Supplemental Digital Content is available in the text
Keywords: biomarker, clinical significance, hepatocellular carcinoma, LTBP2
Abstract
The present study aimed to explore the expression of latent transforming growth factor β binding protein 2 (LTBP2) in patients with hepatocellular carcinoma (HCC) and their correlation to clinicopathologial features.
Serum levels of LTBP2 in 60 patients with HCC, 35 patients with hepatocellular benign tumors, 60 patients with precancerous lesions of HCC, and 60 healthy volunteers were determined by enzyme-linked immunosorbent assay. The expression levels of LTBP2 at messenger RNA (mRNA) and protein levels in 60 cases of HCC and adjacent tissues were detected by quantitative real-time polymerase chain reaction and immunohisochemistry. Statistical analysis was used to analyze the relationship between LTBP2 and clinical characteristics of patients with HCC.
The mRNA and protein levels of LTBP2 were significantly upregulated in HCC tissues compared to adjacent tissues. Additionally, higher serum LTBP2 level was also observed in HCC patients relative to normal controls. Further investigation demonstrated that LTBP2 expression was associated with malignant degree of tumor, tumor progression, tumor differentiation, tumor size, tumor stage and hepatitis virus infection, and has prognostic implications in HCC patients.
LTBP2 might be served as a potential biomarker in diagnosis and treatment of HCC.
Key Points
LTBP2 was highly expressed in HCC patients.
Serum LTBP2 was associated with clinicopathologial features and tumor progression of HCC patients.
LTBP2 mRNA expression in tissues have prognostic implications in HCC patients.
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy, accounting for approximately 80% of total primary liver cancer cases.[1,2] The overall 5-year relative survival rate was only 18% in the United States from 2008 to 2014.[3] Several lines of evidence suggest that the main risk factors for HCC are hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, liver cirrhosis, excessive drinking, and type 2 diabetes. Once clinical signs of HCC are apparent, in some cases the disease has advanced to terminal course, making effective treatment difficult and prognosis unsatisfactory.[4] Therefore, it is of great significance to identify novel biomarkers used for HCC early diagnosis.
It has become increasingly clear that tumor microenvironment is closely related to the occurrence of HCC. Extracellular matrix (ECM) is an essential component of the stromal microenvironment, which provides structural support and biochemical and physical signals for normal cell function maintenance.[5] Latent transforming growth factor β (TGF-β) binding protein 2 (LTBP2) is defined as an ECM protein encoded by the fibrillin/LTBP ECM glycoprotein family, which is expressed in the liver, spleen, lung, and heart. Recently, accumulating evidence has strongly implied that LTBP2 is involved in ECM formation and plays an important role in cell adhesion and elastic fiber aggregation.[6] The LTBP2 gene is located on chromosome 14q24, one of the key tumor-suppressive regions.[7] In recent years, abundant studies regard the underlying roles of LTBP2 in various tumors. For example, Han et al found that LTBP2 protein expression was significantly higher in head and neck squamous cell carcinoma (HNSCC) tissues, and was associated with lymph node metastasis and higher tumor–node-metastasis (TNM) stages, suggesting that LTBP2 served as an independent prognostic biomarker in HNSCC.[8] Moreover, Wang et al[9] reported that LTBP2 protein levels were significantly elevated in pancreatic ductal adenocarcinoma tissues. High levels of LTBP2 were correlated with poor differentiation and advanced TNM stage and predicted worse overall survival (OS) and disease-free survival. da Costa et al investigated the serum levels of LTBP2 in a prospective cohort of 115 patients with chronic liver disease (CLD) from Korea between 1999 and 2001, and found that increased serum LTBP2 was detected in 21 subjects who developed HCC, which improved biomarker-based detection of HBV-related HCC.[10] However, the clinical significance of LTBP2 in HCC remains poorly understood.
The present study was carried out to investigate the relationship between LTBP2 expression levels and the diagnosis of HCC.
