Abstract
Plasma levels of soluble PD-L1 (sPD-L1) have been reported to be an independent prognostic factor in many malignant tumors. The expression of sPD-L1 in nasopharyngeal carcinoma (NPC) has not been reported. The purpose of this study was to evaluate the expression of sPD-L1 and analyze its correlation with clinical characteristics in patients with NPC.
Thirty-five patients with stage I-IVa NPC were included. Plasma samples were obtained pretreatment. The sPD-L1 concentrations were measured by enzyme-linked immunosorbent assay (ELISA). The correlations of sPD-L1 expression with clinical parameters and laboratory data were analyzed.
sPD-L1 was detected in 35 plasma samples, the mean sPD-L1 concentration was 45.47 pg/ml. sPD-L1 was significantly higher in stage III-IVa (50.76 ± 28.15 pg/ml) compared to stage I-II (19.87 ± 11.38 pg/ml) (t = 2.618, P = .013). sPD-L1 was also higher in stage N2–3 (52.03 ± 28.98 pg/ml) than that in N0–1 (32.88 ± 23.75 pg/ml) (t = 2.096, P = .046). Univariate analysis identified that sPD-L1 level positively correlated with clinical stage (r = 0.495, P = .002) and N stage (r = 0.34, P = .046). Multivariate analysis showed the clinical stage was an independent factor affecting sPD-L1 expression.
This is the first report to detect sPD-L1 in NPC. The study indicated sPD-L1 is quantifiable, convenient and easy to obtain. sPD-L1 may serve as a useful biomarker for evaluating tumor progression and therapeutic efficacy of NPC.
Keywords: enzyme-linked immunosorbent assay, nasopharyngeal carcinoma, soluble programmed death ligand 1
1. Introduction
Nasopharyngeal carcinoma (NPC) is a unique type of head and neck cancer (HNC) due to its biological characteristics. It has a special geographic distribution, Epstein-Barr virus (EBV) related etiology, histology.[1] NPC is one of the most common malignant tumors in Southeast Asia. It is well known that NPC is characterized by prevailing EBV infection, which has been implicated in NPC pathogenesis.[2,3] Histologically, NPC can be divided into a keratinizing subtype and a non-keratinizing subtype, the latter of which is most prevalent in endemic areas.[4] Although it is highly sensitive to radiotherapy and chemotherapy, treatment failure for NPC remains nearly 30%.[5] Therefore, better and novel therapies for NPC are urgently warranted.
Programmed death ligand 1(PD-L1) is a critical molecule that inhibits immune responses through its receptor, programmed death-1(PD-1), which is expressed on different immune cells.[6] It is acknowledged that the PD-1/PD-L1 axis plays a crucial role in tumor progression by altering the status of immune surveillance.[7] Recently, the emergence of anti-PD-L1/PD-1 immunotherapy has provided significant clinical improvements in the treatment of various malignant tumors, including HNC.[8–10] For PD-L1-positive recurrent or metastatic NPC, an anti-PD-1 antibody, pembrolizumab, is now as an option in the updated 2018 National Comprehensive Cancer Network (NCCN) guidelines.[11] As a marker for anti-PD1/PD-L1 treatment, PD-L1 expression is detected in tumors using tissue samples obtained by invasive biopsy. Additionally, PD-L1 expression is heterogeneous within tumors and changes dynamically during the treatment.[12] Monitoring the changes in PD-L1 clinically becomes challenging. In addition to being membrane-bound on tumor cells, PD-L1 is also present in a soluble form, soluble PD-L1 (sPD-L1), which is found in the peripheral blood of cancer patients.[13] sPD-L1 which is a protein, was originally detected by enzyme-linked immunosorbent assay (ELISA) in human serum or plasma, which is more convenient and less invasive to obtain than biopsy tissues.[14] sPD-L1 may be an ideal biomarker for real-time dynamic changes of PD-L1 in NPC. Previous studies indicated that sPD-L1 is involved in tumor-associated immune suppression, and promotes cancer progression.[15,16] The results from a study by Okuma et al,[17] suggested that sPD-L1 was able to bind to its PD-1 receptor on T cells, consequently impairing anti-tumor immune activity. To date, only 2 studies have reported that sPD-L1 could be detected in HNC.[18,19] In addition, sPD-L1 was detected in the peripheral blood of patients with lung cancer (LC), biliary tract cancer (BTC), renal cell cancer (RCC), pancreatic cancer(PC), and hepatocellular cancer (HCC). These studies reported that the high level of sPD-L1 was associated with poor prognosis.[15–17,20–25] Therefore, sequentially evaluating the sPD-L1 status is a promising method to monitor the therapeutic efficacy and optimize anti-PD-1/PD-L1 immunotherapy strategies.
