Abstract
As a modifiable risk factor for cardiovascular disease, presence of hypertension (HT) necessitates the awareness of asymptomatic organ damage (AOD). The aim of this study was to measure plasma micro RNA-21 (miR-21) and the parameters that reflect AOD such as carotid intima-media thickness (CIMT), microalbuminuria (MAU) in hypertensive patients compared with healthy controls. In addition, the aim of this study was to evaluate plasma miR-21 levels in HT patients with AOD.
This study was designed as a cross-sectional observational study. The study includes 2 groups: 32 patients with HT and 32 healthy controls. First, we compared these 2 groups. Then, to underline the relationship between plasma miR-21 and HT, hypertensive patients were divided into 2 groups: with AOD and without AOD.
Sixteen patients with HT had AOD. MiR-21 levels significantly correlated with clinical systolic and diastolic blood pressure, MAU, C-reactive protein, and CIMT. CIMT, miR-21, and MAU levels were significantly higher in patients with AOD.
Our study showed increased miR-21 levels in HT patients with AOD.
Keywords: asymptomatic organ damage, hypertension, miR-21
1. Introduction
As a modifiable risk factor for cardiovascular, cerebrovascular, and renovascular disease, hypertension (HT) plays an important role in the pathogenesis of vascular damage.[1] Several underlying mechanisms have been suggested for the pathogenesis of HT, including over-activation of the renin–angiotensin–aldosterone system, sympathetic nervous system overdrive, endothelial dysfunction, oxidative stress, and impaired angiogenesis; however, the molecular basis of HT's essential development is still being explored.[2]
Individuals with HT often experience endothelial dysfunction, atherosclerotic processes, and target organ damage.[3,4] Asymptomatic (subclinical) organ damage (AOD) occurs in a significant number of asymptomatic and/or un-medicated hypertensive patients.[5] Though micro RNAs (miRNA) have been found to be involved in several biological processes such as differentiation, proliferation, development, migration, and apoptosis,[6] research has only recently focused on the relationship between miRNAs and HT. Moreover, few studies in the literature report the role of micro RNA-21 (miR-21) specifically in HT.[7]
The aim of this study was to measure plasma miR-21 and the parameters that reflect AOD such as carotid intima-media thickness (CIMT), microalbuminuria (MAU) in hypertensive patients compared with healthy controls. In addition, the aim of this study was to evaluate plasma miR-21 levels in HT patients with AOD.
2. Methods
2.1. Informed consent
Protocol for sample collection was approved by the Local Ethics Committee and was carried out according to the requirements of the Second Declaration of Helsinki. All patients were fully informed about study procedures before providing written consent.
2.2. Study population
2.2.1. Study design
This study was designed as a cross-sectional observational study. The study participants included HT patients who attended our outpatient clinic, and age/gender matched healthy volunteers as a control group between September 2017 and December 2017. The inclusion criteria were as follows: stage 1 HT without any comorbidity, taking alpha-blockers (2/32), B bloker (11/32), diuretic (12/32), and diuretic plus B bloker (7/32). The subjects who meet the inclusion criteria did not have any of the following: low density lipoprotein (LDL) > 130 mg/dL, diabetes mellitus, metabolic syndrome, body mass index > 30 kg/m2, smoking, signs or symptoms of atherosclerotic vascular disease, malignancy, connective tissue diseases, endocrine diseases, or alcoholism. The use of drugs that affect atherosclerotic process (e.g., angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers), lipid metabolism (statins, fibrates), and antioxidant drugs within the previous 12 months were among the exclusion criteria. The control group consisted of normotensive healthy individuals.
At the time of recruitment, all the study subjects had physical examination, blood pressure measurement, and carotid ultrasonography. Fasting plasma samples were collected and levels of plasma miR-21 were measured. Serum glucose, creatinine, lipid parameters, C-reactive protein (CRP), and urine albumin excretion, which are the parameters associated with HT, were also evaluated. Subgroup analysis was applied to HT patients for indicating the relationship of miR-21 (HT patients were divided into 2 groups based on the presence of AOD).
HT was defined as office systolic blood pressure (SBP) of ≥ 140 mm Hg and/or diastolic blood pressure (DBP) of ≥ 90 mm Hg according to The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure guideline. Three measurements for a period of 5 days were taken. The average of these measurements was taken as the SBP and DBP.
Power analysis demonstrated that a total of at least 60 patients (30 patients in each group) are required to achieve 80% power at a 2-sided 5% significance level for distinguishing HT patients from healthy individuals.
