Abstract
To investigate the clinicopathological features and prognostic impact of Fusobacterium nucleatum (F nucleatum) status in patients with colorectal cancer (CRC) and its relationships with microsatellite instability (MSI) status.
Retrospective analysis of consecutive 91 CRC tissues from surgically resected specimens of stage III or high-risk stage II CRC patients who had received curative surgery in Wuhan Union Hospital from January, 2017 to January, 2019 was conducted. F nucleatum DNA was quantitatively measured and classified into 1 of the 2 categories: F nucleatum-high, or F nucleatum-low/negative. The Cox risk ratio model analysis was performed to identify independent risk factors of F nucleatum. F nucleatum-high group was compared with the F nucleatum-low/negative group with respect to clinicopathological features and their relationships with MSI status. Kaplan–Meier method and log-rank test were used for univariate analysis of prognostic factors in patients with CRC.
The number of total lymph node acquisition and positive lymph nodes, neurological invasion, vascular tumor thrombus were higher in F nucleatum-high group (27.44 ± 25.213 vs 20.70 ± 10.141; P = .018; 3.80 ± 7.974 vs 1.74 ± 3.531; P = .001; 68.0% vs 33.3%; P = .003; 60.0% vs 25.8%; P = .002). Moreover, microsatellite mutations were more frequent in patients with F nucleatum-high (84.0% vs 60.6%; P = .034). A higher abundance of F nucleatum in CRC is associated with a shorter survival time. The F nucleatum status, peripheral nerve invasion, vascular tumor thrombus, lymph node metastasis, and TNM staging were related factors affecting the prognosis of patients with CRC. The Cox risk ratio model analysis showed that the F nucleatum (odds ratio [OR] 2.094, 95% confidence interval [CI] 1.178–8.122, P = .032) and MSI status (OR 2.243, 95% CI 1.136–5.865, P = 0.039) were independent prognostic factors.
Intratumoral F nucleatum load has a poor prognostic effect of CRC by increasing nerve invasion, vascular tumor thrombus, and microsatellite mutation.
Keywords: colorectal cancer, Fusobacterium nucleatum, prognosis
1. Introduction
Colorectal cancer (CRC) is the third most common cancer in the world and ranks as 1 of the 5 most common fatal cancers worldwide.[1] Increasing evidence indicates that gut microbiota play various roles in carcinogenesis, prognosis, and treatment response of CRC.[2,3] Among these gut microbiota, Fusobacterium nucleatum has been found to be significantly elevated in tumor tissues and stool specimens of CRC patients relative to those in normal controls.[4,5] In addition, the amount of invasive F nucleatum gradually increases from premalignant adenomatous lesions to carcinomas in the colorectal carcinogenesis pathway.[6,7] There have been many studies investigating the mechanism of F nucleatum instigating and potentiating colorectal tumor by using tumor tissues. It has been reported that the accumulation of F nucleatum in colorectal tumor is partly due to fusobacterial lectin Fap 2[8] and Fad A adhesion,[2] and that F nucleatum can induce the carcinogenesis and development of colorectal tumor by the microRNA-21-mediated pathway[9] or inhibition of host adaptive immunity,[10,11] and so on.
However, there were also some studies producing conflicting results.[12,13] The diagnostic and prognostic characteristics of F nucleatum for CRC have been ambiguous, with sensitivity ranging from 45% to 100%, and specificity ranging from 10% to 92%.[14,15] To explore the effect of F nucleatum load on prognosis of patients with CRC, we designed a study to investigate the relationships between F nucleatum, and clinicopathological features and molecular factors (including kirsten rat sarcoma viral oncogene (KRAS), v-raf murine sarcoma viral oncogene homolog B1 (BRAF), and microsatellite instability [MSI]).
