OBJECTIVES:
Small intestinal bacterial overgrowth (SIBO) can complicate chronic pancreatitis (CP) and interfere with management. Its predisposing factors in CP and treatment response are unknown. In this review, we evaluated factors affecting disease burden.
METHODS:
A computerized search of PubMed and EMBASE databases from inception through May 2019 was done for studies correlating SIBO with CP. Studies were screened, and relevant data were extracted and analyzed. Pooled prevalence, odds ratio (OR), and meta-regression were performed using the random effects model as classically described by Borenstein et al. (2009). SIBO's relation to diabetes mellitus (DM), pancreatic exocrine insufficiency (PEI), narcotic use, and proton-pump inhibitor use was investigated. Treatment response was pooled across studies. P value < 0.05 was considered significant.
RESULTS:
In 13 studies containing 518 patients with CP, SIBO prevalence was 38.6% (95% confidence interval [CI] 25.5–53.5). OR for SIBO in CP vs controls was 5.58 (95% CI 2.26–13.75). Meta-regression showed that PEI and the diagnostic test used were able to explain 54% and 43% of the variance in SIBO prevalence across studies, respectively. DM and PEI were associated with increased SIBO in CP with OR (2.1, 95% CI 1.2–3.5) and OR (2.5, 95% CI 1.3–4.8), respectively. Symptomatic improvement was reported in 76% of patients after SIBO treatment.
DISCUSSION:
SIBO complicates 38% of CP with OR of 5.58 indicating a predisposition for this condition. PEI correlates with SIBO in CP and might play a role in pathophysiology. DM and PEI are associated with increased SIBO in CP. Treatment of SIBO may lead to symptomatic improvement.
INTRODUCTION
Chronic pancreatitis (CP) is an inflammatory disorder involving injury and scarring of the pancreatic exocrine gland, which can also affect endocrine components. It has a global incidence of 10 per 100,000 population (1). CP results in a variety of signs and symptoms, including abdominal pain, weight loss, bloating, steatorrhea, malabsorption, and diarrhea. CP complications include pancreatic exocrine insufficiency (PEI), postpancreatitis diabetes mellitus (DM), and pancreatic cancer. Management of CP is challenging with multiple modalities targeting symptoms and malnutrition through pain management, pancreatic enzyme replacement therapy (PERT), and, in severe cases, surgery including total pancreatectomy. Despite advancements in CP treatment, 43% of patients do not respond to conventional therapy (2).
Recent evidence suggests that pancreatic exocrine dysfunction is a major determinant of intestinal microbiota (3). Changes in the gut microbial composition have been linked to a wide array of disorders spanning infectious (4), autoimmune (5), functional (6), neoplastic (7), and gastrointestinal (GI) pathologies. Multiple reports have linked CP to microbial dysbiosis, mainly small intestinal bacterial overgrowth (SIBO). SIBO has classically been linked to blind-loop–producing GI surgeries; the resulting combination of altered anatomy, altered motility, hypochlorhydria, and exposure to colonic contents creates a favorable environment for bacterial proliferation (8). SIBO is associated with inflammatory bowel disease (9,10), neurological disorders (11,12), celiac disease (13), and irritable bowel syndrome (14). SIBO symptoms include abdominal pain, bloating, diarrhea, and malabsorption, which overlap with CP and make the diagnosis and management of SIBO in CP all the more difficult. Although Capurso et al. (15) showed in 2016 that SIBO affects 36% of patients with CP, we noted a high level of heterogeneity in the reported SIBO prevalence. In addition, their review lacked an analysis of SIBO's response to treatment or of risk factors for SIBO in CP. Moreover, we found that 3 additional studies (16–18) had been published on this subject since then. Therefore, we performed a comprehensive systematic review and meta-analysis employing rigorous statistical analysis using meta-regression models to explain heterogeneity, determine factors affecting disease prevalence, and response to treatment of SIBO in CP.
METHODS
Eligibility criteria
The primary criterion for eligibility was the availability of data on SIBO incidence in clinically diagnosed CP/PEI. Although confounding factors such as alcohol and narcotic use were often reported, because of sparsity of data, studies that did not statistically account for confounders were included. No restrictions were applied on study design, including CP definition adopted by investigators. Adequate description of diagnostic technique of CP (clinical history, imaging and functional studies) and PEI (stool elastase/fat excretion) was required. Adequate description of SIBO diagnosis was required. Neither time period nor language had effect on eligibility. Conference abstracts were considered ineligible.
Search technique
A computerized search of the MEDLINE and EMBASE databases from inception to May 2019 was performed. Search terms were as follows: chronic pancreatitis, pancreatic exocrine insufficiency, breath tests, small intestine, bacterial infections, bacterial overgrowth, lactulose hydrogen, glucose hydrogen, and jejunal aspirate. Duplicates were excluded. Abstracts of remaining articles were reviewed. Unrelated articles were excluded. The remaining articles were reviewed in detail for eligibility criteria.
Data extraction
For each study, data were extracted on year published; study design; number of patients/controls; participant age and sex; etiology of CP; method of CP diagnosis; prevalence of DM, alcohol, proton-pump inhibitor (PPI), and narcotic use; surgical history; PERT; control group characteristics; SIBO diagnostic test description, including substrate dose, test duration, sampling intervals, and positive test criteria; SIBO treatment; and treatment outcomes. Finally, SIBO prevalence in both CP and controls was extracted. Study quality was assessed using the Newcastle-Ottawa scale (NOS) for cross-sectional and case-control studies. Cutoff of 6 or higher was considered good quality, whereas <6 was considered poor quality.
Statistical analysis
Pooled prevalence of SIBO in multiple subsets of patients with CP was calculated. Random effects model was used to calculate pooled prevalence estimates with 95% confidence intervals (CIs). Heterogeneity was assessed using the I2 measure and the Cochran Q-statistic. Odds ratio (OR) of SIBO in CP compared with controls was calculated based on events/total ratios of both groups. The following stratified analyses were conducted to address sources of heterogeneity and determine factors affecting SIBO prevalence in CP: (i) PEI, (ii) DM, (iii) PERT use, (iv) alcoholic CP, (v) mean age, (vi) SIBO diagnostic method, (vii) surgical history, (viii) study quality per NOS, (ix) PPI use, and (x) narcotic use. Meta-regression was performed as classically described (19) using (i)–(iii) (above) as covariates in logistic regression models aimed at uncovering correlations between such covariates and SIBO prevalence, which might explain heterogeneity. Publication bias was assessed for using the Begg and Mazumdar test. Statistical analysis was performed using the software programs Comprehensive Meta-Analysis version 3.3.070 (Biostat, Englewood, NJ) and Review Manager (RevMan) version 5.3.5 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Denmark).
