OBJECTIVES:
Acute kidney injury (AKI) is a common complication in hospitalized patients with cirrhosis which contributes to morbidity and mortality. Improved prediction of AKI in this population is needed for prevention and early intervention. We developed a model to identify hospitalized patients at risk for AKI.
METHODS:
Admission data from a prospective cohort of hospitalized patients with cirrhosis without AKI on admission (n = 397) was used for derivation. AKI development in the first week of admission was captured. Independent predictors of AKI on multivariate logistic regression were used to develop the prediction model. External validation was performed on a separate multicenter cohort (n = 308).
RESULTS:
In the derivation cohort, the mean age was 57 years, the Model for End-Stage Liver Disease score was 17, and 59 patients (15%) developed AKI after a median of 4 days. Admission creatinine (OR: 2.38 per 1 mg/dL increase [95% CI: 1.47–3.85]), international normalized ratio (OR: 1.92 per 1 unit increase [95% CI: 1.92–3.10]), and white blood cell count (OR: 1.09 per 1 × 109/L increase [95% CI: 1.04–1.15]) were independently associated with AKI. These variables were used to develop a prediction model (area underneath the receiver operator curve: 0.77 [95% CI: 0.70–0.83]). In the validation cohort (mean age of 53 years, Model for End-Stage Liver Disease score of 16, and AKI development of 13%), the area underneath the receiver operator curve for the model was 0.70 (95% CI: 0.61–0.78).
DISCUSSION:
A model consisting of admission creatinine, international normalized ratio, and white blood cell count can identify patients with cirrhosis at risk for in-hospital AKI development. On further validation, our model can be used to apply novel interventions to reduce the incidence of AKI among patients with cirrhosis who are hospitalized.
INTRODUCTION
Acute kidney injury (AKI) is an important complication of decompensated cirrhosis that drives poor outcomes (1,2). AKI is present in 20% (3) of patients with cirrhosis admitted to the hospital, and it develops in up to 19% after admission (4). Its development is independently associated with high short-term mortality and leads to significant morbidity after discharge from the hospital (5,6).
Given the significant burden and prognostic implications of AKI, accurate risk stratification in this population is crucial for efforts to prevent, recognize, and treat AKI to improve outcomes. Although a rise in serum creatinine (Scr) is the cornerstone of AKI diagnosis (7), early recognition is hindered as acute changes in Scr are often delayed due to the time required for creatinine to accumulate and equilibrate. In addition, factors such as ascites, malnutrition, and sarcopenia which are common in hospitalized patients with cirrhosis contribute to spurious creatinine-based estimates of renal function and can prevent the early recognition of patients at risk (8). Novel renal biomarkers such as cystatin C and neutrophil gelatinase-associated lipocalin may allow for improved risk assessment (9), but these research tools are expensive and remain far from clinical use (2). Thus, there is a major unmet need for an accurate tool to predict AKI in hospitalized patients with cirrhosis.
An AKI prediction model using readily available objective clinical parameters could be valuable to identify patients at high risk for AKI. Accurate identification of high-risk patients will allow for early intervention and appropriate allocation of hospital resources to improve patient care and outcomes. Furthermore, development of a prediction model could aid clinical trial design for AKI prevention by identifying patients at highest risk.
Thus, this study develops a simple, objective model to predict AKI development in hospitalized patients with cirrhosis. To identify those at risk promptly, we examined data collected within the first 24 h of hospitalization from a large prospective cohort of hospitalized patients. Further validation of the predictive model was performed on an independent external cohort.
PATIENTS AND METHODS
Study design
We created a model to predict in-hospital AKI using a prospective cohort of patients with cirrhosis who were nonelectively admitted to Indiana University Hospital. The Indiana University Hospital is the largest referral and academic tertiary care hospital in the state of Indiana. External validation of the prediction model was performed using a multicenter retrospective cohort of hospitalized patients with cirrhosis. The diagnosis of cirrhosis was made based on clinical parameters involving laboratory tests, endoscopic or radiologic evidence of cirrhosis, evidence of decompensation (hepatic encephalopathy, ascites, variceal bleeding, jaundice), and liver biopsy if available. Those who met inclusion criteria (see section on “Inclusion Criteria”) were included for the analysis. The derivation and validation of the prediction model was designed according to the TRIPOD guidelines (10). The protocol was approved by the Institutional Review Board at our center.