2. Materials and methods
2.1. Clinical and pathological data
From January, 2017 to January, 2019, 91 patients with stage III or high-risk stage II CRC were subjected to thorough history taking and clinical examination, and their clinical and pathological information was collected from the database of Wuhan Union Hospital in China. Age, sex, tumor location, and gross tumor type, pT/pN categories, tumor grade, lymphovascular invasion, perineural invasion were identified from the patient medical reports. This study protocol was approved by the ethics committee of our college. All patients signed an informed consent regarding their understanding of the procedure and its potential complications, and also their approval of participation in the research. All participating subjects were fully informed of the study and the associated risks before signing an informed consent form, and the principles outlined by the Declaration of Helsinki (2013) were adopted in this study.
2.2. Inclusion and exclusion criteria
The inclusion criteria for the case selection were age greater than 18 years, adenocarcinoma histology without neuroendocrine or squamous cell component, stage III or high-risk stage II according to pathological staging, complete resection (R0) of the primary tumor with tumor-free resection margins, and the completion of at least 6 cycles of adjuvant Calcium Folinate Fluorouracil OXaliplatin chemotherapy or 4 cycles of adjuvant Capecitabine and Oxaliplatin therapy. The criteria for high-risk stage II were tumor invasion into visceral peritoneum or direct invasion into adjacent organ structures (pT4), clinical obstruction or perforation, poorly differentiated or undifferentiated histology (G3/G4), lymphovascular invasion, and perineural invasion. The patients who received preoperative neoadjuvant chemotherapy and/or radiotherapy (especially patients with rectal cancer) and patients with a history of other malignancy within 5 years were excluded.
2.3. DNA extraction and mutation analysis
Tumor DNAs were extracted from formalin-fixed, paraffin-embedded (FFPE) tissues containing more than 50% tumor cells using the QIAamp DNA Mini Kit (Qiagen). Detection of KRAS exon 2 hotspot mutations and BRAF V600E mutations was performed.
2.4. Quantitative PCR for F nucleatum
Genomic DNA extraction from FFPE tissues of the 91 CRCs and quantitative polymerase chain reaction (qPCR) for F nucleatum, using a commercial QIAmp DNA Mini Kit (QIAGEN, Hilden, Germany), according to the manufacturer's instructions. DNA was stored at −80°C, until use. DNA amplification was performed in a final volume of 20 μL (SYBR Premix EX Taq II [2X] [TLi RNaseH Plus]), 20 pmol of forward and reverse primers, and 2 μL of extracted DNA with ABI 7500 (Applied Biosystem). The thermo cycling program was as follows: 35 cycles of 95°C for 5 seconds and 60°C for 34 seconds with an initial cycle of 95°C for 10 minutes, and a primer pair-specific annealing temperature for 60 seconds. A melting curve was used to evaluate the presence of primers-dimers. In brief, the following primers and probes targeting the 16S rRNA gene DNA sequence of F nucleatum and the reference gene (prostaglandin transporter [PGT]) were used: F nucleatum forward primer, 5’-CAACCATTACTTTAACTCTACCATG-TTCA-3’; F nucleatum reverse’ primer, 5’-GTTGACTTTACAG-AAGGAGATTATGTAAAAATC-3’; F nucleatum FAM probe, 5’-GTTGACTTTACAGAAGGAGATTA-3’; PGT forward primer, 5’-ATCCCC AAAGCACCTGGTT-T-3’; PGT reverse primer, 5’-AGAGGCCAAGATAGTCCTGGTAA-3’; PGT VIC probe, 5’-CCATCCATGTC-CTCATCTC-3’. The PCR conditions were 95°C for 10 minutes followed by 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute. To compare the F nucleatum DNA amounts between tumor DNA samples, the relative values (2−ΔCt) calculated from the threshold cycle (Ct) values for F nucleatum normalized to PGT were used. The qPCR method was validated using serially-diluted F nucleatum genomic DNA samples (25586D-5; ATCC, Manassas, VA). F nucleatum-positive CRCs were further classified into 2 subgroups (F nucleatum-high or F nucleatum-low) using a cut-off median value of 2−ΔCt.