2. Materials and methods
2.1. Participants and samples
HCC and adjacent normal tissue (2–5 cm from the outer tumor margin) samples, as well as, plasma samples were obtained from consecutive patients with HCC (n = 60) who underwent surgery at Ningbo Traditional Chinese Medicine Hospital during January 2016 to December 2016. All the patients were followed up through clinical visits and regular phone calls. We also collected plasma samples from 60 age- and sex-matched normal controls, 35 patients with benign hepatic tumors including hepatic hemangioma (HCH) (n = 15), focal nodular hyperplasia (FNH) (n = 15), and hepatocellular adenoma (HCA) (n = 5) and 60 patients with precancerous lesions related to either high-grade dysplastic nodule (DNs) (n = 20), liver cirrhosis (LC) (n = 20), or chronic hepatitis B (CHB) (n = 20) before surgery. Besides, we recorded clinicopathological features, including sex, age, hepatitis virus infection, tumor size, tumor grade, TNM stages, and serum alpha-fetoprotein (AFP) levels. This study was approved by the Ethics Committee of Ningbo Traditional Chinese Medicine Hospital, and informed consent was obtained in writing from all participants.
2.2. RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was extracted from the frozen tissue samples using the TRizol reagents (Applied Biosystems, Carlsbad, CA) according to the manufacturer's instructions, and then reverse-transcribed into complementary DNA using a reverse transcription kit (Takara, Shiga, Japan). Primer sequences of LTBP2 and β-actin were as follows: forward primer 5′-CGGTGATTGAGAATGGCCAG-3′ and reverse primer 5′-GTATTCACACACTCCGCGTC-3′ for human LTBP2 and forward primer 5′-CCTGGCACCCAGCACAAT-3′ and reverse primer 5′-GGGCCGGACTCGTCATACT-3′ for human β-actin. The messenger RNA (mRNA) expression levels of LTBP2 were determined by quantitative real-time polymerase chain reaction (qRT-PCR) performing on 7500 FAST real-time PCR System (Applied Biosystems). The mRNA expression level of LTBP2 in adjacent tissues was set as 1 to calculate the relative expression level of LTBP2 mRNA in HCC tissues. When the relative expression level of LTBP2 mRNA in HCC tissues was greater than 1, it indicated high expression; otherwise, it indicated low expression.
2.3. Immunohistochemical staining for LTBP2
Formaldehyde-fixed, paraffin-embedded tissues were cut into 5-μm sections. The glass slides were dried in an incubator at 65°C for 30 minutes, dewaxed in xylene, and dehydrated in an ethanol series. Sections were heated in microwave oven in 0.3% citrate buffer, treated with 3% H2O2 deionized water and then incubated with primary antibody against LTBP2 (cat. no ab121193; 1:200 dilution; Abcam, Cambridge, UK) overnight at 4°C. The slides were subsequently incubated with secondary antibody (Invitrogen, Carlsbad, CA) at 37°C for 30 minutes, washed twice with PBS and incubated with diaminobenzidine (Invitrogen) for immunolabeling. After hematoxylin counterstain and ethanol dehydration, the sections were sealed by neutral gum with cover glass and examined under an optical microscope. The positive cells were defined as the presence of yellowish or brown particles in the nucleus or cytoplasm.
2.4. Enzyme-linked immunosorbent assay test
Approximately 3 mL fasting venous blood samples were extracted from each subject, and collected in sterile tube without anticoagulant. Plasma was separated by centrifugation at 2000 rpm for 20 minutes and stored at −80°C until analysis. The serum levels of LTBP2 were measured using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) following the manufacturer's instructions.
2.5. Statistical analysis
All data were expressed as mean ± standard deviation if normally distributed, or as median if otherwise. Statistical analyses were conducted using SPSS version 22.0 (SPSS, Chicago, IL), and a P-value <.05 was considered to be statistically significant. Two-sided Student t test or Mann–Whitney U test was employed to analyze differences between 2 groups. Kaplan–Meier curve was performed for survival analysis.
