Abstract
Ionizing radiation can induce deoxyribonucleic acid (DNA) methylation pattern change, and ionizing radiation-induced oxidative damage may also affect DNA methylation status. However, the influence of low-dose ionizing radiation, such as occupational radiation exposure, on DNA methylation is still controversial.
By investigating the relationship between occupational radiation exposure and DNA methylation changes, we evaluated whether radiation-induced oxidative damage was related to DNA methylation alterations and then determined the relationship among occupational radiation level, DNA methylation status, and oxidative damage in interventional physicians.
The study population included 117 interventional physicians and 117 controls. We measured global methylation levels of peripheral blood leukocyte DNA and expression level of DNA methyltransferase (Dnmts) and homocysteine (Hcy) in serum to assess the DNA methylation status of the body. We measured 8-hydroxy-2′-deoxyguanosine (8-OHDG) and 4-hydroxynonenal (4-HNE) levels as indices of oxidative damage. Relevance analysis between multiple indices can reflect the relationship among occupational radiation exposure, DNA methylation changes, and oxidative damage in interventional physicians.
The expression levels of Dnmts, 4-HNE, and 8-OHDG in interventional physicians were higher than those in controls, while there was no statistical difference in total DNA methylation rate and expression of Hcy between interventional physicians and controls. Total cumulative personal dose equivalent in interventional physicians was positively correlated with the expression levels of Dnmts, 8-OHDG, and 4-HNE. The expression levels of 8-OHDG in interventional physicians were negatively correlated with global DNA methylation levels and positively correlated with the expression levels of Hcy.
Occupational radiation exposure of interventional physicians has a certain effect on the expression of related enzymes in the process of DNA methylation, while ionizing radiation-induced oxidative damage also has a certain effect on DNA methylation. However, there was no evidence that dose burden of occupational exposure was associated to changes of DNA methylation status of interventional physicians, since it is rather unclear which differences are observed among the effects produced by radiation exposure and oxidative damage.
Keywords: DNA methylation, interventional physicians, occupational exposure, oxidative damage
1. Introduction
With the guidance of a medical imaging equipment, interventional radiology is a series of techniques used in the diagnosis and treatment of various diseases with the use of a catheter, guidewire, and other equipment based on imaging and clinical diagnoses. Interventional physicians are exposed to a certain dose of radiation in the work environment when performing surgery near patients close to the X-ray bulb with a high distribution of scattered rays. Most occupational exposures involve low-dose ionizing radiation (LDIR). The latest scientific definition of LDIR, published by UNSCEAR in 2010, defines it as a radiation dose <200 mGy or dose rate <0.1 mGy min−1 (average dose rate ≥1 hours) in irradiation dose of X-rays or γ-rays outside.[1] The linear no-threshold model is a classic model used to evaluate ionizing radiation risk. However, the influence of LDIR on the body is still controversial.
Deoxyribonucleic acid (DNA) methylation plays an important role in the life cycle, and the body is in the state of methylation balance, which is an important guarantee for the reasonable regulation of gene expression and maintenance of the stability of genetic material and other life activities. Ionizing radiation can induce DNA methylation pattern change including global genome DNA hypomethylation and hypermethylation of gene promoter, which is associated with genomic instability and proto-oncogenes activation.[2–7] The mechanism of DNA methylation abnormality caused by ionizing radiation is still unclear, but several studies suggested that ionizing radiation can indirectly ionize the water in the cells to generate a large number of reactive oxygen species (ROS) such as H• and OH•, which was the main factors that affects DNA methylation.[8–9] Occupational irradiation, in which interventional physicians are exposed to, is classified as LDIR. However, the influence of LDIR on DNA methylation is still controversial. The analysis of the association between the methylation status of human DNA and occupational exposure of interventional physicians will provide clues for further understanding of the effect of LDIR on human DNA methylation. Homocysteine (Hcy) is an important product in the process of DNA methylation and also affects the methylation status of the body's DNA by combining with ROS.[10] In this study, the expression levels of DNA methyltransferase (Dnmt) and Hcy and the total DNA methylation rate were used to evaluate the effect of occupational LDIR exposure on the human DNA methylation status in interventional physicians.
Moreover, sustained occupational LDIR exposure can produce large amounts of ROS, which will lead to an imbalance in the oxidation and antioxidant effects in the body, and the oxidative damage caused by LDIR is closely related to the DNA methylation status. ROS can attack the 8th carbon atom of guanine base, and the generated 8-hydroxy-2′-deoxyguanosine (8-OHDG) can inhibit DNA methylation of cytosine bases and induce DNA hypomethylation.[8] ROS will cause DNA damage, and the DNA damage response gene will activate Dnmt during the DNA repair process, leading to hypermethylation of gene-specific promoter.[11–13] In this study, the oxidative stress state of interventional physicians was evaluated through the most stable end product of oxidative stress lipid peroxidation in vivo, 4-hydroxynonenal (4-HNE). By analyzing the association of 8-OHDG and 4-HNE with DNA methylation indices, the oxidative stress effect of occupational LDIR exposure on the methylation status of the body was evaluated.
