Abstract
Objective:
We performed a meta-analyisis to evaluate the efficacy of maintenance dexamethasone against acute or delayed chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetic risk chemotherapy regimen.
Methods:
PubMed, Embase, and Cochrane Library were searched for eligible studies. Data comparing maintenance dexamethasone with single-dose dexamethasone during the acute, delayed, and overall phase of CINV were extracted. Overall risk ratio (RR) was used to estimate the efficacy and adverse effects.
Results:
Nine studies were included. In delayed phase, maintenance dexamethasone has similar efficacy to single-dose dexamethasone for no emetic episodes (RR, 1.06; 95% confidence interval [CI], 1.00–1.14), complete response (RR, 1.04; 95% CI, 0.98–1.11), complete control (RR, 1.07; 95% CI, 0.98–1.16), and total control (RR, 1.06; 95% CI, 0.91–1.23). In overall phase, maintenance dexamethasone has similar efficacy to single-dose dexamethasone for no emetic episodes (RR, 1.02; 95% CI, 0.94–1.11), complete response (RR, 1.02; 95% CI, 0.95 -1.09), complete control (RR, 1.03; 95% CI, 0.94–1.13), total control (RR, 1.05; 95% CI, 0.90–1.23), and no rescue medication (RR, 1.07; 95% CI, 0.97–1.19). Maintenance dexamethasone was only superior to single-dose dexamethasone for no rescue medication during delayed phase (RR, 1.10; 95% CI, 1.01–1.21, P = .034). The incidence of hiccup was observed higher in maintenance dexamethasone group (RR = 3.16, 95% CI, 1.12–8.92).
Conclusion:
The single-dose dexamethasone regimen offers high and similar overall control of symptoms as the maintenance dexamethasone regimen in this population. Multiple-day dexamethasone was suitable for patients who used rescue medication during the delayed phase.
Keywords: anti-emetic, cancer, chemotherapy, dexamethasone
1. Introduction
Chemotherapy-induced nausea and vomiting (CINV) has a deleterious influence on the performance status and health-related quality of life of patients receiving chemotherapy.[1] CINV consists of 2 phases: acute-phase CINV occurs within 24 hours of the initial chemotherapy administration, whereas delayed-phase CINV can last for up to 120 hours after chemotherapy administration.[2] The mechanisms and pharmacophysiological pathways are different between acute and delayed phase of CINV[3,4]; hence, distinct medication strategies are maneuvered. In a study of 1910 patients receiving highly emetogenic chemotherapy (HEC) or moderately emetogenic chemotherapy (MEC), Tamura et al reported that acute nausea of CINV could be well processed (20.8% with HEC and 6.7% with MEC). However, half of the patients reported suffering from delayed nausea (49.4% with HEC and 41.7% with MEC).[5] CINV in delayed phase remains a crucial challenge in tumor treatment.
Despite the advance in supportive care that occurred with the introduction of the first-generation serotonin (5-HT3)-receptor antagonists (eg, granisetron, ondansetron, and dolasetron), up to 70% of patients with tumor receiving HEC agents suffered from nausea and vomiting after chemotherapy.[6] Palonosetron, a newer second-generation 5-HT3, has a longer half-time (about 40 hours) and a higher binding affinity to 5-HT3 receptors than first generation of 5-HT3 antagonists.[7] Palonosetron is demonstrated to be more efficient and beneficial than first generation of 5-HT3 antagonists against delayed emesis.[8] Dexamethasone is effective against CINV, combined with a 5-HT3 antagonist. For high emetic risk regimens, triple antiemetic therapy using aprepitant (a NK-1 receptor antagonist), 5-HT3 antagonist, and dexamethasone is recommended.[9]
Dexamethasone was recommended for several days to control CINV associated with HEC and MEC by current guideline of NCCN (http://www.nccn.org). However, Ito et al[10] reported that dexamethasone on days 2 and 3 could be spared when combined with NK-1 receptor antagonist and palonosetron in HEC. In several studies, researchers compared the efficacy of maintenance dexamethasone regimen to single-dose dexamethasone regimen. The present analysis was conducted to enhance evidence for the comparison of maintenance dexamethasone regimen and single-dose dexamethasone regimen.
2. Methods
2.1. Search strategy
We systematically searched the literature on PubMed, Embase, and Cochrane Library (from the beginning of 1992 to February, 2018). The search strategy was based on “dexamethasone,” “chemotherapy,” AND “vomit OR emesis” as keywords or MeSH terms (medical subject heading terms). All titles and abstracts were screened to select eligible articles independently by 2 reviewers (Y-LG and JR). Trials not published in English were excluded in present analysis. Each eligible study must meet the following criteria: studies to compare the efficacy of maintenance dexamethasone versus single-dose dexamethasone; maintenance dexamethasone and single-dose dexamethasone used for CINV; and sufficient variables for figuring risk ratio (RR) of complete response (CR). When studies had overlapping cohorts, we only included the one with largest number of participants.
2.2. Definition of outcomes
The primary endpoint was the prop risk ration of patients achieving a CR. CR was defined as no rescue medication and no emetic episodes. Secondary endpoints included the percentage of patients achieving either a complete control (CC: no emetic episodes, no rescue medication, and no significant nausea), total control (TC: no emetic episodes, no rescue medication, and no nausea), taking no rescue medications or no emesis during the acute, delayed, and overall phase.
2.3. Data extraction
Two reviewers (Y-LG and J-MX) extracted the data from total potential studies independently. The following information from each included studies was captured: first author's name, publication year, mean age, emetogenicity, the detailed treatment regimens, the number of patients in maintenance dexamethasone group, and single-dose dexamethasone group and antiemetic response.
2.4. Quality assessment
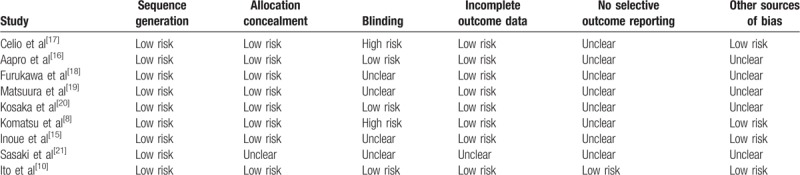
The quality of eligible trials was assessed by 2 reviewers (Y-LG and JR) according to Cochrane Collaboration Reviewers’ Handbook for Systematic Reviews of Interventions. Sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting were evaluated independently. The quality assessment of eligible studies is described in Table 1.
