diabetes mellitus, dyslipidemia, and malignancy. Medication history included current use of antihypertensive drugs, hypoglycemic agents, and lipid-lowering drugs. Smoking status was categorized as never, past, or current smoker. Alcohol consumption status was categorized as either nonheavy (≤20 g/d) or heavy (>20 g/d). Regular exercise was defined as exercising ≥3 times/wk at a moderate intensity. The participants' weights and heights were measured while wearing light clothing and with bare feet, respectively. Weight and height were determined to the nearest 0.1 kg and 0.1 cm, respectively; the BMI was calculated as weight (kg) divided by height (m) squared (kg/m2). Waist circumference was measured in a horizontal plane at the midpoint between the inferior margin of the last rib and the superior iliac crest. BP was measured, using an automated BP monitor (Dinamap PRO 100; GE Healthcare, Milwaukee, WI), with the participant seated, after >5 minutes of quiet rest.
After a ≥12 hours fast, blood samples were collected in the morning and analyzed at the hospital laboratory. Serum levels of glucose, total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), and HDL-C were measured using enzymatic colorimetric and liquid-selective detergent methods with Hitachi 7600 (Hitachi, Tokyo, Japan). Serum glucose levels were measured using the hexokinase/glucose-6-phosphate dehydrogenase method with a Hitachi 7600 Modular Dp-110 autoanalyzer (Hitachi, Tokyo, Japan). Serum high-sensitivity C-reactive protein (hsCRP) concentrations were measured using an immunoturbidimetric assay (CRPL3; Roche Diagnostics, Indianapolis, IN). Plasma insulin levels were measured using a radioimmunoassay method with the Packard Cobra II 5010 (Packard Instrument, Baltimore, MD). The inter- and intra-assay coefficients of variation for quality control specimens were <5% for all blood variables. HOMA-IR was used to evaluate insulin resistance, which was calculated as follows: (fasting insulin [μU/mL] × fasting glucose [mg/dL])/405 (21).
Esophagogastroduodenoscopy
Experienced board-certified gastroenterologists performed each endoscopy using a gastroscope (Olympus GIF-Q260; Olympus Medical Systems, Tokyo, Japan). Thirty-four experienced, board-certified, gastroenterologists performed the EGD. They were endoscopists who have completed gastroenterology fellowship. For the 34 gastroenterologists, the median year of graduation from the medical school was 1997 (range, 1989–2003). The primary endpoint was the presence of erosive esophagitis based on the EGD examination. Erosive esophagitis was defined as the presence of definite mucosal breaks (erosions) and was classified according to the Los Angeles classification system (22).
Statistical analysis
Continuous variables are reported as means ± SDs, while categorical variables are presented as percentages. Continuous variables were compared between groups using one-way analysis of variance; categorical variables were compared using the χ2 test. Descriptive statistics were used to summarize the baseline characteristics of the participants by BMI category. Obesity was categorized using Asian-specific criteria (23): underweight, BMI < 18.5 kg/m2; normal weight, BMI of 18.5–22.9 kg/m2; overweight, BMI of 23.0–24.9 kg/m2; and obese, BMI ≥ 25.0 kg/m2 or the Korean Society for the Study of Obesity (waist circumference ≥90 cm in men and ≥85 cm in women) (24). The primary endpoint was the development of incident erosive esophagitis. Participants were followed from the baseline examination until the development of erosive esophagitis or the last health examination among those who did not develop erosive esophagitis.
Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for erosive esophagitis comparing BMI categories at baseline with the normal-weight category or comparing waist circumference categories at baseline (binary or tertile). We used 3 models, with increasing levels of adjustment, to account for potential confounders. Model 1 was adjusted for age (/yr) and sex. Model 2 was further adjusted for smoking status (never/past vs current smoker), alcoholic intake (non-heavy vs heavy), regular exercise (yes vs no), and history of gastroesophageal reflux disorder (GERD) or acid suppressants use (yes vs no). Model 3 was further adjusted for metabolic variables, including FBG, systolic BP, triglyceride, HDL-C, HOMA-IR, and hsCRP as a systemic inflammatory marker, to account for the possible mediation by these metabolic risk factors of the association between obesity and erosive esophagitis. We evaluated the association between BMI categories and risk of erosive esophagitis in the subgroup without central obesity (≥90 cm for men and ≥85 cm for women). The association between waist circumference and risk of erosive esophagitis was also evaluated in the subgroup without general obesity (BMI < 25 kg/m2). Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC); and a P value < 0.05 was considered statistically significant.