To our knowledge, up to now, circulating sPD-L1 has not been investigated in patients with NPC. This study explored the expression of PD-L1 in the peripheral blood of NPC patients, and analyzed its correlation with clinical characteristics and laboratory data.
2. Materials and methods
2.1. Patients information
In the study, patients with histologically proven NPC without distant metastases were enrolled between January 2016 and December 2017. Before any treatment, all patients underwent the following examinations: a complete head and neck exam, nasopharyngeal fiberoptic examination, biopsy of primary site or fine needle biopsy of the neck, computed tomography (CT) and magnetic resonance imaging (MRI) with contrast of skull base to neck, chest CT with contrast, and/or 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (18F-FDG PET/CT), EBV/DNA testing. The clinical stage of all patients was determined according to the AJCC 8th edition classification system. Peripheral blood samples were obtained from 35 NPC patients. The related clinical data of these patients were collected including gender, age, Tumor-Nodal-Metastasis (TNM) stage, histological subtypes, EBV status, performance Status (PS), and therapy. The study was approved by the Shandong Cancer Hospital, which is affiliated with Shandong University, and informed consent was obtained from the patients.
2.2. Treatment
Definitively radiotherapy alone was used for stage I disease and a combination of radiotherapy and chemotherapy was used for stage II-IVa disease.
2.2.1. Radiotherapy
All patients received a CT simulation scan at the radiotherapy position. The images included plain and enhanced CT scans. The scope of each scan was from the top of the head to 2 cm below subclavicle head (thickness: 3 mm per slice). The gross tumor volume (GTV) was defined as the primary tumor (GTVp), and metastatic lymph nodes (GTVn). Contouring was performed under the Varian planning system. All patients received intensity-modulated radiotherapy (IMRT), and a daily treatment using 2.0 Gy fractions. The prescribed doses for the NPC patients were applied as follows:
-
(1)
planning target volume (PTV) of GTVp and GTVn: 70 Gy/35 fractions;
-
(2)
PTV of the high-risk clinical target volume: 60 Gy/30 fractions;
-
(3)
PTV of the low-risk clinical target volume: 50 Gy/25 fractions.
Radiation planning required that 95% of the PTV be encompassed by 95% of the isodose.
2.2.2. Chemotherapy regimen
Concurrent chemotherapy was administered weekly (cisplatin 40 mg/m2, day 1) or every 3 weeks (cisplatin 75 mg/m2, day 1–3) during radiotherapy course. Induction chemotherapy consisted of cisplatin (75 mg/m2) with docetaxel (75 mg/m2) every 3 weeks for 3 cycles. Adjuvant chemotherapy (cisplatin 75 mg/m2, day 1–3, 5-fuorouracil 3.0 g/m2, continuous infusion into vein 120 hours) was administered every 3 weeks for 3 cycles.
2.2.3. Volume calculation
The volumes of primary tumor and metastatic lymph nodes were calculated automatically by software using the Varian planning system. If there were 2 or multiple metastatic lymph nodes in the neck, the volume of metastatic lymph nodes was the sum of all.
2.3. Blood sample collection and detection of sPD-L1
Plasma samples (7.5 ml) were obtained before any treatment was provided. The blood samples were delivered to the laboratory, and to remove blood cells, and plasma tubes were centrifuged at 3000 rounds per minute for 10 minutes at room temperature to remove blood cells. Plasma specimens were aliquoted and stored at -80°C.
For the detection of sPD-L1 in patients’ plasma, a commercially available ELISA kit was used (Abcam, #ab214565 Human PD-L1 Simple Step ELISA Kit) according to the manufacturer specifications manual. The sPD-L1 level was calculated using standard curves. The minimum detectable level of sPD-L1 was 2.91 pg/ml and the detection range was 21.87–1400 pg/ml. The sPD-L1-positive level was defined as a concentration ≥21.87 pg/ml, and the sPD-L1-negtive level was defined as a concentration <21.87 pg/ml. Each sample was analyzed in duplicate. The intra-assay and inter-assay coefficients of variation were below 10%.
2.4. Blood cell counts and EBV detection
Complete blood cell counts, including white blood cells (WBC), red blood cells (RBC), hemoglobin (HB), platelets (PLT), neutrophils, monocytes, and lymphocytes were measured by an automated hematology analyzer (XE-5000, Sysmex, Kobe, Japan) at the central laboratory of Shandong Cancer Hospital and Institute. Each lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) were calculated by dividing the absolute lymphocyte count (ALC) by the absolute monocyte count (AMC), the absolute neutrophil count (ANC) by the ALC, and the absolute platelet count (PLC) by the ALC, respectively.