2.5. Microsatellite status determination
Mismatch repair (MMR) tumor status was determined by immunohistochemistry (IHC) or by MSI testing when IHC was indeterminate in accordance of the Bethesda criteria and as previously described for each trial.[16,17] MSI phenotype tumors were defined as presenting with the loss of 1 or more MMR proteins’ expression by IHC or exhibiting high-level tumor DNA MSI (MSI-H) on MSI testing. Microsatellite-stable phenotype tumors were defined by normal MMR protein expression in IHC, or MSS or low-level MSI (MSI-L) status on MSI testing.
2.6. Statistical analysis
All data were analyzed using the statistical package SPSS version 23.0. All measurement data were represented as (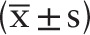 ± s). The paired t test was used in the group, and the analysis of variance was used in the group. The count data were expressed in percentage (%), and the data were processed by chi-square test, and P < .05 was considered statistically significant. The Cox risk ratio model analysis was performed to identify independent risk factors of F nucleatum. F nucleatum-high group was compared with the F nucleatum-low/negative group with respect to clinicopathological features and their relationships with MSI status. Kaplan–Meier method and log-rank test were used for univariate analysis of prognostic factors in patients with CRC.
± s). The paired t test was used in the group, and the analysis of variance was used in the group. The count data were expressed in percentage (%), and the data were processed by chi-square test, and P < .05 was considered statistically significant. The Cox risk ratio model analysis was performed to identify independent risk factors of F nucleatum. F nucleatum-high group was compared with the F nucleatum-low/negative group with respect to clinicopathological features and their relationships with MSI status. Kaplan–Meier method and log-rank test were used for univariate analysis of prognostic factors in patients with CRC.
3. Results
3.1. Baseline patient characteristics
We retrospectively analyzed the information of 91 patients hospitalized for CRC from January, 2017 to January, 2019. They are divided into 2 groups according to the F nucleatum status, F nucleatum-high 25 patients, and F nucleatum-low 66 patients. The qPCR for F nucleatum is shown in Fig. 1.
Figure 1.
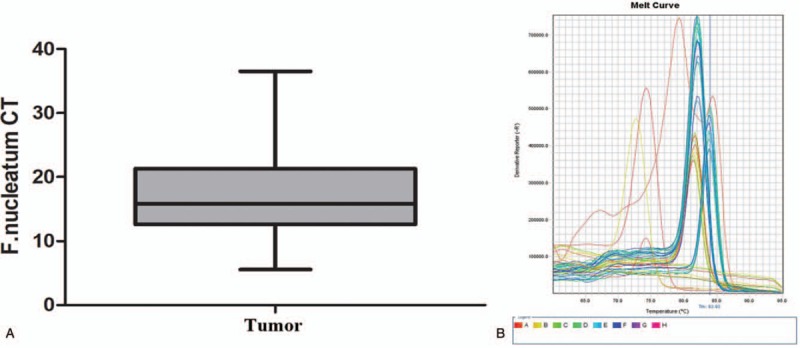
The qPCR for Fusobacterium nucleatum in tumor. (A) F nucleatum CT; (B) melting curve.
We compared the basic characteristics of the patients (age, sex, BMI), and the result showed that there is no statistical significance between the 2 groups (P = .298, P = .364, P = .638). The proportion of F nucleatum-high tumors was highest among descending colon cancers, whereas that of F nucleatum-high tumors was lowest among ascending colon cancers (36.0% and 13.3%, respectively) (Fig. 2). There were 3 cases of hypertension, 1 case of diabetes mellitus, and 1 case of hypertension with diabetes mellitus in F nucleatum-high group, whereas 5 cases of hypertension, 1 case of diabetes mellitus, 1 case of coronary heart disease, 1 case of diabetes mellitus, hypertension, and coronary heart disease, 1 case of cerebral infarction with coronary heart disease, 1 case of cirrhosis with hypertension, and 1 case of hypertension with coronary heart disease in F nucleatum-low group.