3. Results
3.1. The expression of LTBP2 and its relationship with survival rate of patients with HCC
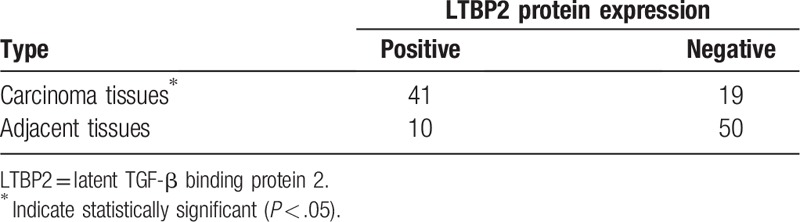
The qRT-PCR analysis demonstrated that the mRNA expression levels of LTBP2 were significantly upregulated in HCC tissues as compared with the adjacent nontumor tissues (P < .01; Fig. 1A). The high LTBP2 mRNA expression rate in HCC tissues (42/60) was significantly higher than that in paracarcinoma tissues (11/60) (P < .05; Table 1). Immunohisochemistry showed that LTBP2 protein expression in HCC tissues was markedly higher than that in corresponding adjacent normal tissues (Fig. 1B). The positive rate of LTBP2 protein expression in HCC tissues (41/60) was significantly higher than that in corresponding paracarcinoma tissues (10/60) (P < .05; Table 2). Consistently, the serum LTBP2 level in the HCC group was significantly higher than in the normal controls (P < .05; Fig. 1C). The survival rate of patients with LTBP2-high expression (13.3%) was significantly lower than in those with LTBP2-low expression (30.0%) (P = .023; Fig. 1D). A significant positive correlation was observed between the serum and mRNA levels of LTBP2 in HCC patients (Supplementary Fig. 1).
Figure 1.
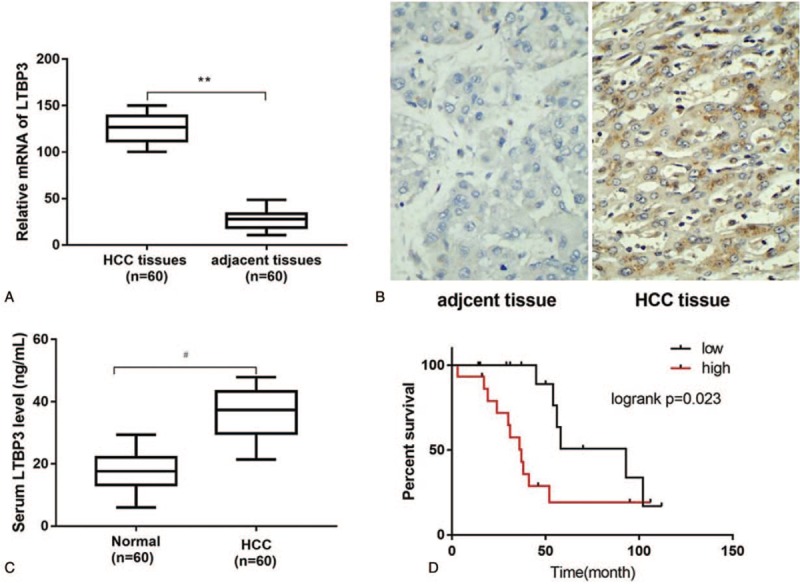
Aberrant expression of LTBP2 in HCC patients. (A) The mRNA expression levels of LTBP2 determined by qRT-PCR in HCC tissues (n = 60) and adjacent nontumor tissues (n = 60); (B) Photographs of LTBP2 immunohistochemical staining in HCC tissues and adjacent non-tumor tissues; (C) The serum LTBP2 levels in patients with HCC and normal controls (n = 60) using ELISA; (D) Kaplan–Meier curves of survival rate differed between high and low LTBP2 expression in HCC patients. ∗∗P < .01 versus adjacent nontumor tissues; #P < .05 versus normal controls. ELISA = enzyme-linked immunosorbent assay, HCC = hepatocellular carcinoma, LTBP2 = latent TGF-β binding protein 2, mRNA = messenger RNA, qRT-PCR = quantitative real time polymerase chain reaction.
Table 1.
LTBP2 mRNA expression in primary hepatocellular carcinoma tissues and adjacent tissues (n = 60).