RESULTS
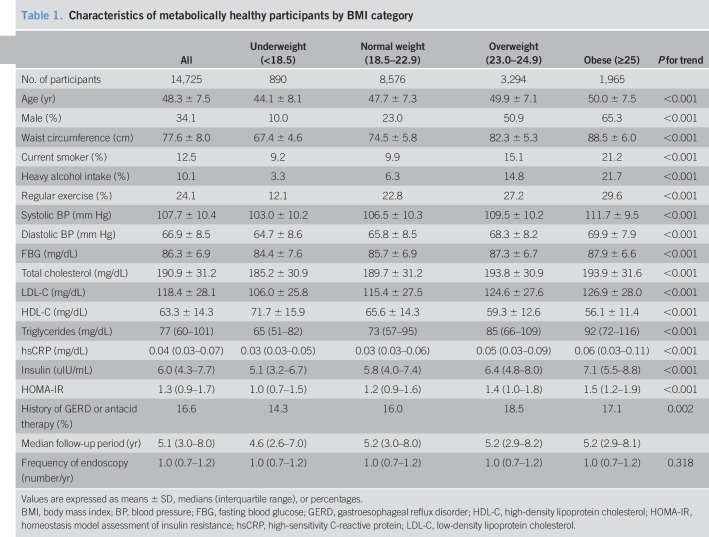
The mean (SD) age of the 14,725 metabolically healthy participants was 48.3 (7.5) years. The baseline characteristics of the participants are shown in Table 1; according to BMI categories, significant differences were observed. The median follow-up time for each of the groups was as follows: 4.6 years for underweight, 5.2 years for normal, 5.2 years for overweight, and 5.2 years for obese participants. The annual frequency (number/yr) of endoscopic examinations performed during the study period was 1.0 (interquartile range, 0.7–1.2) in all groups. Participants in higher BMI categories were more likely to be older, male, current smoker, heavy alcohol drinker, and more likely to exercise regularly. Age, waist circumference, systolic and diastolic BP, FBG, total cholesterol, low-density lipoprotein cholesterol, triglycerides, hsCRP, insulin, and HOMA-IR increased gradually across BMI categories, whereas levels of HDL-C decreased.
Table 1.
Characteristics of metabolically healthy participants by BMI category
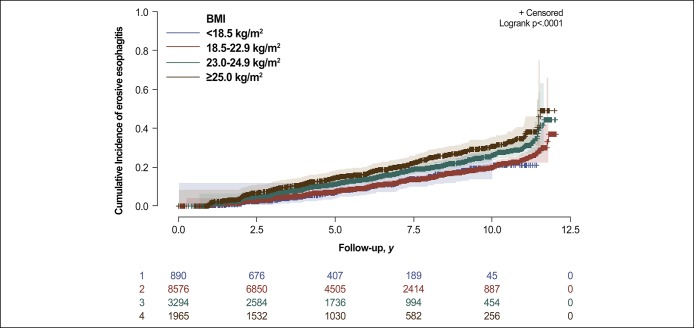
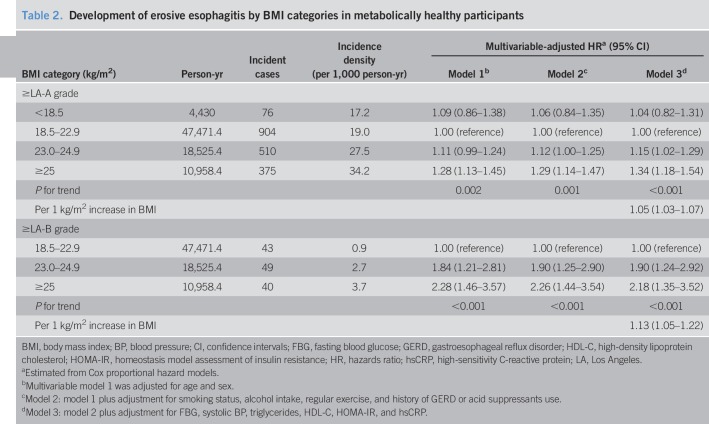
During 81,385.2 person-years of follow-up, 1,865 participants developed erosive esophagitis (incidence rate, 22.9 per 1,000 person-years). In our cohort of metabolically healthy participants, increasing baseline BMI categories showed a positive association with the incidence of erosive esophagitis (Figure 2). The incidence rates (per 1,000 person-years) of erosive esophagitis for each of the BMI categories were 17.2 for underweight, 19.0 for normal weight, 27.5 for overweight, and 34.2 for obese participants (Table 2). In multivariable model 1, adjusted for age and sex, HRs (95% CIs) for incident erosive esophagitis comparing underweight, overweight, and obese participants with normal weight participants were 1.09 (0.86–1.38), 1.11 (0.99–1.24), and 1.28 (1.13–1.45), respectively (P for trend, 0.002). In model 2 adjusted for age, sex, smoking status, alcohol intake, regular exercise, and history of GERD or acid suppressants use, the adjusted HRs (95% CIs) for incident erosive esophagitis comparing underweight, overweight, and obese participants with normal weight participants were 1.06 (0.84–1.34), 1.12 (1.00–1.25), and 1.29 (1.14–1.47), respectively (P for trend, 0.001). To evaluate whether the increased risk of erosive esophagitis with increased BMI was mediated by metabolic risk factors in MHO participants, we conducted additional analyses adjusting for FBG, systolic BP, triglycerides, HDL-C, HOMA-IR, and hsCRP (model 3). After adjusting for the metabolic variables, the association remained significant (model 3). The association between MHO and the incidence of erosive esophagitis at LA-B or higher grade was also observed (adjusted HR, 2.18; 95% CI, 1.35–3.52). In addition, when BMI was introduced as a continuous variable in regression models, adjusted HR associated with a 1 kg/m2 increase was 1.05 (1.03–1.07) for erosive esophagitis ≥ LA-A and 1.13 (1.05–1.22) for erosive esophagitis ≥ LA-B.
Figure 2.
Cumulative incidence of erosive esophagitis, by BMI category at baseline, among metabolically healthy participants. BMI, body mass index.
Table 2.
Development of erosive esophagitis by BMI categories in metabolically healthy participants
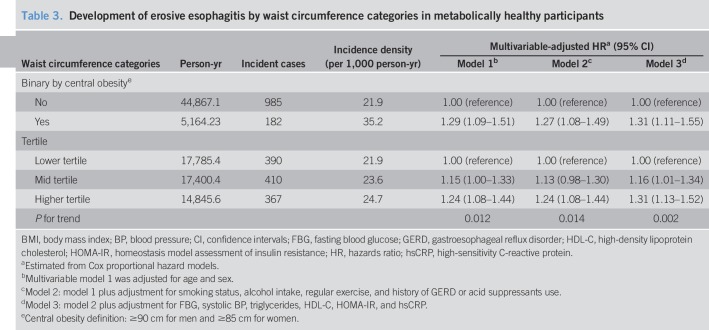
We performed additional analyses to explore whether the association was consistent when obesity criteria based on central obesity by waist circumference were used. MHO by waist circumference categories was still associated with an increased risk of erosive esophagitis (Table 3). In model 2 adjusted for age, sex, smoking status, alcohol intake, and regular exercise, and history of GERD or acid suppressants use, the adjusted HR (95% CI) for incident erosive esophagitis was 1.27 (1.08–1.50) in participants with central obesity compared to those without central obesity. In the multivariable analysis, the adjusted HRs (95% CIs) for developing erosive esophagitis comparing participants with mid and higher tertiles of waist circumference with those with lower tertile were 1.15 (1.00–1.32) and 1.24 (1.07–1.43), respectively (P for trend, 0.014).
Table 3.