Figure 2.
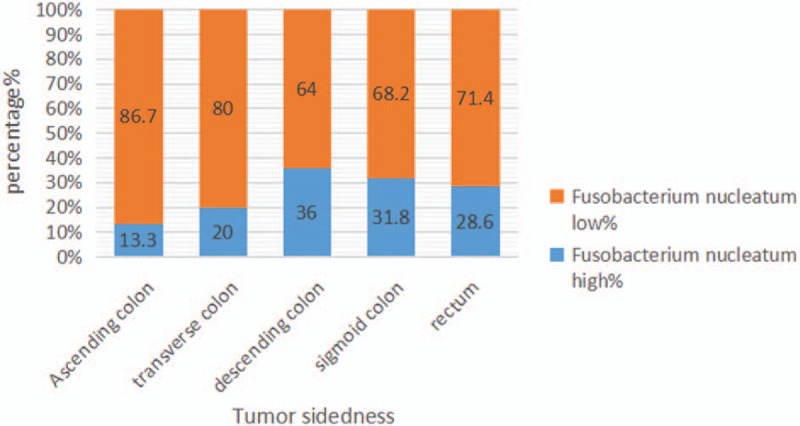
Different proportions of Fusobacterium nucleatum-high versus F nucleatum-low colorectal cancers according to tumor sidedness.
The relationship between clinicopathological and F nucleatum status showed that there were no significant differences in the location of tumors, the differentiation, T stage, N stage, and TNM stage (AJCC 7th edition) between the 2 groups (P = .549, P = .689, P = .066, P = .373, P = .64). However, the number of total lymph node acquisition and positive lymph nodes were higher in F nucleatum-high group (27.44 ± 25.213 vs 20.70 ± 10.141; P = .018; 3.80 ± 7.974 vs 1.74 ± 3.531; P = .001). Moreover, we found that nerve invasion and vascular tumor thrombus were more common in patients with F nucleatum-high (P = .003, P = .002). By analyzing the relationship between F nucleatum status and molecular characteristics, no significances were observed between F nucleatum, and KRAS and BRAF mutation, but microsatellite mutation was more frequent in F nucleatum-high group (84.0% vs 60.6%; P = .001) (Table 1).
Table 1.
Characteristics of stage II/III CRCs according to the Fusobacterium nucleatum status.

3.2. Survival prognostic factors
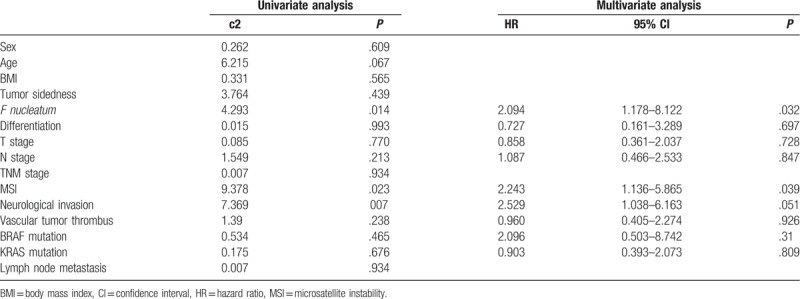
Kaplan–Meier method and log-rank test were used for univariate analysis of prognostic factors in patients with CRC. There was significant difference in overall survival (OS) between the F nucleatum-high and F nucleatum-low/negative groups (log-rank P = .038) (Fig. 3). A higher abundance of F nucleatum in CRC is associated with a shorter survival time. The F nucleatum status, peripheral nerve invasion, vascular thrombus, lymph node metastasis, and TNM staging were related factors affecting the prognosis of patients with CRC (Table 2). The Cox risk ratio model analysis showed that the F nucleatum (odds ratio [OR] 2.094, 95% confidence interval [CI] 1.178–8.122, P = .032) and MSI status (OR 2.243, 95% CI 1.136–5.865, P = .039) were independent prognostic factors in patients with CRC (Table 2).