Table 1.
Quality assessment of included studies.
2.5. Statistical analysis
Pooled estimates of odds ratio (OR) were chosen, whereas the events rate was <1%.[11] Otherwise, pooled estimates of RR were selected. A heterogeneity test was examined using Q statistics.[12] When heterogeneity was negative, which was defined as I2 <50%, fixed-effects model[13] was utilized; otherwise, the random-effects model[14] was utilized. Egger linear regression and funnel plot were used to measure potential publication bias. If the P value of Egger linear regression was <0.05 or the funnel plot was asymmetrical, it indicated that publication bias may exist. All analyses in this study were processed with the program Stata, version 13.0 (StataCorp LP).
2.6. Subgroup analysis
The primary subgroup analysis was conducted to evaluate the differences between chemotherapy emetogenicities and risk levels. We sorted studies according to chemotherapy emetogenicities. All participants included in this analysis were classified as moderate risk or high risk according to the antiemesis guidelines of the National Comprehensive Cancer Network (NCCN, http://www.nccn.org). We conducted a second subgroup analysis by classifying studies based on antiemetic regimens, age categories, and sex.
2.7. Ethics statement
This article is a secondary data processing of previously published studies. No human or animal experiments were conducted. Ethical approval was not necessary.
3. Results
3.1. Eligible trials
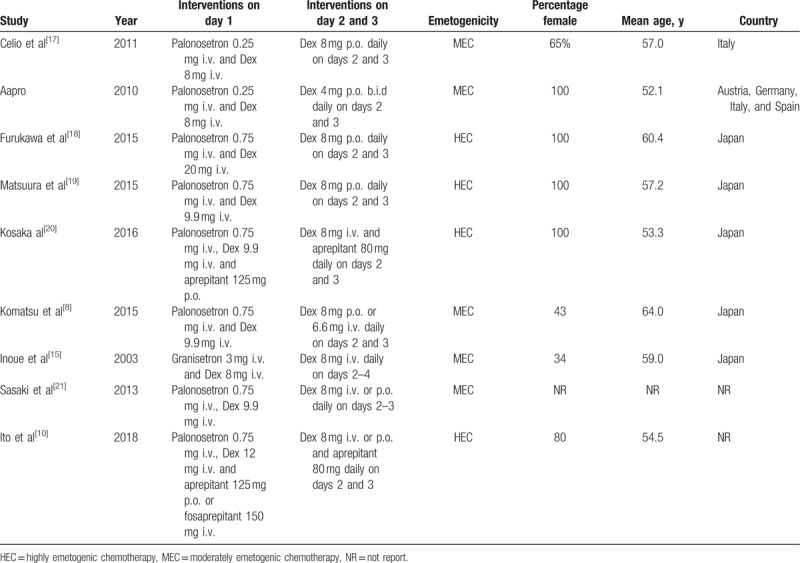
Nine studies,[8,10,15–21] with 1968 cases were included in our meta-analysis (The flowchart was presented in PRISMA Flow Diagram). Three trials[22–24] were excluded because of repetitive data. Considering the heterogeneity and progress of chemotherapy and treatment options, 1 trial[25] was excluded. Among the 1968 patients, 981 patients were treated with maintenance dexamethasone and 987 patients were treated with single-dose dexamethasone. The characteristics of included trials were listed in Table 2.
Table 2.
Summary of the main characteristics of all eligible studies.
3.2. Pooled efficacy of maintenance dexamethasone versus single-dose dexamethasone
3.2.1. Efficacy—acute phase
Because both groups are treated with the same antiemetic regimens within the initial 24 hours, the efficacy of antiemetic regimens was not significantly different during the acute phase (Fig. 1).
Figure 1.
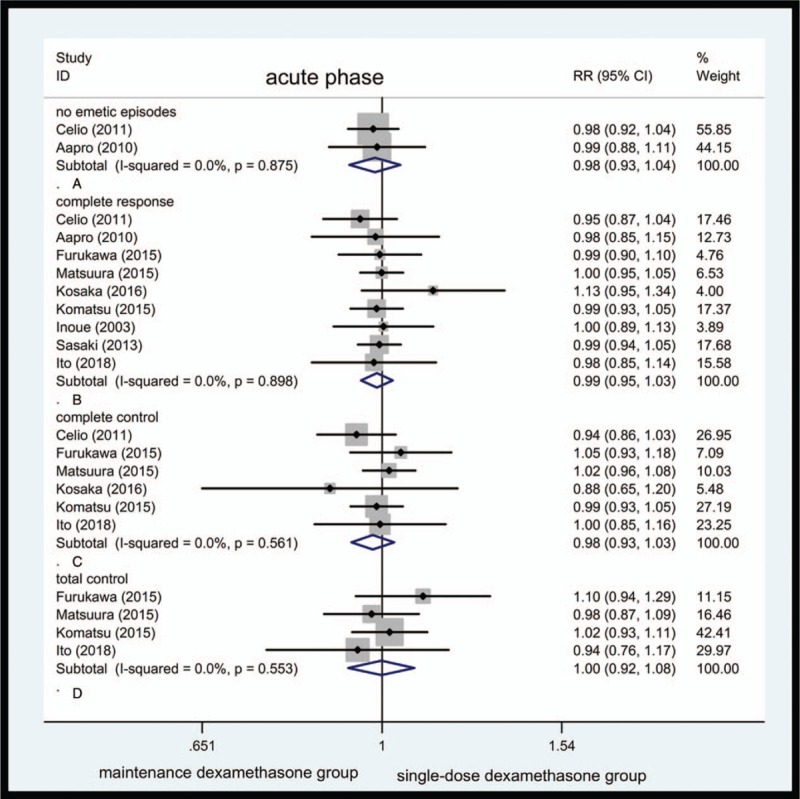
Forest plot showing pooled results for maintenance dexamethasone versus single-dose dexamethasone in acute phase. Pooled risk ratio for no emetic episodes (A). Pooled risk ratio for complete response (B). Pooled risk ratio for complete control (C). Pooled risk ratio for total control (D).
