OBJECTIVES:
Data on long-term natural history of microscopic colitis (MC), including collagenous (CC) and lymphocytic colitis (LC), are lacking.
METHODS:
All new cases of MC diagnosed in the Somme area, France, between January 1, 2005, and December 31, 2007, were prospectively included. Colonic biopsies from all patients were reviewed by a group of 4 gastrointestinal pathologist experts to assess the diagnosis of CC or LC. Demographic and clinical data were retrospectively collected from diagnosis to February 28, 2017.
RESULTS:
One hundred thirty cases of MC, 87 CC and 43 LC, were included (median age at diagnosis: 70 [interquartile range, 61–77] and 48 [IQR, 40–61] years, respectively). The median follow-up was 9.6 years (7.6; 10.6). By the end of the follow-up, 37 patients (28%) relapsed after a median time of 3.9 years (1.2; 5.0) since diagnosis, without significant difference between CC and LC (30% vs 26%; P = 0.47). Twenty patients (15%) were hospitalized for a disease flare, and 32 patients (25%) presented another autoimmune disease. Budesonide was the most widely used treatment (n = 74, 59%), followed by 5-aminosalicylic acid (n = 31, 25%). The median duration of budesonide treatment was 92 days (70; 168), and no adverse event to budesonide was reported. Sixteen patients (22%) developed steroid dependency and 4 (5%) were corticoresistant. No difference in the risk of digestive and extradigestive cancer was observed compared with the general population. None of the death (n = 25) observed during the follow-up were linked to MC. In multivariate analysis, age at diagnosis (HR, 1.03; 95% confidence interval, 1.00–1.06; P = 0.02) and budesonide exposure (HR, 2.50; 95% confidence interval, 1.11–5.55; P = 0.03) were significantly associated with relapse.
DISCUSSION:
This population-based study showed that after diagnosis, two-third of the patients with MC observed long-term clinical remission. Age at diagnosis and budesonide exposure were associated with a risk of relapse.
INTRODUCTION
Microscopic colitis (MC), described for the first time in 1976 (1), is now recognized as a common cause of chronic diarrhea. MC includes 2 histologic entities: collagenous colitis (CC) and lymphocytic colitis (LC). A French study performed between 2005 and 2007 in the Somme department found a mean annual incidence of 7.9/105 inhabitants for the MC (5.3/105 for the CC and 2.6/105 for the LC) (2). This incidence was similar to that recorded for Crohn's disease (7.4/105) during the same period in the same area and using the same data source (EPIMAD registry).
CC and LC share the same clinical characteristics such as watery chronic diarrhea, abdominal pain, or weight loss (3,4). They occur more frequently in women and after the age of 60 years (5). The exact etiology of MC remains unknown. It is assumed that the occurrence of MC may be related to an inadequate mucosal immune response against luminal agents (6,7). The role of microbiota (8), genetic (9,10), hormones (11), or bile acids malabsorption (12) was also reported. Several therapeutic classes (e.g., proton-pump inhibitors, selective serotonin reuptake inhibitors, nonsteroidal anti-inflammatory drugs) were also associated with the occurrence of MC (13). Budesonide is now recognized as the treatment of choice in induction and maintenance (14), although in clinical practice a variety of treatments of debatable efficiency is used (2).
Long-term natural history of MC is unknown. Current data come from cohorts of expert centers (15–18), exposed to significant selection bias, or from general population cohorts with limited follow-up (19–21). The objectives of this population-based study is to evaluate the long-term natural history of MC diagnosed in the Somme area between 2005 and 2007, using a unique private-public network, and to assess the long-term remission rate, the treatments efficiency, and the risk of complications.
PATIENTS AND METHODS
Population
All patients with a new diagnosis of MC in the French department of the Somme (568,086 inhabitants, estimated by the Institut national de la statistique et des études économiques INSEE 2008) between January 1, 2005, and December 31, 2007, were prospectively included. Incident cases were reported by the 4 pathology units of the area. Diagnosis was made according to the international definitions: CC was characterized by the presence of a thickening of the subepithelial collagen band (>10 μm) associated with a variable intraepithelial lymphocytosis and LC was defined by more than 20 intraepithelial lymphocytes per 100 surface epithelial cells (22). A systematic histologic blind review by a group of 4 pathologist experts confirmed the diagnosis (2). Only patients living in the Somme area at the time of diagnosis were included.
