Abstract
This study presents the postoperative pregnancy rate of women with recurrent endometriosis and evaluates the predictive value of the endometriosis fertility index (EFI) for the pregnancy.
A total of 107 women who wished to conceive after surgery for recurrent endometriosis from January 2007 to December 2016 were included. The EFI score was calculated postoperatively. The receiver operator characteristic (ROC) curve was plotted to determine the most promising contributor to predicting pregnancy, and Kaplan–Meier (K–M) analysis was used to estimate the cumulative pregnancy rate (CPR).
A total of 61 pregnancies were registered in 58 women and the remaining 49 patients failed to become pregnant. The EFI score was strongly associated with the postoperative fertility prognosis. The CPRs during the first 2 and 3 years postoperatively were 51.86% and 66.38%, respectively, and increased to 71.98% within the first 5 years postoperatively in patients with EFI scores ≥5. However, the CPR was 26.00% during the first 2 years after surgery in individuals with EFI scores <5, and there was no increase in the CRP thereafter.
Women suffering from recurrent endometriosis still experienced a probability of natural pregnancy, especially patients with EFI scores ≥5. The EFI score had good predictive power for postoperative pregnancy in these patients.
Keywords: cumulative pregnancy rate, endometriosis fertility index, recurrent endometriosis, spontaneous pregnancy
1. Introduction
Endometriosis is an enigmatic condition affecting women of childbearing age and can lead to difficulty in decision-making among even experienced clinicians.[1] Conservative surgery is regarded as the first-line therapy for women with endometriosis, however, completely removing endometriosis lesions is impossible. As stated,[2] recurrent endometriosis after surgery remains a critical issue because up to 50% of patients struggle with postoperative endometriosis recurrence during the subsequent 5 years.
Previous studies[3,4] have revealed that endometriosis negatively impacts fertility. Given that many patients still have fertility requirements upon endometriosis relapse, addressing fecundity has become a vexing problem. As the removal of recurrent endometriosis lesions becomes even more challenging and as more normal ovarian tissue is lost in the second surgery, fertility is heavily damaged. Thus, determining the expectant fertility management strategy for these patients with recurrent endometriosis is an even more serious challenge to physicians.
The optimal postoperative fertility management remains unknown and fertility guidelines always rely on theoretical considerations or empirical observations. There is a keen debate surrounding the optimal management strategy (achieving pregnancy spontaneously or by assisted reproductive technology, ART) for patients with pregnancy intentions. Some experts argue[5] that ART is the principal recommendation and choice for postoperative pregnancy in patients suffering from endometriosis. However, others consider[6] that it is not necessary to perform ART for all individuals because some patients can become pregnant spontaneously. Therefore, an accurate estimate of the effect of fertility counseling for patients with recurrent endometriosis who attempt expectant fertility remains a relevant issue that needs to be addressed.
Empirical predictions regarding fertility management always incorporates age, duration of infertility, prior pregnancy, extent of endometriosis and ovarian reserve and function.[7,8] However, there are few prognostic scales or indicators for predicting the pregnancy rate when patients experience endometriosis recurrence. The endometriosis fertility index (EFI)[9] is commonly employed as a clinical tool to counsel patients with infertility. Nevertheless, previous studies have suggested that EFI should be externally validated for the prediction of both non-ART[10] and ART outcomes.[11] Up to now, no reported research has validated the value of the EFI in the population of recurrent endometriosis individual who wish to have a child. Therefore, our present study was designed to investigate the fertility outcomes and identify the predictive value of the EFI for spontaneous pregnancy after resection of recurrent endometriosis.
2. Materials and methods
This study was approved by the Ethics Review Board Women's Hospital Zhejiang University School of Medicine, and informed consent was obtained from all patients. In the present study, we included 107 patients who were finally diagnosed with recurrent endometriosis by surgery from January 2007 to December 2016. The hospital records were thoroughly reviewed to obtain detailed information on age, body mass index (BMI), previous surgery, the recurrence interval after the initial surgery, pregnancy history and other surgical details. Additionally, EFI scores were calculated for all patients. The EFI staging system accounted for historical factors (including the patient's age, duration of infertility, and previous pregnancy) and surgical factors (including the American Fertility Society [AFS] total score, AFS endometriosis score, and the least function [LF] score). LF scores were the sum of the lowest scores on the bilateral adnexa, including the fallopian tubes, tubal fimbriae, and ovaries.
Patients were contacted by using a telephone questionnaire that addressed
-
(1)
the desire to conceive after surgery;
-
(2)
the method of conception (spontaneous or ART)
-
(3)
the interval between the surgery and pregnancy; and
-
(4)
the number of pregnancies and subsequent outcomes.