2. Materials and methods
2.1. Study population
The study population involved 117 physicians who had been engaged in interventional surgery for >3 years, with a stable status of interventional work, and 117 controls who were not radiologists of the same sex, aged ±3 years, and working in B-mode ultrasound, electrocardiogram, and other auxiliary medical departments. Information regarding age, smoking status, drinking habits, medical history, and duration of work was obtained via personal interviews. Subjects with a history of chronic disease such as coronary heart diseases, myocardial infarction, stroke, hypertension, diabetes mellitus, liver and kidney diseases, and other organic diseases that seriously affected the detection indices were excluded, and the subjects had no emergent events, such as fever and medication use during the period of venous blood collection. Official personal dosimetry records were collected for each interventional physician during the entire working period to obtain the personal dose equivalent.
This study was approved by research ethics committee of the Hwamei Hospital, Chinese Academy of Sciences, obtained the ethical approval protocol (No. PJ-NBEY-KY-2019-095-01). The last follow-up was censored on 22 December, 2018. The informed consent was waived because of the retrospective nature of the study.
3. Methods
3.1. Sample collection and pretreatment
In this study, 5 mL of ethylenediaminetetraacetic acid anticoagulant venous blood was collected from the subjects in the morning, and DNA was extracted from 400 μL sample with the whole blood DNA mini kit (Simgen, Germany). DNA concentration was determined by NanoDrop 2000C nucleic acid protein detector (Thermo Scientific, Waltham, MA) and stored in the refrigerator at −20°C. The rest of the blood samples were centrifuged for 30 minutes in the 1-14 high-speed centrifuge (Sigma, Osterode am Harz, Germany) at 3000 r/min (radius, 19.2 cm) to separate the serum and were stored in the refrigerator at −80°C for inspection.
3.1.1. Detection of total DNA methylation rate by high-performance liquid chromatography
The DNA sample was hydrolyzed according to the specifications of the One-Step DNA Hydrolysis Kit (Epiquik, Farmingdale, NY). To determine the detecting conditions of high-performance liquid chromatography (Agilent, Santa Clara, CA), the chromatographic column used was Kromasil C18 250 mm × 4.6 mm, mobile phase of water:methanol (v:v) = 7:3, flow rate was 1 mL/min, sample amount was 20 μL, wavelength was 285 nm, and column temperature was 30°C. Different concentrations of 2′-deoxycytidine (2-dc) (TCI, Japan) and 5-methyl-2′-deoxycytidine (5-dmc) (Sigma) as standard products were obtained to determine the standard equation. DNA hydrolysis sample was determined, and concentrations of 2-dc and 5-dmc and total DNA methylation rate in the sample were calculated according to the formula c5-dmC/(c2-dC + c5-dmC) × 100%.
3.1.2. Detection of DNA methylation index and oxidative damage marker by enzyme labeling
Standards and serum samples were handled according to the specifications of the Dnmt Activity/Inhibition Assay Kit (Epigentek, Farmingdale, NY), human Hcy enzyme-linked immunosorbent assay test kit (R&D Systems, Minneapolis, MN), human 8-OHDG kit (R&D Systems), and human 4-HNE kit (R&D Systems). The wavelength of 450-nm enzyme marker was set, and the optical density D-values of Dnmt, Hcy, 8-OHDG, and 4-HNE were determined in the microplate reader according to the requirements of the kit. The concentration of the sample indices was calculated according to the standard curve in the specification.
3.2. Statistical analysis
Experimental and baseline data of the study objects were entered into Excel 2010, which were imported into SPSS 17.0 software for statistical analysis. The measurement data were tested for normality, and quantitative data with approximate normality were expressed as average and standard deviation. The comparison of classifying data was conducted with the χ2 test, mean comparison between the 2 groups using the t test, mean comparison among multiple groups with 1-way analysis of variance F test, and pairwise comparison between 2 groups with least-significant difference method. The test level was set to α = 0.05 in this study.
4. Results
4.1. General characteristics of the study populations
The study population was composed of 172 men (73.5%) and 62 women (26.5%). The age range of interventional physicians was 28 to 50 years, while the age range of controls was 27 to 53 years. There was no difference in lifestyle factors, including smoking and drinking habits, between the 2 groups. General characteristics of the study populations are presented in Table 1. The average work duration in interventional physicians was 7.92 ± 3.57 years, and the total cumulative occupational exposure personal dose equivalent in 2016 to 2017 was 2.333 ± 1.052 mSv.
Table 1.
Demographic characteristics of participants 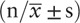 .
.
4.2. Comparison of DNA methylation indices and oxidative damage biomarkers
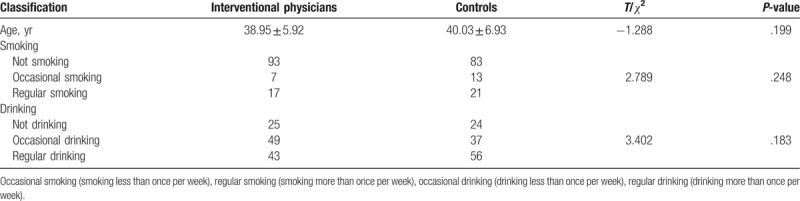
The expression levels of Dnmts (P = .014), 4-HNE (P = .044), and 8-OHDG (P = .028) in interventional physicians were higher than those in the controls, and the difference was statistically significant. The total DNA methylation rate (P = .053) in interventional physicians was lower than that in controls, and the expression levels of Hcy (P = .052) in interventional physicians were higher than those in controls, but the difference was not statistically significant, as shown in Table 2.