Inclusion criteria
The study inclusion criteria were the following:
Availability of a baseline Scr as defined by the International Club of Ascites (7).
Availability of the following laboratory studies within 24 h of admission: complete blood count, basic metabolic profile, hepatic panel, and international normalized ratio (INR).
Patients excluded from the analysis were those who did not meet the inclusion criteria as well as the following: those who had previously undergone liver or kidney transplant, had AKI on admission, and had acute or chronic requirement of hemodialysis at the time of admission, and those with missing laboratory measurements within 24 h of admission.
Definition of AKI
AKI was defined by the Kidney Disease Improving Global Outcomes guideline (11), which has been endorsed by the International Club of Ascites (7) for patients with cirrhosis. Because urine output documentation can be unreliable (7), only the rise in Scr ≥ 0.3 mg/dL or ≥ 1.5× baseline was used to define the in-hospital development of AKI. The primary outcome was AKI development up to 7 days after admission. AKI beyond 7 days was not included in the primary outcome because of the practical difficulty in predicting AKI later in the hospitalization based on the admission data. Stages of AKI were defined by Kidney Disease Improving Global Outcomes (11) staging system which has also been endorsed by the International Club of Ascites: stage 1, increase in Scr > 0.3 mg/dL or increase in Scr ≥ 1.5–2× baseline; stage 2, increase in Scr > 2×–3× increase from baseline; stage 3, increase in Scr >3× from baseline or Scr > 4.0 mg/dL with an acute increase ≥ 0.3 mg/dL or initiation of hemodialysis.
Derivation cohort
Patients who were prospectively enrolled from June 2014 to October 2018 and met the inclusion criteria were included for the analysis. The etiology of cirrhosis, history of complications related to cirrhosis, demographics, reason for hospitalization, daily vital signs, and laboratory data (complete blood count, metabolic panel, and hepatic panel) from the day of admission to day 14 of hospitalization were collected. In addition, the status of infections (on admission and during the hospitalization), phenotype of AKI (prerenal azotemia, acute tubular necrosis, hepatorenal syndrome type 1, and other), precipitants of AKI (large-volume paracentesis, excessive use of diuretics, spontaneous bacterial peritonitis (SBP), non-SBP infections, portal hypertensive-related bleeding, intravenous contrast, and other), and 30-day mortality details were collected. The etiology of cirrhosis was categorized into viral hepatitis C (HCV), HCV and alcohol, alcohol, nonalcoholic steatohepatitis, and other. Cirrhosis severity was calculated using the Model for End-Stage Liver Disease (MELD) (12) and Child-Turcotte-Pugh (13) scores. Acute on chronic liver failure (ACLF) and its severity (grades 1–3) were defined by the CLIF Consortium Organ Failure Score (14).
Validation cohort
Patients who were admitted nonelectively to 4 safety-net hospitals (Baylor College of Medicine Medical Center, Boston University Medical Center, Eskenazi Medical Center, and John Stroger Hospital of Cook County Health) in 2012 were screened for inclusion criteria. Details of this cohort have been previously described (15). Data collected were analogous to the derivation cohort, with the exception of phenotype of AKI, ACLF details, precipitants of AKI, and mortality.
Context of use
The current study was designed to create a prediction model for AKI development in hospitalized patients with cirrhosis. The potential decisions to be made based on the model are the intensity of kidney function monitoring, potential AKI prophylaxis with volume administration, inclusion criteria for preventive clinical trial design, and avoidance of certain nephrotoxins.