Development of erosive esophagitis by waist circumference categories in metabolically healthy participants
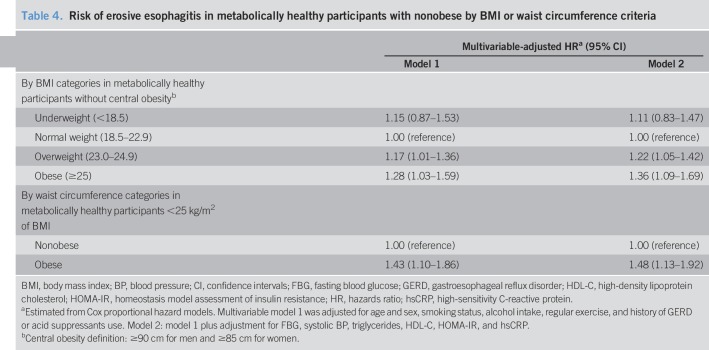
The association between BMI and the risk of erosive esophagitis was observed even among the participants without central obesity (Table 4). In this subgroup, the adjusted HRs (95% CIs) for incident erosive esophagitis comparing overweight and obese with normal-weight participants were 1.17 (1.01–1.36) and 1.28 (1.03–1.59), respectively. In addition, the association between waist circumference and the risk of erosive esophagitis was observed among the participants without general obesity (BMI ≥ 25 kg/m2). The adjusted HR (95% CI) for erosive esophagitis comparing central obesity with normal waist circumference was 1.43 (1.10–1.86).
Table 4.
Risk of erosive esophagitis in metabolically healthy participants with nonobese by BMI or waist circumference criteria
DISCUSSION
In this cohort study of metabolically healthy adults who underwent upper gastrointestinal tract endoscopies, we found that overweight and obese participants were at a higher risk of developing erosive esophagitis than metabolically healthy normal weight participants. This association was consistent after adjustment for metabolic risk factors. In addition, the association between MHO and incident erosive esophagitis was evident even among the subgroup without central obesity. The association between waist circumference categories and the development of erosive esophagitis was also shown. Thus, our findings indicate that MHO is not a harmless condition and it can induce the development of erosive esophagitis.
A previous study of healthy men and women also reported that MHO is associated with an increased risk of erosive esophagitis (18). The previous study has a limitation of a cross-sectional design, which precludes the determination of causality; furthermore, metabolically healthy individuals were defined as those with fewer than 2 metabolic abnormalities. The study also included overweight individuals in the reference group. In contrast, we used a very strict definition for the metabolically healthy status, defined as not having any metabolic abnormalities, including no increase in insulin resistance. Further, we compared MHO participants with metabolically healthy, normal-weight participants in the reference category. Even though we used the very strict definition of metabolic health plus the restriction to normal-weight participants in the reference category, we still found an association between MHO and incident erosive esophagitis that persisted after adjusting for metabolic components, even among participants without central obesity.
Previous evidence has shown a significant association between reflux esophagitis and obesity, especially central obesity (25–27). Although the precise mechanisms linking obesity and reflux esophagitis are not yet fully elucidated, multiple mechanisms have been implicated to account for this association. Central obesity, which is typically measured in terms of waist circumference, waist-hip ratio, or visceral adiposity, seems to be a more important predictor of reflux esophagitis than is general obesity (28). This may be due to the mechanical effect of the increased pressure gradient caused by visceral adiposity inducing reflux esophagitis in individuals with central obesity (10,29). Recently, a new perspective was suggested that reflux esophagitis is also mediated by a metabolic pathway (30–32). Further, esophageal inflammation involving a cytokine-mediated pathway, rather than reflux, has been proposed as a mechanism underlying the pathogenesis of reflux esophagitis (33). This is supported by several studies that have reported positive association between metabolic abnormalities and reflux esophagitis (13,16,18,34–36). However, obesity and metabolic unhealthiness are correlated and may have confounded the outcome. In our study, obesity or central obesity was a risk factor for erosive esophagitis, even in individuals without metabolic unhealthiness. This suggests that general obesity or central obesity by itself may be a sufficient risk factor for erosive esophagitis. Our results reinforced the classic view that obesity or central obesity increases the risk of reflux esophagitis. This is most likely because of the increased intraabdominal pressure, increased transient lower esophageal sphincter relaxation and esophageal acid exposure brought on by obesity or central obesity.
Several limitations need to be considered when interpreting the results of our study. First, we used BMI as a measure of obesity; however, BMI does not contain information on the distribution and composition of fat and lean tissues. If the MHO participants had higher proportion of lean mass, then the association between the MHO phenotype and the risk of erosive esophagitis may have been attenuated. However, we also used waist circumference as a measure of obesity, which is more correlated with the distribution and accumulation of fat tissue than BMI. The association between waist circumference categories in metabolically healthy individuals and the development of erosive esophagitis was also evident. Second, we used a single measurement of BMI at baseline and did not incorporate changes in BMI in the analysis. Third, interobserver variations in the endoscopic diagnoses of erosive esophagitis were not evaluated. However, experienced board-certified gastroenterologists performed the endoscopies, and erosive esophagitis was clearly defined; the same classification system was used for all of the participants. Fourth, although we measured several important confounders in the multivariable analysis, we cannot exclude the possibility of residual confounding due to unmeasured parameters such as dietary variables and socioeconomic status. Finally, this study focused on healthy participants who underwent routine health checkups; thus, our findings may not be generalized to other populations.