2.3. Asymptomatic organ damage
AOD inclusion criteria were: CIMT (IMT > 0.9 mm) or plaque, MAU (30–300 mg per 24 hour), chronic kidney disease with estimated glomerular filtration rate (eGFR) 30 to 60 mL/min/1.73 m2 body surface area (BSA), electrocardiographic left ventricular hypertrophy (LVH) (Sokolow–Lyon index > 3.5 mV; RaVL > 1.1 MV; Cornell voltage duration product > 244 mV), and echocardiographic LVH (left ventricular mass (LVM) index: men > 115 g/m2, women > 95 g/m2 (BSA)). The existence of at least one of these parameters indicated AOD.[8]
2.4. Carotid ultrasonography
The extracranial carotid arteries were sonographically investigated using standard protocol. Sonography was performed with a color Doppler ultrasound unit (LOGIQ 9, GE Healthcare, WA) equipped with a 5 to 10 MHz linear transducer. A single radiologist, blind to patient groups, performed and evaluated all ultrasonographic measurements. CIMT greater than 0.9 mm is indicative of atherosclerosis and predictive of an increased risk of cardiovascular disease.[8] The presence of 1 or more CIMT > 0.9 mm in 1 major area (left and/or right common carotid arteries, carotid bulb, internal carotid arteries) was considered as indicative of increased CIMT.
2.5. Biochemical assays
Serum high-sensitive CRP levels were measured using a nephelometric method (Immage 800 Beckman Coulter). Albumin excretion in 24 hours urine samples was measured using Roche Hitachi P800 (Roche, Mannheim, Germany) with an ALBT2 microalbumin kit, and the mean value was calculated as daily albumin excretion. Albumin excretion of ≤ 30 mg per 24 hours was accepted as normoalbuminuria. Creatinine clearance (CrCl) was calculated using the Cockcroft-Gault formula: [CrCl (mL/min per 1.73m2) = [140 − age (years)] × body weight (kg) /(72xCr) × (0.85, if female)].
2.6. Electrocardiographic LVH (Sokolow–Lyon index)
All patients had a standard 12-lead electrocardiogram recorded at 25 mm/s and 1 mV/cm. LVH was defined as a Sokolow–Lyon voltage amplitude of (SV1 + RV5 or RV6) ≥35 mV, a Cornell voltage of (SV3 + RaVL) ≥28 mV for men and ≥20 mV for women, or a Cornell product of [(SV3 + RaVL) × QRS duration] ≥2440 mV ms.[8]
2.7. Echocardiographic LVH (LVM index)
Left ventricular dimensions [interventricular septal diastolic thickness (IVSTd), left ventricular posterior wall diastolic thickness (LVPWTd), and left ventricular end-diastolic diameter (LVDd)] were measured at the end of diastole with M-mode using leading edge to leading edge convention. LVM was calculated according to the Devereux adjusted formula: LVM = 0.8 × 1.04 × [(LVDd + LVPWTd + IVSTd)3 – (LVDd)3] + 0.6 g. LVM index was defined as LVM divided by BSA (LVM/BSA, g/m2) or by height2.7 (LVM/H2.7, g/m2.7). BSA was calculated according to the formula: BSA = 0.6 × height (m) + 0.0128 × weight (kg) − 0.1529. Echocardiographic LVH criteria was determined according to the arterial HT guideline of ESC 2013.[8]
2.8. Plasma collection, ribonucleic acid (RNA) isolation, and measuring level of miR-21
Peripheral blood samples of participants were collected into Vacutainers (with EDTA) in the morning after a 12-hour fasting period. Samples were then centrifuged at 3000 g for 10 minutes. After phase separation, plasma samples free of blood cells were collected and divided into 2 aliquots, snap frozen, and stored at −80°C until RNA isolation. Total RNA from plasma samples was isolated using miRNeasy Mini Kit (Qiagen, Germany) following the manufacturer's protocol. The purities and concentrations of RNA samples were measured spectrophotometrically using NanoDrop ND-2000c (Thermo Fisher Scientific, Inc, Wilmington, DE).