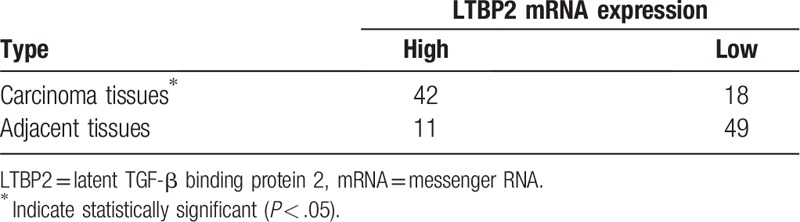
Table 2.
LTBP2 protein expression in primary hepatocellular carcinoma tissues and adjacent tissues (n = 60).
3.2. Association between serum LTBP2 expression and clinicopathological features
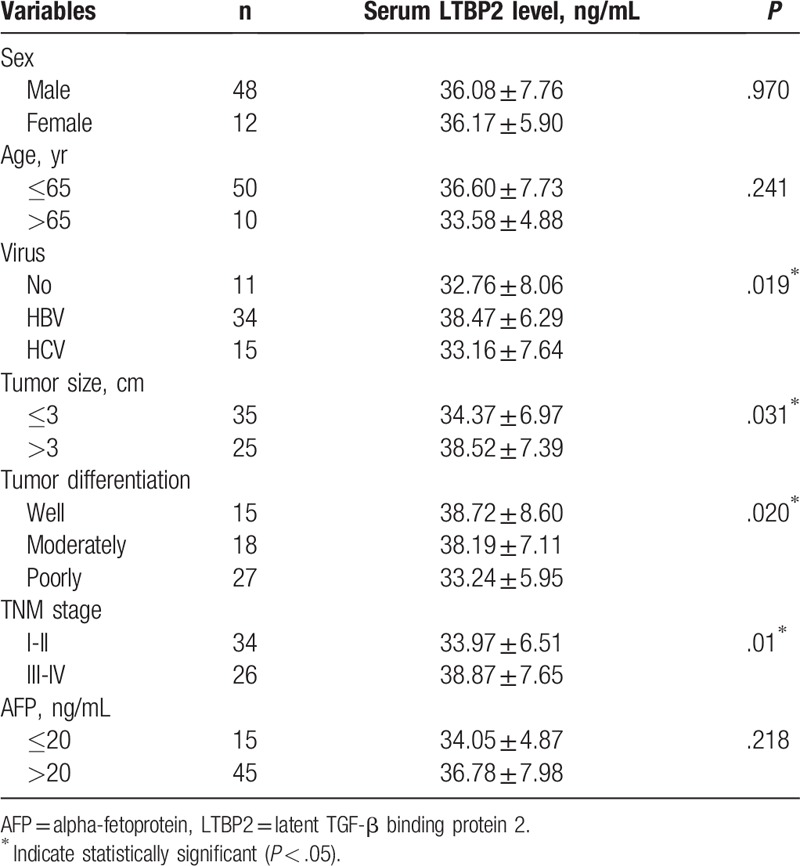
We next analyzed the potential correlation between serum LTBP2 level and clinicopathological characteristics of HCC. In contrast to HCC patients, the serum LTBP2 levels were significantly low in HCH, FNH and HCA patients (P < .05; Fig. 2A). In addition, serum LTBP2 levels were gradually high from normal controls, CHB, high-grade DNs and LC to HCC (P < .05; Fig. 2B). Furthermore, HCC patients with poorly differentiated tumors, tumor size >3 cm and TNM stage III/IV had higher serum levels of LTBP2 compared to patients with well or moderately differentiated tumors (P < .05; Fig. 2C and Table 3), tumor size ≤3 cm (P < .05; Fig. 2D and Table 3) and TNM stage I/II (P < .05; Fig. 2E and Table 3). Higher levels of LTBP2 were also observed in HCC patients infected with HBV as compared with those without hepatitis virus infection or with HCV infection (P < .05; Fig. 2F and Table 3). However, no significant difference was found between serum LTBP2 level and AFP level in HCC patients. Taken together, serum LTBP2 expression in patients with HCC was associated with malignant degree of tumor, tumor progression, tumor differentiation, tumor size, tumor stage and hepatitis virus infection.