No emetic episodes were seen. Only 2 studies reported vomiting incidence. Forty-four of 330 cases in maintenance dexamethasone group and 40 of 314 cases in single-dose dexamethasone group suffer from vomiting. Overall RR for no emetic episodes was 0.98 (95% CI, 0.93–1.04, Fig. 1A). It failed to achieve a statistical significance. The heterogeneity was not observed; the fixed-effects model was preferred (Table 1).
CR: 9 studies reported CR in 808 of 981 cases in maintenance dexamethasone group and 822 in 987 cases in single-dose dexamethasone group. The overall RR for CR was 0.99 (95% CI, 0.95–1.03, Fig. 1B). The heterogeneity of CR for this comparison was not observed.
CC: 6 studies documented CC in 509 of 644 cases in maintenance dexamethasone group and 527 of 651 cases in single-dose dexamethasone group. Pooled RR for CC was 0.98 (95% CI, 0.93–1.03, Fig. 1C).
TC: Only 4 studies were available for this variable. Three-hundred four of 442 cases in maintenance dexamethasone and 310 of 450 cases in single-dose dexamethasone group showed TC. The RR for TC was 1.00 (95% CI, 0.92–1.08, Fig. 1D).
No rescue medication was taken. No article recorded this variable; thus, no calculations could be made.
3.2.2. Efficacy—delayed phase
Values between 24 and 120 hours after initial chemotherapy were pooled and forest plot was listed in Figure 2.
Figure 2.
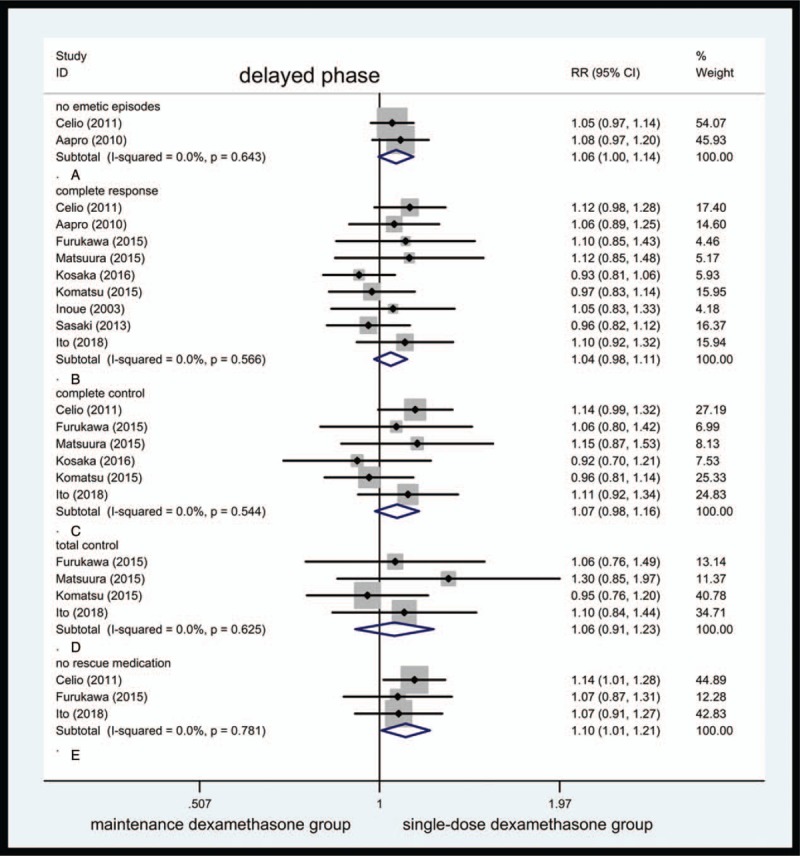
Forest plot showing pooled results for maintenance dexamethasone versus single-dose dexamethasone in delayed phase. Pooled risk ratio for no emetic episodes (A). Pooled risk ratio for complete response (B). Pooled risk ratio for complete control (C). Pooled risk ratio for total control (D). Pooled risk ratio for no rescue medication (E).
No emetic episodes were seen. Only 2 studies reported vomiting incidence, with pooled RR of no emetic episodes being 1.06 (95% CI, 1.00–1.14, Fig. 2A); no statistical differences were observed in this outcome (P = .065). A total of 272 of 310 cases in maintenance dexamethasone group and 259 of 314 cases in single-dose dexamethasone group reported no emetic episodes. The heterogeneity was not observed in this comparison.
CR: 9 clinical trials enclosed these variables. Nine studies reported CR in 665 of 981 cases and 641 of 987 cases in maintenance dexamethasone group and single-dose dexamethasone group. The pooled RR for CR was 1.04 (95% CI, 0.98–1.11, Fig. 2B).
CC: 6 studies reported CR in 419 of 644 cases in maintenance dexamethasone group and 397 of 652 cases in single-dose dexamethasone group. The pooled RR for CC was 1.07 (95% CI, 0.98–1.16, Fig. 2C).
TC: Only 4 studies were available for this variable. A total of 197 of 442 cases in maintenance dexamethasone and 190 of 450 cases in single-dose dexamethasone group showed TC. The pooled RR for TC was 1.06 (95% CI, 0.91–1.23, Fig. 2D).
No rescue medication was taken. Three enrolled studies documented this values, sum of 2 groups had 802 cases. A total of 287 patients in maintenance dexamethasone group and 267 in single-dose dexamethasone group did not require rescue medication. Above this comparison, heterogeneity was not observed, with RR being 1.10 (95% CI, 1.01–1.21, Fig. 2E). The pooled RR for no rescue medication was statistically different (P = .034) despite only 3 trials reporting this variable.
3.2.3. Efficacy—overall phase
Variables for the total period were pooled and forest plot was listed in Figure 3.
Figure 3.