Figure 3.
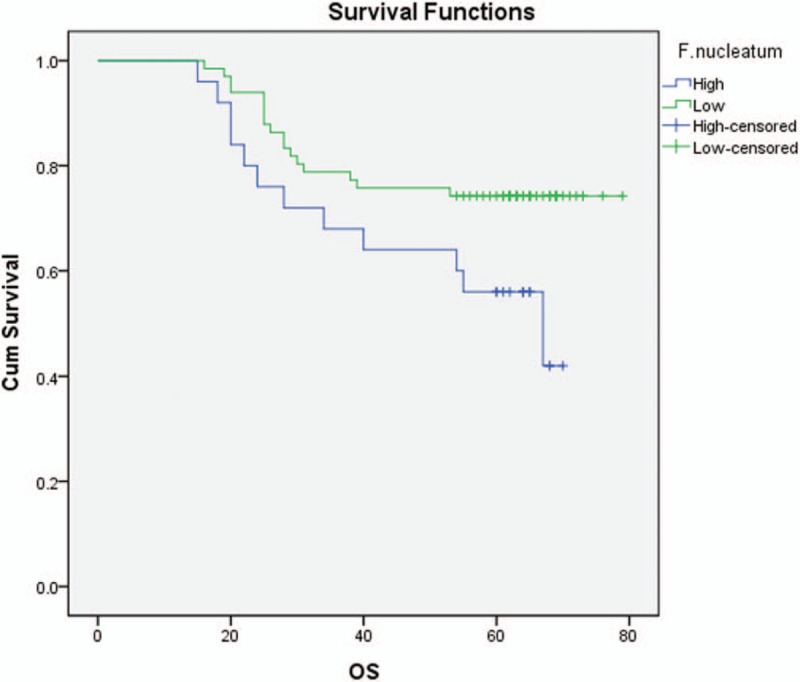
Kaplan–Meier survival analysis. Statistical difference in overall survival was evident between Fusobacterium nucleatum-high and Fusobacterium nucleatum-low subgroups in the overall 91-stage colorectal cancer patients.
Table 2.
Univariate and multivariate analyses of prognostic factors in colorectal cancer.
4. Discussion
Fusobacterium nucleatum is a Gram-negative obligate anaerobe bacterium in the oral cavity and plays a role in several oral diseases, including periodontitis and gingivitis.[12,18] Recently, several studies have reported that the level of F nucleatum is significantly elevated in human colorectal adenomas and carcinomas compared with that in adjacent normal tissue.[19,20] It has been validated that MSI-H is not a predictive factor because it is not related with better survival in patients with stage II and III colon cancer with adjuvant chemotherapy.[21,22] We decided to investigate the relationships between F nucleatum, and clinicopathological features and molecular factors (including KRAS, BRAF, and MSI).
Microsatellites or short tandem repeats correspond to short sequences of bases (from 1 to 6 bases), which are repeated throughout coding and noncoding regions of the genome. In these regions, the DNA polymerase tends to make more mistakes during the DNA synthesis by inserting additional bases. MSI increases mutation rates coming from defects in the DNA MMR by 100 to 1000 times.[23,24] Approximately 15% of colorectal adenocarcinomas are associated with MSI: 2.5% coming from a genetic inheritance and 12.5% being a sporadic one.[25] Sporadic MSI is caused by epigenetic silencing of the MLH1 promoter by methylation, which is associated with a somatic BRAF V600E mutation. Hereditary MSI commonly happens due to autosomal dominant mutations in MMR. Critical analysis of the best available evidence in the literature allows us to conclude that stage II and III colon cancer with MSI-H is associated with a statistically significant better disease-free survival and OS compared with stage II and III colon cancer with low microsatellite instability (MSI-L) or microsatellite stability.[26,27] The survival in untreated patients with stage II or III colorectal adenocarcinoma and MSI-H was better than that in untreated patients with stage II or III colorectal adenocarcinoma and MSS.[28] In our study, the greater amount of F nucleatum has a significant association with MSI-L/MSS.
