Abstract
Background:
The aim of this study was to observe the effect and safety of Heyan Kuntai Capsule (HYKT) on glucose and lipid metabolism in patients with polycystic ovary syndrome (PCOS).
Methods:
Hundred patients with PCOS were randomly divided into HYKT group (n = 50) and placebo groups (n = 50) in which the individuals were treated with HYKT and its placebo continuously for 6 months. Meanwhile, all participants received health education (such as exercise and diet). The primary outcomes were serum sex hormone levels, a series of blood lipid, fasting and postprandial 2 hours blood glucose. Body mass index (BMI), waist–hip ratio (WHR), insulin, homeostatic model assessment of insulin resistance (HOMA-IR), and insulin-sensitive index (ISI) were also observed. In addition, adverse events were recorded to evaluate the drug safety.
Results:
After treatment, the BMI and WHR of all the patients were decreased. The fasting and postprandial 2 hours blood glucose levels were significantly declined when treated with HYKT, which were not observed in the placebo group. Similarly, serum sex hormones including luteinizing hormone (LH), LH/follicle-stimulating hormone (FSH), and testosterone were lowered after treated with HYKT instead of the placebo. Besides, blood lipids outcomes such as total cholesterol, triglyceride, and low-density lipoprotein cholesterol, as well as insulin and HOMA-IR were decreased with significance in HYKT group when compared with those in the placebo group, whereas high-density lipoprotein cholesterol and ISI increased obviously.
Conclusion:
HYKT showed the effect on ameliorating the glucose and lipid metabolism disorder and improving insulin resistance and increase insulin sensitivity of PCOS patients, which is similar to insulin sensitizing agent.
Keywords: extension of curative effect, glucose and lipid metabolism, insulin resistance, kuntai capsule, polycystic ovary syndrome
1. Introduction
Polycystic ovary syndrome (PCOS) is a common disease occurred to the women worldwide in their fertile ages, characterized by menstrual dysfunction, androgen excess, and polycystic ovaries, leading to anovulatory subfertility, obesity, and insulin resistance. At present, PCOS is more likely to be considered as the result of genetic and environmental factors,[1] although the exact pathogenesis remains unknown.
Nowadays, the primary treatments for PCOS are lifestyle changes and medications, aiming to control the weight, regulate menstrual cycle, reduce serum androgen level, ameliorate insulin resistance, and induce ovulation. However, because of the short persistence time and side effects, the treatment should not be used for a long time and patients are difficult to accept.[2] In China, Traditional Chinese Medicine (TCM), characterized by holism and treatment based on pattern identification, has the great potential in this disease. One of the Chinese patented medicine Heyan Kuntai capsule (HYKT) consists of prepared Dihuang (Radix Rehmanniae), Huanglian (Rhizoma Coptidis), Baishao (Radix Paeoniae Alba), Huangqin (Radix Scutellariae Baicalensis), Ejiao (Colla Corii Asini), and Fuling (Poria). In this context, we carried out this double-blinded, randomized, placebo-controlled clinical trial to determine the role of HYKT in the treatment of PCOS and to evaluate its efficacy on insulin resistance.
2. Materials and methods
2.1. Ethics
This study was approved by medical ethics committee of affiliated hospital of Jiangxi university of Traditional Chinese Medicine. The informed consent was obtained from all included patients.
2.2. General information
Hundred subjects of PCOS patients were collected in the second affiliated hospital of Jiangxi University of Traditional Chinese Medicine or Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine from September 2014 to November 2015. When the participants were enrolled, health education, such as aerobic exercise, and low-fat diet, were carried out in both 2 groups. Based on safety considerations, patients (n = 28), who did not have a dual-phase basal body temperature in consecutive 3 months or sustained high temperature in 7∼8 days with the progesterone <3 ng/mL, were orally given progesterone 200 mg/2 capsules 1 day, for continuous 6 days (Progesterone Soft Capsules, alias LaiTing, xinchang pharmaceutical factory of Zhejiang pharmaceutical Co. Ltd., Zhunzi H20040982, batch number: 140605.) to draw blood.