Table 2.
Comparison of DNA methylation indices and oxidative damage biomarkers between interventional physicians and controls.
4.3. Comparison of DNA methylation indices and oxidative damage biomarkers of interventional physicians with different annual effective doses
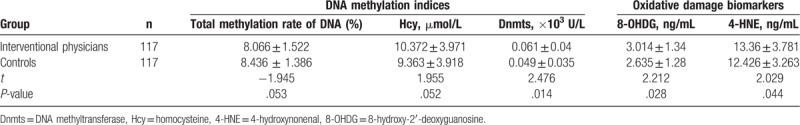
As for the expression levels of Dnmts and 4-HNE, they were statistically significantly different among interventional physicians with different annual effective doses (note: the annual effective dose in this study refers to the annual average of total cumulative personal dose equivalent in 2016–2017), as shown in Table 3. The pairwise comparison between different annual effective dose groups, showed that the expression levels of Dnmts and 4-HNE were diverse (Dnmts, P = .000, P = .000, P = .042; 4-HNE, P = .000, P = .000, P = .042). As the annual effective dose increased, the average value of Dnmts and 4-HNE increased successively. Moreover, the groups with annual effective dose >2 mSv were associated to a higher expression level of 8-OHDG than that with an annual effective dose <1 mSv.
Table 3.
Comparison of DNA methylation and oxidative damage indices among interventional physicians of different annual average effective doses.
The correlation analysis among annual effective dose, DNA methylation indices, and oxidative damage biomarkers showed that the annual effective dose in interventional physicians was positively correlated with Dnmts (r = 0.481, P = .000), 8-OHDG (r = 0.274, P = .003), and 4-HNE (r = 0.384, P = .000) levels. No statistical correlation was found between annual effective dose and total DNA methylation rate and Hcy level, as shown in Figures 1 and 2 for the distribution among the indices.
Figure 1.
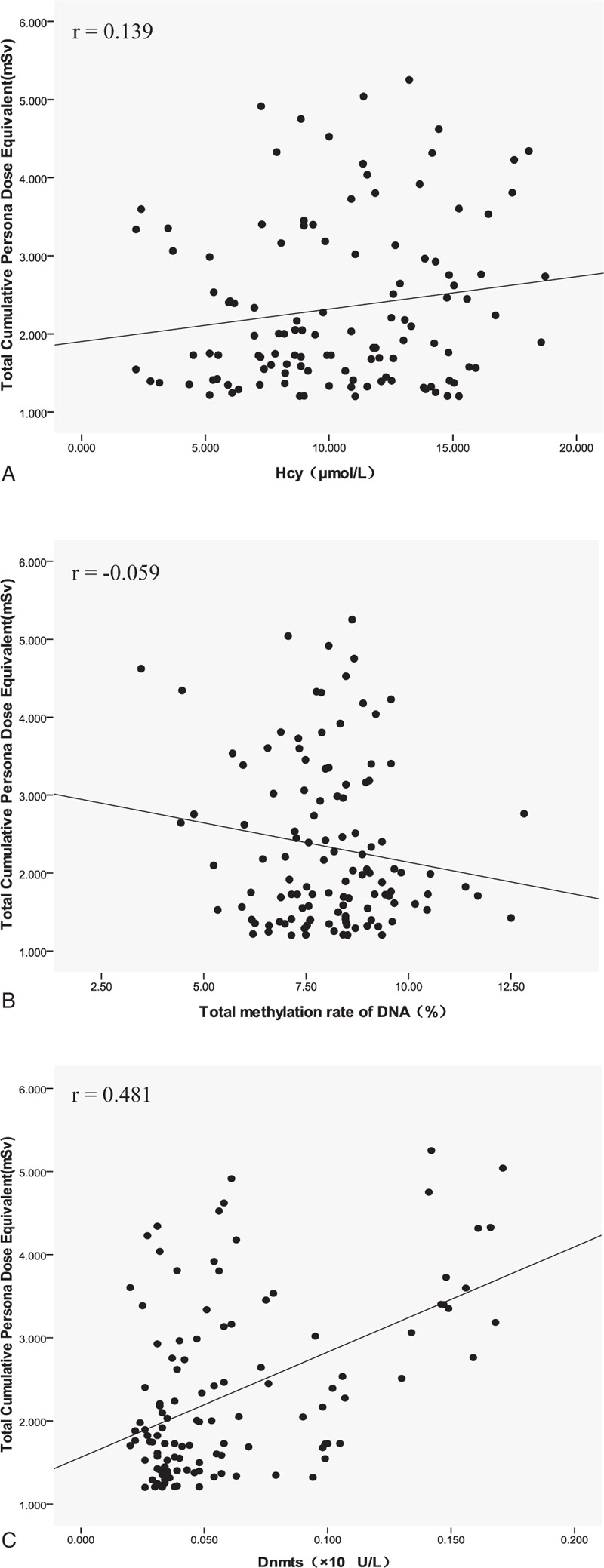
The scatter distribution between total cumulative personal dose equivalent from 2016 to 2017 and DNA methylation indices in interventional physicians. (A) Total cumulative personal dose equivalent versus Hcy level. (B) Total cumulative personal dose equivalent versus total methylation rate DNA (%). (C) Total cumulative personal dose equivalent versus Dnmts level. Dnmts = DNA methyltransferase, Hcy = homocysteine.