Data collection
The methodology of the EPIMAD registry was used for this study (23). The data at diagnosis were collected from the medical records of the 27 gastroenterologists of the Somme area (see Acknowledgments) (2). Data of follow-up were retrospectively collected until February 28, 2017, from the records of gastroenterologists and general practitioners who were in charge of the patients. The following variables were collected at diagnosis: date of first symptoms, date of diagnosis (date of first colonoscopy leading to the diagnosis), type of symptoms, weight, height, diagnosis site (university hospital, general hospital, private practice), associated autoimmune disease (AI), consumption of medication at risk of MC (13), and smoking status. Data collected until the end of the follow-up were change in diagnosis (CC, LC), number of relapses, time to relapse, number of hospitalizations related to MC, duration of hospitalization, digestive or extradigestive cancer, and the date and cause of death if applicable. For each colonoscopy, the presence of mucosal lesions such as erythema, erosions, ulcerations, and adenoma or colorectal cancer was recorded. Treatments of each relapse were collected: budesonide, systemic corticosteroid, immunosuppressant, 5-aminosalicylic acid (5-ASA), antimotility agent, antisecretory drug, cholestyramine, antibiotic, probiotic, no treatment, and discontinuation of medication at risk of MC. The clinical remission was defined by cessation of diarrhea (<3 stools/d). Relapse was defined by recurrence of diarrhea or other symptoms present at the diagnosis of MC. Steroid dependence was defined as the inability to decrease to less than 3 mg/d for budesonide or 10 mg/d for systemic corticosteroid or relapse within 3 months after discontinuation of therapy. Steroid resistance was defined as the persistence of diarrhea despite 9 mg/d of budesonide or 0.75 mg/kg of systemic corticosteroid for 4 weeks.
Statistical analysis
Quantitative variables were expressed as median and interquartile range, and qualitative variables were expressed as absolute number and percentages. The cumulative probabilities and 95% confidence intervals (CI) were calculated using the Kaplan-Meier method with accompanied log-rank test. Risk factors for relapse were investigated by a univariate and multivariable Cox proportional hazard model. Parameters with a P value < 0.05 in the univariate analyses were introduced in the multivariate model. The level of statistical significance was defined a priori as a value of P ≤ 0.05. Standardized incidence ratio (SIR) and 95% CI were calculated by the exact Poisson method of Owen. Only cancers occurring during the follow-up were taken into account. Reference data were obtained from the Somme Cancer Registry covering the same area and period. Calculation of the expected cases of deaths and cancers was gender and age adjusted. The data were analyzed with R Software (3.2.4 version).
Ethics
This work was part of the EPIMAD registry, recognized by the National Registry Committee and registered by the CNIL (authorization No. 97107, May 14, 1997), and was part of the Hospital Clinical Research Program, PHRC 2004.
RESULTS
At diagnosis
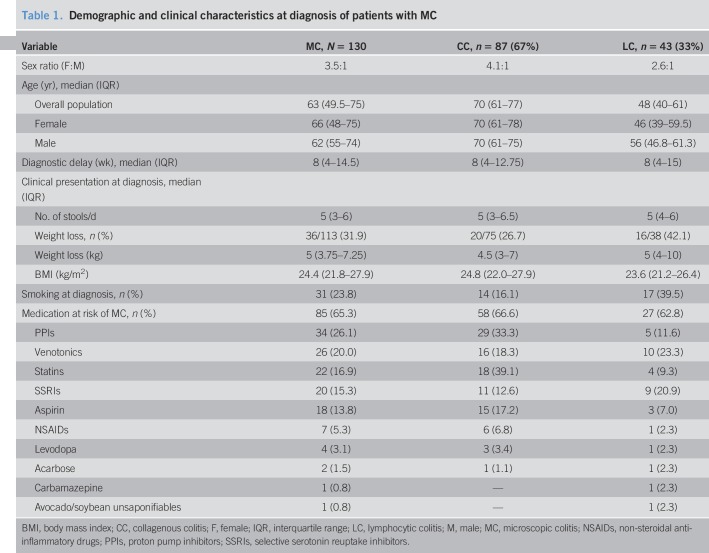
One hundred thirty patients with MC, 87 with CC and 43 with LC, have been recorded. The median age at diagnosis of MC was 63 years (interquartile range, 49–75), 70 years for CC and 48 years for LC. The median time between symptoms onset and diagnosis was 8 weeks (4,14). The median number of stools per day was 5 (3,6), whereas one-third of the patients (36, 32%) had weight loss at diagnosis. Twenty-four percent of patients were active smokers, 16.5% in the CC and 40.5% in the LC group. At diagnosis, 85 patients (66%) were exposed to medication at risk of MC. The most common drugs at risk were proton-pump inhibitors (27%), venotonics (20%), statins (17%), selective serotonin reuptake inhibitors (16%), and aspirin (14%) (Table 1).