Figure 2.
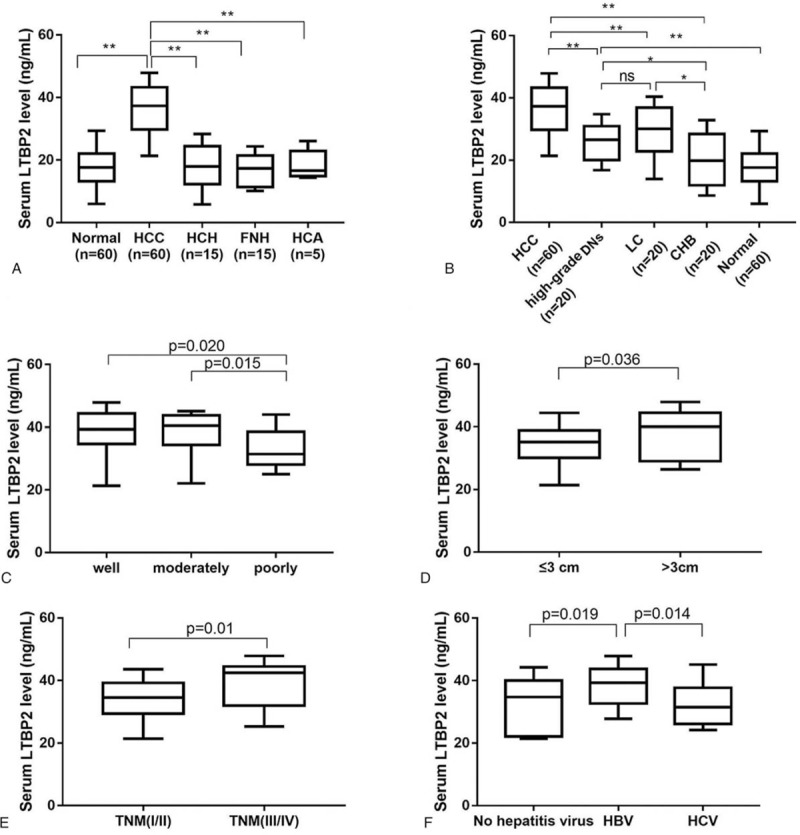
Serum LTBP2 levels in patients with HCC according to clinicopathologic characteristics. (A) The serum LTBP2 levels in patients with HCC (n = 60), HCH (n = 15), FNH (n = 15) and HCA (n = 5) and normal controls (n = 60); (B) Serum LTBP2 in patients with HCC, high grade DNs (n = 20), LC (n = 20) and CHB (n = 20) and normal controls (n = 60); (C) Serum LTBP2 in HCC patients with well (n = 15), moderately (n = 18) and poorly (n = 27) differentiated tumors; (D) Serum LTBP2 in HCC patients with tumor size >3 cm (n = 25) and ≤3 cm (n = 35); (E) Serum LTBP2 in HCC patients at TNM stage I/II (n = 34) or III/IV (n = 26); (F) Serum LTBP2 in HCC patients with no hepatitis virus infection (n = 11), HBV infection (n = 34) and HCV infection (n = 15). ∗P < .05 and ∗∗P < .01. DNs = dysplastic nodules, CHB = chronic hepatitis B, FNH = focal nodular hyperplasia, HCA = hepatocellular adenoma, HCC = hepatocellular carcinoma, HCH = hepatic hemangioma, HCV = hepatitis C virus, LC = liver cirrhosis, LTBP2 = latent TGF-β binding protein 2, TNM = tumor–node-metastasis.
Table 3.
Correlation between LTBP2 expression and clinicopathological parameters of primary hepatocellular carcinoma.