2.3. Diagnostic criteria
Based on the revision of the European Society for reproductive and embryo medicine and the American Society for reproductive medicine in 2003 proposed the Rotterdam standard[3] diagnosis of PCOS: oligomenorrhea or amenorrhoea; transvaginal ultrasound indicated unilateral or bilateral ovarian antral follicle which diameter is 2–9 mm count greater than 12 and (or) ovarian volume is more than 10 mL; clinical or biochemical hyperandrogenism. Two of the three criteria meet and other causes of hyperandrogen are excluded.
2.4. Inclusion criteria
Subjects were diagnosed with PCOS by Rotterdam criteria[3] and untreated with any drug within the last 3 months. In addition, the enrolled participants were willing to accept the method of treatment and actively cooperate with the visits and complete the clinical observation.
Based on the Guiding Principles of Clinical Research on the Traditional Chinese Medicine to Treat Irregular Menstruation,[4] eligible patients should also have the following symptoms, all of which are the indications of Kuntai capsules: delayed menstrual cycle, less menstruation or amenorrhea, or menstrual cycle disorder, long marriage sterile; red tongue body; thready rapid pulse; facial acne, fleshy, and hairy; feverish sensation over the palm and sole and tidal reddening of the face; soreness and weakness of waist and knees, dizziness, and tinnitus; or thirsty and constipation.
2.5. Exclusion criteria
The participants were excluded when they met the following criteria: combined with hyperprolactinemia, atypical congenital adrenal hyperplasia, thyroid gland disease, Cushing syndrome, tumor-secreting androgen, diabetes, or other diseases causing endocrine disorders; hormones or other drugs have been used in the past 3 months, including Chinese medicinal formulae and Chinese patent drug which may affect the results; psychiatric patients; people who were allergic to this drug; the duration of treatment was <6 months or incomplete information which would affect the efficacy of judgment; did not agree to sign the informed consent form; in the last 6 weeks had pregnancy history, abortion or childbearing; had a history of breastfeeding in the last 6 months; patients with other special diseases could not tolerate or adhere to the test.
2.6. Study drugs and methods
HYKY and its placebo capsules were provided by the Guiyang Xintian pharmaceutical Co. Ltd. The lot number was 140201. All enrolled participants were randomly divided as 1:1 into 2 groups, HYKT and placebo group. In each group, 50 PCOS patients were orally taken HYKT and placebo, respectively, 3 times a day, 4 capsules each time, continuous medication for 6 months.
2.7. Observation indexes and measurements
Physical examination, that is height, weight, waist and hip circumference were measured accurately to within 0.01 m, 0.01 kg, and 0.1 cm. Waist circumference was the perimeter of the waist. Hip circumference was the largest meridian of hip. The subjects were weighted with light clothes and no shoes.
2.8. Tests for hormone levels
Patients diagnosed with PCOS may receive blood samples on the same day or the next day if they have no developmental follicles. If the ultrasound shows signs of developmental follicles, blood samples may be taken from day 3 to day 5 of the menstrual cycle (natural menstrual cycle or progesterone blood draw). Testosterone (T), luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol (E2) and prolactin (PRL) levels were measured.
2.9. Insulin resistance index
Homeostatic model assessment of insulin resistance (HOMA-IR) is closely correlated with fasting insulin (FINS) and fasting blood glucose (FBG), which are evaluated by the formula as the following: HOMA-IR = (FBG × FINS)/22.5). PCOS patients were diagnosed as having insulin resistance by defining that HOMA-IR is ≥2.69 according to the national diabetes cooperation survey data, >4 percentile on China people's HOMA-IR.[5]
2.10. Tests for Glucose metabolism indexes
Glucose tolerance test (OGTT) and insulin release test were performed. Patirnts were instructed to fast for 8 hours or more. Fasting insulin and fasting blood glucose were measured in fasting blood samples at 8:00 next morning. Then patients immediately took 75 g of glucose orally and blood was collected 120 minutes after glucose loading. The blood insulin and the blood glucose levels were determined by chemiluminescence. insulin resistance index was calculated as (HOMA-IR) = (FBG × FINS)/22.5 and insulin sensitivity index (ISI) = 1/(FINS × FBG).