This study also has several strengths. First, it was a cohort study, with a relatively large sample size, and the exclusion of baseline erosive esophagitis cases allowed us to identify a temporal relationship, which is not usually possible in cross-sectional studies. Additional strengths include the use of high-quality standardized anthropometric measurements, the incorporation of an epidemiological questionnaire regarding lifestyle factors, and the inclusion of various laboratory studies.
In conclusion, this study showed that excess body weight or increase in waist circumference was associated with an increased risk of erosive esophagitis, in the absence of a metabolic unhealthy state. This association was consistent after adjustment for metabolic risk factors. Even in the absence of central obesity, MHO was a risk factor for erosive esophagitis. Therefore, physicians should adequately address the increased risk of erosive esophagitis in MHO individuals and counsel them about the importance of maintaining healthy weight and lifestyle.
CONFLICTS OF INTEREST
Guarantor of the article: Hyuk Lee, MD, PhD.
Specific author contributions: Study concept and design: H.L. Acquisition, analysis, or interpretation of data: T.J.K. and H.L. Writing and Drafting of the manuscript: T.J.K. Critical revision of the manuscript for important intellectual content: Y.W.M., B.-H.M., J.H.L., H.J.S., P.-L.R., and J.J.K. Statistical analysis: S.-Y.B. and K.K. All authors approved the final submission.
Financial support: None.
Potential competing interests: None.
Study Highlights.
WHAT IS KNOWN
✓ Obesity and metabolic abnormalities are risk factors for some diseases, including reflux esophagitis.
✓ However, MHO, that is, persons who have obesity without metabolic abnormalities is controversial on the health implications.
✓ The risk of erosive esophagitis among MHO individuals is unknown.
WHAT IS NEW HERE
✓ MHO phenotype was associated with an increased risk of incident erosive esophagitis.
✓ Obesity can increase the risk of erosive esophagitis, regardless of metabolic abnormalities.
TRANSLATIONAL IMPACT
✓ Physicians should adequately address the increased risk of erosive esophagitis in MHO individuals and counsel them about the importance of maintaining healthy weight.
REFERENCES
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179–87 e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2014;63:871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol 2011;8:340–7. [DOI] [PubMed] [Google Scholar]
- 4.Turati F, Tramacere I, La Vecchia C, et al. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013;24:609–17. [DOI] [PubMed] [Google Scholar]
- 5.Friedenberg FK, Xanthopoulos M, Foster GD, et al. The association between gastroesophageal reflux disease and obesity. Am J Gastroenterol 2008;103:2111–22. [DOI] [PubMed] [Google Scholar]
- 6.Yadlapati R, Pandolfino JE, Alexeeva O, et al. The reflux improvement and monitoring (TRIM) program is associated with symptom improvement and weight reduction for patients with obesity and gastroesophageal reflux disease. Am J Gastroenterol 2018;113:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelstein ZR, Farrow DC, Bronner MP, et al. Central adiposity and risk of Barrett's esophagus. Gastroenterology 2007;133:403–11. [DOI] [PubMed] [Google Scholar]
- 8.Rubenstein JH, Kao JY, Madanick RD, et al. .Association of adiponectin multimers with Barrett's oesophagus. Gut 2009;58:1583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anggiansah R, Sweis R, Anggiansah A, et al. The effects of obesity on oesophageal function, acid exposure and the symptoms of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2013;37:555–63. [DOI] [PubMed] [Google Scholar]
- 10.Robertson EV, Derakhshan MH, Wirz AA, et al. Central obesity in asymptomatic volunteers is associated with increased intrasphincteric acid reflux and lengthening of the cardiac mucosa. Gastroenterology 2013;145:730–9. [DOI] [PubMed] [Google Scholar]
- 11.Souza RF, Huo X, Mittal V, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 2009;137:1776–84. [DOI] [PubMed] [Google Scholar]
- 12.Lee H. Weight management as a treatment option for gastroesophageal reflux disease: A mechanical or metabolic rescuer? Gut Liver 2018;12:607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung SJ, Kim D, Park MJ, et al. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: A cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut 2008;57:1360–5. [DOI] [PubMed] [Google Scholar]