Figure 2.
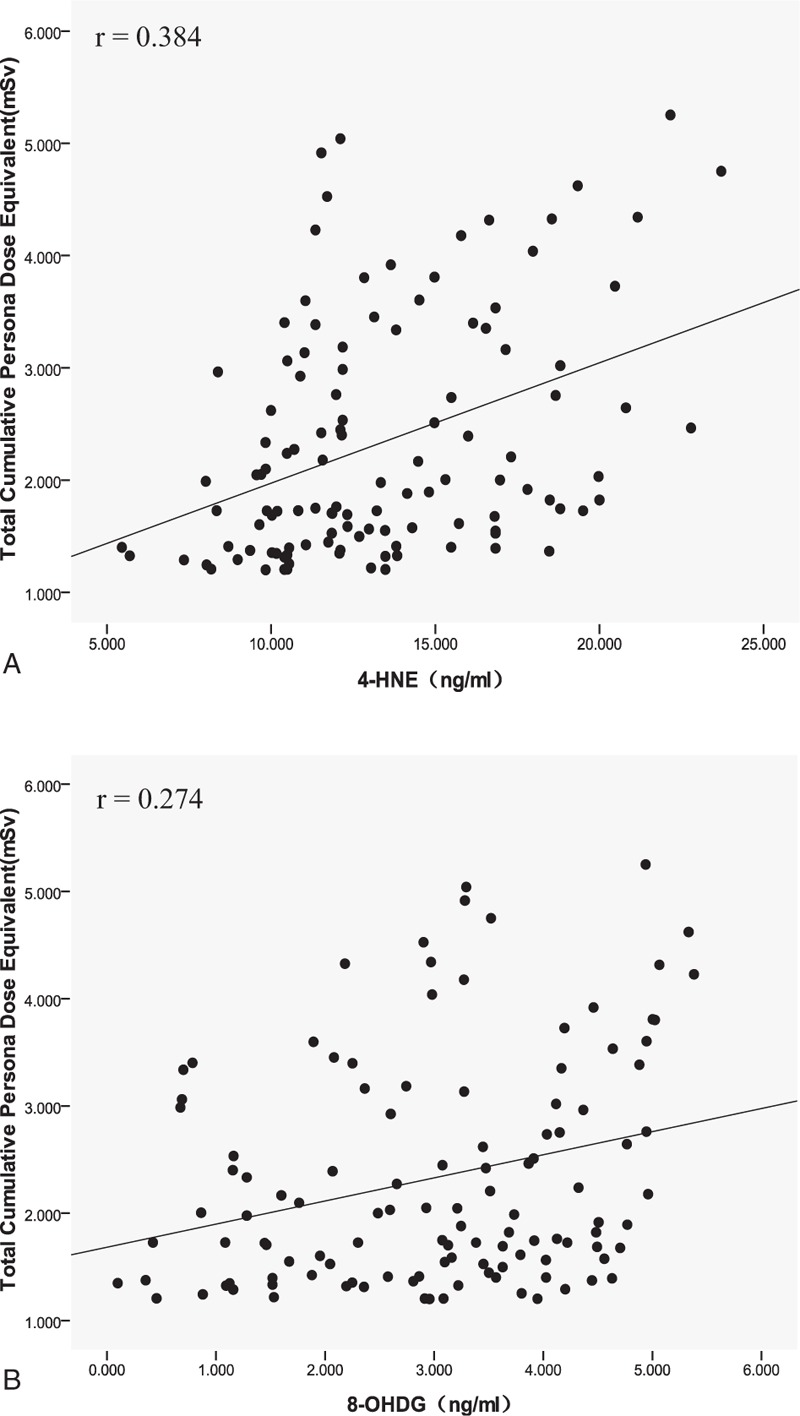
The scatter distribution between total cumulative personal dose equivalent from 2016 to 2017 and oxidative damage biomarkers in interventional physicians. (A) Total cumulative personal dose equivalent versus 4-HNE level. (B) Total cumulative personal dose equivalent versus 8-OHDG level. 4-HNE = 4-hydroxynonenal, 8-OHDG = 8-hydroxy-2′-deoxyguanosine.