2.11. Test for fasting lipids
Total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured and evaluated.
2.12. Safety indexes
Blood and urine routine test; electrocardiograph (ECG); liver and renal functions (alanine amino shift enzyme, aspartate aminotransferase, total bilirubin, blood urea nitrogen, creatinine) inspection; recorded the systemic and local adverse reactions and side effects.
2.13. Statistical analysis
All data were analyzed using SPSS 20.0 (SPSS Inc, Chicago, IL). Quantitative variables were presented as mean ± standard deviation ( ± s). Paired sample t tests were performed to compare measurement data before and after treatment. An independent t test was used to compare measurement data between the 2 groups. The rank test was applied to non-normal distribution, variance, and rank data. Qualitative variables were presented as cases or proportions. The χ2 test was performed to compare data between the 2 groups. All statistical tests were 2-sided and significant level was ɑ = 0.05. P < .05 indicated a significant difference.
± s). Paired sample t tests were performed to compare measurement data before and after treatment. An independent t test was used to compare measurement data between the 2 groups. The rank test was applied to non-normal distribution, variance, and rank data. Qualitative variables were presented as cases or proportions. The χ2 test was performed to compare data between the 2 groups. All statistical tests were 2-sided and significant level was ɑ = 0.05. P < .05 indicated a significant difference.
3. Results
3.1. General information
A total of 95 subjects were 18- to 33 years’ old (mean age: 24.69 ± 3.55). The menarche age ranged from 11 to 16 (13.83 ± 1.16) years. Body weight was 46 to 77 kg (60.49 ± 6.96). The average age of participants in HYKT group was 24.67 ± 3.92 years, average menarche age was 13.85 ± 1.20 years, and average body mass index (BMI) was 23.30 ± 2.26 kg/m2, whereas the average age of those in placebo group was 24.72 ± 3.18 years, average menarche age was 13.81 ± 1.14 years, and average BMI was 23.28 ± 1.84 kg/m2. The general information between the 2 groups was comparable (P > 0.05). During the period of observation, 2 cases in HYKT group and 3 cases in the placebo group were lost.
3.2. The effect of HYKT on the clinical metabolic signs and sex hormone levels
As shown in Table 1, the BMI and waist–hip ratio (WHR) in both groups were decreased obviously after the treatment when compared with those before treatment (P < .05). Serum sex hormones including LH, LH/FSH and T levels were declined in HYKT group significantly (P < .05) which was not observed in the placebo group (P > 0.05). when compared with the placebo group, the BMI, WHR, LH, LH/FSH, and T were significantly improved in HYKT group (P < .05).
Table 1.
The comparison of BMI, WHR, LH, LH/FSH, T in HYKT, and placebo groups (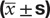 ± s).
± s).
3.3. The effect of HYKT on the blood lipid metabolism indexes
The TC, TG, LDL-C levels in both groups were decreased obviously compared with those in the pretreatment (P < .05), whereas the HDL-C was increased (P < .05). Compared with the placebo group, the TC, TG, LDL-C, and HDL-C were better with significance after treated with HYKT (P < .05, Table 2).
Table 2.
The comparison of TC, TG, HDL-C, LDL-C in both groups (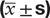 ± s).
± s).