- 14.Primeau V, Coderre L, Karelis AD, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35:971–81. [DOI] [PubMed] [Google Scholar]
- 15.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 2008;168:1617–24. [DOI] [PubMed] [Google Scholar]
- 16.Niigaki M, Adachi K, Hirakawa K, et al. Association between metabolic syndrome and prevalence of gastroesophageal reflux disease in a health screening facility in Japan. J Gastroenterol 2013;48:463–72. [DOI] [PubMed] [Google Scholar]
- 17.Wu P, Ma L, Dai GX, et al. The association of metabolic syndrome with reflux esophagitis: A case-control study. Neurogastroenterol Motil 2011;23:989–94. [DOI] [PubMed] [Google Scholar]
- 18.Baeg MK, Ko SH, Ko SY, et al. Obesity increases the risk of erosive esophagitis but metabolic unhealthiness alone does not: A large-scale cross-sectional study. BMC Gastroenterol 2018;18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- 20.Kim TJ, Sinn DH, Min YW, et al. A cohort study on helicobacter pylori infection associated with nonalcoholic fatty liver disease. J Gastroenterol 2017;52:1201–10. [DOI] [PubMed] [Google Scholar]
- 21.Kim TJ, Shin HY, Chang Y, et al. Metabolically healthy obesity and the risk for subclinical atherosclerosis. Atherosclerosis 2017;262:191–7. [DOI] [PubMed] [Google Scholar]
- 22.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: Clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anuurad E, Shiwaku K, Nogi A, et al. The new BMI criteria for Asians by the regional office for the Western Pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J Occup Health 2003;45:335–43. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Park HS, Kim DJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract 2007;75:72–80. [DOI] [PubMed] [Google Scholar]
- 25.Corley DA, Kubo A. Body mass index and gastroesophageal reflux disease: A systematic review and meta-analysis. Am J Gastroenterol 2006;101:2619–28. [DOI] [PubMed] [Google Scholar]
- 26.El-Serag H. The association between obesity and GERD: A review of the epidemiological evidence. Dig Dis Sci 2008;53:2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray L, Johnston B, Lane A, et al. Relationship between body mass and gastro-oesophageal reflux symptoms: The Bristol Helicobacter Project. Int J Epidemiol 2003;32:645–50. [DOI] [PubMed] [Google Scholar]
- 28.Singh S, Sharma AN, Murad MH, et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1399–412 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YY, McColl KE. Disruption of the gastroesophageal junction by central obesity and waist belt: Role of raised intra-abdominal pressure. Dis Esophagus 2015;28:318–25. [DOI] [PubMed] [Google Scholar]
- 30.Boeckxstaens G, El-Serag HB, Smout AJ, et al. Symptomatic reflux disease: The present, the past and the future. Gut 2014;63:1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilg H, Moschen AR. Visceral adipose tissue attacks beyond the liver: Esophagogastric junction as a new target. Gastroenterology 2010;139:1823–6. [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Lim Y, Chi S, et al. Relationship between obesity and development of erosive reflux disease: A mediation analysis of the role of cardiometabolic risk factors. Sci Rep 2017;7:6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunbar KB, Agoston AT, Odze RD, et al. Association of acute gastroesophageal reflux disease with esophageal histologic changes. JAMA 2016;315:2104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu CS, Wang PC, Chen JH, et al. Increasing insulin resistance is associated with increased severity and prevalence of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2011;34:994–1004. [DOI] [PubMed] [Google Scholar]
- 35.Pointer SD, Rickstrew J, Slaughter JC, et al. Dietary carbohydrate intake, insulin resistance and gastro-oesophageal reflux disease: A pilot study in European- and African-American obese women. Aliment Pharmacol Ther 2016;44:976–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tai CM, Lee YC, Tu HP, et al. The relationship between visceral adiposity and the risk of erosive esophagitis in severely obese Chinese patients. Obesity (Silver Spring) 2010;18:2165–9. [DOI] [PubMed] [Google Scholar]