4.4. Relationship between DNA methylation indices and oxidative damage biomarkers
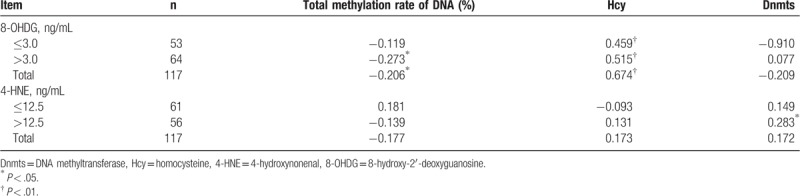
8-OHDG level was negatively correlated with total DNA methylation rate but positively correlated with Hcy level. No statistical correlation was found among other indices. According to the expression level of oxidative damage biomarkers, 4-HNE level >12.5 ng/mL was positively correlated with Dnmts level, as shown in Table 4 for correlation between indices.
Table 4.
Pearson correlation analysis between DNA methylation indices and oxidative damage biomarkers.
5. Discussion
DNA methylation plays an important role in developmental processes, imprinting cell proliferation, and the maintenance of genome stability.[4–7] Recently published studies indicate that ionizing radiation exposure affects DNA methylation patterns, such as gene-specific DNA methylation and DNA methylation of repetitive elements.[14] LDIR conditionally shows stimulatory effects in various cells and organisms, contrasting with detrimental effects induced at high doses. Whether LDIR affects DNA methylation is still controversial. One study has revealed that there was a time window for LDIR to change the methylation status of the body. Acute LDIR induced transient whole-genome DNA hypomethylation and gene-specific promoter hypermethylation after 2 hours in the blood, but DNA hypomethylation gradually recovered in a month, and gene-specific promoter hypermethylation was relatively stable.[15] In our study, no statistical difference was found in the total DNA methylation rate between the interventional physicians and controls, and no statistically significant difference was found in the total DNA methylation rate of different irradiation doses in the comparison of DNA methylation indices of irradiated subjects at different annual effective doses in interventional physicians. Our study shows that DNA methylation effects of LDIR may be dose-dependent, which suggested that extremely LDIR had little effect on DNA methylation status.
However, what is the ionizing radiation dose threshold for DNA methylation? The International Commission on Radiological Protection (ICRP) permitted dose for occupational exposure is 20 mSv per year. However, no studies have suggested that this permitted dose affects the body's biochemical reactions, such as DNA methylation. A study on LDIR and DNA methylation of power plant workers shows occupational exposure to low-dose radiation could affect DNA methylation levels. The average annual effective dose of power plant workers was 5.3 mSv,[7] while the dose in our study was 1.167 mSv. However, our data on interventional worker's average annual effective dose may underestimate the occupational exposure dose in power plant workers. The estimation method of the effective dose in interventional physicians, such as double dosimetry, is based on the ICRP Publication No. 103.[16] The method requires intervention workers to wear one dosimeter at the front of the human torso in the lead protective clothing, and another dosimeter is worn on the outer collar of the apron. However, in the actual intervention, the lead glass protective screen will be placed in front of the torso of the intervention worker. This will result in underestimation of the monitoring data.
LDIR had a certain influence on the expression of methylation-related enzymes. Long-term induced adaptive response by LDIR can stimulate the increase in the expression of Dnmt1 and methyl-CpG binding protein and formation of heterochromatin.[17] In this study, the expression level of Dnmts in interventional physicians was higher than that in controls, and the expression levels of Dnmts were different among various annual effective dose groups of interventional physicians. As the annual effective dose increased, the average value of Dnmts increased successively. The correlation analysis also indicated that there was a statistically positive correlation between Dnmts and annual effective dose. However, whether LDIR may have an effect on the expression of Dnmts still needs further evidence.
The majority of observed biological effects mediated by LDIR occur due to the generation of ROS via ionization of water molecules.[18] The degree of influence of LDIR on the oxidative stress state of the body is controversial. In the study of Koc et al, the serum level of thiol in radiologists was lower than that in controls, but no statistical difference was found between the groups with total mercaptogen levels. This result suggested that LDIR could affect the oxidative stress state of the body but would not alter the oxidation-antioxidant balance.[19] Our study compared the different oxidative damage biomarkers in interventional physicians and controls, and the expression levels of 8-OHDG and 4-HNE in interventional physicians were higher than those in controls, which indicated that LDIR in the work environment of interventional physicians had an impact on the oxidative stress state of the body. As the annual effective dose increased, the average value of 4-HNE and 8-OHDG in interventional physicians increased successively, although the expression levels of 8-OHDG had no statistically significant difference among interventional physicians of different annual effective dose groups. Whether dose thresholds existed in LDIR lipid and DNA peroxidation damage remained to be further studied. The correlation analysis showed that there was a statistically positive correlation between the expression levels of 8-OHDG and 4-HNE and the effective dose, which further confirmed that LDIR had some influence on the oxidative stress state of the body.