EBV, including EBV-VCR IgA and EBV-Rta IgG was measured using ELISA at our central laboratory. EBV positivity was defined as Ig A and/or IgG positive. EBV negative was defined as the negative for both Ig A and IgG.
2.5. Statistical analysis
All data were analyzed using IBM SPSS software version 23.0 (SPSS Inc., Chicago, IL). A descriptive statistical analysis was performed and the results were reported as the mean ± standard deviation (SD) for continuous variable and categorical variables were presented as rates. The difference in PD-L1 levels between groups was evaluated by t test. Univariate analysis to identify clinical factors associated with sPD-L1 expression was performed by using Pearson correlation coefficient test or Spearman rank correlation coefficient test. For multivariate analysis, the forward Wald procedure was performed using a logistic regression model containing all statistically significant variables in univariate analysis. Standard error of measurement was used to determine the 95% confidence interval. A 2-sided P value <.05 was used to evaluate the significance of the data. Figures were made by GraphPad Prism version 7.00 for Mac, GraphPad Software, San Diego, CA.
3. Results
3.1. General information
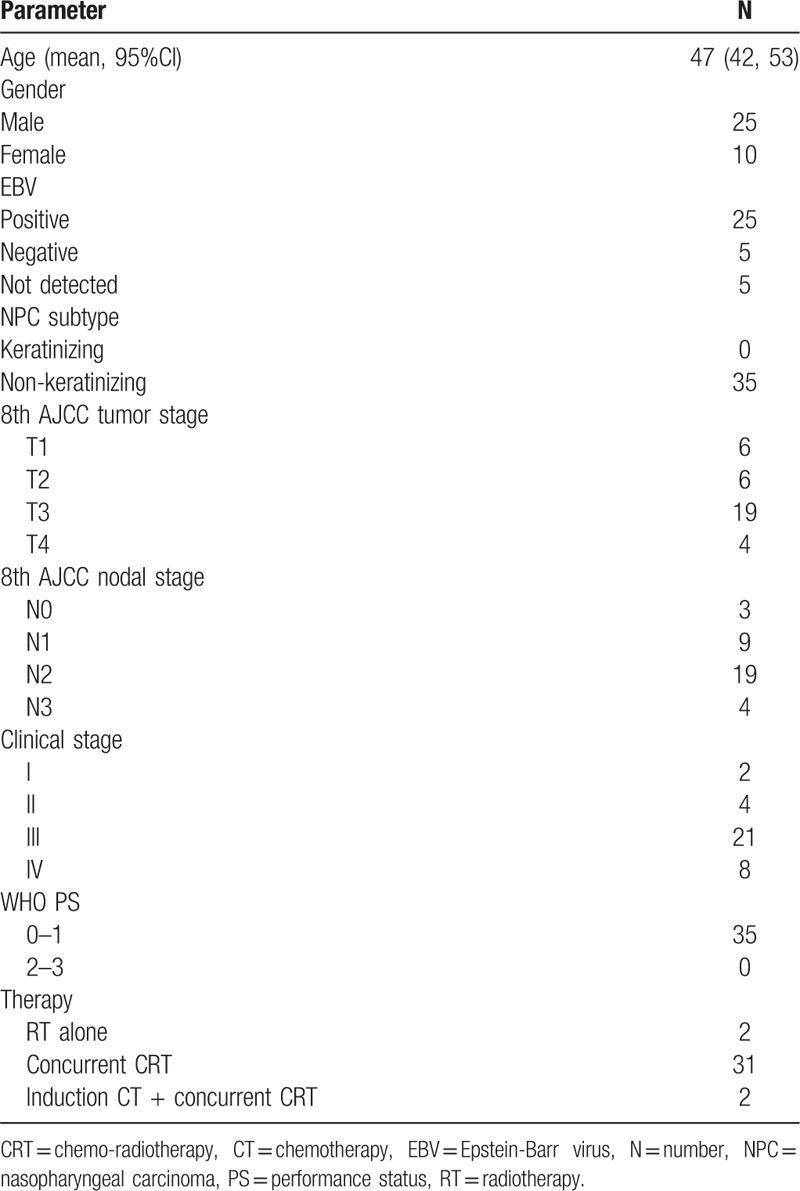
A total of 35 NPC patients with clinical stage I-IVa (2 in stage I, 4 in stage II, 21 in stage III, 8 in stage IVa) were analyzed. Twenty-five patients were males and 10 patients were females, and the mean age was 47 years old (range from 18 to 76 years old). All tumors were classified as non-keratinizing phenotype [World Health Organization (WHO) II/III]. The patients and tumor characteristics are summarized in Table 1. Two patients with stage I disease received definitively radiotherapy alone and 33 patients with stage II-IVa disease received radiotherapy combined with chemotherapy. Of the 33 patients, 2 patients received induction chemotherapy following concurrent chemo-radiotherapy, and 31 patients received concurrent chemo-radiotherapy following adjuvant chemotherapy.