RESULTS
Our search identified 2,236 references. Initial screening included review of titles to exclude duplicates, and subsequently, abstracts were screened for relevance. A total of 2,192 studies were excluded as duplicates, conference abstracts, or unrelated to the review subject. This resulted in 44 studies that were reviewed in detail, 13 of which met our eligibility criteria. Only 8 studies compared SIBO event rates in CP vs controls and were included in the comparative analysis (Figure 1).
Figure 1.
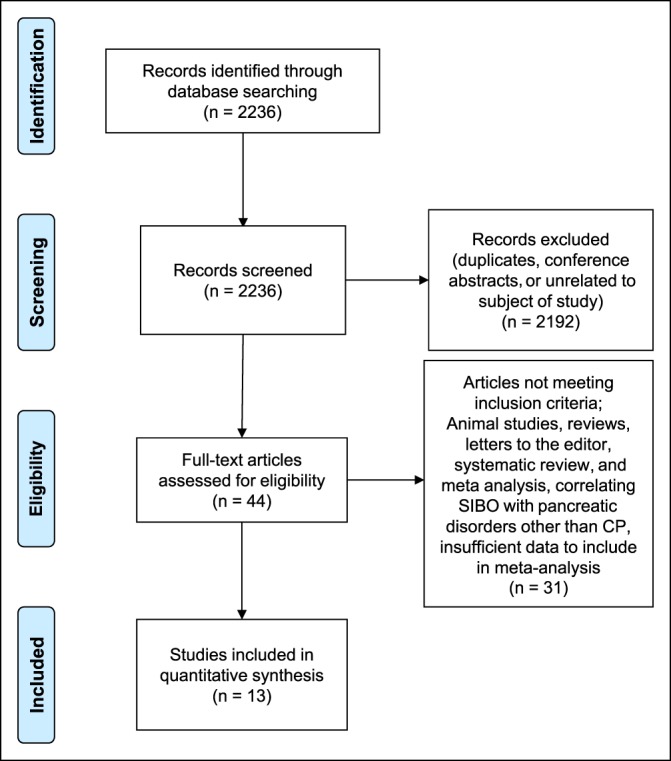
Flow diagram of reference allocation. CP, chronic pancreatitis; SIBO, small intestinal bacterial overgrowth.
Study breakdown
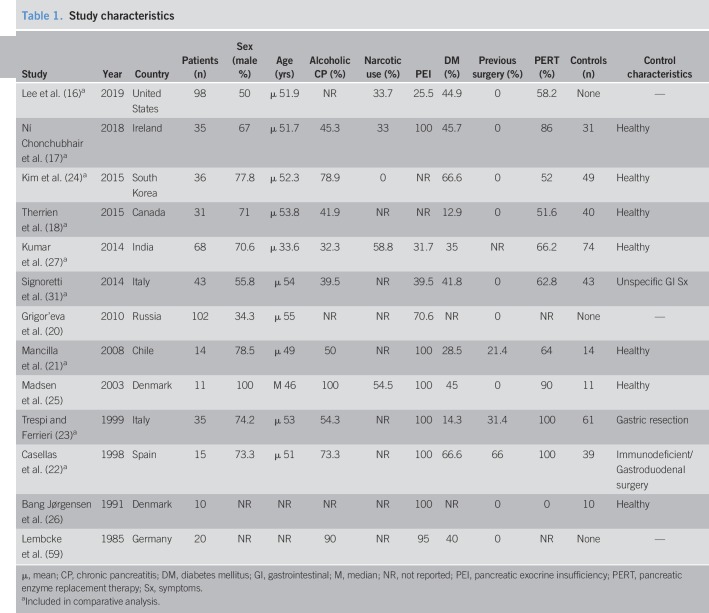
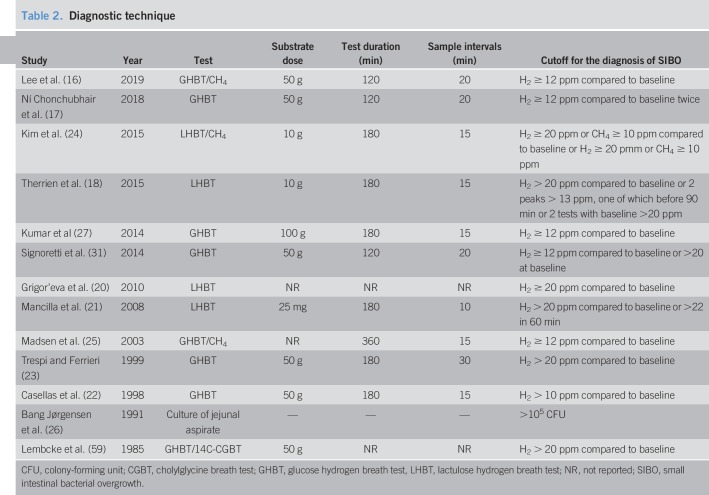
The studies were conducted in 11 countries; 6 European, 2 Asian, 1 South American, and 2 North American. They were published between 1985 and 2019. Twelve studies were published in English, 1 in Russian (20), and 1 in Spanish (21). Ten case-control and 3 cross-sectional studies examining 518 patients with CP and 372 controls were included. Study characteristics are depicted in Table 1. Three studies included CP patients with a history of gastroduodenal or pancreatic surgery (gastrectomy, vagotomy, pancreaticoduodenectomy, and Puestow procedure) (21–23), and 2 of those also included surgical patients in the control group (22,23). Eight studies used glucose hydrogen breath test (GHBT), 4 used lactulose hydrogen BT (LHBT), and 1 used jejunal aspirate culture. Three studies combined breath methane with LHBT/GHBT (16,24,25). Test techniques are listed in Table 2. CP diagnostic method is listed in Table S2 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A93). The individual studies found no association between patient-age, sex, or PEI and SIBO prevalence. Only 2 studies (16,17) found DM and PERT to be more prevalent in SIBO patients. Nine of 13 studies were of high quality on the NOS, and the remaining 4 were considered of poor quality (Table S1, see Supplementary Digital Content 1, http://links.lww.com/CTG/A93). Sensitivity analysis excluding poor-quality studies did not significantly alter analysis results.
Table 1.
Study characteristics
Table 2.