Previous data indicate that high intratumoral F nucleatum load might be associated with poor prognostic effect.[19,20] Some researchers have demonstrated that F nucleatum modulates the tumor immune microenvironment while promoting CRC development.[29] Recently, it has been confirmed that biomarkers such as immune antibodies, miRNA, tumor-associated macrophages, and tumor-infiltrating T-cell subsets play a significant role in F nucleatum-associated CRC.[30,31] A previous study has shown that lymph node metastases are present in 52 out of 88 (59.1%) cases with a high abundance of F nucleatum and in 0 out of 13 (0%) subjects with a low abundance of F nucleatum, which indicates that a high abundance of F nucleatum is associated with CRC progression and metastasis.[32] Some researchers have also reported that the load of F nucleatum DNA in CRC tissue is correlated with higher CRC-specific mortality and that F nucleatum DNA may serve as a potential poor prognostic biomarker.[33] Fusobacterium was shown to be enriched in the mucosa-adherent microbiota and have the ability to adhere to and invade human epithelial and endothelial cells.[34,35] Recently, several researchers have suggested that F nucleatum is a pathogenic bacterium rather than a bacterium that promotes colorectal carcinogenesis.[36]
This study's limitations deserve commentary. First, the proportion of F nucleatum-positive cases in CRCs by qPCR analysis has been variable according to different investigations (8.6%–74%).[36] In our results, F nucleatum DNA was detected in 25 out of 91 cases (27.5%). The reason for variability in the F nucleatum-positive rate in CRCs is unclear, but tissue quality might be a critical factor for this discrepancy. Recently, Lee et al[19] found that the tissue fixation method could affect different results of F nucleatum qPCR analysis. Therefore, it can be inferred that F nucleatum-positive rate by qPCR method could be variable, depending on tissue fixation method and tissue storage time. Second, our study cohort was retrospectively collected. The results of the present analysis will hopefully lead to a prospective randomized study with the ultimate goal of a centralized national program for prognostic relevance of F nucleatum in CRCs and its relationships with MSI status.
Author contributions
Conceptualization: Yanglong Chen, Ying Lu, Yanling Li.
Data curation: Yanglong Chen.
Formal analysis: Yanglong Chen.
Investigation: Yanglong Chen.
Project administration: Ying Lu.
Resources: Ying Lu, YuTing Ke.
Software: Ying Lu, YuTing Ke.
Supervision: YuTing Ke, Yanling Li.
Validation: Yanling Li.
Visualization: YuTing Ke, Yanling Li.
Writing – original draft: Yanling Li.
Writing – review & editing: Yanling Li.
Footnotes
Abbreviations: CRC = colorectal cancers, F nucleatum = Fusobacterium nucleatum, FFPE = formalin-fixed, paraffin-embedded, IHC = immunohistochemistry, MMR = mismatch repair, MSI = microsatellite instability, OS = overall survival, PGT = prostaglandin transporter.
How to cite this article: Chen Y, Lu Y, Ke Y, Li Y. Prognostic impact of the Fusobacterium nucleatum status in colorectal cancers. Medicine. 2019;98:39(e17221).