Two μL of total RNA from each sample were reverse transcribed into first strand DNA (cDNA) using “miScript II RT Kit” according to the manufacturer's instructions (Qiagen, Germany). Custom miScript miRNA PCR Array (Qiagen, Germany) was utilized to evaluate the expression level of the miR-21. miRNA expression was normalized to RNU6B. Quantitative RT-PCR was carried out using miScript SYBR Green PCR Kit (Qiagen, Germany) according to the manufacturer's protocol in a Roche LightCycler480-II real-time thermal cycler (Roche, Switzerland). Each experiment was performed in duplicate. The relative quantification analysis was done using the delta-delta-Ct method.[9]
2.9. Statistical analysis
A sample size of n = 29 per group is required to provide 80% power to detect a difference in the mean relative expression levels with a significance of 0.001 (2-sided α). The normal distribution of data was tested by the 1-sample Kolmogorov–Smirnov test. Statistical analyses were performed using SPSS 22.0 software for Windows (SPSS Inc, Chicago, IL). All statistical comparisons were performed using the Student t test. The unpaired t test was also validated using the nonparametric Mann–Whitney U tests. Chi-square tests or Fisher exact tests were applied in the comparison of categorical variables. The values were expressed as mean ± standard deviation (SD) or the median and the interquartile range (IQR, range from the 25th to the 75th percentile). Pearson's correlation was used for numerical data. Spearman's correlation was used for nominal data. To assess the diagnostic accuracy, we performed receiver operating characteristic (ROC) curve analysis. The area under the ROC curve (AUC) was then estimated. P < .05 values were considered statistically significant.
3. Results
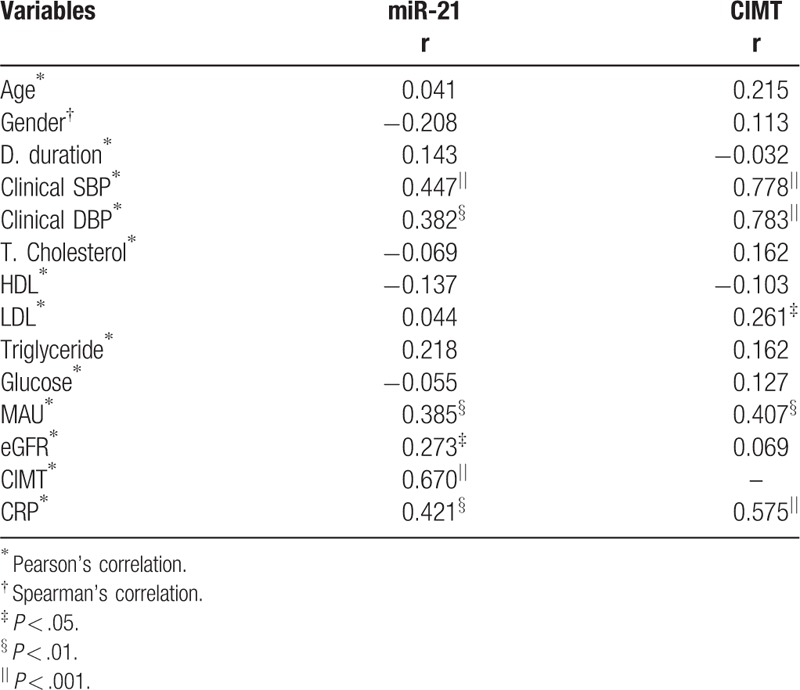
A total of 128 hypertensive patients were screened, and the 32 patients who fulfilled requirements for inclusion and exclusion criteria were included in the study. Thirty-two subjects without HT were enrolled in the control group. The demographic data and laboratory parameters are summarized in Table 1. Age and gender distribution was similar among groups (P > .05, each). The office SBP and DBP levels were significantly higher in hypertensive patients than in the control group (P < .001, both). There were no significant differences in the lipid parameters, glucose levels, and eGFR between the HT and control groups (P > .05, each). CRP, MAU levels, and CIMT measurements were significantly higher in the HT group than in the control group (P < .001, P = .005, and P < .001, respectively). Plasma miR-21 showed increased relative expression in the HT group compared with the control group (P = .001). The results of the correlation analysis are presented in Table 2. Plasma miR-21 level had significant and positive correlation with the office SBP and DBP, MAU, eGFR, CIMT, CRP. CIMT measurements positively correlated with office SBP and DBP, LDL, and MAU (Table 2).
Table 1.
Demographic, clinic, and laboratory characteristics of the HT and control groups.