Statistical analysis
Patients were stratified based on the development of AKI or not, and their characteristics on admission were compared. Continuous variables were presented as mean ± SD and median with interquartile range (IQR) where appropriate. Categorical variables were presented as percentages. Differences across the groups with respect to categorical variables were analyzed using χ2 test and Fisher Exact test, whereas continuous variables were analyzed using the nonparametric Kruskal-Wallis test and student t test. A 2-sided nominal P value of less than or equal to 0.05 was considered significant.
Derivation of the AKI prediction model
Variables of clinical significance were considered for the multivariate analysis. These were baseline Scr, admission Scr, white blood cell count (WBC), mean arterial pressure, INR, admission sodium, admission total bilirubin, admission albumin, presence of infection on admission, ascites, age, and gender (Table 1). The variables were ordered based on univariate significance by fitting a logistic regression model and added into the multivariate model using a forward selection procedure. Highly correlated variables were compared and included only once. Model selection was based on minimizing Akaike information criterion and maximizing area underneath the receiver operator curve (AUROC) or concordance c-statistic, with priority given to the lowest Akaike information criterion. The final model was then applied to the validation cohort, and AUROC analysis was performed for the prediction of AKI. The diagnostic performance of the model in both derivation and validation cohorts was evaluated using sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy at 90% sensitivity, 90% specificity, and the maximum Youden index. The final model was then compared with the MELD-Na and the CLIF Consortium Acute Decompensation (CLIF-AD) (16) scores via DeLongs method. All analyses were performed in SAS version 9.4.
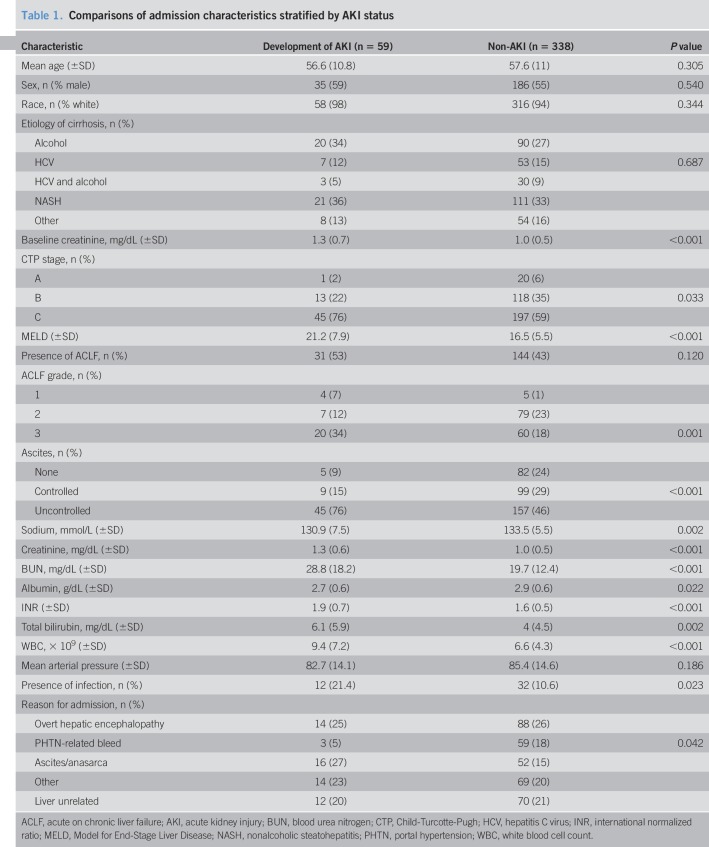
Table 1.
Comparisons of admission characteristics stratified by AKI status
RESULTS
Derivation cohort
A total of 627 patients were enrolled during the study period. After excluding those who did not meet the inclusion criteria (229 patients in total: 191 had AKI on admission and 38 had missing admission labs), a total of 397 patients were included for the analysis (59 with AKI development [14%] and 338 without AKI development).