Diagnostic technique
Prevalence of SIBO in CP
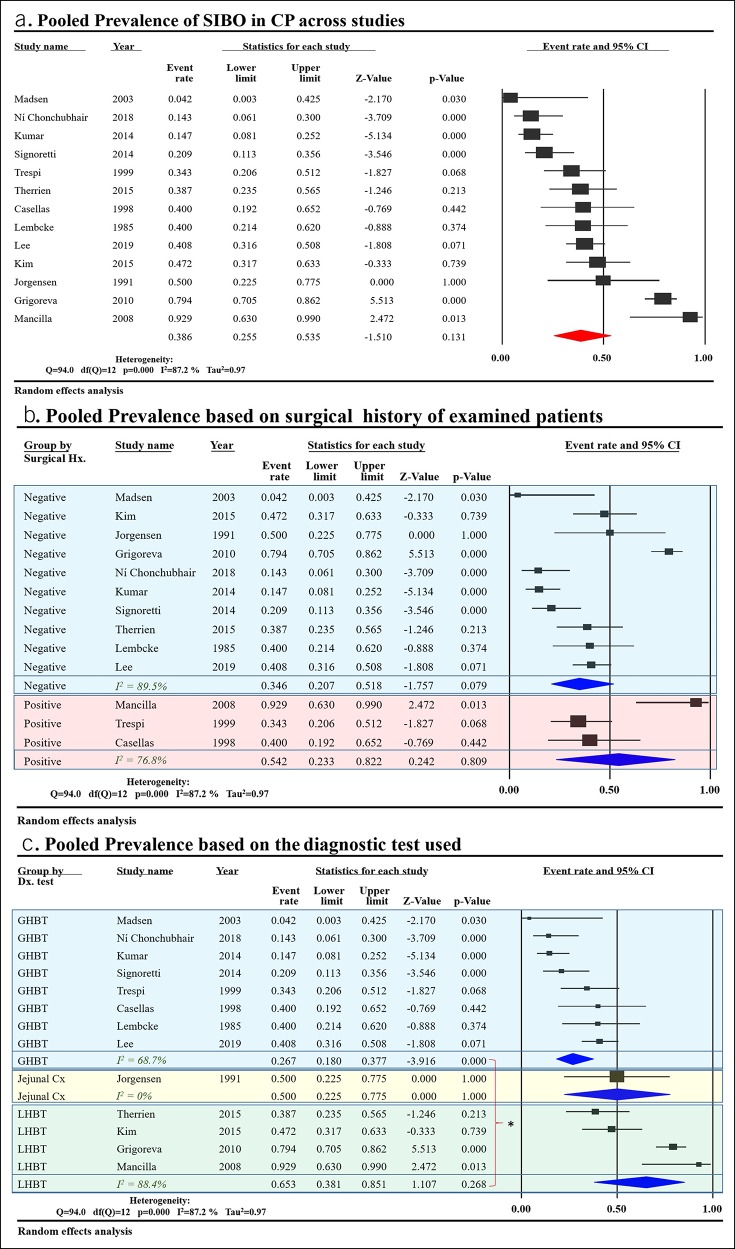
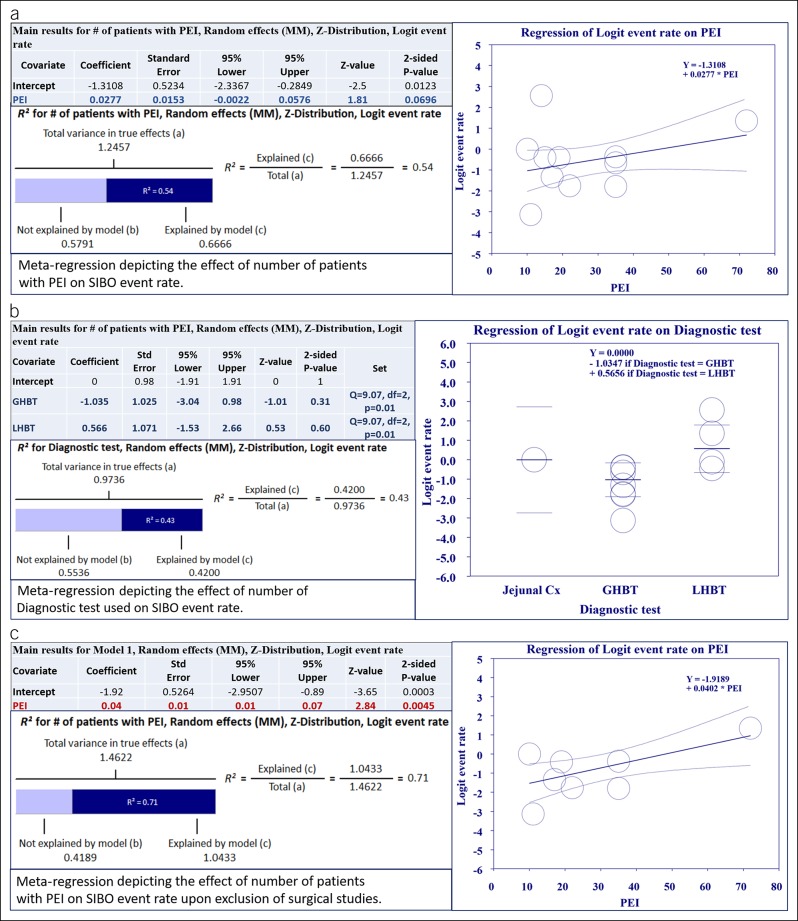
The studies reported prevalence in a range from 0%–93%. Pooled prevalence of SIBO in CP across the 13 included studies was 38.6% (95% CI 25.5%–53.5%) with considerable heterogeneity (I2 = 87%) (Figure 2a) compared with 9.9% (95% CI 4.9%–19%) in controls. The studies were subgrouped based on the inclusion of subjects with a GI surgical history. Pooled prevalence was 34.6% (95% CI 20.7%–51.8%) in nonsurgical studies and 54.2% (95% CI 23.3%–82.2%) in surgical ones (Figure 2b). When subgrouping based on the diagnostic test used, pooled prevalence in 8 studies using GHBT was 26.7% (95% CI 18.0%–37.7%) compared with 65.3% (95% CI 38.1%–85.1%) in the 4 studies using LHBT (Figure 2c). The one study using jejunal aspirate cultures showed a prevalence of 50% (26). To further understand the sources of heterogeneity between studies, we performed a meta-regression analysis taking into account covariates such as surgical history of subjects, number of patients with PEI, DM, PERT, alcoholic etiology of CP, age, and diagnostic test used. Only “number of patients with PEI” and “diagnostic test used” were able to explain the observed variance. Meta-regression models of these 2 covariates were able to explain 55% and 43% of the variance in prevalence between the studies, respectively (Figure 3a,b).
Figure 2.