We collected all the information regarding spontaneous pregnancy for this study. For women who become pregnant, clinical pregnancies were only taken into account when they were confirmed by ultrasound examination. The exclusion criteria for the study were as follows: a history of hysterectomy; a history of bilateral salpingectomy; no wish to conceive after surgery; unavailability for follow up; tubal obstruction or male infertility; and achievement of pregnancy by ART.
Statistical analysis and preparation of figures were undertaken using Graph Pad Prism version 6.00 Windows (GraphPad Software, San Diego, CA). Statistical analysis was based on the Student t test or ANOVA test. A receiver operator characteristic (ROC) curve was plotted to determine the value for pregnancy prediction and the Kaplan–Meier (K–M) analysis was used to assess the cumulative pregnancy rate (CPR). For all analyses, values of P < .05 were considered significant.
3. Results
3.1. Clinical characteristics and fertility outcomes
From January 2007 to December 2016, a total of 7644 patients with endometriosis were admitted to our hospital, and 246 (3.21%) women were diagnosed with recurrent endometriosis. Of the total number of consecutive patients diagnosed with recurrent endometriosis, 111 patients (45.12%) had no reproductive requirement to actively avoid pregnancy, and a total of 135 women (54.87%) were seeking a pregnancy after fertility-sparing resection of endometriosis. Of the 135 patients seeking to become pregnant, we excluded 11 patients who were lost to follow-up and 17 individuals who had completed pregnancy by ART. Hence, as a result of these exclusions, a total of 107 women who attempted to conceive were contacted with a mean follow-up time of 6.71 ± 3.62 years, and the clinical characteristics of the study are summarized in Table 1. Among the 107 patients, a total of 61 pregnancies were registered in 58 women (54.21%), and the remaining 49 (45.79%) patients did not achieve pregnancy.
Table 1.
Patient characteristics of the study population (N = 107).

3.2. Data for the 107 women who tried to conceive
In a comparison of patients who became pregnant with those who did not (Table 2), no statistically relevant differences between the 2 groups were found in terms of age at surgery, BMI or AFS stage. However, there were material differences in the AFS total score, AFS endometriosis score and LF score between both groups. Moreover, the recurrence interval after surgery was remarkably longer, and the EFI score was significantly higher in the group of patients who became pregnant than in the group of patients who did not.
Table 2.
Comparison of patients between pregnant and not pregnant after surgery (N = 107).

3.3. Predictive evaluations of the EFI score
As shown by ROC analysis for the AFS endometriosis score (Fig. 1), AFS total score, LF score and EFI score, the EFI score was associated with the highest area under the curves (AUC), suggesting that the EFI was highly associated with pregnancy. The best cut-off point of the EFI score was 5.5 (sensitivity: 87.76%, specificity: 63.79%), and the Youden index was 0.515 (Youden index = sensitivity + specificity−1). We classified the cases into 2 groups according to the best cut-off point: the EFI ≥ 5 group and the EFI < 5 group.
Figure 1.
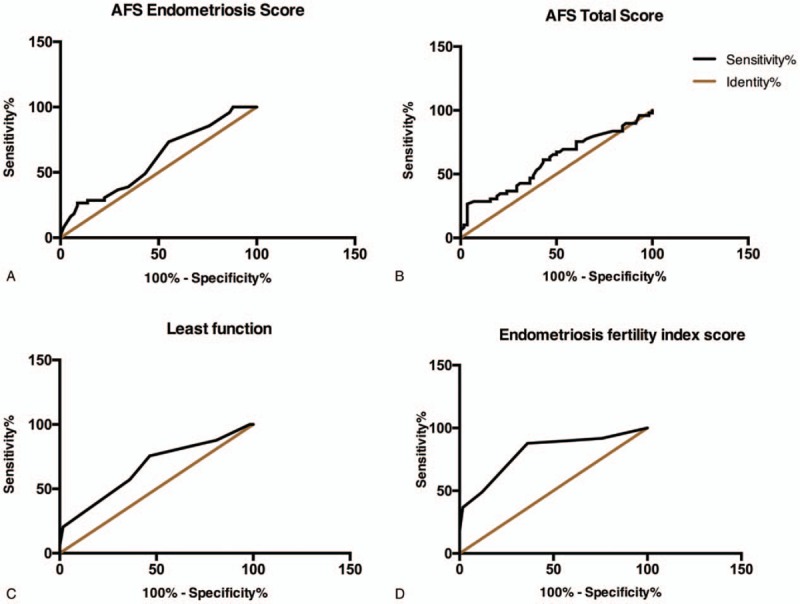
A. AUC of the AFS endometriosis score = .601 (95% CI .49–.71), Std. Error = .06, P = .07; B. AUC of the AFS total score = .60 (95% CI .49–.71), Std. Error = .06, P = .07; C. AUC of the least function score = .665 (95% CI .56–.77), Std. Error = 0.05, P = .003; D. AUC of the endometriosis fertility index (EFI) score = .80 (95% CI 0.72–0.89), Std. Error = 0.04, P < .001. AFS = American Fertility Society, AUC = Area under the curves.