Patient characteristics
The mean age of the entire cohort was 57.3 ± 10.8 years. Fifty-seven percent were men, with a mean MELD score of 17.2 ± 6.1. Nonalcoholic steatohepatitis (33%), alcohol (27%), and HCV (15%) were the most common etiologies of cirrhosis. Those who developed AKI had a significantly higher baseline Scr, admission Scr, admission total bilirubin, admission INR, admission blood urea nitrogen, admission WBC, and admission MELD score compared with the non-AKI group (Table 1). The presence of infection was found to be significantly higher in the AKI group compared with the non-AKI group (21% and 11%, respectively; P = 0.023). The AKI group had more admissions for ascites/anasarca and fewer admissions for portal hypertensive-related bleeding (Table 1).
One hundred seventy-five patients had ACLF on admission, of which 31 patients developed AKI. There were no differences between the 2 groups for the presence of ACLF on admission (54% AKI development vs 42% no AKI development; P = 0.120) (Table 1). However, there were differences across ACLF grades between the 2 groups (P = 0.001), with a higher percentage of patients having ACLF grade 3 who developed AKI (34%) vs patients who did not develop AKI (18%; P = 0.003).
AKI development details and hospitalization outcomes
The median (IQR) time to AKI development was 4 (3–5) hospital days. Ninety-five percent of patients had stage 1 AKI at the time of diagnosis, followed by 5% for stage 2. The most frequent precipitant for AKI identified was non-SBP infections (32% overall), followed by excessive use of diuretics (19%). SBP was found to be the cause of AKI in 7% of patients.
In those who had stage 1 AKI at the time of diagnoses, 68% stayed at stage 1 and the rest progressed to stages 2 and 3 (16% and 10%, respectively). The most frequent phenotype of AKI was prerenal at 52%, followed by acute tubular necrosis at 19% and type 1 hepatorenal syndrome at 3%. Ten percent of patients with AKI required hemodialysis during the hospitalization. Compared with the non-AKI group, patients who developed AKI had significantly higher in-hospital mortality (16% vs 2%; P < 0.001) and 30-day mortality (28% vs 6%; P < 0.001).
Validation cohort
A total of 733 patients were screened for inclusion criteria. Four hundred twenty-five patients were excluded (170 patients with AKI on admission and 255 patients with missing admission data), leaving 308 patients for the analysis (41 with AKI development [13%] and 267 without AKI). Most of them were men (73%), and the mean MELD score was 15.9 ± 5.9. Similar to the derivation cohort, patients who developed AKI had higher baseline Scr, admission Scr, admission total bilirubin, admission INR, admission blood urea nitrogen, admission WBC, and admission MELD score compared with the non-AKI group (see Table 1, Supplementary Digital Content, http://links.lww.com/CTG/A92). In addition, the presence of infection was found to be significantly higher in the AKI development group (42%) compared with the non-AKI group (15%) (P ≤ 0.0001). Comparisons between the derivation and validation cohorts can be found in Supplementary Table 2 (Supplementary Digital Content, http://links.lww.com/CTG/A92).
The median (IQR) time to AKI development was 2 (1–3) days, and 73%, 20%, and 8% had stage 1, 2, and 3 at the time of AKI diagnosis. Thirty-one percent of patients diagnosed at stage 1 progressed to higher stages of AKI, and 63% of patients with stage 2 AKI progressed to stage 3. Seven percent of patients required hemodialysis.