3.3. Diagnostic significance of LTBP2 mRNA expression in patients with primary HCC
We further analyzed the mRNA expression of LTBP2 in tissue samples, which were matched to serum samples. As shown in Fig. 3A, the expression of LTBP2 at mRNA level was gradually high from NC, CHB, high-grade DNs and LC to HCC (P < .05). The mRNA levels of LTBP2 in patients with poorly differentiated HCC, tumor size of more than 3 cm, TNM stage III-IV and HBV infection were observably higher than those with well/moderately differentiated tumors (P < .05; Fig. 3B), tumor size ≤3 cm (P < .05; Fig. 3C), TNM stage I-II (P < .05; Fig. 3D) and no hepatitis virus infection or HCV infection (P < .05; Fig. 3E). Collectively, our data suggested that elevated LTBP2 expression at mRNA and protein levels as well as in serum was closely associated with the clinicopathological characteristics of HCC patients.
Figure 3.
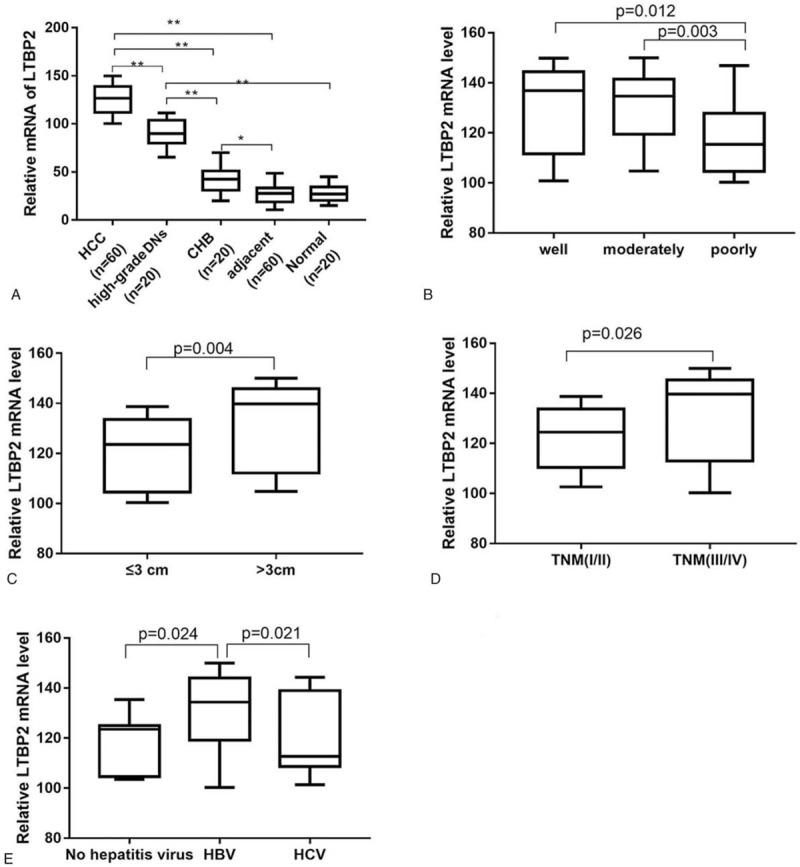
The mRNA LTBP2 expression in patients with HCC according to clinicopathologic characteristics. (A) LTBP2 mRNA expression in patients with HCC, high grade DNs (n = 20), LC (n = 20) and CHB (n = 20) and normal controls (n = 60); (B) LTBP2 mRNA in HCC patients with well (n = 15), moderately (n = 18) and poorly (n = 27) differentiated tumors; (C) LTBP2 mRNA in HCC patients with tumor size >3 cm (n = 25) and ≤3 cm (n = 35); (D) LTBP2 mRNA in HCC patients at TNM stage I/II (n = 34) or III/IV (n = 26); (E) LTBP2 mRNA in HCC patients with no hepatitis virus infection (n = 11), HBV infection (n = 34) and HCV infection (n = 15). ∗∗P < .01. DNs = dysplastic nodules, CHB = chronic hepatitis B, HCA = hepatocellular adenoma, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, LC = liver cirrhosis, LTBP2 = latent TGF-β binding protein 2, mRNA = messenger RNA, TNM = tumor–node-metastasis.