Then, the Kaplan–Meier (K–M) estimator was used to estimate the CPR. The CPR was significantly higher in the EFI ≥ 5 group than in the EFI < 5 group (Fig. 2). The probabilities of conceiving during the first 24 and 36 months postoperatively were 51.86% and 66.38%, respectively, and the probability increased to 71.98% within the first 60 months postoperatively in the EFI ≥ 5 group. However, the CPR was 26.00% during the first 2 years after surgery in individuals with EFI scores <5, and there was no increase in the EFI in subsequent years.
Figure 2.
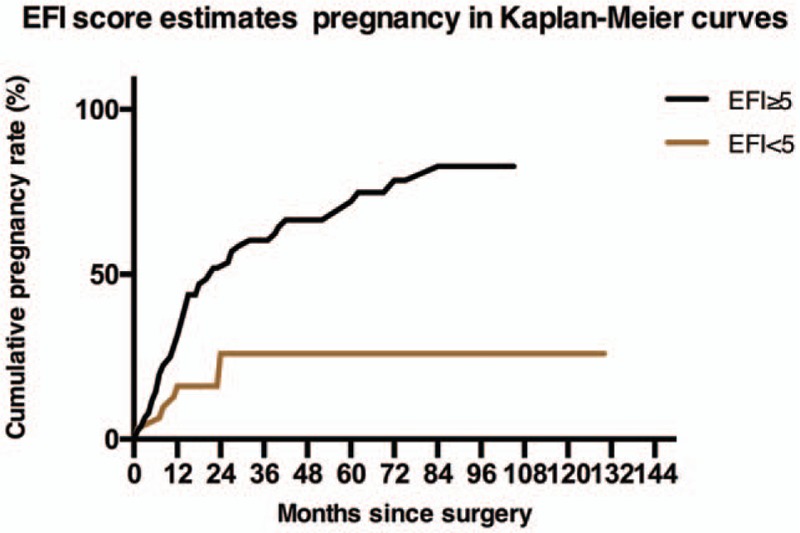
The Kaplan–Meier analysis showed that the cumulative pregnancy rate was statistically significantly different between the EFI ≥ 5 group and the EFI < 5 group (Gehan-Breslow-Wilcoxon test, X2 = 8.65, 95% CI 1.57–4.74, P = .003). EFI =Endometriosis fertility index.
4. Discussion
Our study showed that 107 women with recurrent endometriosis wished to conceive after surgery. There were 61 pregnancies that were achieved spontaneously among the 58 women, and the rate of miscarriages in this group was 21.31%. A previous study concluded an increased risk of spontaneous abortion in women with endometriosis.[12] In general, an inference of experimental data suggests that the endometria of women with endometriosis may be altered and is thus more prone to causing abortion for several reasons. First, the eutopic endometrium is resistant to the selective actions of progesterone that affect downstream P target genes and ultimately leads to decidualization [13] ; Second, the detrimental effects of the inflammatory process related to endometriosis can impede the progression of early embryos during the first trimester of pregnancy [14]; Third, alterations in humoral and cell-mediated immunity in the endometrium have been discussed in the context of pregnancy failure in women with endometriosis.[15,16] However, these possibilities are difficult to demonstrate and remained speculative. More evidence is needed.
In an impressively large survey[17] that included 13090 singleton births among 8922 women diagnosed with endometriosis, a higher preterm birth rate (6.78%) was observed in women with endometriosis than in those without endometriosis (4.98%). Consistent with the above study, our results showed that a rate of nearly 8.2% for preterm births, which indicated a high risk of preterm birth in patients with recurrent endometriosis. It has been hypothesized that the association between endometriosis and preterm birth may be attributable to multiple causes, including alterations in inflammatory mediators; hypermethylations of the progesterone receptor gene and decidual senescence; and vascular abnormalities that may contribute to the labor initiation and preterm birth.[18,19] However, these hypotheses need additional research to provide irrevocable scientific validation.