AKI prediction model
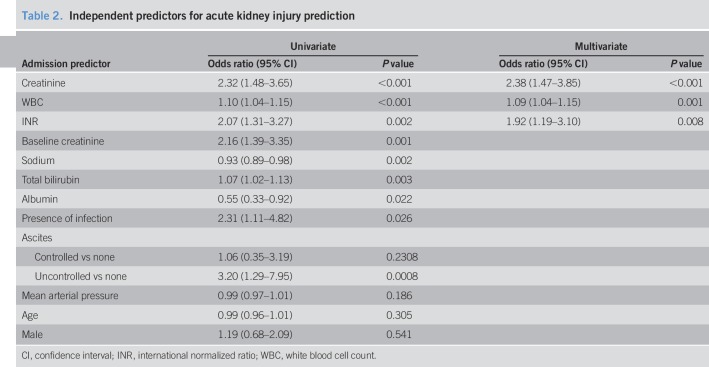
The admission variables that were found to be significant for AKI development on univariate analysis in the derivation cohort are listed in Table 2. On multivariate analysis, admission Scr (OR: 2.38 per 1 mg/dL increase [95% CI: 1.47–3.85]), INR (OR: 1.92 per 1 unit increase [95% CI: 1.92–3.10]), and WBC (OR: 1.09 per 1 × 109/L increase [95% CI: 1.04–1.15]) were independently associated with AKI. Using these variables, a logistic regression model was created for prediction:
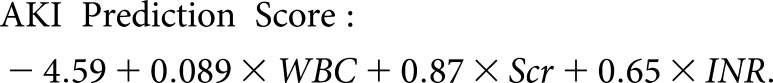 |
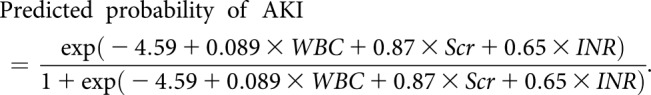 |
Table 2.
Independent predictors for acute kidney injury prediction
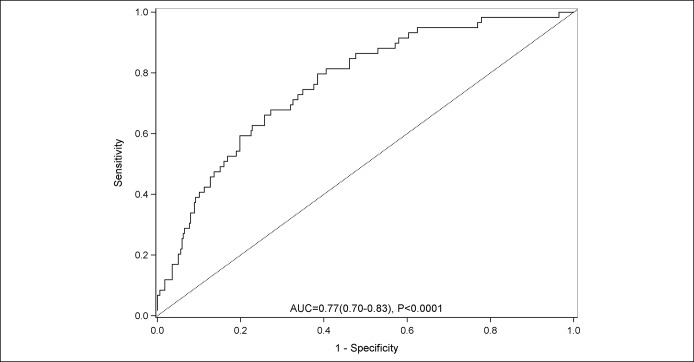
The AUROC for prediction was found to be 0.77 (95% CI: 0.70, 0.83; P < 0.0001) (Figure 1), and the Akaike information criterion was 298. The AKI prediction model was found to have a numerically higher AUROC than MELD-Na score (0.71 [95% CI: 0.63, 0.79]) and CLIF-AD score (0.73 [95% CI: 0.66, 0.80]), although the differences were not statistically significant (P = 0.08 vs MELD-NA and P = 0.13 vs CLIF-AD).
Figure 1.
Area underneath the receiver operating curve for the prediction of acute kidney injury in the derivation cohort.
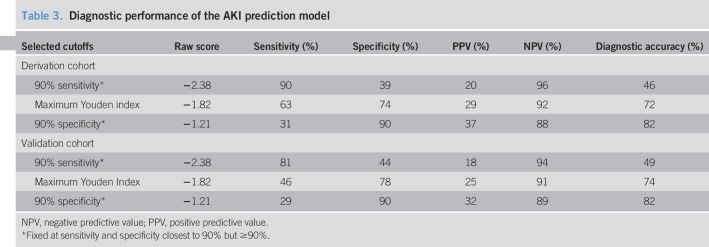
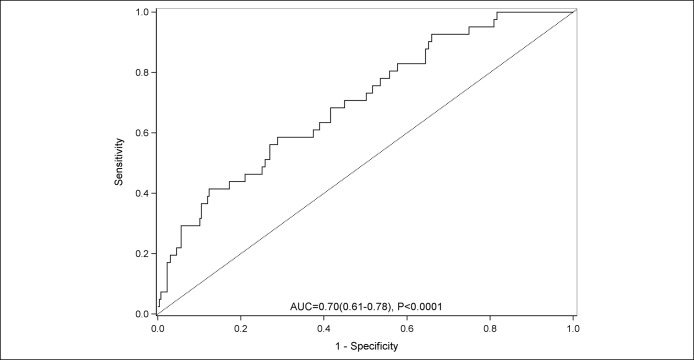
The diagnostic performance of the AKI prediction model is shown in Table 3. With the sensitivity and specificity fixed at 90%, the negative predictive value was high at 96% and 88%, respectively. The diagnostic accuracy was highest with a cutoff > −1.21 (specificity at 90%) at 82%. When the logistic regression AKI prediction model from the derivation cohort was applied to the validation cohort, the AUROC was 0.70 (95% CI: 0.61, 0.78; P < 0.0001) (Figure 2). Similar to the derivation cohort, a score of > −1.21 had a diagnostic accuracy at 82%, with a specificity of 90%.