4. Discussion
Recent studies have shown that tumor microenvironment plays a pivotal role in the development and progression of HCC. The tumor microenvironment includes mesenchymal cells such as fibroblasts, vascular cells, infiltrated immune cells, and noncellular components consisting of soluble cytokines and solid ECM.[11] The tumor microenvironment of HCC is characterized by the infiltration of tumor-associated macrophages (TAM) and T cells. Such tumor microenvironment contribute to TAM polarization into M2 macrophages expressed cytokines such as IL-10 and TGF-β, which in turn promote the aggregation of regulatory T cells and the formation of immune responses of T helper 17 cells.[12]
ECM is a dynamic, complex environment providing multiple signals that mediate important cellular functions such as proliferation, migration, differentiation, and death.[13] LTBP2 is a member of the fibrillin/LTBP superfamily of ECM proteins characterized by a repeated domain structure. The fibrillin/LTBP superfamily is structurally conducive to matrix formation and also regulate the biological effects of TGF-β superfamily members. However, the key mechanism by which TGF-β signaling pathway inhibits HCC progression via inhibiting tumor growth and increasing proliferation of liver stem cells has been elucidated.[14] Suri et al conformed that LTBP2 knockdown on ECM genes expression and apoptosis in trabecular meshwork cells was mediated by TGF-β signaling pathway activation.[15]
In recent years, abundant studies provided strong evidence that LTBP2 exhibits tumor-promoting functions in different types of cancer. For instance, Wang et al demonstrated that LTBP2 expression was upregulated in gastric cancer (GC) tissues and cell lines, and was associated with poor OS in patients with TNM I/II and TNM III/IV. Mechanistic studies demonstrated that silencing of LTBP2 suppressed the proliferation, migration, invasion, and epithelial-mesenchymal transition in GC cells.[16] Wan et al displayed that knockdown of LTBP2 inhibited the proliferation, invasion, EMT phenotype, and activation of the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) pathway in thyroid carcinoma cells.[17]
In the present study, the diagnostic values of LTBP2 in patients with HCC was estimated. The results of this study suggested that LTBP2 mRNA and protein expression levels were upregulated in HCC tissues than in para-carcinoma tissues. Serum LTBP2 was higher in HCC patients compared with the normal controls and HCH, FNH, and HCA patients. Furthermore, the serum LTBP2 was positively correlated with mRNA levels of LTBP2 in HCC patients. High expression of LTBP2 predicted poor OS in patients with HCC. Additionally, ELISA assay was used to analyze serum LTBP2 expression and the result revealed that LTBP2 increased gradually from healthy controls, CHB, LC to high-grade DNs and HCC. These data indicated that LTBP2 increased gradually along with the deepening of hepatic impairment during the progression of benign liver diseases to precancerous lesions and HCC. An important outcome of the present study was that LTBP2 expression was related to poor differentiation, tumor size (>3 cm) and advanced TNM stages and HBV infection, while these clinicopathological factors were highly correlated with aggression and metastasis of HCC.[18] In summary, we concluded that LTBP2 expression seems to be associated with the progression of CLD to precancerous lesions and HCC. A high level of LTBP2 could be a good biomarker for diagnosis and prognosis in patients with HCC.
Author contributions
Investigation: Jinchun Chen, Guosheng Gao, Xingtao Ye, Jun Zhou, Jianjun Lin.
Methodology: Guosheng Gao, Xingtao Ye, Jun Zhou, Jianjun Lin.
Validation: Hui Wang.
Writing – original draft: Jinchun Chen.
Writing – review and editing: Hui Wang.
Supplementary Material
Footnotes
Abbreviations: AFP = alpha-fetoprotein, CHB = chronic hepatitis B, CLD = chronic liver disease, DNs = dysplastic nodules, ECM = extracellular matrix, ELISA = enzyme-linked immunosorbent assay, FNH = focal nodular hyperplasia, GC = gastric cancer, HBV = hepatitis B virus, HCA = hepatocellular adenoma, HCC = hepatocellular carcinoma, HCH = hepatic hemangioma, HCV = hepatitis C virus, HNSCC = head and neck squamous cell carcinoma, LC = liver cirrhosis, LTBP2 = latent TGF-β binding protein 2, OS = overall survival, qRT-PCR = quantitative real-time polymerase chain, TAM = tumor-associated macrophages, TGF-β = transforming growth factor β, TNM = tumor–node-metastasis.