Previous studies have resulted in conflicting results regarding the risk and increased rate of caesarean delivery in women with endometriosis.[18,20] The risk of caesarean section in patients with recurrent endometriosis was 55.8% in the present analysis, which was dramatically different from the rate of approximately 43% that was registered at our hospital during the last 5 years. However, a definitive conclusion could not be drawn from our data because the data were limited by the generally low number of pregnant women included, and robust evidence from a large- sample study is required.
In the comparison of women who became pregnant to those who did not become pregnant, the findings in the study suggested some impressive considerations: a longer recurrent interval from the first surgery to the endometriosis relapsed value was observed in patients became pregnant, which implied the lower invasiveness of the recurrent endometriosis with less impairment of fertility in these patients. In contrast, higher AFS total scores and AFS endometriosis scores were calculated in patients who did not become pregnant, suggesting the higher invasiveness of endometriosis and consequent damage to fertility potential. Moreover, patients who became pregnant were associated with higher LF and EFI scores, indicating that the LF and EFI could be promising predictors for postoperative pregnancy in recurrent endometriosis. Notably, no distinction was observed based on the AFS stage between the groups in the present study, which was consistent with a previous research finding that the AFS stage classification was not associated with postoperative pregnancy rates.[21] For this reason, our findings suggested that AFS grades were not associated with fertility outcomes.
According to the ROC analysis, the EFI score was the most promising contributor to predicting postoperative pregnancy for recurrent endometriosis because the EFI score was associated with the highest AUC. These results suggested that the chances of spontaneous pregnancy were highly correlated with the EFI score, and a higher EFI score might be associated with a better fertility prognosis. Furthermore, the CPR was 51.86% at 2 years postoperatively, rising to 66.38% at 3 years with an EFI score ≥ 5. These results suggested that all efforts should be made to achieve a spontaneous pregnancy during the first 3 years following the initial surgery. We also observed that the CPR continued to increase to 71.98% at 5 years, suggesting that patients still had an additional chance of pregnancy even after 3 years. Hence, appropriate fertility counseling could be offered for patients in this regard, since the optimal fertility management would allow the avoidance of a costly and invasive fertility protocol, particularly in poor resource areas where access to ART is difficult or women cannot afford to have ART. Nonetheless, for women with a poor EFI score of <5, we found that 26.00% of the pregnancies occurred in the first 2 years and that no women became pregnant in subsequent years. Thus, it would appear to be crucial for clinicians to provide an ART recommendation to refer to these women as soon as possible to optimize their chances of pregnancy. The main limitation of this study was its retrospective design. All fertility information included was highly selected, and the small sample size and the single-center research may be insufficient to reveal all aspects related to postoperative pregnancy associated with recurrent endometriosis. Nonetheless, we believe that our data should provide novel insight into fertility management for recurrent endometriosis and encourage future research regarding this field.
5. Conclusions
Our results suggest that spontaneous conception can be favored in cases with a high EFI score (≥ 5) within 5 years postoperatively. However, in women with a low EFI score (<5), one needs to emphasize that they may benefit from rapid ART procedures due to a poor probably of spontaneous pregnancy. The EFI was validated as an objective scoring system to predict postoperative pregnancy for patients with recurrent endometriosis who attempt to become pregnant spontaneously. It also offers a useful tool to counsel couples for personalized fertility management about a reasonable time before seeking ART.
Author contributions
Conceptualization: Yong Zhou, Ruijin Wu.
Data curation: Zhengyun Chen.
Formal analysis: Yong Zhou, Chaolu Chen.
Investigation: Li Lin, Enchun Li.
Software: Li Lin.
Validation: Zhengyun Chen, Yuan Wang.
Writing – original draft: Yong Zhou.
Writing – review & editing: Chaolu Chen, Enchun Li.
Footnotes
Abbreviations: AFS = American Fertility Society, ART = assisted reproductive techniques, AUC = area under curves, BMI = body mass index, CPR = cumulative pregnancy rate; EFI = endometriosis fertility index, K-M = Kaplan–Meier, LF = least function, ROC = receiver operator characteristic.
How to cite this article: Zhou Y, Lin L, Chen Z, Wang Y, Chen C, Li E, Wu R. Fertility performance and the predictive value of the endometriosis fertility index staging system in women with recurrent endometriosis: a retrospective study. Medicine. 2019;98:39(e16965).