Table 3.
Diagnostic performance of the AKI prediction model
Figure 2.
Area underneath the receiver operating curve for the prediction of acute kidney injury in the validation cohort.
DISCUSSION
In this study, we have derived and externally validated a predictive model for the development of AKI in hospitalized patients with cirrhosis. Our model is based on 3 objective measures that are widely available in clinical practice (Scr, INR, and WBC) and can therefore be applied in a variety of hospitalized settings. The AUROC for prediction of AKI in the derivation cohort was 0.77, and the AUROC in an independent validation cohort was 0.70.
The pathophysiology of AKI in patients with cirrhosis is complex and often related to renal vasoconstriction secondary to the effective hypovolemia that occurs in advanced cirrhosis and portal hypertension (17). Furthermore, it is increasingly recognized that AKI is associated with systemic and intrarenal inflammation (18–21). Patients with advanced cirrhosis have higher levels of circulating endotoxins, possibly due to bacterial translocation across the intestinal wall (22,23), thus promoting the production of many proinflammatory cytokines, substances that cause peripheral and splanchnic vasodilation (24,25), as well as intrarenal endothelial dysfunction and cell damage (20,26). Each of these pathophysiologic elements is indirectly captured by the variables in our predictive model. For example, a high WBC, a surrogate of systemic inflammation, is a known independent predictor of mortality in patients with advanced cirrhosis and is a driver for the development of ACLF (19,27). An elevated WBC has also been associated with renal dysfunction in patients with SBP (28,29) and alcoholic hepatitis (30). The other 2 components of the risk score were INR and Scr. INR is a well-known marker of progressive liver failure and therefore worsening portal hypertension (12,13) which has been linked to renal impairment (17,31,32). Furthermore, despite its limitations, we found Scr to have the highest OR of 2.38 for 1 mg/dL increase. Our findings are in line with other studies (33–35), where higher Scr is independently associated with AKI development and poor renal outcomes.
There are several limitations to our study. First, although urine output is not considered a part of the diagnostic criteria for AKI by the International Club of Ascites (7), we were not able to compare our risk score with early urine output. Urine output should be evaluated in future studies because it has been shown to be sensitive for incident AKI (36). Similarly, we were unable to compare our model with novel kidney biomarkers (e.g., cystatin C, neutrophil gelatinase-associated lipocalin) for risk assessment. Thus, future studies are also needed to assess these kidney biomarkers to substantiate their roles as clinical tools and to assess if the addition of biomarkers enhances prediction. Last, because only 5% of patients had AKI stage 2 or higher at the time of diagnosis, we were unable to determine if our model is applicable to predict higher stages of AKI. In contrast to these weaknesses, the study benefits from the use of a multicenter external validation cohort, with numerous significant differences compared with the derivation cohort. These differences highlight the potential generalizability of the model, which may be applied in diverse settings to predict AKI.
The ability to risk stratify patients early during their hospitalization has several important implications for clinical management. For example, a patient without AKI on admission with an INR of 2.5, WBC of 15, and Scr 2.0 would have an AKI risk score of 0.11, corresponding to a 7-day risk of AKI of 53% with >80% diagnostic accuracy. By contrast, a patient with an INR of 1.7, WBC of 10, and Scr of 0.8 would have an AKI risk score of −1.89, corresponding to a much lower risk of 13%. Hence, knowledge of individual patient risk for AKI may allow the treating physician to personalize the intensity of laboratory monitoring and to be mindful in the use of intravenous diuretics or iodinated contrast. Furthermore, risk stratification could provide a standardized approach for future clinical trials focusing on AKI prevention. Therefore, though not yet proven, the model has a clear potential to influence therapeutic or prognostic decisions in the future. An example of this would be early administration of albumin or early hepatorenal syndrome-directed therapy (albumin, terlipressin, etc.) in a patient at high risk to develop hepatorenal syndrome. Our model of AKI prediction calculator is available at http://gihep.com/calculators/hepatology/aki-in-hospitalized-patients-with-cirrhosis/.