PP of SIBO in CP data: (a) Shows PP across the 12 included studies. (b) Shows PP in studies subgrouped by inclusion of patients with a surgical history concomitant with CP. As indicated by overlap in CI, there was no significant difference between PP in studies which included CP patients with a surgical history and those which excluded surgical patients. (c) Shows PP in studies subgrouped by the diagnostic test used. As indicated by nonoverlap in CI, the use of LHBT results in a significantly higher PP of SIBO in CP than GHBT (*P < 0.05). CI, confidence interval; CP, chronic pancreatitis; GHBT, glucose hydrogen breath test; LHBT, lactulose hydrogen breath test; PP, pooled prevalence; SIBO, small intestinal bacterial overgrowth.
Figure 3.
Meta-regression results for PP analysis: (a) Meta-regression exploring the relationship between the SIBO event rate in CP and number of patients with PEI. As indicated in the accompanying table, correlation was not statistically significant. Despite that, the regression model was able to explain 54% (R2) of between-study variance in PP. (b) Meta-regression exploring the relationship between the SIBO event rate and the diagnostic test used. As indicated in the accompanying table, the correlation was statistically significant. The logistic regression model was able to explain 43% (R2) of between-study variance in PP. (c) Meta-regression exploring the relationship between the SIBO event rate in CP and number of patients with PEI after exclusion of studies with surgical patients. As indicated in the accompanying table, the correlation was statistically significant and able to explain 71% (R2) of between-study variance in PP. R2 calculation: (a) To compute the total variance (of all studies about the grand mean), we run the regression with no covariates. (b) To compute the variance not explained by the model (of all studies about the regression line), we run the regression with the covariates. (c) The difference between these values gives us the variance explained by the model. CP, chronic pancreatitis; GHBT, glucose hydrogen breath test; LHBT, lactulose hydrogen breath test; PEI, pancreatic exocrine insufficiency; PP, pooled prevalence; SIBO, small intestinal bacterial overgrowth.
A sensitivity analysis excluding studies with surgical patients was performed; 8 studies remained and showed a significant correlation of SIBO prevalence with number of patients with PEI. This model was able to explain 71% of variance (Figure 3c).
Factors affecting SIBO in CP
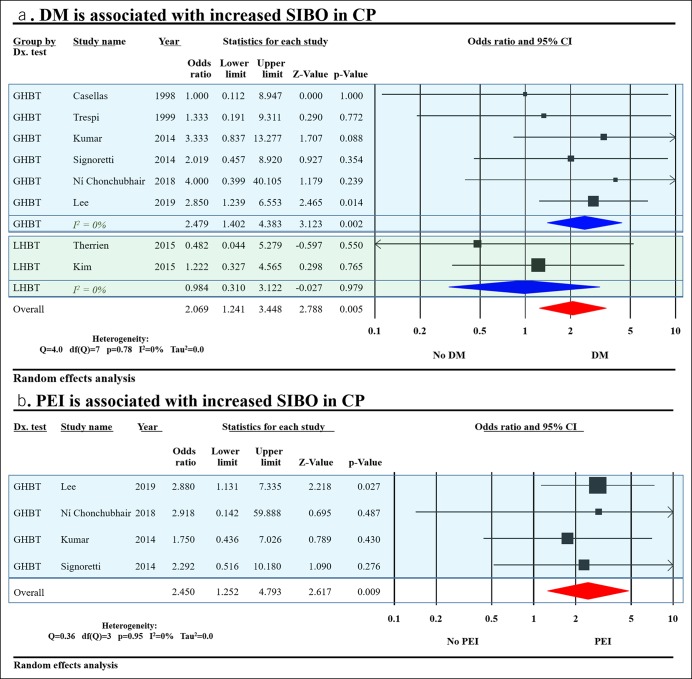
Eight studies evaluated the effect of DM on SIBO in CP and found it was associated with increased SIBO with OR (2.1, 95% CI 1.2–3.5) (Figure 4a). After stratification by the diagnostic test used, DM was found to be associated with increased SIBO in patients tested with GHBT only and not in those tested with LHBT. Four studies evaluated the effect of PEI on SIBO in CP, and it was associated with increased SIBO with OR (2.5, 95% CI 1.3–4.8) (Figure 4b). Further analysis of studies evaluating the effects of PPI, narcotic use, and PERT did not yield significant increase in SIBO (Figure S2A-C, see Supplementary Digital Content 1, http://links.lww.com/CTG/A93).
Figure 4.
Factors affecting SIBO in CP: (a) DM was associated with increased SIBO in CP. (b) PEI was associated with increased SIBO in CP. CI, confidence interval; CP, chronic pancreatitis; DM, diabetes mellitus; GHBT, glucose hydrogen breath test; LHBT, lactulose hydrogen breath test; PEI, pancreatic exocrine insufficiency; SIBO, small intestinal bacterial overgrowth.
SIBO in CP compared with controls
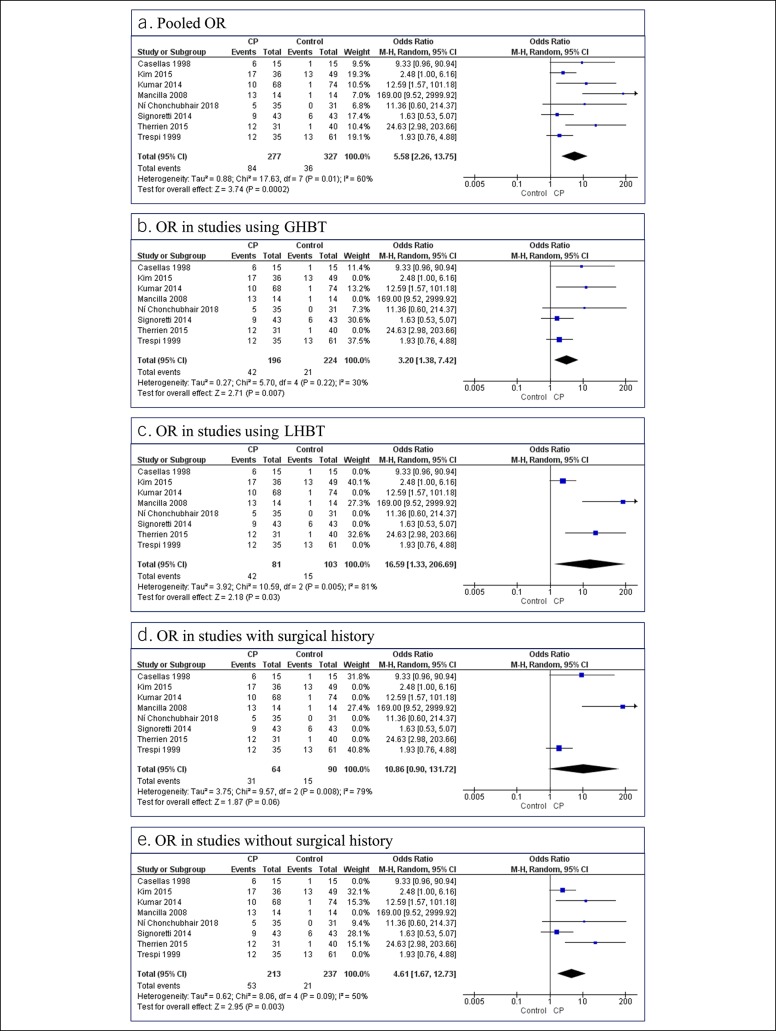
Eight studies compared the event rate of SIBO in CP with controls and were included in this analysis. The OR for a positive test in CP vs controls was 5.58 (95% CI 2.26–13.75) (I2 = 60%) (Figure 5a). Subgroup analysis showed an OR of 16.6 (95% CI 1.33–206.69) (I2 = 81%) for SIBO in CP in 3 studies using LHBT, compared with 3.2 (95% CI 1.38–7.42) (I2 = 30%) in 5 studies using GHBT (Figure 5b,c). Upon subgrouping based on surgical history, 3 studies using patients with a surgical history showed an OR of 10.86 (95% CI 0.90–131.72) (I2 = 79%), whereas 5 studies excluding patients with a surgical history yielded an OR of 4.61 (95% CI 1.67–12.73) (I2 = 50%) (Figure 5d,e).