Table 1.
Clinicopathological features in NPCs.
3.2. Detection of sPD-L1 in patients before treatment
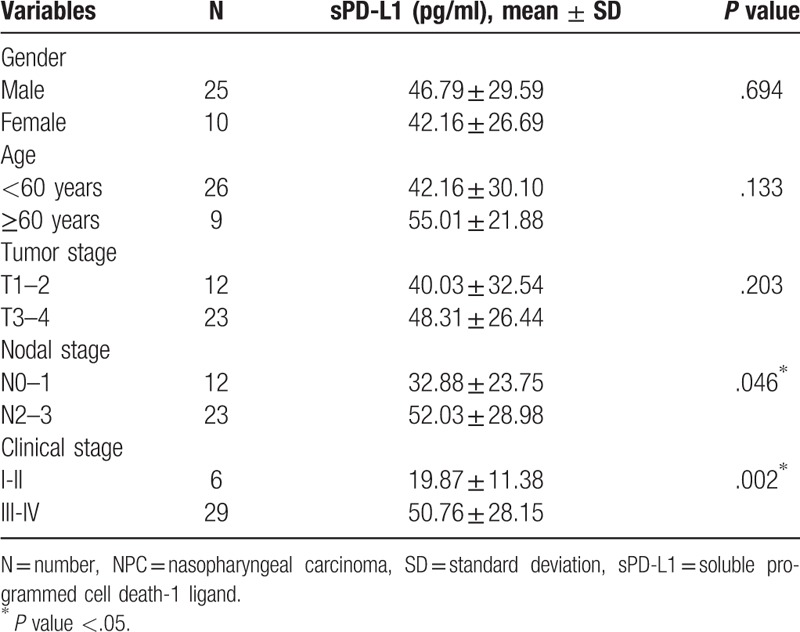
Of the 35 patients, sPD-L1 was quantified, and 29 patients (83%) were within the detection range (21.87–1400 pg/ml), which was defined as sPD-L1 positive, while 6 patients (17%) were below the lower limit of detection (sPD-L1 < 21.87 pg/ml), which was defined as sPD-L1 negative. The mean plasma concentration (mean ± SD) of sPD-L1 in all patients was 45.47 ± 28.48 pg/ml (median: 36.36; range: 6.44–114.97 pg/ml).
3.3. The association between sPD-L1 and clinical features
The concentration of sPD-L1 was higher in stage III-IVa (50.76 ± 28.15 pg/ml) compared to stage I-II (19.87 ± 11.38 pg/ml) (t = 2.618, P = .013) (see Fig. 1A). The sPD-L1 level was positively correlated with the clinical stage, when it was sub-divided into stage III-IVa and stage I-II (r = 0.495, P = .002). The concentration of sPD-L1 was higher in stage N2–3 (52.03 ± 28.98 pg/ml) than stage N0–1 (32.88 ± 23.75 pg/ml) (t = 2.096, P = .046) (see Fig. 1B). The level of sPD-L1 positively correlated with the N stage (r = 0.34, P = .046). No difference in sPD-L1 level between stage T3–4 (48.31 ± 26.44 pg/ml) and stage T1–2 (40.03 ± 32.54 pg/ml) (t = 0.812, P = .422) (see Fig. 1C) was found. The correlation between sPD-L1 and the T stages was not identified (r = 0.221, P = .203). There was no association between sPD-L1 level and gender or age neither (both P > .05). The associations between the plasma levels of sPD-L1 and the clinical features of the patients are summarized in Table 2.
Figure 1.