In conclusion, in this large prospective study of hospitalized patients with cirrhosis, the development of in-hospital AKI is common and confers a poor prognosis. Our model for AKI prediction is based on 3 widely available laboratory parameters; it captures the pathophysiologic elements of AKI, and it may identify patients at high risk for AKI. The use of this model may allow for closer monitoring and early treatment to prevent AKI in those at risk. Ultimately, future studies are required to demonstrate whether this risk score can be used to improve clinical outcomes.
CONFLICTS OF INTEREST
Guarantor of the article: Eric S. Orman, MD.
Specific author contributions: K.R.P., M.S.G., N.P.C., and E.S.O.: study concept and design. K.R.P., E.S.O., C.X., and M.S.G.: data analysis. K.R.P., E.S.O., and C.X.: manuscript preparation. All authors reviewed the manuscript.
Financial support: This publication was made possible, in part, with support from the Indiana Clinical and Translational Sciences Institute funded, in part, by Award Number KL2TR001106 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. Research reported in this publication was supported, in part, by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K23DK109202 (E.S.O.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential competing interests: Dr Naga Chalasani had paid consulting activities with the following companies in last 12 months: Abbvie, Shire, NuSirt, Afimmune, Axovant, Allergan, Madrigal, Coherus, Siemens, and Genentech. He has received research support from Lilly, Galectin, Gilead, Exact Sciences, and Cumberland. No disclosures for the other authors.
Study Highlights.
WHAT IS KNOWN
✓ In-hospital AKI is a common complication of cirrhosis which leads to significant morbidity and mortality.
✓ There are no existing tools to predict in-hospital AKI in this population. Creation of a prediction model may help guide treatment and improve outcomes.
WHAT IS NEW HERE
✓ Admission laboratory values (white blood cell count, creatinine, and international normalized ratio) were used to derive the AKI prediction model. The model had an AUROC of 0.77 (95% CI: 0.70–0.83; P < 0.001) for AKI prediction.
✓ When validated in a separate multicenter cohort, the AUROC for AKI prediction was 0.70 (95% CI: 0.61–0.78; P < 0.001).
✓ Our AKI prediction model may allow for prevention and timely treatment of AKI to improve patient outcomes.
TRANSLATIONAL IMPACT
✓ Our AKI prediction model may allow for prevention and timely treatment of AKI to improve patient outcomes.
✓ Furthermore, risk stratification via our AKI model could provide a standardized approach for future clinical trials focusing on AKI prevention.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A92
REFERENCES
- 1.Wong F, O'Leary JG, Reddy KR, et al. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology 2013;145:1280–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piano S, Brocca A, Angeli P. Renal function in cirrhosis: A critical review of available tools. Semin Liver Dis 2018;38:230–41. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 2008;48:2064–77. [DOI] [PubMed] [Google Scholar]
- 4.Huelin P, Piano S, Sola E, et al. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute-on-chronic liver failure. Clin Gastroenterol Hepatol 2017;15:438–45 e5. [DOI] [PubMed] [Google Scholar]
- 5.O'Leary JG, Levitsky J, Wong F, et al. Protecting the kidney in liver transplant candidates: Practice-based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transpl 2016;16:2516–31. [DOI] [PubMed] [Google Scholar]
- 6.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 workgroup. Nat Rev Nephrol 2017;13:241–57. [DOI] [PubMed] [Google Scholar]
- 7.Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. Gut 2015;64:531–7. [DOI] [PubMed] [Google Scholar]
- 8.Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol 2016;65:809–24. [DOI] [PubMed] [Google Scholar]
- 9.Markwardt D, Holdt L, Steib C, et al. Plasma cystatin C is a predictor of renal dysfunction, acute-on-chronic liver failure, and mortality in patients with acutely decompensated liver cirrhosis. Hepatology 2017;66:1232–41. [DOI] [PubMed] [Google Scholar]