Figure 5.
(a–e) Results of comparative analysis: All 8 studies included in the comparative analysis are listed in all figures for reference. Excluded studies were removed from forest plots. CI, confidence interval; CP, chronic pancreatitis; GHBT, glucose hydrogen breath test; LHBT, lactulose hydrogen breath test; OR, odds ratio.
Publication bias was assessed for using 2 funnel plots (Figure S1, see Supplementary Digital Content 1, http://links.lww.com/CTG/A93) and the Begg and Mazumdar test. No publication bias was found.
Clinical presentation and response to treatment
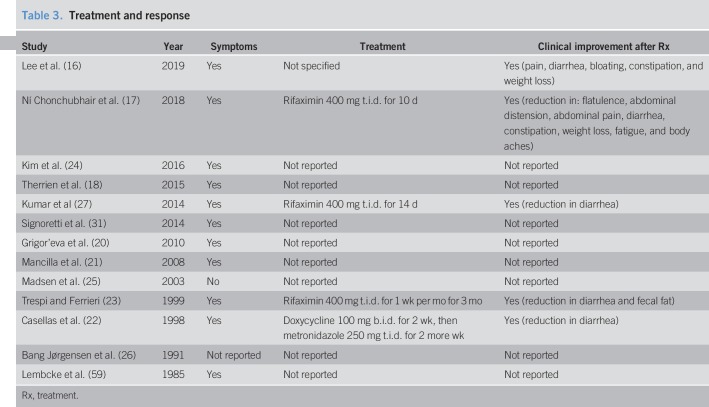
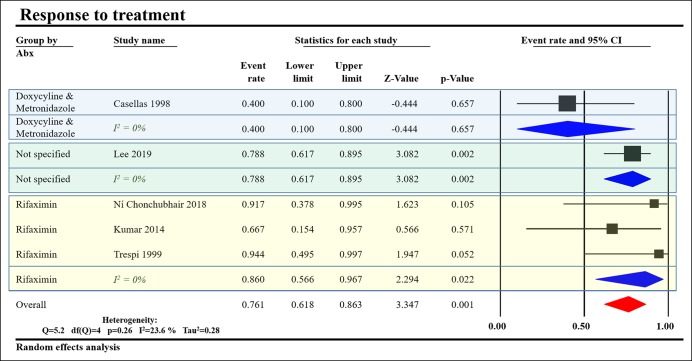
All studies except that by Madsen et al. (25) reported symptoms in the SIBO group refractory to PERT. Five studies reported a trial of antibiotics, 3 studies used rifaximin (17,23,27), Casellas et al. (22) used doxycycline followed by metronidazole, and Lee et al. (16) used an unspecified antibiotic regimen (Table 3). We pooled the data from these studies to show that 86% of patients treated with rifaximin showed symptomatic improvement compared with 40% of those treated with doxycyline and metronidazole (Figure 6).
Table 3.
Treatment and response
Figure 6.
Response to treatment based on the type of antibiotic used. Treatment with rifaximin showed a higher response than doxycycline and metronidazole. Subgrouping by antibiotic regimen was able to eliminate heterogeneity in treatment response as evidenced by drop of I2 from 38.3% to 0% in all subgroups. CI, confidence interval.
DISCUSSION
This meta-analysis provides the latest evidence on the association between SIBO and CP. Although SIBO prevalence was highly heterogeneous, it was more likely to be present in patients with CP than controls with an OR of 5.58 (95% CI 2.26–13.75). The type of the diagnostic test used significantly affected SIBO prevalence with LHBT, showing a higher prevalence than GHBT. Studies including patients with a surgical history had higher SIBO prevalence, although this was not statistically significant. We found DM and PEI to be associated with significant increases in SIBO in CP. In addition, we note that PEI correlates with SIBO prevalence and explains 54%–71% of the heterogeneity noted (Figure 3a–c). We found symptomatic improvement in 86% of patients treated with rifaximin compared with 40% of those treated with doxycycline and metronidazole (Figure 6), suggesting an alternative treatment strategy for patients with CP unresponsive to PERT.
The etiology of SIBO in CP is unclear. CP-associated intestinal dysmotility can lead to small bowel stasis (28–30), thereby increasing the likelihood of SIBO. The increased consumption of alcohol and narcotics in patients with CP was also suggested (18). In this review, 6 studies reported on alcoholic CP's relationship to SIBO (16–18,21,23,31), and only Ní Chonchubhair et al. (17) found it to have higher SIBO rates than nonalcohol CP. Narcotic use, investigated in 3 studies (16,17,27), showed no relation to SIBO prevalence (Figure S2C, see Supplementary Digital Content 1, http://links.lww.com/CTG/A93). In addition, the use of PPI is common in CP and might be a risk factor for SIBO, which was evaluated by 3 studies (16,17,31) in this review showing no significant relation to SIBO prevalence (Figure S2B, see Supplementary Digital Content 1, http://links.lww.com/CTG/A93). DM is associated with SIBO (32) and can affect up to 83% of patients with CP (33). We found it was associated with increased SIBO in CP (Figure 5a). Whether DM is reflective of pancreatic endocrine dysfunction leading to increased risk of SIBO is yet to be verified. Although the decline in pancreatic trypsin can disrupt the activation of defensin, hindering pancreatic antibacterial activity (34,35), the inhibition of pancreatic proteolytic enzymes does not impair this antibacterial activity (34). In addition, in canine models of PEI, PERT did not prevent intestinal microbial disruption (36). However, substituting cathelicidin-related antimicrobial peptide in mice deficient in acinar Orai1 protected them from fatal bacterial overgrowth (37). This suggests a direct effect of antimicrobials secreted by pancreatic acini and independent of digestive enzymes, which is consistent with recent findings by Frost et al. (3) showing exocrine pancreatic function to be a significant determinant of gut microbial load and diversity. In this review, we found PEI to be associated with increased SIBO in CP. We also found PEI to correlate well with SIBO prevalence, suggesting that antimicrobial pancreatic secretions might play a role in the pathogenesis of SIBO in CP. These results raise the question of whether direct endocrine/exocrine pancreatic gland destruction in CP is more important for SIBO development than external factors such as PPI, narcotics, or PERT.