Table 1.
Demographic and clinical characteristics at diagnosis of patients with MC
Follow-up
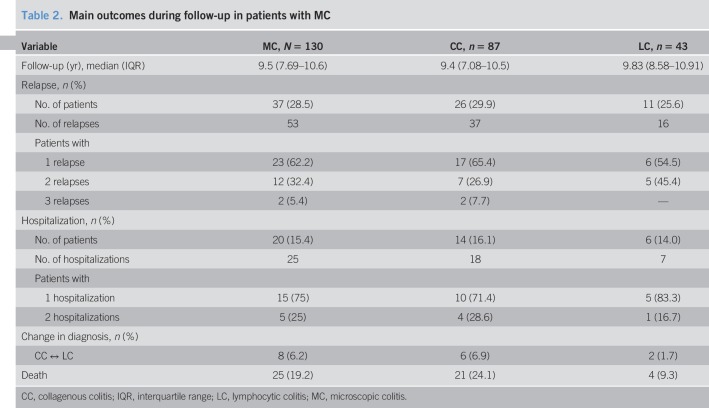
The median duration of follow-up was 9.6 years (7.6; 10.6) (Table 2). Most patients were followed by private gastroenterologists (80%). Thirty-seven patients with MC (28.5%) experienced a total of 53 relapses. The cumulative probability of relapse was 3.2% (0.1%–6.3%) at 1 year, 20.8% (13.0%–27.9%) at 5 years, and 23.6% (15.3%–31.0%) at 7 years (Figure 1a). The median time to first relapse was 3.9 years (1.2; 5). At 7 years, the probability of relapse was 25% in CC and 19% in LC (P = 0.47) (Figure 1b). Eight patients (6%) changed their diagnosis during follow-up, 6 from CC to LC and 2 from LC to CC. Twenty-five patients (19%) died during the follow-up; no death was related to MC.
Table 2.
Main outcomes during follow-up in patients with MC
Figure 1.
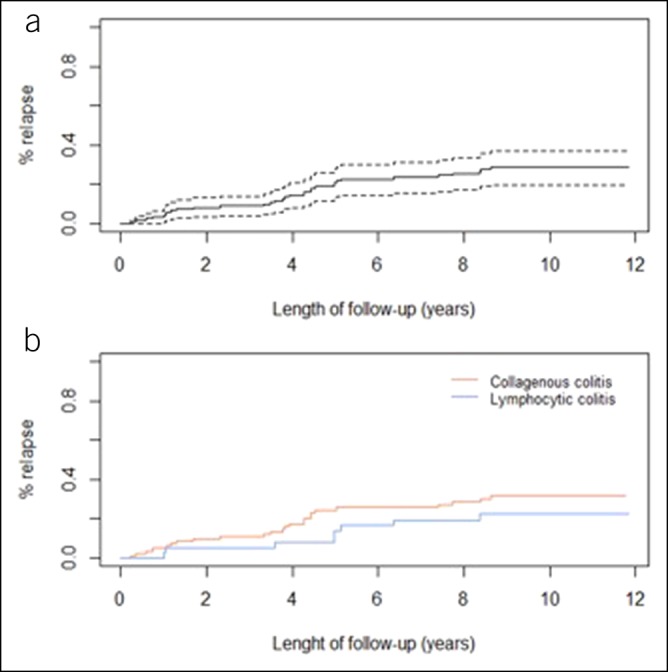
Cumulative probability of first relapse for microscopic colitis (a) and for collagenous colitis or lymphocytic colitis (b).