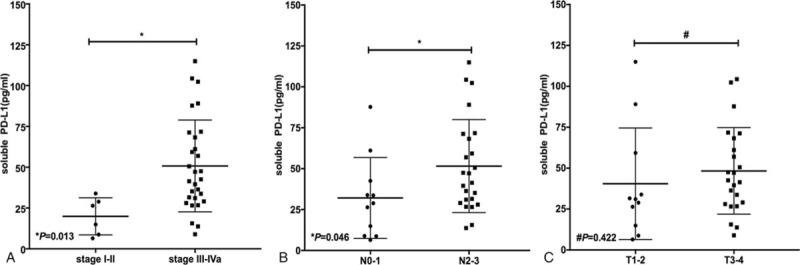
Soluble programmed death-ligand 1 (sPD-L1) levels in nasopharyngeal carcinoma (NPC) patients with different clinical stages, N and T stages. A: in comparison of sPD-L1 levels with clinical stages I-II and III-IVa. B: sPD-L1 levels with different N stages (N0–1 vs N2–3). C: sPD-L1 levels with different T stages (T1–2 vs T3–4). Significant differences between the groups are marked by asterisk. Non-significant differences are marked by a hash. The horizontal lines within the data signify the mean ± standard deviation (SD).
Table 2.
Associations between plasma concentrations of sPD-L1 and clinical variables in NPCs.
3.4. The association between sPD-L1 and volumes of primary tumor or metastatic lymph nodes
The mean volume of the primary tumor was 40.24 mm3 (range: 10.4–101.9 mm3) in the 35 patients. No positive correlation between sPD-L1 level and the volume of the primary tumor (P = .549) was found. There were 31 patients (31/35) with lymph node metastasis. The mean volume of metastatic lymph nodes was 17.22 mm3 (range: 0.5–57.7 mm3). There was no correlation between sPD-L1 level and metastatic lymph nodes volumes (P = .179). When further analyzing the volume of primary tumor and metastatic lymph nodes together, no association with sPD-L1 level was identified neither (P = .112).
3.5. The association between sPD-L1 level and blood cell counts or EBV
The mean counts of WBC, RBC, HB, PLT, neutrophils, monocytes, and lymphocytes were (6.29 ± 1.84) × 109/L, (4.67 ± 0.57) × 1012/L, (136 ± 16)g/L, (268 ± 74) × 109/L, (4.37 ± 1.6) × 109/L, (0.57 ± 0.19) × 109/L, (1.26 ± 0.67) × 109/L, respectively. The means of NLR, LMR, and PLR values were (4.71 ± 3.21), (2.51 ± 1.66), (290 ± 203), respectively. No correlation was found between sPD-L1 level and blood cell counts or between sPD-L1 level and LMR, NLR, or PLR (all P > .05) (Table 3).
Table 3.
Associations between sPD-L1 level and blood cell counts or EBV level.
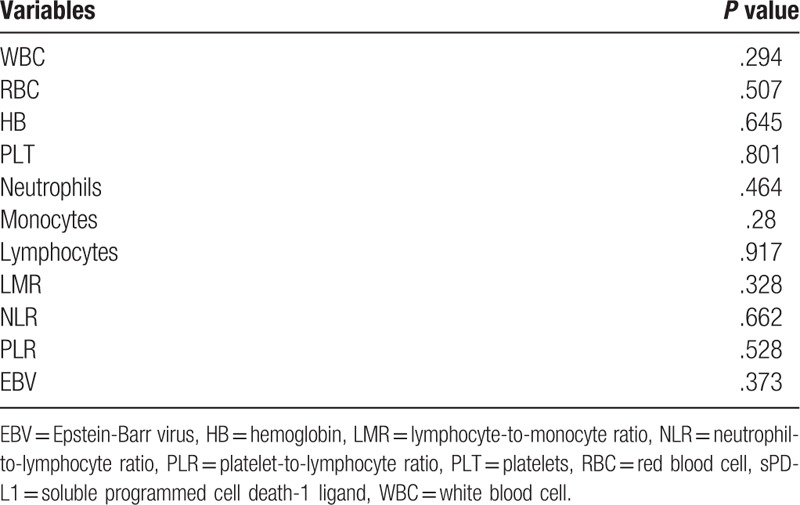
In the 35 NPC patients, 30 patients had detectable EBV levels, of which 25 patients were positive, and 5 patients were negative. The relationship between sPD-L1 level and the EBV level was not identified (P = .373) (Table 3).
3.6. Univariate and multivariate analyses
Univariate analysis identified that clinical stage and N stage were correlated with sPD-L1 expression. A multivariate regression model with forward stepwise analysis including the above variables showed that only the clinical stage was an independent factor affecting sPD-L1 expression. Adjusted R2 = 0.147.