All but one of the studies in this review used BTs to diagnose SIBO. LHBT showed significantly higher prevalence of SIBO in CP than GHBT (Figure 2c). This might be the result of accelerated intestinal transit delivering lactulose to colonic bacteria earlier than expected (18,38). Although GHBT is inherently more reliable as glucose is fully absorbed in the small intestine, glucose malabsorption rather than SIBO can result in positive GHBT (39–41). Despite attempts to standardize BT (42), they are still confounded by multiple technical issues, including the size of carbohydrate load, the osmotic and transit-accelerating effects of a highly concentrated substrate solution, test duration, and the adherence to dietary and other restrictions imposed by the test (42). Jejunal aspirate culture has long been the gold standard for SIBO diagnosis (43). It was used in one study (26) in our review. However, it also has its setbacks because of inaccessibility to the distal small bowel, potential for contamination, and false negatives for obligate anaerobes (44–46). In addition, when comparing culture to polymerase chain reaction analysis of jejunal aspirates, only 24.4% of jejunal bacteria are able to grow on standard media, raising questions about the ability of jejunal aspirate cultures to accurately reflect the intestinal bacterial load (41).
This degree of unreliability of current diagnostic techniques is reflected in the considerable heterogeneity among studies in this review. Accounting for differing results among diagnostic methods was able to explain 55% of the variance in SIBO prevalence in CP. Recent innovation using a swallowed capsule was able to measure in real time the concentrations of intraluminal gases such as H2, CO2, O2, and CH4. This technology provides much higher sensitivity than BT (47). Its clinical utility in SIBO diagnosis is yet to be verified. Therefore, although GHBT might provide practical and more reliable testing than LHBT, the diagnosis of SIBO in CP remains an elusive enterprise. Bacterial metabolomics and nucleic acid amplification techniques offer hope for more reliable testing in the near future.
CP is associated with malnutrition due to malabsorption of d-xylose (48,49), glucose, folate (50), and neutral amino acids (51), as well as maldigestion due to PEI. Despite adequate doses of PERT, 43% of patients remain symptomatic (2). SIBO may result in malnutrition because of increased consumption of carbohydrates, proteins, and vitamins, as well as malabsorption of micronutrients resulting in lasting nutritional consequences (28,52,53). Signoretti et al. (31) reported on the association of SIBO in CP with nutritional status and found patients with CP complicated by SIBO to have significantly higher levels of folate, which is likely related to increased intestinal bacterial production of the compound (54). However, they had lower levels of vitamin D, although that only trended toward significance with a P value of 0.08. Lee et al. (16) found patients with SIBO in CP to have a significantly lower serum albumin level. They also found zinc deficiency, a known complication of CP (55,56), to be significantly more common in SIBO. Frost et al. (3) and Gubergrits et al. (28) found CP/PEI to alter intestinal bacterial composition, shifting the balance among species favoring some over others. These findings suggest that selective bacterial proliferation could be more important than mere numbers (8) when considering SIBO manifestations in CP. In addition, Ní Chonchubhair et al. (17) found patients with SIBO and CP to have more weight loss than their SIBO-negative counterparts. These results suggest SIBO might play a considerable role in malnourished CP patients.
Five studies in this review assessed response to SIBO treatment (Table 3). Our analysis showed a better response to rifaximin than doxycycline and metronidazole, suggesting rifaximin treatment might be of benefit to patients with CP unresponsive to PERT. Moreover, this response supports that SIBO is not merely associated with CP but also contributes to the clinical syndrome. Further studies to prove normalization of tests after antibiotic treatment would further support this role.
Celiac disease is associated with CP/PEI (57) and SIBO (13). In this review, only 2 studies (16,18) reported on celiac disease in their patient population. Whether this is a strong confounding factor potentially explaining the refractory symptoms and malabsorption seen in CP remains unknown. Löhr et al. (58) summarized PEI treatment guidelines to recommend testing for celiac disease after failure of PERT at double the recommended dose and adequate acid suppression to control symptoms.
Although this subject has previously been presented in a systematic review by Capurso et al. (15), in this review, we provide the broadest and latest evidence on the prevalence, diagnosis, and treatment of SIBO in CP by including data from 4 additional studies and expanding on the analysis to factor in modifiers of SIBO prevalence in CP. Our rigorous analytic techniques using meta-regression were able to explain the heterogeneity among studies providing valuable insight into the possible role of PEI in SIBO in CP and diagnostic discrepancies. Moreover, we provide the first analysis in the literature into the outcomes of SIBO treatment in CP.
The limitations of our study include the retrospective nature of the included literature, which incurs associational data. Suboptimal design and quality of available evidence manifested in failure of most studies to address confounders such as concomitant celiac disease and the unreliability of LHBT. We also noted heterogeneity in diagnostic testing and control group characteristics. These limitations have likely affected our conclusions; new research must take them into consideration.
In summary, under current diagnostic guidelines, SIBO complicates 38.6% (95% CI 25.5%–53.5%) of CP with an OR of 5.58 (95% CI 2.26%–13.75), indicating a predisposition for this condition. Although the etiology of SIBO in CP remains unknown, emerging evidence suggests a role for pancreatic exocrine dysfunction and DM as manifested by the association of PEI/DM with increased SIBO in CP. Discrepancies between diagnostic methods for SIBO are clear and pose a diagnostic dilemma. However, as the search and optimization of diagnostic testing continues, GHBT remains the test of choice. Our study shows antibiotic treatment of SIBO is associated with improved clinical outcomes (Table 3, Figure 6), suggesting an alternative strategy to treatment of patients with CP unresponsive to PERT.
CONFLICTS OF INTEREST
Guarantor of the article: Bara El Kurdi, MD.