Hospitalization
Twenty-five MC-related hospitalizations were observed in 20 patients (15%), including 9 patients (36%) at diagnosis (Table 2). The cumulative probability of hospitalization was 7.7% (3.0%–12.2%) at 1 year, 10.2% (4.8%–15.3%) at 5 years, and 14.7% (8.2%–20.8%) at 7 years, with no significant difference between CC and LC (P = 0.6) (see Fig. 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A91). The reasons for hospitalization were diarrhea (100%) with nocturnal stools (12%), hypokalemia (32%), abdominal pain (16%), rectal bleeding (8%), dehydration (8%), and anal incontinence (4%). The median duration of hospitalization was 10 days (6,14).
Associated autoimmune diseases
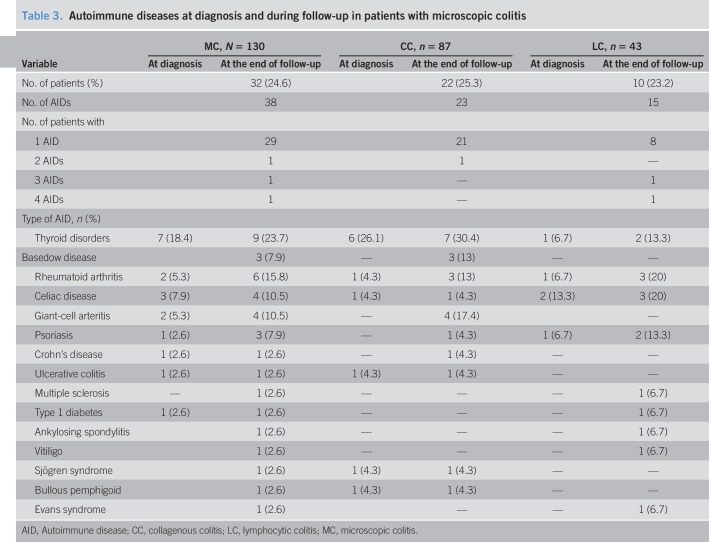
At diagnosis, 18 patients (15%) had AI disease. At the end of follow-up, 32 patients (25%; CC, 25%; LC, 23.2) presented 38 associated autoimmune diseases. The most frequent were thyroid disorders (24%), rheumatoid arthritis (16%), celiac disease (15.8%), and giant-cell arteritis (10.5%) (Table 3).
Table 3.
Autoimmune diseases at diagnosis and during follow-up in patients with microscopic colitis
Dysplasia and cancer
Twelve patients (9%) had one or more colonic adenomas detected during the follow-up, 4 of them (33%) with high-grade dysplasia. No case of colorectal cancer was observed (SIR: 0; 95% CI: 0–0.28). Twenty neoplasias were observed in 17 patients (11%). Fifteen of them were diagnosed after MC diagnosis. No increased risk of overall cancer was observed compared with the general population (SIR: 0.83; 95% CI: 0.44–1.27). (see Tables 2 and 3, Supplementary Digital Content 1, http://links.lww.com/CTG/A91).
Endoscopic lesions
At diagnosis, colonoscopy was performed for 121 patients (94%) and rectosigmoidoscopy for 9 patients (6%). During a median follow-up of 9.6 years (7.6–10.6), 70 colonoscopies and 40 rectosigmoidoscopy were performed again. Erythema was observed in 17 patients (13.0%) and in 23 endoscopies (9.5%) with no significant difference between CC (14.9%, n = 13) and LC (8.3%, n = 4) (P = 0.42). Superficial ulcerations were observed in 2 patients and in 0.4% (n = 2) of endoscopies, with no difference between LC (n = 1) and CC (n = 1). These lesions were located in the sigmoid (61.1%), left colon (44.4%), transverse colon (33.3%), rectum (27.8%), and right colon (27.8%). No cases of perforation have been reported.