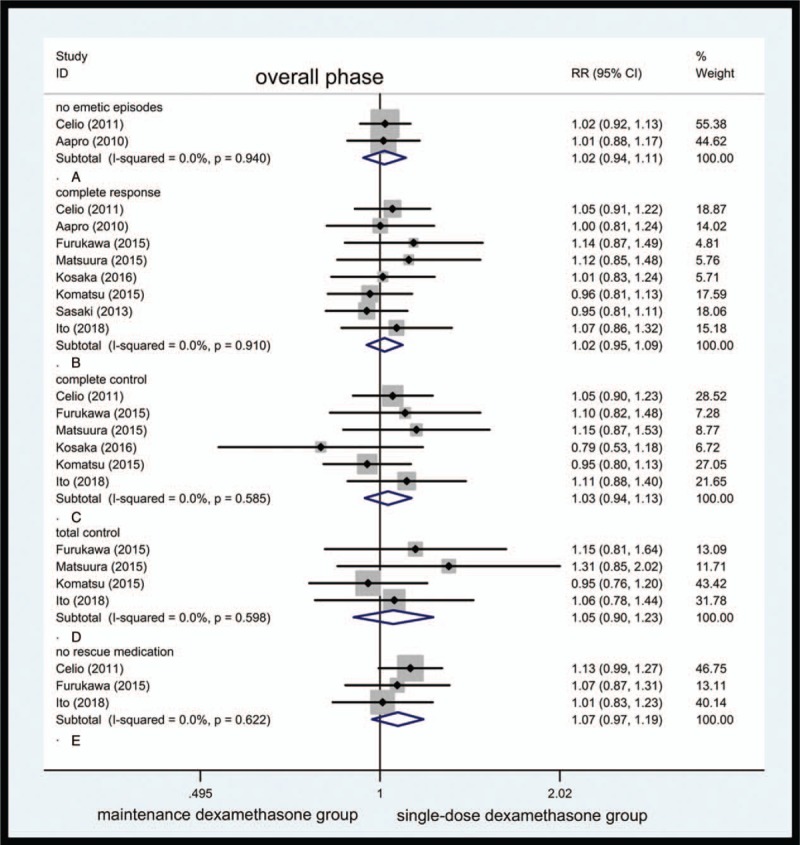
Forest plot showing pooled results for maintenance dexamethasone versus single-dose dexamethasone in overall phase. Pooled risk ratio for incidence of no emetic episodes (A). Pooled risk ratio for complete response (B). Pooled risk ratio for complete control (C). Pooled risk ratio for total control (D). Pooled risk ratio for no rescue medication (E).
No emetic episodes were seen. Two studies reported vomiting incidence, where 243 of 310 cases did not vomit in maintenance dexamethasone group and 242 of 314 cases did not vomit in single-dose dexamethasone group at least once during the above duration. The pooled RR for no emetic episodes was 1.02 (95% CI, 0.94–1.11, Fig. 3A). The heterogeneity for the above comparison was of nonexistence.
CR: 8 clinical trials reported the desired variables. A total of 582 of 946 cases in maintenance dexamethasone group and 576 of 954 cases in single-dose dexamethasone group showed CR. The pooled RR for CR was 1.02 (95% CI, 0.95–1.09, Fig. 3B).
CC: 6 clinical trials reported the CC 375 of 644 patients in maintenance dexamethasone group and 368 of 652 patients in single-dose dexamethasone group reported CC. The RR for CC was 1.03 (95% CI, 0.94–1.13, Fig. 3C). The heterogeneity was not observed in this comparison.
TC: Only 4 studies were available for this variable. One hundred eighty-two of 442 cases in maintenance dexamethasone and 176 of 450 cases in single-dose dexamethasone group behaved TC. The RR for TC was 1.05 (95% CI, 0.90–1.23, Fig. 3D).
No rescue medication was taken. Two hundred sixty-oneof 396 patients in maintenance dexamethasone group and 250 of 406 patients in single-dose dexamethasone group did not require additional rescue antiemetic, with RR being 1.07 (95% CI, 0.97–1.19, Fig. 3E).
3.3. Subgroup analyses
Among subgroup emetogenicity analysis, maintenance dexamethasone has similar efficacy to single-dose dexamethasone in both HEC and MEC group during the acute (Fig. 4A), delayed (Fig. 4B), and overall phase (Fig. 4C). The RR for CR in MEC was 0.98 (95% CI 0.94–1.02, Fig. 4A) and the RR for CR in HEC was 1.01 (95% CI, 0.93–1.09, Fig. 4A) in acute phase. The RR for CR in MEC was 1.03 (95% CI, 0.96–1.11, Fig. 4B) and the RR for CR in HEC was 1.07 (95% CI 0.96–1.20, Fig. 4B) in delayed phase. The RR for CR in MEC was 0.99 (95% CI, 0.91–1.08, Fig. 4C) and the RR for CR in HEC was 1.08 (95% CI, 0.95–1.23, Fig. 4C) in overall phase.
Figure 4.