- 10.Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD statement. Ann Intern Med 2015;162:55–63. [DOI] [PubMed] [Google Scholar]
- 11.KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012;Suppl 1–138. [Google Scholar]
- 12.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464–70. [DOI] [PubMed] [Google Scholar]
- 13.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–9. [DOI] [PubMed] [Google Scholar]
- 14.Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol 2014;61:1038–47. [DOI] [PubMed] [Google Scholar]
- 15.Mukthinuthalapati V, Akinyeye S, Fricker ZP, et al. Early predictors of outcomes of hospitalization for cirrhosis and assessment of the impact of race and ethnicity at safety-net hospitals. PLoS One 2019;14:e0211811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jalan R, Pavesi M, Saliba F, et al. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol 2015;62:831–40. [DOI] [PubMed] [Google Scholar]
- 17.Stadlbauer V, Wright GA, Banaji M, et al. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology 2008;134:111–9. [DOI] [PubMed] [Google Scholar]
- 18.Wong F, Pappas SC, Boyer TD, et al. Terlipressin improves renal function and reverses hepatorenal syndrome in patients with systemic inflammatory response syndrome. Clin Gastroenterol Hepatol 2017;15:266–72 e1. [DOI] [PubMed] [Google Scholar]
- 19.Claria J, Stauber RE, Coenraad MJ, et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology 2016;64:1249–64. [DOI] [PubMed] [Google Scholar]
- 20.Shah N, Mohamed FE, Jover-Cobos M, et al. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int 2013;33:398–409. [DOI] [PubMed] [Google Scholar]
- 21.Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014;41:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol 2014;60:197–209. [DOI] [PubMed] [Google Scholar]
- 23.Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014;60:940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tazi KA, Moreau R, Herve P, et al. Norfloxacin reduces aortic NO synthases and proinflammatory cytokine up-regulation in cirrhotic rats: Role of Akt signaling. Gastroenterology 2005;129:303–14. [DOI] [PubMed] [Google Scholar]
- 25.Yang YY, Liu H, Nam SW, et al. Mechanisms of TNFalpha-induced cardiac dysfunction in cholestatic bile duct-ligated mice: Interaction between TNFalpha and endocannabinoids. J Hepatol 2010;53:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah N, Dhar D, El Zahraa Mohammed F, et al. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J Hepatol 2012;56:1047–53. [DOI] [PubMed] [Google Scholar]
- 27.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–37. 1437 e1–9. [DOI] [PubMed] [Google Scholar]
- 28.Navasa M, Follo A, Filella X, et al. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: Relationship with the development of renal impairment and mortality. Hepatology 1998;27:1227–32. [DOI] [PubMed] [Google Scholar]
- 29.Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 1999;341:403–9. [DOI] [PubMed] [Google Scholar]
- 30.Altamirano J, Fagundes C, Dominguez M, et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol 2012;10:65–71 e3. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-del-Arbol L, Urman J, Fernandez J, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology 2003;38:1210–8. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-del-Arbol L, Monescillo A, Arocena C, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology 2005;42:439–47. [DOI] [PubMed] [Google Scholar]
- 33.Wong F, O'Leary JG, Reddy KR, et al. Acute kidney injury in cirrhosis: Baseline serum creatinine predicts patient outcomes. Am J Gastroenterol 2017;112:1103–10. [DOI] [PubMed] [Google Scholar]
- 34.Wong F, Reddy RK, O'Leary JG, et al. Impact of chronic kidney disease on outcomes in cirrhosis. Liver Transpl 2019;25:870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belcher JM, Garcia-Tsao G, Sanyal AJ, et al. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology 2013;57:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amathieu R, Al-Khafaji A, Sileanu FE, et al. Significance of oliguria in critically ill patients with chronic liver disease. Hepatology 2017;66:1592–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.