Treatment of MC
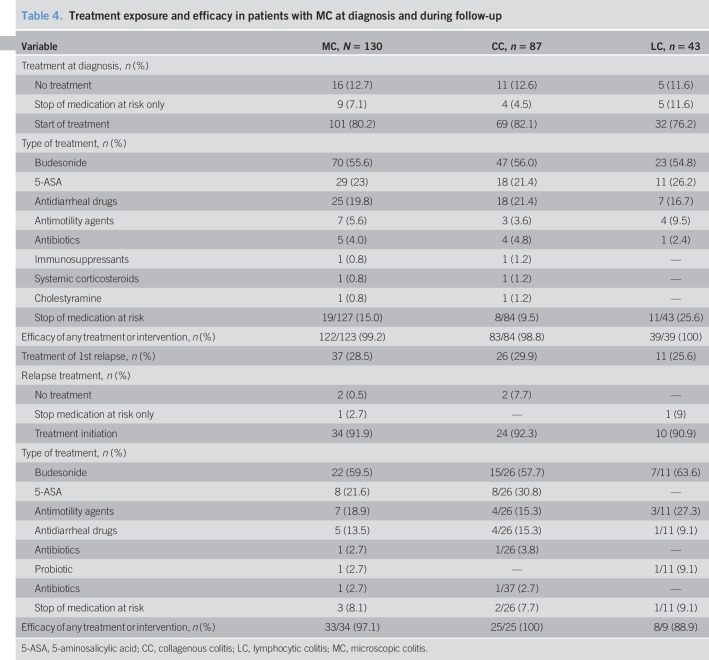
At diagnosis, 70 patients (55.6%) were initially treated with budesonide, 29 (23%) with 5-ASA, 32 (25.4%) with antidiarrheal drugs, 5 (4%) by antibiotics, and 1 (0.8%) by cholestyramine. Only 1 patient (0.8%) was treated with systemic corticosteroids. Of the 85 patients who had a medication at risk of MC, it was stopped in 22 patients (26%). It was the only intervention in 9 patients (7%). Sixteen patients (13%) received no treatment. The efficacy of first-stage treatment was 99% (Table 4).
Table 4.
Treatment exposure and efficacy in patients with MC at diagnosis and during follow-up
Among the 37 patients who relapsed, the distribution of treatments was similar. Two patients (5%) had no treatment, and 1 patient had only stopped a potential medication at risk. Twenty-two (60%) patients were treated by budesonide with a median duration of 145 days (interquartile range, 90–270) and 8 (21.6%) by 5-ASA. The initial efficacy of treatment for the first relapse was 97%.
Overall, the cumulative probability of exposure to budesonide was 55.6% (46.0%–63.5%) at 1 year, 57.3% (47.7%–65.1%) at 5 years, and 58.2% (48.6%–66.0%) at 7 years. The median duration of initial treatment with budesonide was 92 days (70; 168). Thirty patients (40.5% of patients treated with budesonide) required more than one course of budesonide and 4 (5.4%) had budesonide resistance. One patient was effectively treated with azathioprine for 2.5 years for resistance to budesonide. Three patients (2.4%) were exposed to systemic corticosteroids. Symptomatic treatment (antimotility, antisecretory, or other antidiarrheal drugs) was initiated in 39% of patients. It was effective in 69% of cases (Table 4).
No side effects have been reported with budesonide. Five patients (16%) had to stop mesalazine for adverse effects (acute pancreatitis, hepatic and hematologic toxicity, allergic reaction, digestive intolerance). The only patient treated with azathioprine developed an ENT squamous cell carcinoma.
Factors associated with relapse
In the multivariable analysis, the age at diagnosis (HR, 1.03; 95% CI, 1.00–1.06; P = 0.02) and treatment with budesonide at diagnosis (HR, 2.50; 95% CI, 1.11–5.55; P = 0.03) were associated with the risk of relapse. Gender, smoking at diagnosis, discontinuation of medication at risk of MC, or delay in the diagnosis were not associated with relapse (see Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A91).
DISCUSSION
In this large French population-based study of MC, with a median follow-up of nearly 10 years, we report a relapse rate of 30% and a hospitalization rate of 15%. A quarter of patients had an associated AI disease. Budesonide was prescribed in 60% of patients and was effective in 95% of them. Twenty percent of patients treated with budesonide developed steroid dependence and 5% steroid resistance.