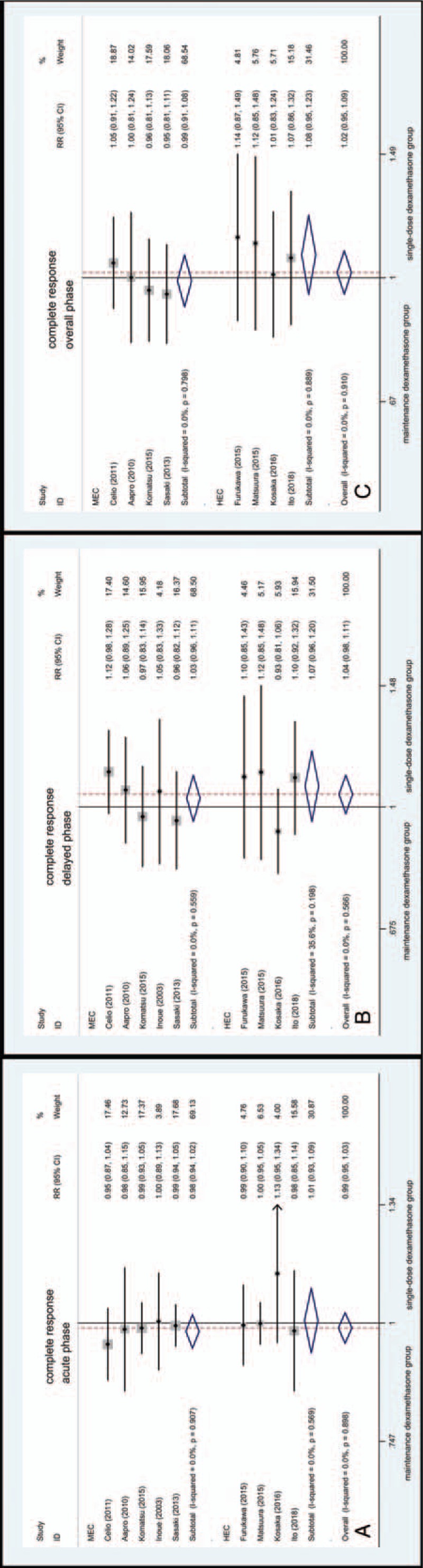
Forest plot for complete response of subgroup analysis of the emetogenic potential.
Among subgroup antiemetic regimens analysis, statistical significance favoring maintenance dexamethasone was not found during the acute (Fig. 5A), delayed (Fig. 5B), and overall phase (Fig. 5C). The RR for CR in palonosetron group was 1.01 (95% CI, 0.93–1.09, Fig. 5C) in overall phase. The RR for CR in aprepitant group was 1.05 (95% CI, 0.89–1.24, Fig. 5C) in overall phase.
Figure 5.
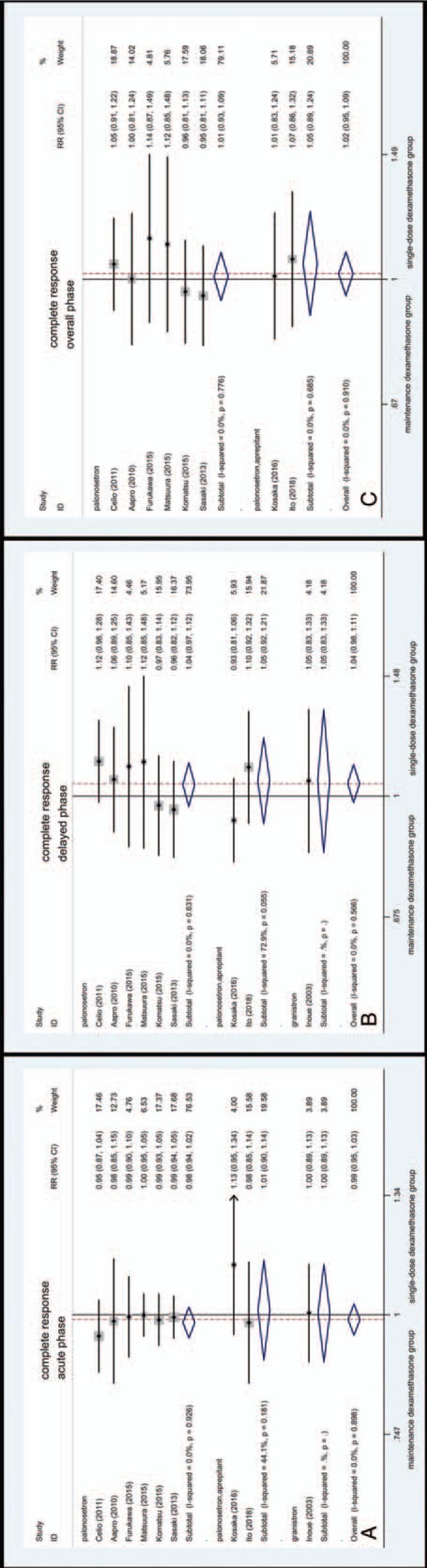
Forest plot for complete response of subgroup analysis of antiemetic regimens.
Upon the subgroup analysis of age categories or sex, CR rates showed no difference between treatment groups (Fig. 6).
Figure 6.
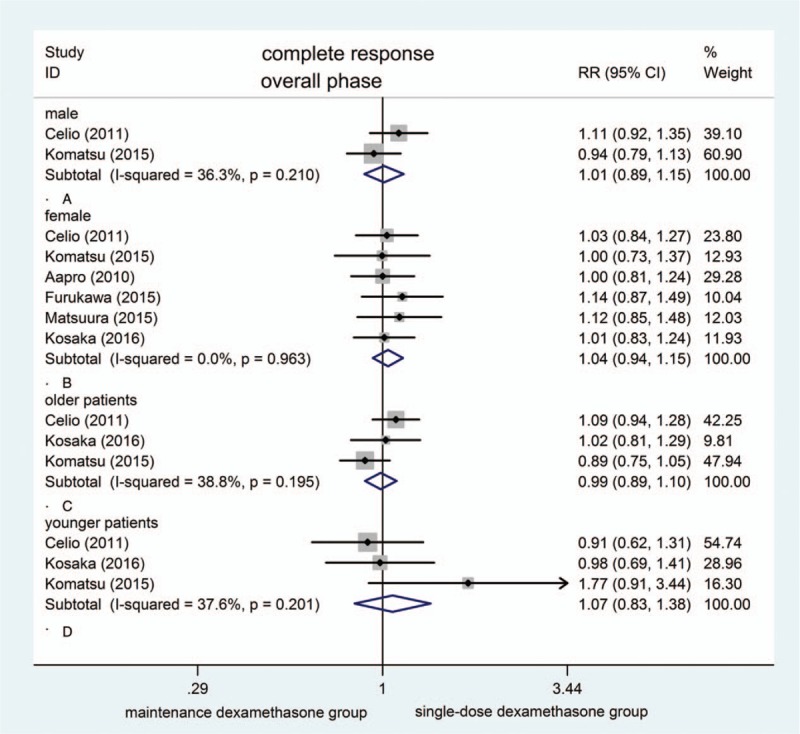
Forest plot for complete response of subgroup analysis of age categories and sex.