The statistics in the study had been supported by the epidemiologist of Ye Ding who was from School of Public Health Zhejiang Chinese Medical University. This study was supported by Natural Science Foundation of Zhejiang Province (LY18H040004, Q19H040054 and LY14H040007) and National Natural Science Foundation of China (No. 81170547), granted from Gynecology Innovation Branch of Zhejiang Province and Zhejiang Health Department Project (2014KYA250).
The authors have no conflicts of interests to disclose.
References
- [1].Ludovico M, Chiara DT, Mara DF, et al. Surgery versus expectant management in patients with endometrioma who seek pregnancy. Journal of Endometr Pelvic Pain Disord 2017;9:135–8. [Google Scholar]
- [2].Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update 2009;15:441–61. [DOI] [PubMed] [Google Scholar]
- [3].Evans MB, Decherney AH. Fertility and Endometriosis. Clin Obstet Gynecol 2017;60:497–502. [DOI] [PubMed] [Google Scholar]
- [4].Young K, Fisher J, Kirkman M. Endometriosis and fertility: women's accounts of healthcare. Hum Reprod 2016;31:554–62. [DOI] [PubMed] [Google Scholar]
- [5].Tanbo T, Fedorcsak P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet Gynecol Scand 2017;96:659–67. [DOI] [PubMed] [Google Scholar]
- [6].Leone RMU, Ferrero S, Mangili G, et al. A systematic review on endometriosis during pregnancy: diagnosis, misdiagnosis, complications and outcomes. Hum Reprod Update 2016;22:70–103. [DOI] [PubMed] [Google Scholar]
- [7].Roman H, Chanavaz LI, Ballester M, et al. High postoperative fertility rate following surgical management of colorectal endometriosis. Hum Reprod 2018;33:1669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Haydardedeoglu B, Zeyneloglu HB. The impact of endometriosis on fertility. Womens Health 2015;11:619–23. [DOI] [PubMed] [Google Scholar]
- [9].Maheux LS, Nesbitt HE, Deans R, et al. Endometriosis fertility index predicts live births following surgical resection of moderate and severe endometriosis. Hum Reprod 2017;32:2243–9. [DOI] [PubMed] [Google Scholar]
- [10].Tomassetti C, Geysenbergh B, Meuleman C, et al. External validation of the endometriosis fertility index (EFI) staging system for predicting non ART pregnancy after endometriosis surgery. Hum Reprod 2013;28:1280–8. [DOI] [PubMed] [Google Scholar]
- [11].Boujenah J, Cedrin DI, Herbemont C, et al. Use of the endometriosis fertility index in daily practice: a prospective evaluation. Eur J Obstet Gynecol Reprod Biol 2017;219:28–34. [DOI] [PubMed] [Google Scholar]
- [12].Santulli P, Marcellin L, Menard S, et al. Increased rate of spontaneous miscarriages in endometriosis-affected women. Hum Reprod 2016;31:1014–23. [DOI] [PubMed] [Google Scholar]
- [13].Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007;148:3814–26. [DOI] [PubMed] [Google Scholar]
- [14].Benagiano G, Brosens I, Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update 2014;20:386–402. [DOI] [PubMed] [Google Scholar]
- [15].Jiang Y, Chen L, Taylor RN, et al. Physiological and pathological implications of retinoid action in the endometrium. Endocrinol 2018;236:169–88. [DOI] [PubMed] [Google Scholar]
- [16].Hutter S, Heublein S, Knabl J, et al. Macrophages: are they involved in endometriosis, abortion and preeclampsia and how? Nippon Med Sch 2013;80:97–103. [DOI] [PubMed] [Google Scholar]
- [17].Stephansson O, Kieler H, Granath F, et al. Endometriosis, assisted reproduction technology, and risk of adverse pregnancy outcome. Hum Reprod 2009;24:2341–7. [DOI] [PubMed] [Google Scholar]
- [18].Petraglia F, Arcuri F, de Ziegler D, et al. Inflammation: a link between endometriosis and preterm birth. Fertil Steril 2012;98:36–40. [DOI] [PubMed] [Google Scholar]
- [19].Reis FM, Petraglia F, Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update 2013;19:406–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Uccella S, Cromi A, Agosti M, et al. Fertility rates, course of pregnancy and perinatal outcomes after Laparoscopic ureterolysis for deep endometriosis: a long-term follow-up study. J Obstet Gynaecol 2016;36:800–5. [DOI] [PubMed] [Google Scholar]
- [21].Palmisano GP, Adamson GD, Lamb EJ. Can staging system for endometriosis based on anatomic location and lesion type predict pregnancy rate? Int J Fertil Menopause Stud 1993;38:241–9. [PubMed] [Google Scholar]