Herein, the association of MC with autoimmune diseases is described. However, we found a less frequent association than previously reported (28%–43%) (3,24,25). These associations were mainly reported by studies of expert centers exposed to important selection bias. Moreover, unlike these studies, we excluded type 2 diabetes from the autoimmune diseases spectrum. Few studies have reported diagnosis change between CL and CC over time. In our study, 8 patients changed their diagnosis during follow-up, 6 (13%) from CC to LC and 2 (5%) from LC to CC. However, only half of the patients had a second endoscopy with new biopsies during follow-up. For these reasons, we may have underestimated the rate of change in diagnosis over time. A recent Danish cohort reports similar trends, with a changed to a different MC subgroup in, respectively, 6% and 9% of CC and LC after 2 years of follow-up. One objective of our study was to assess the risk of colorectal cancer in patients with MC. Chronic inflammation is responsible for colonic neoplasia in Crohn's disease or ulcerative colitis (26). During the 10 years of follow-up, no colorectal cancer has been detected. Two studies have reported a decreased risk of colorectal cancer in patients with MC (27,28). One of the hypotheses mentioned was the more frequent colonoscopy in these patients, allowing earlier diagnosis and treatment of preneoplastic lesions. We used data of the Somme cancer registry and observed no increased or decreased risk of digestive or extradigestive cancer in patients with MC.
Budesonide was the most used treatment in our cohort. Its high efficacy is comparable with data from the literature (clinical remission: 80%–86%) (14,29,30). We observed a high rate of steroid dependence (22%), which is similar to that reported in Crohn's disease and ulcerative colitis (31). By contrast, a population-based study of MC conducted by Gentile et al. (19) reported a 64% dependence on corticosteroids. However, the study included smaller patient population, and more than 20% of patients were treated with systemic corticosteroids. The same study found a rate of steroid resistance for budesonide of 3.5%.
In our study, more than 1 in 4 patients experienced a relapse. This relapse rate is consistent with data from several prospective studies (25%–61%) (17,21,29,32). These results may support the interest of maintenance treatment with budesonide (14). As demonstrated by a randomized controlled clinical trial, a 12-month treatment with budesonide is effective and well tolerated. Identifying factors predicting relapse would identify patients who could benefit from such treatment. In our study, older age at diagnosis and budesonide exposure at diagnosis were associated with relapse. A post-hoc analysis of 4 randomized studies conducted in 2013 (32) identified the following risk factors of disease relapse: number of stools at diagnosis above 5, a delay at diagnosis of more than 1 year, and the absence of maintenance treatment by budesonide. In our study, the association between budesonide treatment at first flare and risk of relapse was observed. Comparable results have been observed in Crohn's disease or ulcerative colitis where the first course of corticosteroid could be a surrogate marker of disease severity. New prospective studies will be needed to assess the risk of relapse.
Our study has several strengths. First, histologic confirmation by a group of 4 pathologists was systematically blinded to initial results. Second, our cohort is the largest among the population-based cohorts (15,16,19,20), except for the study by Fernández-Bañares (21) which followed 184 patients with MC but only over a period of 28 months. The duration of follow-up of our study is so far the longest reported in the literature. The number of variables identified, the cross-referencing of data through several sources (university hospital, private gastroenterologists, general practitioner), and the methodology of data collection validated by the EPIMAD register reinforce the validity of the collected data. One of the limitations of our study is the retrospective data collection. Furthermore, it was demonstrated in previous studies that there is a similarity between symptoms of MC and irritable bowel syndrome (IBS), with 38%–58% of patients with MC fulfilling IBS criteria (33,34). This leads to difficulty in individualizing an authentic flare of MC from IBS symptoms.
In our large population-based cohort with a median follow-up of 10 years, two-third of patients with MC observed long-term clinical remission after diagnosis. A high prevalence of autoimmune disease has been observed, but no increased risk of cancer was observed. Fifteen percent of patients were hospitalized due to MC. Budesonide was the most used and most effective treatment.
CONFLICTS OF INTEREST
Guarantor of the article: Mathurin Fumery, MD.
Specific author contributions: J.L. and M.F.: Study concept and design. J.L.: Acquisition of data. D.D., H.S., J.L., M.F., H.B.K., and O.G.: Analysis and interpretation of data. J.L. and M.F.: Drafting of the manuscript. C.G.R., G.S., H.S., D.D., E.N.K., J.L.D., F.B., C.Y., D.C., H.B.K., O.G.: Critical revision of the manuscript for important intellectual content. M.F., J.L.: Approval of the final manuscript.
Financial support: None.