The authors have no conflicts of interest to disclose.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013;14:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016;65:1973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 2012;22:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012;22:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ito M, Kanno S, Nosho K, et al. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int J Cancer 2015;137:1258–68. [DOI] [PubMed] [Google Scholar]
- [7].Flanagan L, Schmid J, Ebert M, et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur J Clin Microbiol Infect Dis 2014;33:1381–90. [DOI] [PubMed] [Google Scholar]
- [8].Abed J, Emgard JE, Zamir G, et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe 2016;20:215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-kappaB, and up-regulating expression of microRNA-21. Gastroenterology 2017;152:851–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gur C, Ibrahim Y, Isaacson B, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015;42:344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol 2015;1:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Amitay EL, Werner S, Vital M, et al. Fusobacterium and colorectal cancer: causal factor or passenger? Results from a large colorectal cancer screening study. Carcinogenesis 2017;38:781–8. [DOI] [PubMed] [Google Scholar]
- [13].Xie YH, Gao QY, Cai GX, et al. Fecal Clostridium symbiosum for noninvasive detection of early and advanced colorectal cancer: test and validation studies. EBioMedicine 2017;25:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zeller G, Tap J, Voigt AY, et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol Syst Biol 2014;10:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liang Q, Chiu J, Chen Y, et al. Fecal bacteria act as novel biomarkers for noninvasive diagnosis of colorectal cancer. Clin Cancer Res 2017;23:2061–70. [DOI] [PubMed] [Google Scholar]
- [16].Taieb J, Zaanan A, Le Malicot K, et al. Prognostic effect of BRAF and KRAS mutations in patients with stage III colon cancer treated with leucovorin, fluorouracil, and oxaliplatin with or without cetuximab: a post hoc analysis of the PETACC-8 trial. JAMA Oncol 2016;2:643–53. [DOI] [PubMed] [Google Scholar]
- [17].Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tahara T, Yamamoto E, Suzuki H, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res 2014;74:1311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee DW, Han SW, Kang JK, et al. Association between Fusobacterium nucleatum, pathway mutation, and patient prognosis in colorectal cancer. Ann Surg Oncol 2018;25:3389–95. [DOI] [PubMed] [Google Scholar]
- [20].Yamaoka Y, Suehiro Y, Hashimoto S, et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J Gastroenterol 2018;53:517–24. [DOI] [PubMed] [Google Scholar]
- [21].Taieb J, Le Malicot K, Shi Q, et al. Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst 2016;109: doi: 10.1093/jnci/djw272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609–18. [DOI] [PubMed] [Google Scholar]
- [23].Lee V, Le DT. Efficacy of PD-1 blockade in tumors with MMR deficiency. Immunotherapy 2016;8:1–3. [DOI] [PubMed] [Google Scholar]
- [24].Dudley JC, Lin MT, Le DT, et al. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res 2016;22:813–20. [DOI] [PubMed] [Google Scholar]
- [25].Klingbiel D, Saridaki Z, Roth AD, et al. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial. Ann Oncol 2015;26:126–32. [DOI] [PubMed] [Google Scholar]
- [26].Gavin PG, Colangelo LH, Fumagalli D, et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clin Cancer Res 2012;18:6531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim KP, Kim JE, Hong YS, et al. Paired primary and metastatic tumor analysis of somatic mutations in synchronous and metachronous colorectal cancer. Cancer Res Treat 2017;49:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Des Guetz G, Schischmanoff O, Nicolas P, et al. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur J Cancer 2009;45:1890–6. [DOI] [PubMed] [Google Scholar]
- [29].Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013;14:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang HF, Li LF, Guo SH, et al. Evaluation of antibody level against Fusobacterium nucleatum in the serological diagnosis of colorectal cancer. Sci Rep 2016;6:33440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Waniczek D, Lorenc Z, Snietura M, et al. Tumor-associated macrophages and regulatory T cells infiltration and the clinical outcome in colorectal cancer. Arch Immunol Ther Exp (Warsz) 2017;65:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li YY, Ge QX, Cao J, et al. Association of Fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World J Gastroenterol 2016;22:3227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Han YW, Redline RW, Li M, et al. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun 2004;72:2272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen W, Liu F, Ling Z, et al. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One 2012;7:e39743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Han YW, Shi W, Huang GT, et al. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect Immun 2000;68:3140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol 2018;10:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