The increase in ROS in the body can change the epigenetic gene regulation mechanism,[20–22] and the change in DNA methylation was the most important factor. ROS-mediated DNA damage can increase the expression of Dnmt3a and Dnmt3b, resulting in specific hypermethylation of the body's gene promoter.[11] LDIR leads to mitochondrial DNA hypomethylation, which was mainly caused by the effect of ionizing radiation on the mitochondria, producing a large amount of ROS. In order to repair mitochondrial DNA damage, methyltransferase accumulates in the mitochondria, which leads to the lack of methyltransferase in the nucleus. It is unclear whether LDIR produces genome-wide hypo- or hypermethylation.[23] Studies have shown that oxidization of reduced glutathione (GSH) to GSSH inhibits synthesizing S-adenosyl methionine (SAM) and oxidative stress-mediated inhibition of SAM results in genomic hypomethylation.[24] This study analyzed the correlation between DNA methylation index and oxidative damage biomarkers, showing that the expression level of 8-OHDG was negatively correlated with the total DNA methylation rate but positively correlated with Hcy level. The reason may be that the interaction with ROS and Hcy leads to the gradual depletion of SAM in the methionine cycle, and the decrease in the active methyl donor leads to the decrease in the total methylation rate of DNA.[10] This study suggests a possible mechanism of action of LDIR, the occupational exposure dose in interventional physicians may not have reached the dose threshold for changing the methylation status of DNA, and there may be a process in which DNA is slightly hypomethylated, which will enhance Dnmt. Expression accelerates the body's methionine cycle, which leads to an increase in Hcy level. While the increased amount of ROS in ionizing radiation affects the DNA methylation process, ROS can attack the 8th carbon atom of guanine base, which will increase the expression level of 8-OHDG, as shown in Figure 3. No statistical correlation was found between the expression level of 4-HNE and DNA methylation index. However, after grouping the expression levels of 4-HNE, it was found that 4-HNE level >12.5 ng/mL was positively correlated with Dnmts level, suggesting that there was a correlation between oxidative damage biomarkers and DNA methylation indices, while the change in 8-OHDG expression level may affect the methylation status of the body.
Figure 3.
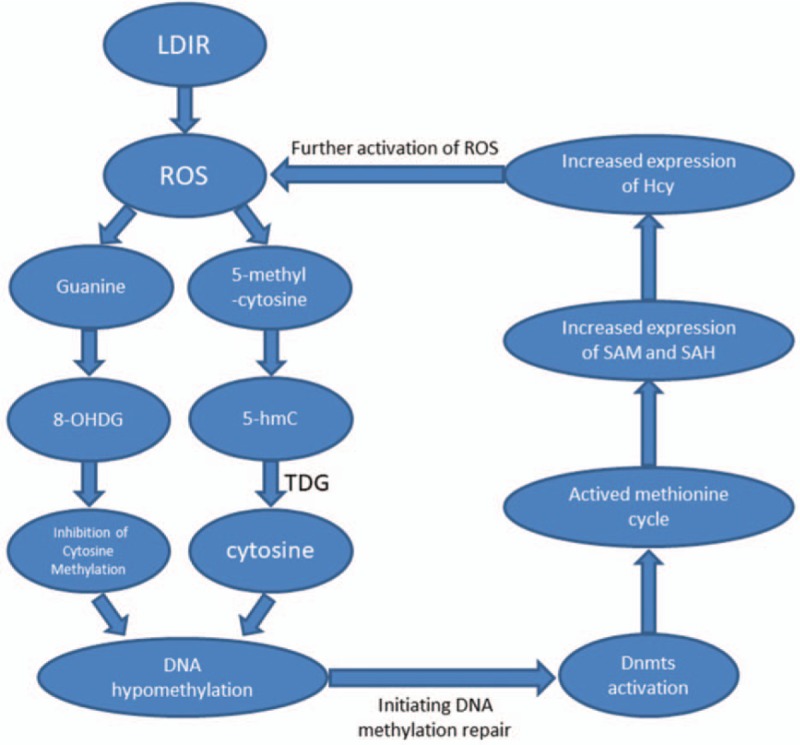
LDIR can ionize and stimulate the body's hydrogen peroxide and produce a large number of ROS (HO, HO 2, ROO , etc). ROS can attack the 8th carbon atom of guanine base, and 8-hydroxydeoxyguanosine could inhibit DNA methylation of cytosine base and induce DNA hypomethylation.[7] The production of ROS leads to the activity of oxygenases. 5-Methylcytosine can produce 5-hydroxymethylcytosine under the action of Tet dioxygenase and further oxidize to 5-cytosine carboxyl group. Under the action of TDG, the decarboxylation group is reduced to cytosine, which induces DNA hypomethylation. [8] When LDIR did not reach the dose threshold, the body-initiated DNA methylation repair, Dnmts activation led to methionine cycling activity, SAM and SAH expression increased, and Hcy expression increased. Hcy further activates ROS and forms a cycle. Dnmts = DNA methyltransferase, Hcy = homocysteine, LDIR = low-dose ionizing radiation, ROS = reactive oxygen species, TDG = thymidine DNA glycosylase.