3.4. The effect of HYKT on the glucose metabolism
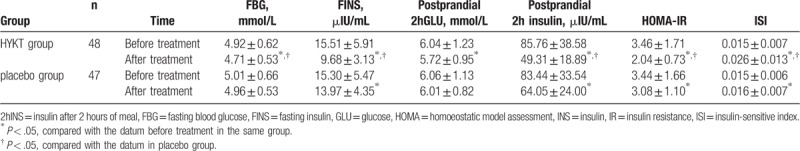
As illustrated in Table 3, the FINS, insulin after 2 hours of meal (2hINS), and HOMA-IR levels in both groups were decreased (P < .05), whereas ISI increased compared with pretreatment (P < .05). The FBG and 2hGLU levels were declined obviously after treated with HYKT (P < .05), which were not observed in the placebo group (P > .05). After treatment, the FBG, FINS, 2hINS, and HOMA-IR in Kuntai group were better than those in placebo group (P < .05). However, no significant difference was detected in 2hGLU between HYKT and the placebo groups P > .05).
Table 3.
The comparison of FBG, FINS, 2hGLU, 2hINS, HOMA-IR, and ISI in both groups (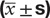 ± s).
± s).
3.5. Comparison of IR between 2 groups
Before treatment, the total incidence of IR was 78.95% (75/95), in which 77.08% (37/48) and 80.85% (38/47) occurred in HYKT and placebo group, respectively. After 2 courses of treatment (6 months), the total incidence of IR reduced to 46.32% (44/95) among all subjects, in which the incidence of IR in HYKT group was 22.92% (11/48) whereas that in the placebo group remained to 70.21% (33/47).
3.6. The missed follow-up rate and adverse reaction rates
This study included 100 subjects and 5 cases were lost during the research. The missed follow-up rate was 5%. A total of adverse events rate was 11.58% (11/95), in which 3.16% (3/48) and 8.42% (8/47) occurred in HYKT and the placebo groups, showing that fewer adverse events were found in HYKT group than that in the placebo group.
4. Discussion
The results of this study showed that HYKT can effectively ameliorate the insulin resistance by reducing the fasting and postprandial insulin levels, as well as improving HOMA-IR and ISI. Besides, HYKT can directly improve abnormal glucose and lipid metabolism in patients with PCOS by decreasing serum total cholesterol, triglyceride, and low-density lipoprotein, as well as increasing the high-density lipoprotein. At the same time, with the gradual balance of insulin levels, fasting and postprandial blood glucose also gradually stabilized.
In addition to the high blood fat problem, BMI, WHR, and other indicators had been also improved. It is found that the LH, LH/FSH, T, and other gonadal hormone levels can be reduced by HYKT capsule, although HYKT had no obvious estrogen-like effects.[6] Instead, the results of this study indicated a potential role of HYKT in the metabolism of glucose and lipid.
PCOS is a multisystem disease, and IR is the central link of PCOS pathological and physiological processes.[7] Insulin, binding to insulin receptor, through the cross-membrane transfer of the signal, participates in normal follicular development to regulate ovarian function. If insulin in the blood circulation is reduced, the follicle cells which have the function of encapsulating the follicle, producing androgen and transforming androgen into estrogen, will reduce the production of androgen because of lacking high insulin stimulation.[8] It will improve the level of gonadal hormone in PCOS. Therefore, adjusting the hyperinsulinemia and improving IR in PCOS patients is the key to treat infertility caused by PCOS.[8] But the disease is complex and diverse and there are still many problems that cannot be solved. So further researches are needed in the future.
HYKT capsule, a Chinese patented drug, is widely used to treat the menopausal syndrome. Huanglian in this formula has similar effects of berberine component of Rhizoma Coptidis. The study of Wu et al[9] also pointed out that berberine (berberine hydrochloride) has the function of increasing insulin sensitivity, meanwhile it can reduce body weight and fat. By reducing the lipid toxicity of free fatty acid and the deposition of the tissue triacylglycerol, berberine can reduce the damage of islet beta cells, improve islet function, and promote insulin secretion to decrease blood glucose. Low-density cholesterol receptors in liver cells can also be regulated by berberine. And it can effectively reduce blood fat, which can improve the endocrine and metabolic disturbance in PCOS women[10,11] and significantly improve insulin resistance in women with PCOS.[12] Huanglian is also commonly used in the treatment of diabetes. Moreover, acidic polysaccharides in Fuling can improve the disorder of serum cholesterol metabolism and cytokines level,[13] and triterpenoid in Fuling can accelerate the process of adipocyte differentiation,[14] which plays the role of insulin sensitizer in cells.[15] Above all, the whole mechanism of HYKT mutually fits with the pathogenesis characteristics of PCOS.