4. Discussion
Although PD-1 inhibitor is now as an option in recurrent or metastatic NPC patients, the objective response rate (ORR) of patients with PD-L1-positive patients was only 25.9%. Therefore, it is significantly important to find a biomarker for selecting the patients who might benefit from anti-PD1/PD-L1 immunotherapies. The levels of sPD-L1 have been reported to be an independent prognostic factor in many malignant tumors that are susceptible to immunotherapy targeting the PD-1 axis.[16,20] One meta-analysis focused on the prognostic significance of sPD-L1 in 8 cancers and 1102 cancer patients.[14] It was shown that a higher level of sPD-L1 was associated with poor outcomes. sPD-L1 may serve as a potential biomarker for cancer immunotherapy. To the best of our knowledge, sPD-L1 expression in NPC patients has not been reported. The present study was the first to detect sPD-L1 levels by ELISA in patients with NPC. The mean plasma levels of sPD-L1 in all patients enrolled was 45.47 pg/ml. Twenty-nine samples (83%) were determined to be positive and 6 samples (17%) were determined to be negative when using the recommended cut-off value (21.47 pg/ml) in the test reagent manuals. For LC patients, Okuma et al[17] reported that sPD-L1 was detected in all plasma samples, and the mean concentration was 6950 pg/ml (median 7930 pg/ml; range: 2300–20000 pg/ml). Oher studies detected the expression of sPD-L1 using serum samples. In 158 advanced BTC patients, sPD-L1 was detected in all samples and the median level was 1200 pg/ml (range: 500–2100 pg/ml).[21] The median level of sPD-L1 in 172 RCC patients was 230 pg/mL (range: 0–4400 pg/mL), among which 165 patients (96%) had detectable sPD-L1.[16] A Study by Kruger et al[26] showed that the median sPD-L1 level was 12 pg/mL (range: 7–632 pg/mL) in 41 advanced PC patients, of which 26 cases (63.4%) were detectable. In 215 HCC patients, the median sPD-L1 level was 500 pg/ml (range: 30–6040 pg/ml), while only 40 patients (18.6%) had sPD-L1 levels among the detection range (160–10000 pg/ml).[20] The above studies indicated that sPD-L1 was detectable both in the plasma and the serum. However, the difference in sPD-L1 expression between plasma and serum or which sample type is better has not yet been compared. The concentrations of sPD-L1 varied (ranging from 12 to 6950 pg/ml) among cancers. The most likely explanation is that different types of cancer have different sPD-L1 basic levels, which may be affected by different infiltrative immunocytes or different expressions of other immunosuppressant molecules. The other possible reasons may be different antibodies manufactured by different companies, and the different sample sizes.
Previous studies suggested that sPD-L1 was correlated with the progression of cancer. In the present study, it was found that the level of sPD-L1 was higher in advanced clinical stages (III-IVa) compared to those with early stages (I-II). Further analysis showed that the sPD-L1 level was positively correlated with the clinical stage, which suggested that sPD-L1 might be an indicator of the extent of tumor invasion. The data on the correlation between sPD-L1 and clinical stage were inconsistent in previous studies. A study by Finkelmeier et al,[20] found that the level of sPD-L1 with stage C or D was significantly higher than that in stage A or B in HCC patients according to the BCLC staging system, and the sPD-L1 levels positively correlated with stages of HCC. A study by Cheng et al[27] showed that the level of sPD-L1 in patients with advanced stages (III-IV) was higher than those at early stages (I-II) in non-small cell cancer, and a positive association was observed between sPD-L1 levels and clinical stages. Another study also enrolled LC patients, but no difference in sPD-L1 level was found among different stages.[17] Further analysis showed that there was no correlation between sPD-L1 levels and the clinical stage. A possible reason for the inconsistent results may be that the patients enrolled in the study by Cheng et al were stage I-IV, while those enrolled by Okuma et al were stage IIIB-IV. Additionally, we found that the N stage (when grouped into N0–1 and N2–3) was positively correlated with the sPD-L1 level. The study by Weber et al[18] also demonstrated that the increased PD-L1 expression in peripheral blood was significantly associated with lymph node metastases (N+ vs. N0) in oral squamous cell carcinoma. It was suggested that the sPD-L1 level might be an indicator of the existence of metastatic disease. We additionally analyzed the correlation between sPD-L1 level and tumor burden. Unfortunately, the sPD-L1 level was not correlated with tumor volume, metastatic lymph nodes volume, or both volumes together. This result was consistent with those from a previous study, which found no correlation between the high level of PD-L1 staining and the nasopharynx the gross tumor volume.[28] Apart from this, other similar reports have not yet been found.