The occupational exposure dose in interventional physicians is the highest among all medical radiologists. Studies have suggested that occupational exposure does have an impact on interventional physicians, such as morphological and functional alterations in the dermal microcirculation,[25] chromosomal damage,[26] and radiation-induced cataract.[27] It is necessary to provide appropriate protection mechanisms for interventional physicians. Radiation protection is the primary link to reduce the dose burden in interventional workers, including radiation protection and reduced operating time during the interventional procedure. Freestanding adjustable over-table shields are currently the main facility for main beam protection. Scattered beam protection equipment includes lightweight disposable lead-free drapes, ceiling-suspended shields, and mobile freestanding shields. Ergonomic double-layer composite lightweight apron can be used to improve surgical comfort and reduce surgical time.[28] Our study suggests that the dose of occupational exposure in interventional physicians may be lower than the dose threshold that affects DNA methylation status. However, the oxidative damage indices in interventional physicians in our study were higher than those in controls. If the workload of interventional physicians continues to increase, it may affect the DNA methylation status of the body. If appropriate supplementation of folic acid and vitamins would repair DNA oxidative damage and keep the body's DNA methylation in a normal state, of course, such interventions still require further research.
Our study has some limitations. First, based on the study results, LDIR activates the expression of Dnmts, promotes the acquisition of methyl monomer by methionine cycle, and restores the body's DNA methyl group. The Hcy level slightly increased. However, this hypothesis lacks key evidence. The expression level of folic acid in the body during different occupational doses will provide the basis for the consumption of methyl monomer in the body. Second, in our study, only long-term indices of oxidative damage markers were considered, which only reflect the increase in oxidative damage end products in the body and cannot reflect the changes in the body's antioxidant capacity. Therefore, it is impossible to determine the occupational exposure dose in the interventional staff. Short-term oxidative stress indicators such as superoxide dismutase, catalase, and GSH peroxidase were not included in the study. Limited to the factors such as funding, there are some shortcomings in the study, but this study analyzes the relationship between occupational exposure and biomarkers at the molecular level, which provides a reference for the early prevention and intervention of occupational oxidative damage.
6. Conclusions
The results of this study show that occupational irradiation has a certain influence on the expression of relevant enzymes in the process of DNA methylation and oxidative damage induced by ionizing radiation has a certain influence on DNA methylation. Low doses of occupational irradiation are insufficient to significantly alter DNA methylation in interventional physicians. Based on the results of this study, oxidative stress-mediated by LDIR leads to hypomethylation of the body's DNA, and the compensatory expression of Dnmts has a positive effect on the methylation status of the body's DNA before the decompensation and replenishment of the body's methyl donors. Moreover, this study only considers the effects of the work environment, age, medication use, and smoking and alcohol consumption on DNA methylation test values. Due to the lack of information on sample sources, heredity, living environment, diet, and other factors were not included, which may have some influence on the extrapolation of the study results, which should be refined and improved in future research.
Acknowledgments
The authors would like to thank Rica Joy, editor of Editage, and the reviewers for their constructive comments and English polishing guidance.
Author contributions
Conceptualization: Dandan Zhang.
Investigation: Qun Zhang, Aihong Wang.
Methodology: Linyan Qu.
Project administration: Peng Yan.
Writing – original draft: Bin Chen, Qi Dai.
Writing – review and editing: Yinhua Jin, Dandan Zhang.
Footnotes
Abbreviations: 4-HNE = 4-hydroxynonenal, 8-OHDG = 8-hydroxy-2′-deoxyguanosine, DNA = deoxyribonucleic acid, Dnmts = DNA methyltransferase, GSH = glutathione, Hcy = homocysteine, LDIR = low-dose ionizing radiation, ROS = reactive oxygen species, SAM = S-adenosyl methionine.
How to cite this article: Chen B, Dai Q, Zhang Q, Yan P, Wang A, Qu L, Jin Y, Zhang D. The relationship among occupational irradiation, DNA methylation status, and oxidative damage in interventional physicians. Medicine. 2019;98:39(e17373).
BC and QD contributed equally to this work.
This study was sponsored by the Health and Medical Science and Technology Scheme of Zhejiang Province (2019KY587, 2017KYB601), China, and the Science and Technology Scheme of Ningbo (2015A610194, 2016A610180), China.
The authors have no conflicts of interest to disclose.