Modern medical supports that IR is the core of PCOS pathogenesis. In our study, the incidence of IR was 78.95%, which is similar to the recognized results of previous studies. In the early period of PCOS, the main problems were reproductive disorder, such as menstrual disorder, infertility, threatened abortion, premature birth, and so on. However, the development of the PCOS in the late period will be particularly concerned about the metabolic disease. The clinical diversity and complexity of PCOS bring modern women many negative effects, including the reproductive and endocrine aspects and a number of long-term complications caused by metabolic disorders, which are concerned by both medical workers and patients.
According to the early clinical research by Liang et al,[16,17] PCOS patients mostly have the symptoms, which can be effectively improved by taking HYKT. In this study, patients with PCOS in placebo group also have a certain improvement in the IR and lipid metabolism. This may have a positive correlation with the unified health education when patients enrolled. All subjects were asked to have regular aerobic exercise and dietary structure optimization (such as fasting visceral, high lipid food, and so on), which helped PCOS patients improve the metabolic index. In this study, the statistical safety examination and adverse events showed that the patients in Kuntai group had occasional mild abdominal distension but could be tolerated and be able to ease, which showed that HYKT was safe having no obvious side effects.
In conclusion, HYKT has similar effect to insulin sensitizing agent, which can significantly improve insulin resistance and increase insulin sensitivity in patients with PCOS and effectively regulate the glucose and lipid metabolism disorder. Moreover, HYKT is safe and convenient to patients. It is interesting to treat the metabolism of PCOS by Traditional Chinese Medicine Kutai capsule.
Author contributions
Conceptualization: Ruining Liang, Zhen Liu, Xuemei Peng.
Data curation: Zhen Liu, Pei Fan.
Formal analysis: Ruining Liang, Peishuang Li.
Funding acquisition: Ruining Liang.
Investigation: Zhen Liu.
Methodology: Zhen Liu, Peishuang Li, Pei Fan, Ling Xu, Meinan Zhang.
Resources: Peishuang Li, Xueyan Sun.
Software: Peishuang Li, Pei Fan, Ling Xu, Meinan Zhang.
Supervision: Ruining Liang.
Validation: Xueyan Sun, Jiahua Peng, Xuemei Peng.
Visualization: Jiahua Peng, Xuemei Peng.
Writing – original draft: Zhen Liu.
Writing – review & editing: Ruining Liang.
Footnotes
Abbreviations: BMI = Body mass index, E2 = estradiol, ECG = electrocardiograph, FBG = fasting blood glucose, FINS = fasting insulin, FSH = follicle stimulating hormone, GLU = glucose, HDL-C = high-density lipoprotein cholesterol, HOMA = homoeostatic model assessment, HYKT = Heyan Kuntai Capsule, INS = insulin, IR = insulin resistance, ISI = insulin-sensitive index, LDL-C = low-density lipoprotein cholesterol, LH = luteinizing hormone, OGTT = glucose tolerance test, PCOS = polycystic ovary syndrome, PRL = prolactin, T = testosterone, TC = total cholesterol, TCM = Traditional Chinese Medicine, TG = triglyceride, WHR = waist–hip ratio.
How to cite this article: Liang R, Liu Z, Li P, Fan P, Xu L, Sun X, Peng J, Peng X, Zhang M. Kuntai capsules improve glucolipid metabolism in patients with polycystic ovary syndrome (PCOS). Medicine. 2019;98:39(e16788).
RL and ZL contributed equally to this work.
The authors report no conflicts of interest.
This study is supported by National Natural Science Foundation of China (No. 81560783) and Jiangxi province science and technology plan projects (No. 20141BBG70056).