Peripheral blood cells, including WBC, neutrophils, and lymphocytes, which are known as inflammatory factors, were considered to be related to the change in immune microenvironment. This change may lead to an imbalance between tumor-promoting inflammatory and antitumor immune status. In this study, the findings did not show the correlation was found between sPD-L1 and WBC, neutrophils, and lymphocytes. Takahashi et al[22] also analyzed the association between sPD-L1 levels and WBC and neutrophils in metastatic or recurrent gastric cancer. The results showed that patients with a high WBC had a higher sPD-L1 levels than those patients with a low WBC. The patients with a high neutrophil count also had a higher sPD-L1 level than those with a low neutrophil count level. The data on the relationship between sPD-L1 levels and inflammatory factors are limited and need to be further explored. Additionally, it is well known that NPC is a virus-driven malignancy that is characterized by prevailing EBV infection.[29] Unfortunately, there was no association between sPD-L1 and EBV levels in this study, which was similar to the results of the previous studies.[28,30] Fang et al,[31] found that PD-L1 expression was higher in EBV positive NPC cell lines than in EBV negative cell lines. It was also suggested that PD-L1 expression could be increased by EBV-induced latent membrane protein 1 (LMP1). Although the PD-L1-mediated immune escape mechanism was related to EBV status in NPC, the expression of PD-L1 in tissue samples may result from a variety of factors. Further studies are needed to verify the relationship between them.
There are some limitations to our study. First, it has a relatively small number of patients, and further studies with a large number of patients are needed to verify these findings. Second, further research is needed to compare PD-L1 expression in paired specimens of the same individual's peripheral blood and tumor tissue. Furthermore, the correlation between sPD-L1 level and the prognosis, as well as the value in predicting relapse of NPC patients, need to be further investigated. Studies with a larger sample size and further follow-up are needed to confirm these observations and to evaluate the predictive value of sPD-L1.
In conclusion, sPD-L1 was detectable in the plasma of NPC. Circulating sPD-L1 is quantifiable, convenient and easy to obtain in NPC patients. sPD-L1 was an indicator of the extent of tumor invasion and the existence of metastatic disease. sPD-L1 may serve as a useful biomarker for evaluating the treatment efficacy and prognosis of NPC with further follow up.
Acknowledgment
We thank the patients included in the current study.
Author contributions
Conceptualization: Man Hu, Jinming Yu.
Data curation: Jia Yang, Xinbin Bai, Bingjie Fan.
Formal analysis: Jia Yang, Bingjie Fan.
Funding acquisition: Jinming Yu.
Investigation: Ji Ma.
Methodology: Xingchen Ding, Ji Ma.
Supervision: Li Xie, Jinming Yu.
Writing – original draft: Jia Yang.
Writing – review & editing: Jia Yang, Man Hu.
Footnotes
Abbreviations: 18F-FDG PET/CT = 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography, BTC = biliary tract cancer, CT = computed tomography, CTV = clinical target volume, EBV = Epstein-Barr virus, ELISA = enzyme-linked immunosorbent assay, GTV = gross tumor volume, GTVn = GTV of metastasis lymph nodes, GTVp = GTV of primary tumor, HB = hemoglobin, HCC = hepatocellular cancer, HNC = head and neck cancer, IMRT = intensity-modulated radiotherapy, LC = lung cancer, LMP1 = latent membrane protein 1, LMR = lymphocyte-to-monocyte ratio, MRI = magnetic resonance imaging, NCCN = National Comprehensive Cancer Network, NLR = neutrophil-to-lymphocyte ratio, NPC = nasopharyngeal carcinoma, ORR = objective response rate, PC = pancreatic cancer, PD-1 = (programmed death-1), PD-L1 = programmed death ligand 1, PLR = platelet-to-lymphocyte ratio, PLT = platelets, PS = performance status, PTV = planning target volume, RBC = red blood cell, RCC = renal cell cancer, SD = standard deviation, sPD-L1 = soluble PD-L1, TNM = tumor-nodal-metastasis, WBC = white blood cell, WHO = World Health Organization.
How to cite this article: Yang J, Hu M, Bai X, Ding X, Xie L, Ma J, Fan B, Yu J. Plasma levels of soluble programmed death ligand 1 (sPD-L1) in WHO II/III nasopharyngeal carcinoma (NPC). Medicine. 2019;98:39(e17231).
This work was supported by the grant from the National Key Research and Development Plan (2018YFC1313201).
The authors have no conflicts of interests to disclose.