Specific author contributions: Conception and design: B.E.K., M.M.L, and V.P.S. Screening of the literature: all authors. Data analysis: B.E.K, S.B., M.E.I., A.B., and V.P.S. Writing the manuscript: B.E.K. and V.P.S. Critical review of the manuscript: all authors.
Financial support: The following grants were used to cover publication fees: DK092460 and DK119646 from the NIDDK, at the NIH, and PR151612 from the department of Army for V.P.S.
Potential competing interests: None.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A93
REFERENCES
- 1.Xiao AY, Tan MLY, Wu LM, et al. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016;1:45–55. [DOI] [PubMed] [Google Scholar]
- 2.Domínguez-Muñoz JE, Iglesias-García J, Iglesias-Rey M, et al. Optimising the therapy of exocrine pancreatic insufficiency by the association of a proton pump inhibitor to enteric coated pancreatic extracts. Gut 2006;55:1056–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frost F, Kacprowski T, Rühlemann M, et al. Impaired exocrine pancreatic function associates with changes in intestinal microbiota composition and diversity. Gastroenterology 2019;156:1010–5. [DOI] [PubMed] [Google Scholar]
- 4.Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: Pathogenesis and host defence. Nat Rev Microbiol 2016;14:609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014;146:1489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy PJ, Cryan JF, Dinan TG, et al. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J Gastroenterol 2014;20:14105–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hold GL. Gastrointestinal microbiota and colon cancer. Dig Dis 2016;34:244–50. [DOI] [PubMed] [Google Scholar]
- 8.Quigley EMM. The spectrum of small intestinal bacterial overgrowth (SIBO). Curr Gastroenterol Rep 2019;21:3. [DOI] [PubMed] [Google Scholar]
- 9.Greco A, Caviglia GP, Brignolo P, et al. Glucose breath test and Crohn's disease: Diagnosis of small intestinal bacterial overgrowth and evaluation of therapeutic response. Scand J Gastroenterol 2015;50:1376–81. [DOI] [PubMed] [Google Scholar]
- 10.Ricci JER, Jr, Chebli LA, Ribeiro TCDR, et al. Small-intestinal bacterial overgrowth is associated with concurrent intestinal inflammation but not with systemic inflammation in Crohn's disease patients. J Clin Gastroenterol 2018;52:530–6. [DOI] [PubMed] [Google Scholar]
- 11.Tan AH, Mahadeva S, Thalha AM, et al. Small intestinal bacterial overgrowth in Parkinson's disease. Parkinsonism Relat Disord 2014;20:535–40. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Liu G, Duan Y, et al. Prevalence of small intestinal bacterial overgrowth in multiple sclerosis: A case-control study from China. J Neuroimmunol 2016;301:83–7. [DOI] [PubMed] [Google Scholar]
- 13.Losurdo G, Marra A, Shahini E, et al. Small intestinal bacterial overgrowth and celiac disease: A systematic review with pooled-data analysis. Neurogastroenterol Motil 2017;29. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Kim JJ, Zhang Y, et al. Prevalence and predictors of small intestinal bacterial overgrowth in irritable bowel syndrome: A systematic review and meta-analysis. J Gastroenterol 2018;53:807–18. [DOI] [PubMed] [Google Scholar]
- 15.Capurso G, Signoretti M, Archibugi L, et al. Systematic review and meta-analysis: Small intestinal bacterial overgrowth in chronic pancreatitis. United European Gastroenterol J 2016;4:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee AA, Baker JR, Wamsteker EJ, et al. Small intestinal bacterial overgrowth is common in chronic pancreatitis and associates with diabetes, chronic pancreatitis severity, low zinc levels, and opiate use. Am J Gastroenterol 2019;1 (http://Insights.ovid.com/crossref?an=00000434-900000000-99691). Accessed June 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ní Chonchubhair HM, Bashir Y, Dobson M, et al. The prevalence of small intestinal bacterial overgrowth in non-surgical patients with chronic pancreatitis and pancreatic exocrine insufficiency (PEI). Pancreatology 2018;18:379–85. [DOI] [PubMed] [Google Scholar]
- 18.Therrien A, Bouchard S, Sidani S, et al. Prevalence of small intestinal bacterial overgrowth among chronic pancreatitis patients: A case-control study. Can J Gastroenterol Hepatol 2016;2016:7424831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouin M, Hedges LV, Higgins JPT, et al. Introduction to Meta-Analysis. John Wiley & Sons, Ltd: Chichester, UK, 2009. [Google Scholar]
- 20.Grigor'eva IuV, Iakovenko ÉP, Volosheĭnikova TV, et al. The clinical manifestations and duodenal mucosa in the patients with chronic pancreatitis and bacterial overgrowth in the small intestine. Eksp Klin Gastroenterol 2010;29–34. [PubMed] [Google Scholar]
- 21.Mancilla AC, Madrid SAM, Hurtado HC, et al. Small intestine bacterial overgrowth in patients with chronic pancreatitis. Rev Med Chil 2008;136:976–80. [PubMed] [Google Scholar]
- 22.Casellas F, Guarner L, Vaquero E, et al. Hydrogen breath test with glucose in exocrine pancreatic insufficiency. Pancreas 1998;16:481–6. [DOI] [PubMed] [Google Scholar]
- 23.Trespi E, Ferrieri A. Intestinal bacterial overgrowth during chronic pancreatitis. Curr Med Res Opin 1999;15:47–52. [DOI] [PubMed] [Google Scholar]
- 24.Kim DB, Paik CN, Sung HJ, et al. Breath hydrogen and methane are associated with intestinal symptoms in patients with chronic pancreatitis. Pancreatology 2015;15:514–8. [DOI] [PubMed] [Google Scholar]
- 25.Madsen JL, Graff J, Philipsen EK, et al. Bile acid malabsorption or disturbed intestinal permeability in patients treated with enzyme substitution for exocrine pancreatic insufficiency is not caused by bacterial overgrowth. Pancreas 2003;26:130–3. [DOI] [PubMed] [Google Scholar]
- 26.Bang Jørgensen B, Thorsgaard Pedersen N, Worning H. Short report: Lipid and vitamin B12 malassimilation in pancreatic insufficiency. Aliment Pharmacol Ther 1991;5:207–10. [DOI] [PubMed] [Google Scholar]
- 27.Kumar K, Ghoshal UC, Srivastava D, et al. Small intestinal bacterial overgrowth is common both among patients with alcoholic and idiopathic chronic pancreatitis. Pancreatology 2014;14:280–3. [DOI] [PubMed] [Google Scholar]