3.4. Adverse effects
The common adverse effects reported among the included studies were constipation, diarrhea, headache, abdominal pain, hiccup, insomnia, anorexia, erythema, and fatigue. The incidence of hiccup was observed higher in maintenance dexamethasone group (RR = 3.16; 95% CI, 1.12–8.92). These data were only reported in 2 studies. The incidence of diarrhea (RR = 1.38; 95% CI, 0.28–6.64), abdominal pain (RR = 1.27; 95% CI, 0.50–3.24), erythema (RR = 1.13; 95% CI, 0.46–2.78), fatigue (RR = 0.55; 95% CI, 0.24–1.23), constipation (RR = 0.92; 95% CI, 0.63–1.34), headache (RR = 0.89; 95% CI, 0.63–1.25), insomnia (RR = 1.55; 95% CI, 0.77–3.09), and anorexia (RR = 1.17; 95% CI, 0.64–2.14) showed statistical similarity.
3.5. Assessment of publication bias
No emetic episodes were seen. The sample size was too small to perform Egger regression test (only 2 studies were included).
CR: The funnel plot was subjective to evaluate publication bias (Fig. 7A). No publication bias was observed in Egger linear regression because the intercept of Egger regression test was reported at 1.34202 and P value was .302 (Fig. 7B).
CC: Publication bias was not attained in this analysis. The intercept was reported at −0.7597613 with a P value of .633 (Fig. 7C).
TC: No publication bias could be achieved in this comparison. The intercept was reported at 2.96113 (P = .110, Fig. 7D).
Figure 7.
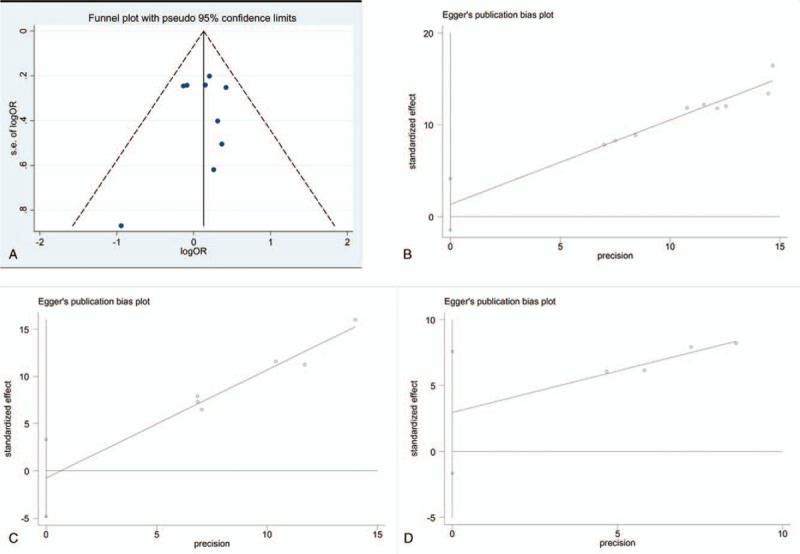
The funnel plot for complete response (A). Egger regression test for complete response (B). Egger regression test for complete control (C). Egger regression test for total control (D).
No rescue medication was taken. Egger regression test was not available owing to the insufficient data.
4. Discussion
John et al showed that dexamethasone offered an obvious superiority against emesis among both the acute and delayed phases.[26] Studies which compared maintenance dexamethasone with single-dose dexamethasone were limited. This analysis was conducted to provide further evidence for the comparison.
Nausea and vomiting are both problems in CINV. We attached more importance to vomiting control as it is more objective to verify, values of nausea were relatively limited. The endpoints of this meta-analysis are no emesis, CR, CC, TC, and taking no rescue medications during the acute, delayed, or overall phase. Incidence of vomiting, CR, CC, and TC did not differ significantly between the maintenance dexamethasone and single-dose dexamethasone during the acute, delayed, or overall phase. Statistical significance favoring maintenance dexamethasone was attained in patients who did not use rescue medication.
The outcomes of present analysis suggest that patients in maintenance dexamethasone group have similar efficacy to those in single-dose dexamethasone group. However, maintenance dexamethasone regimen reduced the need for rescue analgesics when compared with single-dose dexamethasone regimen among the delayed (P = .034).
Some studies indicate that female or younger age is important risk factor for predicting a higher risk for CINV.[27,28] In this analysis, neither age nor sex was essentially associated with overall CR to anti-emetic treatment in subgroup analysis. However, the sample size was relatively limited.
Acute and chronic toxicity of dexamethasone comprise agitation, insomnia, increased appetite, weight gain, gastroesophageal reflux disease, and acne. Adverse effects are of interest but were inadequately reported. In most antiemetic studies, the adverse effects were recorded during the hospitalization. Also, it is difficult to determine whether the adverse effects were attributable to antiemetic treatment or maintenance dexamethasone. Furukawa et al[18] reported that patients accepting the maintenance dexamethasone prescription experienced a statistically significant but not severe higher incidence of insomnia. Vardy et al designed the questionnaire to evaluate the side effects associated with maintenance dexamethasone. In this study, patients experienced moderate to severe side effects with insomnia (45%), indigestion/epigastric discomfort (27%), agitation (27%), increased appetite (19%), weight gain (16%), and acne (15%), in the week following their chemotherapy.[29] The side effects of multiple-day dexamethasone may do more harm than good for patients receiving MEC.[29]
In our analysis, we performed the meta-analysis to evaluate the adverse effects. The incidence of hiccup was higher associated with maintenance dexamethasone. However, this value was recorded in an insufficient number of studies. Future trials should accurately concentrate on the side effects of dexamethasone so as to establish the overall safety of dexamethasone. Maintenance dexamethasone should not be applied in patients with a history of ulcers, hypertension, or diabetes.