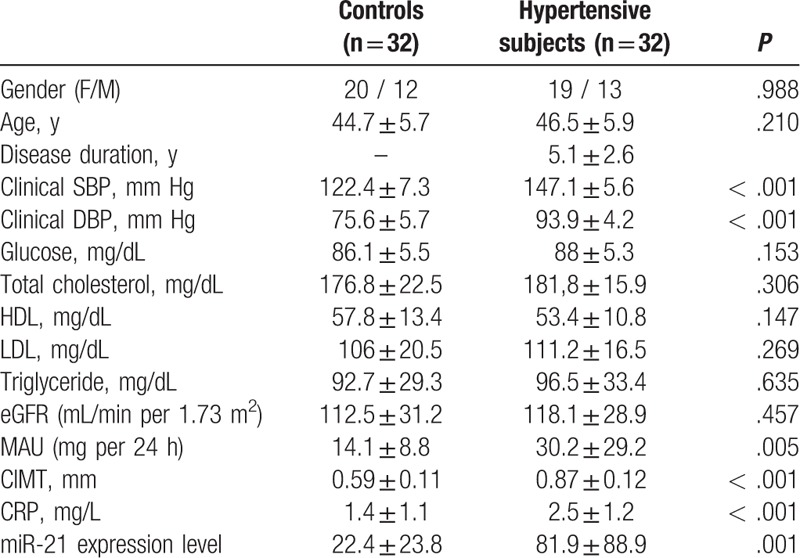
Table 2.
Relationship of miR-21 and CIMT with the other characteristics of patients.
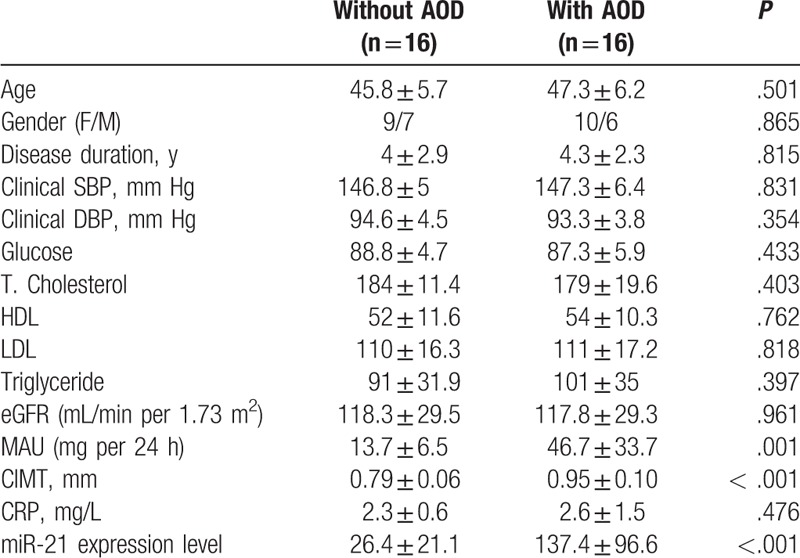
The rate of AOD in hypertensive patients was 50% (16 patients), in the control group, this rate was 6.2% (2 patients) (P < .001) (Table 3). Hypertensive patients were divided into 2 groups based on the presence of AOD: with AOD and without AOD. Table 4 displays characteristics of patients according to the 2 groups. Age and gender distribution, disease duration, clinical SBP and DBP, eGFR, CRP, lipid parameters, glucose did not differ among groups (P > .05, each). However, MAU level and CIMT measurement were significantly higher in the group with AOD than in that without AOD (P = .001, P < .001, respectively). Plasma miR-21 displayed increased relative expression in patients with AOD compared to those without AOD (137.4 ± 96.6 vs 26.4 ± 21.1; P < .001).
Table 3.
Asymptomatic organ damage of the HT and control groups.
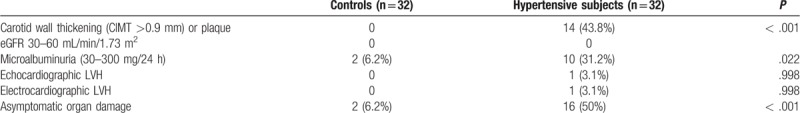
Table 4.
Demographic, clinic, and laboratory characteristics of according to asymptomatic organ damage in the hypertensive group.
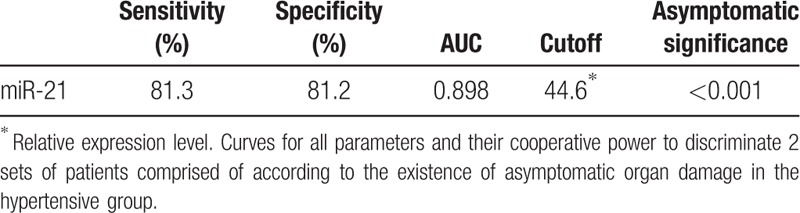
In ROC analysis, the sensitivity of miR-21 was 81.3% and specificity was 81.2% for detecting AOD, AUC was 0.898 and cut-off was 44.6, P < .001 (Table 5).