References
- [1].Tsao SW, Yip YL, Tsang CM, et al. Etiological factors of nasopharyngeal carcinoma. Oral Oncol 2014;50:330–8. [DOI] [PubMed] [Google Scholar]
- [2].Ramayanti O, Juwana H, Verkuijlen SA, et al. Epstein-Barr virus mRNA profiles and viral DNA methylation status in nasopharyngeal brushings from nasopharyngeal carcinoma patients reflect tumor origin. Int J Cancer 2017;140:149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yoshizaki T, Kondo S, Endo K, et al. Modulation of the tumor microenvironment by Epstein-Barr virus latent membrane protein 1 in nasopharyngeal carcinoma. Cancer Sci 2018;109:272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Petersson F. Nasopharyngeal carcinoma: a review. Semin Diagn Pathol 2015;32:54–73. [DOI] [PubMed] [Google Scholar]
- [5].Zhang L, Chen QY, Liu H, et al. Emerging treatment options for nasopharyngeal carcinoma. Drug Des Devel Ther 2013;7:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- [8].Swanson MS, Sinha UK. Rationale for combined blockade of PD-1 and CTLA-4 in advanced head and neck squamous cell cancer-review of current data. Oral Oncol 2015;51:12–5. [DOI] [PubMed] [Google Scholar]
- [9].Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol 2016;34:3838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harrington KJ, Ferris RL, Blumenschein G, Jr, et al. Nivolumab versus standard, single-agent therapy of investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol 2017;18:1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hsu C, Lee SH, Ejadi S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol 2017;35:4050–6. [DOI] [PubMed] [Google Scholar]
- [12].Chan OS, Kowanetz M, Ng WT, et al. Characterization of PD-L1 expression and immune cell infiltration in nasopharyngeal cancer. Oral Oncol 2017;67:52–60. [DOI] [PubMed] [Google Scholar]
- [13].Wei W, Xu B, Wang Y, et al. Prognostic significance of circulating soluble programmed death ligand-1 in patients with solid tumors: a meta-analysis. Medicine (Baltimore) 2018;97:e9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ding Y, Sun C, Li J, et al. The prognostic significance of soluble programmed death ligand 1 expression in cancers: a systematic review and meta-analysis. Scand J Immunol 2017;86:361–7. [DOI] [PubMed] [Google Scholar]
- [15].Rossille D, Gressier M, Damotte D, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia 2014;28:2367–75. [DOI] [PubMed] [Google Scholar]
- [16].Frigola X, Inman BA, Lohse CM, et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res 2011;17:1915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Okuma Y, Hosomi Y, Nakahara Y, et al. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer 2017;104:1–6. [DOI] [PubMed] [Google Scholar]
- [18].Weber M, Wehrhan F, Baran C, et al. PD-L1 expression in tumor tissue and peripheral blood of patients with oral squamous cell carcinoma. Oncotarget 2017;8:112584–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Theodoraki MN, Yerneni SS, Hoffmann TK, et al. Clinical significance of PD-L1(+) exosomes in plasma of head and neck cancer patients. Clin Cancer Res 2018;24:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Finkelmeier F, Canli O, Tal A, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer 2016;59:152–9. [DOI] [PubMed] [Google Scholar]
- [21].Ha H, Nam AR, Bang JH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget 2016;7:76604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Takahashi N, Iwasa S, Sasaki Y, et al. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol 2016;142:1727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang L, Wang H, Chen H, et al. Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget 2015;6:41228–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang H, Wang L, Liu WJ, et al. High post-treatment serum levels of soluble programmed cell death ligand 1 predict early relapse and poor prognosis in extranodal NK/T cell lymphoma patients. Oncotarget 2016;7:33035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nagato T, Ohkuri T, Ohara K, et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother 2017;66:877–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kruger S, Legenstein ML, Rosgen V, et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. Oncoimmunology 2017;6:e1310358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cheng S, Zheng J, Zhu J, et al. PD-L1 gene polymorphism and high level of plasma soluble PD-L1 protein may be associated with non-small cell lung cancer. Int J Biol Markers 2015;30:e364–8. [DOI] [PubMed] [Google Scholar]
- [28].Zhou Y, Shi D, Miao J, et al. PD-L1 predicts poor prognosis for nasopharyngeal carcinoma irrespective of PD-1 and EBV-DNA load. Sci Rep 2017;7:43627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen CJ, Hsu WL, Yang HI, et al. Epidemiology of virus infection and human cancer. Recent Results Cancer Res 2014;193:11–32. [DOI] [PubMed] [Google Scholar]
- [30].Chang AMV, Chiosea SI, Altman A, et al. Programmed death-ligand 1 expression, microsatellite instability, epstein-barr virus, and human papillomavirus in nasopharyngeal carcinomas of patients from the philippines. Head Neck Pathol 2017;11:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fang W, Zhang J, Hong S, et al. EBV-driven LMP1 and IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget 2014;5:12189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]