Current guideline of NCCN (http://www.nccn.org) recommended that dexamethasone should be applied for several days to control CINV associated with HEC and MEC. In the guideline of MASCC/ESMO, a triple regimen consisting of maintenance dexamethasone plus a 5-HT3-receptor antagonist and an NK-1 receptor antagonist for the prevention of CINV owing to nonanthracycline/cyclophosphamide (AC)-based HEC agents was recommended.[30,31] In breast cancer patients treated with aprepitant, 5-HT3-receptor antagonist and dexamethasone on day 1, aprepitant or dexamethasone on day 2 and 3 were suggested.[31] In the guideline of ASCO, a 4-drug regimen consisting of 5-HT3-receptor antagonist, an NK-1 receptor antagonist, olanzapine, and dexamethasone was recommended to patients who are treated with HEC agents. Dexamethasone was only recommended on day 1 associated with AC-based HEC agents and was recommended on day 1 to 4 associated with other HEC agents.[32]
In the subgroup analysis of chemotherapy emetogenicities, we found that maintenance dexamethasone was not superior to single-dose dexamethasone in both HEC and MEC group during the acute, delayed, or overall phase. In the subgroup of antiemetic regimens analysis, antiemetic regimens consist of granisetron (one trial), palonosetron (6 trials), and palonosetron combined with aprepitant (2 trials). We found that maintenance dexamethasone was not superior to single-dose dexamethasone. Aprepitant was only applied in 2 studies which was recommended by guidelines.
In the guidelines of MASCC/ESMO and ASCO, patients who are treated with MEC agents that are known to cause delayed emesis may be recommended dexamethasone on days 2 to 3. This recommendation was based upon the study.[33] However, this study was conducted among the patients receiving AC (now considered HEC) single agent anthracycline, CMF, or carboplatin and it was unclear what percentage of enrolled patients received AC.[34]
Aprepitant has been demonstrated to be effective against CINV in both the acute and delayed phases.[35,36] However, there is no sufficient evidence to compare maintenance dexamethasone with single-dose dexamethasone when both are combined with NK-1 receptor antagonist directly. This analysis suggested that single-dose dexamethasone was effective. Additional studies are required to provide further evidence.
There is no doubt that dexamethasone is an effective and beneficial addition to anti-CINV regiments. In this analysis, we found single-dose dexamethasone regimen was an effective and safe alternative in antiemetic protection. These data may be of help to reduce the excessive utilization of dexamethasone, while still guaranteeing antiemetic effect and minimizing the side effect.
There are limitations among this study. One trials[21] included in this analysis was proceedings paper and only available for abstract. Only 9 clinical trials were reported to compare the efficacy of anti-CINV between maintenance dexamethasone and single-dose dexamethasone regimen. Aprepitant was only applied in 2 studies which was recommended for anti-CINV by all guidelines. CINV definition, types of chemotherapy and treatment options dramatically changed over the years, we excluded the studies before 2003.
The conclusion of this meta-analysis indicated that multiple-day dexamethasone was superfluous unless patients needed rescue medication. Our findings indicate that, irrespective of age, the single-dose dexamethasone regimen offers high and similar overall control of symptoms as the maintenance dexamethasone regimen in this population.
Acknowledgment
Y-lG is particularly grateful to PP Shen, who gave powerful support over the past year.
Author contributions
Conceptualization: Jie Ren.
Data curation: Jia-ming Xie.
Formal analysis: Yan-Lin Gu, Jin-rong Wei.
Investigation: Le-Ning Shao.
Methodology: Jia-ming Xie, Chao Chen.
Project administration: Jin-rong Wei, Chao Chen.
Resources: Jie Ren.
Software: Yan-Lin Gu.
Supervision: Hua Cao.
Visualization: Hua Cao.
Writing – original draft: Yan-Lin Gu.
Writing – review & editing: guoqin jiang.
Footnotes
Abbreviations: AC = anthracycline/cyclophosphamide, CC = complete control, CINV = chemotherapy-induced nausea and vomiting, CR = complete response, HEC = highly emetogenic chemotherapy, MEC = moderately emetogenic chemotherapy, MeSH terms = medical subject heading terms, NCCN = National Comprehensive Cancer Network, OR = odds ratio, RR = risk ratio, TC = total control.
How to cite this article: Gu YL, Xie JM, Ren J, Cao H, Wei JR, Chen C, Shao LN, Jiang GQ. Dexamethasone-sparing regimen is an effective and safe alternative in overall anti-emetic protection. Medicine. 2019;98:39(e17364).
Y-LG, J-mX, and JR contributed equally to this work.
Funding: Suzhou Health Planning Commission's Key Clinical Diagnosis and Treatment Program (LCZX201606), National Natural Science Foundation of China (81873730) and the Jiangsu Women and Children Health Key Discipline Program (FXK201758).
The funding came from Suzhou Health Planning Commission's Key Clinical Diagnosis and Treatment Program (Award ID: LCZX201606), National Natural Science Foundation of China (Award ID: 81873730) and the Jiangsu Women and Children Health Key Discipline Program (Award ID: FXK201758).
The authors report no conflicts of interest.