Table 5.
Sensitivity, specificity, AUC, cutoff, and asymptomatic significance of ROC analysis of parameters.
4. Discussion
In this study, we demonstrated that the level of plasma miR-21 increased in hypertensive patients and was associated with clinical SBP and DBP, MAU, CRP, and CIMT. Plasma miR-21, CIMT, MAU displayed increased relative expression in patients with AOD compared to those without AOD.
Subtle damage to certain organs can be detected in hypertensive patients even before overt clinical events are observed.[10] The 2013 guidelines of the European Society of HT and the European Society of Cardiology (ESH/ESC) emphasize the important role of AOD in determining the cardiovascular risk of patients with HT.[8] Several arguments suggest the search for asymptomatic (or subclinical) organ damage in hypertensive patients to determine the total cardiovascular risk before deciding the treatment strategy. Organ damage has been demonstrated to possess an independent prognostic significance, irrespective of whether it involves the structure and/or function of the heart, brain, kidney, or vessels.[10]
MiRNAs are small, endogenous, noncoding RNA molecules that are approximately 18–22 base pairs long. They induce messenger RNA (mRNA) degradation or their target's translational inhibition through posttranscriptional targeting of mRNAs. They are supposed to regulate the expression of almost 60% of human genes.[6] MiRNAs are proposed as putative biomarkers and therapeutic targets against cardiovascular diseases and HT development. MiR-21 has been reported to play critical roles in angiogenesis, apoptosis, and cardiac and renal fibrosis.[11,12]
Sustained pressure overload of myocardium results in cardiac fibrosis, and miR-21 is overexpressed in response to cardiac stress. Overexpression of miR-21 has been detected in cardiac fibroblasts in failing hearts.[13] Besides, miR-21 level in hypertensive rats was reported to be elevated in comparison with normotensive rats, and exercise training reversed the miR-21 overexpression in hypertensive rats, which indicated that exercise training restored the levels of miR-21 associated with revascularization in HT.[14] In another study, increased miR- 21 level and correlation of its expression with 24-hour DBP were reported in HT patients compared to controls.[6] In our study, we showed that circulating miR-21 is significantly increased in HT patients, and its expression is positively associated with office SBP and DBP levels. Additionally, we found that miR-21 expression is positively associated with CIMT, MAU, eGFR, CRP. Also, according to an experimental study, development of a senescent, arterial, and endothelial cell phenotype featuring induced apoptosis and inflammation could be related to alterations in miRNAs associated with the regulation of these processes.[15]
Although the eGFR levels were similar in both groups, CIMT and MAU were significantly higher in hypertensive patients than healthy controls. There is not strong evidence for this result, as it can be explained by the initiation of the atherosclerotic process in carotid arteries preceding renal involvement. But the current study's results suggest that increased plasma miR-21 expression indicates the presence of atherosclerosis in the early stage of hypertensive patients. The higher level of CRP, which is generally accepted as a biomarker of atherosclerosis, in the hypertensive group with increased CIMT, supports this suggestion.
There were certain limitations of our study. First, the number of study patients was relatively small-sized managed by a single institution and the results need to be supported by larger multicenter studies. Second, we evaluated only 5 parameters for subclinical target organ damage, and third, we could not profile all the miRNAs detected in humans; we only investigated miR-21. Fourth, although there is no difference between glucose levels and lipid parameters among hypertensive patients with AOD and without AOD, antihypertensive drugs (beta-blockers and diuretics) may be effective on glucose levels and lipid parameters. The diuretics may be effective on MAU levels. These effects of used drugs on atherosclerosis could not be excluded. Fifth, these results apply only to HT patients without associated diseases and cannot be generalized to all HT patients. Finally, our study cannot explain causality of the relationship between AOD and other parameters since it was designed as a cross-sectional study.
In conclusion, our study showed increased miR-21 levels in HT patients with AOD. Therefore, its measurement and monitoring might be useful in risk assessment and therapeutic choices in hypertensive patients. However, further and larger studies are required to understand the exact mechanisms underlying these observations in AOD in HT, and confirm these results.
Author contributions
Conceptualization: Erkan Yildirim, Hakan Ucar, Serap Yavuzer, Mahir Cengiz.