References
- [1].UNSCEAR 2010 Report. “Summary of Low-dose Radiation Effects on Health.” Report of the United Nations Scientific Committee on the Effects of Atomic Radiation 2010. United Nations, New York, 2011.6–9. [Google Scholar]
- [2].Kaneda A, Tsukamoto T, Takamuraenya T, et al. Frequent hypomethylation in multiple promoter CpG islands is associated with global hypomethylation, but not with frequent promoter hypermethylation. Cancer Sci 2004;95:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Miousse IR, Chang J, Shao L, et al. Inter-strain differences in LINE-1 DNA methylation in the mouse hematopoietic system in response to exposure to ionizing radiation. Int J Mol Sci 2017;18:1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lima F, Ding D, Goetz W, et al. High LET (56)Fe ion irradiation induces tissue-specific changes in DNA methylation in the mouse. Environ Mol Mutagen 2014;55:266–77. [DOI] [PubMed] [Google Scholar]
- [5].Chaudhry MA, Omaruddin RA. Differential DNA methylation alterations in radiation-sensitive and -resistant cells. DNA Cell Biol 2012;31:908–16. [DOI] [PubMed] [Google Scholar]
- [6].Luzhna L, Ilnytskyy Y, Kovalchuk O. Mobilization of LINE-1 in irradiated mammary gland tissue may potentially contribute to low dose radiation-induced genomic instability. Genes Cancer 2015;6:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee Y, Kim YJ, Choi YJ, et al. Radiation-induced changes in DNA methylation and their relationship to chromosome aberrations in nuclear power plant workers. Int J Radiat Biol 2015;91:142–9. [DOI] [PubMed] [Google Scholar]
- [8].Wu Q, Ni X. ROS-mediated DNA methylation pattern alterations in carcinogenesis. Curr Drug Targets 2015;16:13–9. [DOI] [PubMed] [Google Scholar]
- [9].Xu GL, Wong JM. Oxidative DNA demethylation mediated by Tet enzymes. Natl Sci Rev 2015;3:318–28. [Google Scholar]
- [10].Kloypan C, Srisa-art M, Mutirangura A, et al. LINE-1 hypomethylation induced by reactive oxygen species is mediated via depletion of S-adenosyl methionine. Cell Biochem Funct 2015;33:375–85. [DOI] [PubMed] [Google Scholar]
- [11].Cuozzo C, Porcellini A, Angrisano T, et al. DNA damage, homology-directed repair, and DNA methylation. PLoS Genet 2007;3:e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wei D, Loeken MR. Increased DNA methyltransferase 3b(Dnmt3b)-mediated CpG island methylation stimulated by oxidative stress inhibits expression of a gene required for neural tube and neural crest development in diabetic pregnancy. Diabetes 2014;63:3512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thanan R, Techasen A, Hou B, et al. Development and characterization of a hydrogen peroxide-resistant cholangiocyte cell line: a novel model of oxidative stress-related cholangiocarcinoma genesis. Biochem Biophys Res Commun 2015;464:182–8. [DOI] [PubMed] [Google Scholar]
- [14].Miousse IR, Kutanzi KR, Koturbash I. Effects of ionizing radiation on DNA methylation: from experimental biology to clinical applications. Int J Radiat Biol 2017;93:457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang J, Zhang Y, Xu K, et al. Genomewide screen of DNA methylation changes induced by low dose X-ray radiation in mice. PloS One 2014;9:e90804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Valentin J. The 2007 Recommendations of the International Commission on Radiological Protection[J]. Annals of the ICRP 2007;37:1–332. [DOI] [PubMed] [Google Scholar]
- [17].Ye S, Yuan D, Xie Y, et al. Role of DNA methylation in long-term low-dose gamma-rays induced adaptive response in human B lymphoblast cells. Int J Radiat Biol 2013;89:898–906. [DOI] [PubMed] [Google Scholar]
- [18].Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett 2012;327:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Koc U, Tan S, Ertem AG, et al. Evaluation of thiol-disulphide homeostasis in radiation workers. Int J Radiat Biol 2017;93:705–10. [DOI] [PubMed] [Google Scholar]
- [20].Johnstone SE, Baylin SB. Stress and the epigenetic landscape: a link to the pathobiology of human diseases? Nat Rev Genet 2010;11:806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Franco R, Schoneveld O, Georgakilas AG, et al. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett 2008;266:6–11. [DOI] [PubMed] [Google Scholar]
- [22].Gu X, Sun J, Li S, et al. Oxidative stress induces DNA demethylation and histone acetylation in SH-SY5Y cells: potential epigenetic mechanisms in gene transcription in Abeta production. Neurobiol Aging 2013;34:1069–79. [DOI] [PubMed] [Google Scholar]
- [23].Szumiel I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: the pivotal role of mitochondria. Int J Radiat Biol 2015;91:1–2. [DOI] [PubMed] [Google Scholar]
- [24].Avila JG, Echeverri I, de Plata CA, et al. Impact of oxidative stress during pregnancy on fetal epigenetic patterns and early origin of vascular diseases. Nutr Rev 2015;73:12–21. [DOI] [PubMed] [Google Scholar]
- [25].Wild P, Gauron C, Champion K, et al. Effects of chronic low-dose exposure to ionizing radiation on physician microvascular structure revealed by nail fold capillaroscopy. Radiat Environ Biophys 2016;55:71–9. [DOI] [PubMed] [Google Scholar]
- [26].Zakeri F, Hirobe T, Akbari Noghabi K. Biological effects of low-dose ionizing radiation exposure on interventional cardiologists. Occup Med (Lond) 2010;60:464–9. [DOI] [PubMed] [Google Scholar]
- [27].Milacic S. Risk of occupational radiation-induced cataract in medical workers. Med Lav 2009;100:178–86. [PubMed] [Google Scholar]
- [28].López PO, Dauer LT, Loose R, et al. ICRP Publication 139: Occupational Radiological Protection in Interventional Procedures[J]. Annals of the ICRP 2018;47:1–18. [DOI] [PubMed] [Google Scholar]