References
- [1].Xie X, Gou WL. Obstetrics and Gynecology[M]. 2013;Beijing: People's Medical Publishing House, 359-361. [Google Scholar]
- [2].Zhang J, Xue H, Su J, et al. The clinical observation of the effect of tanshinone on the treatment of polycystic ovary syndrome with hyperandrogenism[J]. Guangxi Medicine 2015;37:767–9. [Google Scholar]
- [3].Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS)[J]. Hum Reprod 2004;19:41–7. [DOI] [PubMed] [Google Scholar]
- [4].Zheng X. The guiding principle of clinical research on new drugs of traditional Chinese medicine (for Trial Implementation)[S]. 2002;Beijing: The Medicine Science and Technology Press of China, 240–241. [Google Scholar]
- [5].Yang W, Yang Z, Li G, et al. With combination of waist to hip ratio (or waist circumference) and blood pressure can predict metabolic syndrome[J]. Chinese Journal of Endocrinology and Metabolism 2005;21:227. [Google Scholar]
- [6].Chen J, Gao H, Li Q, et al. Clinical observation of the KunTai capsule treat 30 cases patients with menopausal syndrome[J]. Journal of Traditional Chinese Medicine 2015;56:231–4. [Google Scholar]
- [7].Wu X. Polycystic ovary syndrome: a primary follicular disorder[J]. Chinese Journal of Practical Gynecology and Obstetrics 2007;23:660–3. [Google Scholar]
- [8].Qi B, Hao S, Hou L, et al. Polycystic ovary syndrome-insulin resistance and the intervention of traditional Chinese medicine[J]. Journal of Traditional Chinese Medicine 2011;52:656–8. [Google Scholar]
- [9].Li L, Li C, Pan P, et al. A single arm pilot study of effects of berberine on the menstrual pattern, ovulation rate, hormonal and metabolic profiles in anovulatory Chinese women with polycystic ovary syndrome[J]. PLoS One 2015;10:e0144072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wei W, Zhao H, Wang A, et al. A clinical study on the short-term effect of berberine in comparison to metformin on the metabolic characteristics of women with polycystic ovary syndrome[J]. Eur J Endocrinol 2012;166:99–105. [DOI] [PubMed] [Google Scholar]
- [11].An Y, Sun Z, Zhang Y, et al. The use of berberine for women with polycystic ovary syndrome undergoing IVF treatment[J]. Clin Endocrinol 2014;80:425–31. [DOI] [PubMed] [Google Scholar]
- [12].Li Y, Ma H, Zhang Y, et al. Effect of berberine on insul in resistance in women with polycystic ovary syndrome: study protocol for a randomized multicenter controlled Trial [J]. Biomed Central Trials 2013;14:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Deng Y. Extraction Process and Pharmacodynamic Studies of poria cocos acidic polysaccharides[D]. Wuhan: Hubei University of traditional Chinese medicine; 2012. [Google Scholar]
- [14].Li TH, Hou CC, Chang CL, et al. Anti-hyperglycemic properties of crude extract and triterpenes from poria cocos[J]. Evid Based Complement Alternat Med 2011;11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ato MS, Ai TT, Unoura YN, et al. Dehydro tramet enolic acid induces preadipocyte differentiation and sensitizes animal models of noninsulin-dependent diabetes mellitus to insulin[J]. Biol Pharm Bull 2002;25:81–6. [DOI] [PubMed] [Google Scholar]
- [16].Liang R, Wu X. Slightly analysis of TCM etiology and pathogenesis of polycystic ovary syndrome[J]. Jiangxi Chinese Medicine 2012;43:3–5. [Google Scholar]
- [17].Liang R, Zhao ML, Li P, et al. Clinical observation of Nourishing Yin, clearing heat, removing blood stasis and dampness treatment on the kidney yin deficiency type of polycystic ovary syndrome[J]. Chin Matern Child Health Care 2014;29:4215–7. [Google Scholar]