Potential competing interests: None.
Study Highlights.
WHAT IS KNOWN
✓ MC is now recognized as one of the most common causes of chronic diarrhea, but long-term natural history of MC is unknown.
WHAT IS NEW HERE
✓ About one-third of patients will relapse, and 15% will be hospitalized.
✓ Twenty-five percent of patients will present an associated autoimmune disease.
✓ MC did not increase risk of digestive and extradigestive cancer.
✓ About one-quarter will present corticodependency.
TRANSLATIONAL IMPACT
✓ MC is a global disease associated with a systemic disease and risk of flare-related hospitalization.
✓ No increased risk of cancer is associated with MC.
Supplementary Material
ACKNOWLEDGMENTS
Collaborators: Fadi Assi, Abselam Bental, Sylvie Dautreme, Mathieu Eoche, Denis Laude, Jean Christophe Prevost, Francois Sevenet, Arsene Papazian, Hubert Mancheron, Alain Rudelli, Bruno Heyman, Michel Wantiez, Gil Cohen, Marine Lagarde, Nicole Reix, Marion Groux, Marthe Chavance-thelu, Morgane Bourgeois Fumery, Richard Delcenserie, Jean-Paul Joly, Vincent Hautefeuille, Justine Thomas, Sami Hakim, Charles Sabbagh.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A91
REFERENCES
- 1.Lindström CG. “Collagenous colitis” with watery diarrhoea—A new entity? Pathol Eur 1976;11:87–9. [PubMed] [Google Scholar]
- 2.Fumery M, Kohut M, Gower-Rousseau C, et al. Incidence, clinical presentation, and associated factors of microscopic colitis in northern France: A population-based study. Dig Dis Sci 2016. [DOI] [PubMed] [Google Scholar]
- 3.O'Toole A, Coss A, Holleran G, et al. Microscopic colitis: Clinical characteristics, treatment and outcomes in an Irish population. Int J Colorectal Dis 2014;29:799–803. [DOI] [PubMed] [Google Scholar]
- 4.Bjørnbak C, Engel PJH, Nielsen PL, et al. Microscopic colitis: Clinical findings, topography and persistence of histopathological subgroups. Aliment Pharmacol Ther 2011;34:1225–34. [DOI] [PubMed] [Google Scholar]
- 5.Tong J, Zheng Q, Zhang C, et al. Incidence, prevalence, and temporal trends of microscopic colitis: A systematic review and meta-analysis. Am J Gastroenterol 2015;110:265–76. [DOI] [PubMed] [Google Scholar]
- 6.Järnerot G, Tysk C, Bohr J, et al. Collagenous colitis and fecal stream diversion. Gastroenterology 1995;109:449–55. [DOI] [PubMed] [Google Scholar]
- 7.Austin LL, Dobbins WO. Studies of the rectal mucosa in coeliac sprue: The intraepithelial lymphocyte. Gut 1988;29:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer H, Holst E, Karlsson F, et al. Altered microbiota in microscopic colitis. Gut 2015;64:1185–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine KD, Do K, Schulte K, et al. High prevalence of celiac sprue-like HLA-DQ genes and enteropathy in patients with the microscopic colitis syndrome. Am J Gastroenterol 2000;95:1974–82. [DOI] [PubMed] [Google Scholar]
- 10.Järnerot G, Hertervig E, Grännö C, et al. Familial occurrence of microscopic colitis: A report on five families. Scand J Gastroenterol 2001;36:959–62. [DOI] [PubMed] [Google Scholar]
- 11.Roth B, Manjer J, Ohlsson B. Microscopic colitis and reproductive factors related to exposure to estrogens and progesterone. Drug Target Insights 2013;7:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Bañares F, Esteve M, Salas A, et al. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci 2001;46:2231–8. [DOI] [PubMed] [Google Scholar]
- 13.Beaugerie L, Pardi DS. Review article: Drug-induced microscopic colitis—Proposal for a scoring system and review of the literature. Aliment Pharmacol Ther 2005;22:277–84. [DOI] [PubMed] [Google Scholar]
- 14.Münch A, Bohr J, Miehlke S, et al. Low-dose budesonide for maintenance of clinical remission in collagenous colitis: A randomised, placebo-controlled, 12-month trial. Gut 2016;65:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan JL, Tersmette AC, Offerhaus GJ, et al. Cancer risk in collagenous colitis. Inflamm Bowel Dis 1999;5:40–3. [DOI] [PubMed] [Google Scholar]