Data curation: Erkan Yildirim, Samir Allahverdiyev, Mahir Cengiz.
Formal analysis: Erkan Yildirim, Serap Yavuzer, Mahir Cengiz.
Investigation: Erkan Yildirim, Serap Yavuzer, Mahir Cengiz.
Methodology: Erkan Yildirim, Emrah Ermis.
Software: Erkan Yildirim, Samir Allahverdiyev.
Supervision: Emrah Ermis, Samir Allahverdiyev, Hakan Ucar, Serap Yavuzer, Mahir Cengiz.
Visualization: Erkan Yildirim, Hakan Ucar, Mahir Cengiz.
Writing – original draft: Erkan Yildirim, Mahir Cengiz.
Writing – review & editing: Hakan Ucar, Serap Yavuzer, Mahir Cengiz.
Footnotes
Abbreviations: AOD = asymptomatic organ damage, AUC = area under curve, BSA = body surface area, CIMT = carotid intima–media thickness, CrCI = creatinine clearance, CRP = C-reactive protein, D = disease, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, F = female, HDL = high-density lipoprotein, HT = hypertension, IVSTd = interventricular septal diastolic thickness, LDL = low-density lipoprotein, LVDd = left ventricular end-diastolic diameter, LVH = left ventricular hypertrophy, LVM = left ventricular mass, LVPWTd = left ventricular posterior wall diastolic thickness, M = male, MAU = microalbuminuria, miR-21 = micro RNA-21, mRNA = messenger RNA, RNA = ribonucleic acid, ROC = receiver operating characteristic, SBP = systolic blood pressure, T = total.
How to cite this article: Yildirim E, Ermis E, Allahverdiyev S, Ucar H, Yavuzer S, Cengiz M. Circulating miR-21 levels in hypertensive patients with asymptomatic organ damage. Medicine. 2019;98:39(e17297).
The authors have no conflicts of interest to disclose.
References
- [1].Kearney PM, Whelton M, Reynolds K, et al. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004;22:11–9. [DOI] [PubMed] [Google Scholar]
- [2].Perlini S, Grassi G. Hypertension-related target organ damage: is it a continuum? J Hypertens 2013;31:1083–5. [DOI] [PubMed] [Google Scholar]
- [3].Yavuzer S, Yavuzer H, Cengiz M, et al. Endothelial damage in white coat hypertension: role of lectin-like oxidized low-density lipoprotein-1. J Hum Hypertens 2014;29:92–8. [DOI] [PubMed] [Google Scholar]
- [4].Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens 2011;24:52–8. [DOI] [PubMed] [Google Scholar]
- [5].Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc 2013;2:87–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cengiz M, Karatas OF, Koparir E, et al. Differential expression of hypertension-associated MicroRNAs in the plasma of patients with white coat hypertension. Medicine (Baltimore) 2015;94:e693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cengiz M, Yavuzer S, Kiliçkiran Avci, et al. Circulating miR-21 and eNOS in subclinical atherosclerosis in patients with hypertension. Clin Exp Hypertens 2015;37:643–9. [DOI] [PubMed] [Google Scholar]
- [8].Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2014;23:3–16. [DOI] [PubMed] [Google Scholar]
- [9].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- [10].Shlomai G, Grassi G, Grossman E, et al. Assessment of target organ damage in the evaluation and follow-up of hypertensive patients: where do we stand? J Clin Hypertens (Greenwich) 2013;15:742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mehta JL. Oxidized or native low-density lipoprotein cholesterol: which is more important in atherogenesis? J Am Coll Cardiol 2006;48:980–2. [DOI] [PubMed] [Google Scholar]
- [12].Asada S, Takahashi T, Isodono K, et al. Downregulation of Dicer expression by serum withdrawal sensitizes human endothelial cells to apoptosis. Am J Physiol Heart Circ Physiol 2008;295:2512–21. [DOI] [PubMed] [Google Scholar]
- [13].Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980–4. [DOI] [PubMed] [Google Scholar]
- [14].Fernandes T, Magalhães FC, Roque FR, et al. Exercise training prevents the microvascular rarefaction in hypertension balancing angiogenic and apoptotic factors: role of microRNAs-16, -21, and -126. Hypertension 2012;59:513–20. [DOI] [PubMed] [Google Scholar]
- [15].Ji R, Cheng Y, Yue J, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res 2007;100:1579–88. [DOI] [PubMed] [Google Scholar]