References
- [1].Sharma R, Tobin P, Clarke SJ. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol 2005;6:93–102. [DOI] [PubMed] [Google Scholar]
- [2].Grunberg SM. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: dosing, efficacy, and tolerability analysis. Ann Oncol 2007;18:233–40. [DOI] [PubMed] [Google Scholar]
- [3].Hesketh PJ. Chemotherapy-induced nausea and vomiting. The New England journal of medicine 2008;358:2482–94. [DOI] [PubMed] [Google Scholar]
- [4].Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med 2016;374:1356–67. [DOI] [PubMed] [Google Scholar]
- [5].Tamura K, Aiba K, Saeki T, et al. Testing the effectiveness of antiemetic guidelines: results of a prospective registry by the CINV Study Group of Japan. Int J Clin Oncol 2015;20:855–65. [DOI] [PubMed] [Google Scholar]
- [6].Hickok JT, Roscoe JA, Morrow GR, et al. Use of 5-HT3 receptor antagonists to prevent nausea and emesis caused by chemotherapy for patients with breast carcinoma in community practice settings. Cancer 1999;86:64–71. [DOI] [PubMed] [Google Scholar]
- [7].Park JW, Jun JW, Lim YH, et al. The comparative study to evaluate the effect of palonosetron monotherapy versus palonosetron with dexamethasone combination therapy for prevention of postoperative nausea and vomiting. Korean J Anesthesiol 2012;63:334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Komatsu Y, Okita K, Yuki S, et al. Open-label, randomized, comparative, phase III study on effects of reducing steroid use in combination with Palonosetron. Cancer Sci 2015;106:891–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Inui N. Antiemetic therapy for non-anthracycline and cyclophosphamide moderately emetogenic chemotherapy. Med Oncol 2017;34:77. [DOI] [PubMed] [Google Scholar]
- [10].Ito Y, Tsuda T, Minatogawa H, et al. Placebo-controlled, double-blinded phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin-1 receptor antagonist and palonosetron in high-emetogenic chemotherapy. J Clin Oncol 2018;36:1000–6. [DOI] [PubMed] [Google Scholar]
- [11].Bradburn MJ, Deeks JJ, Berlin JA, et al. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 2007;26:53–77. [DOI] [PubMed] [Google Scholar]
- [12].Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [13].Kuritz SJ, Landis JR, Koch GG. A general overview of Mantel-Haenszel methods: applications and recent developments. Ann Rev Public Health 1988;9:123–60. [DOI] [PubMed] [Google Scholar]
- [14].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [15].Inoue A, Yamada Y, Matsumura Y, et al. Randomized study of dexamethasone treatment for delayed emesis, anorexia and fatigue induced by irinotecan. Support Care Cancer 2003;11:528–32. [DOI] [PubMed] [Google Scholar]
- [16].Aapro M, Fabi A, Nole F, et al. Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol 2010;21:1083–8. [DOI] [PubMed] [Google Scholar]
- [17].Celio L, Frustaci S, Denaro A, et al. Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multicenter, phase III trial. Support Care Cancer 2011;19:1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Furukawa N, Kanayama S, Tanase Y, et al. Palonosetron in combination with 1-day versus 3-day dexamethasone to prevent nausea and vomiting in patients receiving paclitaxel and carboplatin. Support Care Cancer 2015;23:3317–22. [DOI] [PubMed] [Google Scholar]
- [19].Matsuura M, Satohisa S, Teramoto M, et al. Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following paclitaxel and carboplatin in patients with gynecologic cancers: a randomized, multicenter, phase-II trial. J Obstet Gynaecol Res 2015;41:1607–13. [DOI] [PubMed] [Google Scholar]
- [20].Kosaka Y, Tanino H, Sengoku N, et al. Phase II randomized, controlled trial of 1 day versus 3 days of dexamethasone combined with palonosetron and aprepitant to prevent nausea and vomiting in Japanese breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer 2016;24:1405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sasaki K, Okita K, Yuki S, et al. A randomized phase III trial of palonosetron plus dexamethasone (day 1) versus palonosetron plus dexamethasone (day 1-3) in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy, not including a combination of anthracycline plus cyclophosphamide. Eur J Cancer 2013;49:S267–8. [Google Scholar]
- [22].Matsuzaki K, Ito Y, Fukuda M, et al. Placebo-controlled phase III study comparing dexamethasone on day 1 to on day 1-3 with NKj receptor antagonist and palonosetoron in high emetogenic chemotherapy. J Clin Oncol 2016;34:10019. [DOI] [PubMed] [Google Scholar]
- [23].Fabi A, Nole F, Roila F, et al. Comparison of palonosetron (PALO) plus dexamethasone on day 1, with or without dexamethasone on days 2 and 3, in the prevention of chemotherapy induced nausea and vomiting (CINV) in breast cancer patients treated with anthracycline/cyclophosphamide regimen. Eur J Clin Microbiol Infectious Diseases 2009;16:535–7. [Google Scholar]
- [24].Celio L, Denaro A, Agustoni F, et al. Palonosetron plus 1-day dexamethasone for the prevention of nausea and vomiting due to moderately emetogenic chemotherapy: effect of established risk factors on treatment outcome in a phase III trial. J Support Oncol 2012;10:65–71. [DOI] [PubMed] [Google Scholar]
- [25].Koo WH, Ang PT. Role of maintenance oral dexamethasone in prophylaxis of delayed emesis caused by moderately emetogenic chemotherapy. Ann Oncol 1996;7:71–4. [DOI] [PubMed] [Google Scholar]
- [26].Ioannidis JP, Hesketh PJ, Lau J. Contribution of dexamethasone to control of chemotherapy-induced nausea and vomiting: a meta-analysis of randomized evidence. J Clin Oncol 2000;18:3409–22. [DOI] [PubMed] [Google Scholar]
- [27].Sekine I, Segawa Y, Fau-Kubota K, et al. Risk factors of chemotherapy-induced nausea and vomiting: Index for personalized antiemetic prophylaxis. Cancer Science 2013;104:711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hesketh PJ, Aapro M, Fau-Street JC, et al. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Supportive Care in Cancer 2010;18:1171–7. [DOI] [PubMed] [Google Scholar]
- [29].Vardy J, Chiew KS, Galica J, et al. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 2006;94:1011–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Einhorn LH, Rapoport B, Navari RM, et al. 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following multiple-day chemotherapy, high-dose chemotherapy, and breakthrough nausea and vomiting. Support Care Cancer 2017;25:303–8. [DOI] [PubMed] [Google Scholar]
- [31].Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27:v119–33. [DOI] [PubMed] [Google Scholar]
- [32].Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3240–61. [DOI] [PubMed] [Google Scholar]
- [33].Dexamethasone alone or in combination with ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy. N Engl J Med 2000;342:1554–9. [DOI] [PubMed] [Google Scholar]
- [34].Roila F, Warr D, Hesketh PJ, et al. 2016 updated MASCC/ESMO consensus recommendations: Prevention of nausea and vomiting following moderately emetogenic chemotherapy. Support Care Cancer 2017;25:289–94. [DOI] [PubMed] [Google Scholar]
- [35].Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol 2003;21:4112–9. [DOI] [PubMed] [Google Scholar]
- [36].Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 2005;23:2822–30. [DOI] [PubMed] [Google Scholar]