- 28.Gubergrits NB, Linevskiy YV, Lukashevich GM, et al. Morphological and functional alterations of small intestine in chronic pancreatitis. JOP 2012;13:519–28. [DOI] [PubMed] [Google Scholar]
- 29.Spiller RC, Trotman IF, Higgins BE, et al. The ileal brake—inhibition of jejunal motility after ileal fat perfusion in man. Gut 1984;25:365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordgaard I, Rumessen JJ, Gudmand-Høyer E. Assimilation of wheat starch in patients with chronic pancreatitis: Positive effect of enzyme replacement. Scand J Gastroenterol 1992;27:412–6. [DOI] [PubMed] [Google Scholar]
- 31.Signoretti M, Stigliano S, Valente R, et al. Small intestinal bacterial overgrowth in patients with chronic pancreatitis. J Clin Gastroenterol 2014;48(Suppl 1):S52–55. [DOI] [PubMed] [Google Scholar]
- 32.Rana SV, Malik A, Bhadada SK, et al. Malabsorption, orocecal transit time and small intestinal bacterial overgrowth in type 2 diabetic patients: A connection. Indian J Clin Biochem 2017;32:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malka D, Hammel P, Sauvanet A, et al. Risk factors for diabetes mellitus in chronic pancreatitis. Gastroenterology 2000;119:1324–32. [DOI] [PubMed] [Google Scholar]
- 34.Rubinstein E, Mark Z, Haspel J, et al. Antibacterial activity of the pancreatic fluid. Gastroenterology 1985;88:927–32. [DOI] [PubMed] [Google Scholar]
- 35.Bassi C, Fontana R, Vesentini S, et al. Antibacterial and mezlocillin-enhancing activity of pure human pancreatic fluid. Int J Pancreatol 1991;10:293–7. [DOI] [PubMed] [Google Scholar]
- 36.Isaiah A, Parambeth JC, Steiner JM, et al. The fecal microbiome of dogs with exocrine pancreatic insufficiency. Anaerobe 2017;45:50–8. [DOI] [PubMed] [Google Scholar]
- 37.Ahuja M, Schwartz DM, Tandon M, et al. Orai1-Mediated antimicrobial secretion from pancreatic acini shapes the gut microbiome and regulates gut innate immunity. Cell Metab 2017;25:635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut 2011;60:334–40. [DOI] [PubMed] [Google Scholar]
- 39.Sellin JH, Hart R. Glucose malabsorption associated with rapid intestinal transit. Am J Gastroenterol 1992;87:584–9. [PubMed] [Google Scholar]
- 40.Berean KJ, Ha N, Ou JZ, et al. The safety and sensitivity of a telemetric capsule to monitor gastrointestinal hydrogen production in vivo in healthy subjects: A pilot trial comparison to concurrent breath analysis. Aliment Pharmacol Ther 2018;48:646–54. [DOI] [PubMed] [Google Scholar]
- 41.Sundin OH, Mendoza-Ladd A, Morales E, et al. Does a glucose-based hydrogen and methane breath test detect bacterial overgrowth in the jejunum? Neurogastroenterol Motil 2018;30:e13350. [DOI] [PubMed] [Google Scholar]
- 42.Gasbarrini A, Corazza GR, Gasbarrini G, et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment Pharmacol Ther 2009;29(Suppl 1):1–49. [DOI] [PubMed] [Google Scholar]
- 43.Isaacs PE, Kim YS. The contaminated small bowel syndrome. Am J Med 1979;67:1049–57. [DOI] [PubMed] [Google Scholar]
- 44.Ford AC, Spiegel BMR, Talley NJ, et al. Small intestinal bacterial overgrowth in irritable bowel syndrome: Systematic review and meta-analysis. Clin Gastroenterol Hepatol 2009;7:1279–86. [DOI] [PubMed] [Google Scholar]
- 45.Sachdeva S, Rawat AK, Reddy RS, et al. Small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome: Frequency and predictors. J Gastroenterol Hepatol 2011;26(Suppl 3):135–8. [DOI] [PubMed] [Google Scholar]
- 46.Puri M, Lin HC, Enayati P, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol 2006;290:G1089–95. [DOI] [PubMed] [Google Scholar]
- 47.Ou JZ, Yao CK, Rotbart A, et al. Human intestinal gas measurement systems: In vitro fermentation and gas capsules. Trends Biotechnol 2015;33:208–13. [DOI] [PubMed] [Google Scholar]
- 48.Bank S, Marks IN, Novis BH. Small bowel structure and function in pancreatic steatorrhea. Mt Sinai J Med 1975;42:552–64. [PubMed] [Google Scholar]
- 49.Helman CA, Barbezat GO, Bank S. Jejunal monosaccharide, water, and electrolyte transport in patients with chronic pancreatitis. Gut 1978;19:46–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mössner J, Koch W, Kestel W, et al. Intestinal absorption of folic acid, glucose, sodium and water in chronic pancreatitis. Z Gastroenterol 1986;24:212–7. [PubMed] [Google Scholar]
- 51.Schneider PJ, Kilby A, Rassam UB, et al. Small intestinal absorption of amino acids and a dipeptide in pancreatic insufficiency. Gut 1983;24:818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Stefano M, Veneto G, Malservisi S, et al. Small intestine bacterial overgrowth and metabolic bone disease. Dig Dis Sci 2001;46:1077–82. [DOI] [PubMed] [Google Scholar]
- 53.Corazza P-O, Johansson C, Mellström D, et al. Bone mineral density in patients with small intestinal bacterial overgrowth. Hepatogastroenterology 2003;50:1415–8. [PubMed] [Google Scholar]
- 54.Camilo E, Zimmerman J, Mason JB, et al. Folate synthesized by bacteria in the human upper small intestine is assimilated by the host. Gastroenterology 1996;110:991–8. [DOI] [PubMed] [Google Scholar]
- 55.Vujasinovic M, Hedström A, Maisonneuve P, et al. Zinc deficiency in patients with chronic pancreatitis. World J Gastroenterol 2019;25:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Girish BN, Rajesh G, Vaidyanathan K, et al. Zinc status in chronic pancreatitis and its relationship with exocrine and endocrine insufficiency. JOP 2009;10:651–6. [PubMed] [Google Scholar]
- 57.Balakrishnan MJ. Exocrine pancreatic insufficiency and pancreatitis associated with celiac disease. Pancreapedia: The Exocrine Pancreas Knowledge Base 2018 (reviews/exocrine-pancreatic-insufficiency-and-pancreatitis-associated-with-celiac-disease). Accessed January 31, 2019.
- 58.Löhr JM, Oliver MR, Frulloni L. Synopsis of recent guidelines on pancreatic exocrine insufficiency. United European Gastroenterol J 2013;1:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lembcke B, Kraus B, Lankisch PG. Small intestinal function in chronic relapsing pancreatitis. Hepatogastroenterology 1985;32:149–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.