How to cite this article: Chen J, Wang H, Gao G, Ye X, Zhou J, Lin J. Expression and clinical significance of latent-transforming growth factor beta-binding protein 2 in primary hepatocellular carcinoma. Medicine. 2019;98:39(e17216).
Funding: Key Laboratory of Diagnosis and Treatment of Digestive System Tumors of Zhejiang Province (2019E10020).
JC and GG contributed equally to this work.
Author certify that this manuscript is original and has not been published and will not be submitted elsewhere for publication. The submission has been received explicitly from all co-authors, and authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Malki A, El-Sharkawy A, El SM, et al. Antitumor activities of the novel isosteviol derivative 10C against liver cancer. Anticancer Res 2017;37:1591–601. [DOI] [PubMed] [Google Scholar]
- [2].Yeon LJ, Hoon KY, Hoon RY, et al. Intraoperative radiofrequency ablation for hepatocellular carcinoma in 112 patients with cirrhosis: a surgeon's view. Ann Surg Treat Res 2016;90:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- [4].Xiong Q, Wang J, Bai Y, et al. Recent progress in nanomedicine for hepatocellular carcinoma therapy. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2018;35:314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer 2017;8:761–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vehviläinen P, Hyytiäinen M, Keskioja J. Matrix association of latent TGF-beta binding protein-2 (LTBP-2) is dependent on fibrillin-1. J Cell Physiol 2010;221:586–93. [DOI] [PubMed] [Google Scholar]
- [7].Morén A, Olofsson A, Stenman G, et al. Identification and characterization of LTBP-2, a novel latent transforming growth factor-beta-binding protein. J Biol Chem 1994;269:32469–78. [PubMed] [Google Scholar]
- [8].Han L, Tang MM, Xu X, et al. LTBP2 is a prognostic marker in head and neck squamous cell carcinoma. Oncotarget 2016;7:45052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang C, Wang G, Zhang L, et al. Latent transforming growth factor β binding protein 2 (LTBP2) as a novel biomarker for the diagnosis and prognosis of pancreatic carcinoma. Med Sci Monit 2017;23:3232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].da Costa AN, Plymoth A, Santos-Silva D, et al. Osteopontin and latent-TGF beta binding-protein 2 as potential diagnostic markers for HBV-related hepatocellular carcinoma. Int J Cancer 2015;136:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Itano N, Zhuo L, Kimata K. Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression. Cancer Sci 2010;99:1720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Buonaguro L, Petrizzo A, Tagliamonte M, et al. Challenges in cancer vaccine development for hepatocellular carcinoma. J Hepatol 2013;59:897–903. [DOI] [PubMed] [Google Scholar]
- [13].Xu K, Shuai Q, Li X, et al. Human VE-cadherin fusion protein as an artificial extracellular matrix enhancing the proliferation and differentiation functions of endothelial cell. Biomacromolecules 2016;17:756–66. [DOI] [PubMed] [Google Scholar]
- [14].Mishra L, Banker T, Murray J, et al. Liver stem cells and hepatocellular carcinoma. Hepatology 2010;49:318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Suri F, Yazdani S, Elahi E. LTBP2 knockdown and oxidative stress affect glaucoma features including TGFβ pathways, ECM genes expression and apoptosis in trabecular meshwork cells. Gene 2018;673:70–81. [DOI] [PubMed] [Google Scholar]
- [16].Wang J, Liang WJ, Min GT, et al. LTBP2 promotes the migration and invasion of gastric cancer cells and predicts poor outcome of patients with gastric cancer. Int J Oncol 2018;52:1886–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wan F, Peng L, Zhu C, et al. Knockdown of latent transforming growth factor (TGF) beta-binding protein 2 (LTBP2) inhibits invasion and tumorigenesis in thyroid carcinoma cells. Oncol Res 2016;25:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shimada K, Sano T, Sakamoto Y, et al. A long-term follow-up and management study of hepatocellular carcinoma patients surviving for 10 years or longer after curative hepatectomy. Cancer 2010;104:1939–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