- 16.Bonner GF, Petras RE, Cheong DM, et al. Short- and long-term follow-up of treatment for lymphocytic and collagenous colitis. Inflamm Bowel Dis 2000;6:85–91. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Bañares F, Salas A, Esteve M, et al. Collagenous and lymphocytic colitis. evaluation of clinical and histological features, response to treatment, and long-term follow-up. Am J Gastroenterol 2003;98:340–7. [DOI] [PubMed] [Google Scholar]
- 18.Goff JS, Barnett JL, Pelke T, et al. Collagenous colitis: Histopathology and clinical course. Am J Gastroenterol 1997;92:57–60. [PubMed] [Google Scholar]
- 19.Gentile NM, Abdalla AA, Khanna S, et al. Outcomes of patients with microscopic colitis treated with corticosteroids: A population-based study. Am J Gastroenterol 2013;108:256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sveinsson OA, Orvar KB, Birgisson S, et al. Clinical features of microscopic colitis in a nation-wide follow-up study in Iceland. Scand J Gastroenterol 2008;43:955–60. [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Bañares F, de Sousa MR, Salas A, et al. Impact of current smoking on the clinical course of microscopic colitis. Inflamm Bowel Dis 2013;19:1470–6. [DOI] [PubMed] [Google Scholar]
- 22.Schneider S, Rampal A, Hebuterne X, et al. Microscopic colitis. Gastroenterol Clin Biol 1998;22:431–41. [PubMed] [Google Scholar]
- 23.Gower-Rousseau C, Salomez JL, Dupas JL, et al. Incidence of inflammatory bowel disease in northern France (1988-1990). Gut 1994;35:1433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olesen M, Eriksson S, Bohr J, et al. Lymphocytic colitis: A retrospective clinical study of 199 Swedish patients. Gut 2004;53:536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardi DS, Ramnath VR, Loftus EV, et al. Lymphocytic colitis: Clinical features, treatment, and outcomes. Am J Gastroenterol 2002;97:2829–33. [DOI] [PubMed] [Google Scholar]
- 26.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am J Physiol Gastrointest Liver Physiol 2004;287:G7–17. [DOI] [PubMed] [Google Scholar]
- 27.Tontini GE, Pastorelli L, Spina L, et al. Microscopic colitis and colorectal neoplastic lesion rate in chronic nonbloody diarrhea: A prospective, multicenter study. Inflamm Bowel Dis 2014;20:882–91. [DOI] [PubMed] [Google Scholar]
- 28.Yen EF, Pokhrel B, Bianchi LK, et al. Decreased colorectal cancer and adenoma risk in patients with microscopic colitis. Dig Dis Sci 2012;57:161–9. [DOI] [PubMed] [Google Scholar]
- 29.Miehlke S, Madisch A, Karimi D, et al. Budesonide is effective in treating lymphocytic colitis: A randomized double-blind placebo-controlled study. Gastroenterology 2009;136:2092–100. [DOI] [PubMed] [Google Scholar]
- 30.Miehlke S, Madisch A, Kupcinskas L, et al. Double-blind, double-dummy, randomized, placebo-controlled, multicenter trial of budesonide and mesalamine in collagenous colitis. Gastroenterology 2012;142:S-211. [Google Scholar]
- 31.Faubion WA, Loftus EV, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: A population-based study. Gastroenterology 2001;121:255–60. [DOI] [PubMed] [Google Scholar]
- 32.Miehlke S, Hansen JB, Madisch A, et al. Risk factors for symptom relapse in collagenous colitis after withdrawal of short-term budesonide therapy. Inflamm Bowel Dis 2013;19:2763–7. [DOI] [PubMed] [Google Scholar]
- 33.Abboud R, Pardi DS, Tremaine WJ, et al. Symptomatic overlap between microscopic colitis and irritable bowel syndrome: A prospective study. Inflamm Bowel Dis 2013;19:550–3. [DOI] [PubMed] [Google Scholar]
- 34.Limsui D, Pardi DS, Camilleri M, et al. Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm Bowel Dis 2007;13:175–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.