Abstract
Human papillomavirus (HPV) infection is the leading cause of cervical cancer and precancerous lesions. Knowledge regarding the prevalence and genotype distribution of HPV in women is important to establish strategies for cervical cancer screening and HPV vaccination. This study aimed to evaluate the characteristics of HPV infection in Taizhou, China. HPV genotype of 10,733 women who visited Taizhou People's Hospital from November 2016 to October 2018 was determined using a PCR and hybridization-based detection test. The prevalence of overall, high risk (HR), and low risk (LR) HPV infections was 34.58%, 29.92%, and 10.12%, respectively. Of HPV-positive cases, 2417 (65.13%) were infected with a single HPV genotype and 1294 (34.87%) were infected with multiple HPV genotypes. HPV-52 was the most prevalent genotype (6.21%), followed by HPV-16 (5.33%), HPV-53 (4.03%), HPV-58 (3.89%), and HPV-81 (3.75%). The highest prevalence of HPV infection was found in women aged ≥60 years (40.72%). Furthermore, the prevalence of HPV increased with the severity of cervical lesions. In conclusions, the prevalence and genotype distribution of HPV varied with age and cervical lesions. The findings might serve as a potential reference for guiding cervical cancer screening and vaccine-based HPV prevention in Taizhou.
Keywords: age, cervical cancer, distribution, human papillomavirus, prevalence
1. Introduction
Cervical cancer ranks fourth in both incidence and mortality in cancers diagnosed in women worldwide, accounting for 6.6% of all female cancer cases and 7.5% of all female cancer death.[1] Although great improvement in the health and sanitary conditions, both incidence and mortality rates of cervical cancer are still increasing in China.[2,3] It is deemed necessary to further strengthen cervical cancer screening program.
Human papillomavirus (HPV) infection is the cause of the majority of cervical neoplasia, as well as some other anogenital and oropharyngeal cancers,[4] accounting for approximately 5% of all cancers worldwide.[5] To date, >200 HPV subtypes have been identified, which are classified into high risk (HR) and low risk (LR) types on the basis of their correlation with malignant tumors, especially cervical cancer.[6–10] International Agency for Research on Cancer (IARC) has clearly classified HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-51, HPV-52, HPV-56, HPV-58, and HPV-59 as HR-HPV which account for 95% of all cervical cancer.[11,12] On the other hand, LR-HPV subtypes such as 6, 11, can cause visible genital warts and early or low cervical epithelial cell changes. HPV vaccine has become a major progress in preventing cervical disease and cancer. The current vaccines were developed based on epidemiological data from Europe and the United States, especially for HPV-16 and -18, whereas the prevalence and genotype distribution of HR-HPV vary by race and country, and even the most common HPV genotypes may vary by the region in the same country.[13–15] As the 3 HPV vaccines have been approved by the Chinese government, it is necessary to understand the HPV genotype distribution in different regions of China to help improve cervical screening and vaccination. In the present study, we aimed to investigate the prevalence and genotype distribution of HPV among women in Taizhou, Jiangsu Province, China.
2. Materials and methods
2.1. Subjects
This retrospective study was designed to analyze the results of cervical HPV test of 10,733 women who visited Taizhou People's Hospital from November 2016 to October 2018. The patients were aged from 16 to 85 years and the mean age was 40 years. Patients who had history of cervical intraepithelial neoplasia (CIN), cervical cancer, and hysterectomy were excluded. No gynecological examination, vaginal drug administration, and sex life were performed within 3 days before the examination. Of 10,733 women, 4865 cases simultaneously or subsequently underwent cervical cytology. Cytological specimens were blinded and evaluated by experienced pathologists according to the Bethesda System. The samples were classified as: negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells of undetermined significance (ASCUS), atypical squamous cells-cannot exclude high-grade squamous intraepithelial neoplasia (ASC-H), low-grade squamous intraepithelial neoplasia (LSIL), high-grade squamous intraepithelial neoplasia (HSIL), atypical glandular cells (AGC), or squamous cell carcinoma (SCC). Furthermore, 343 patients were diagnosed as cervical intraepithelial neoplasia or cervical cancer by cervical biopsy or conization. This study was approved by the Ethics Committee of Taizhou people's Hospital.
2.2. HPV genotyping
HPV isolates were genotyped using the PCR-RDB HPV genotyping for 23 types kit (Yaneng Biotech, Shenzhen, China), which can identify 17 HR-HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) and 6 LR-HPV types (6, 11, 42, 43, 81, and 83). HPV DNA was extracted, amplified, and genotyped according to the manufacturers’ protocol. Furthermore, sterile water and specimens with known HPV genotypes were used as the negative and positive controls, respectively.
2.3. Statistical analyses
All statistical analyses were performed by using SPSS V22.0 (SPSS, Chicago, IL). Differences between groups were examined using the chi-square test or Fisher exact probability test. A P value of <.05 was considered statistically significant.
3. Results
3.1. General characteristics
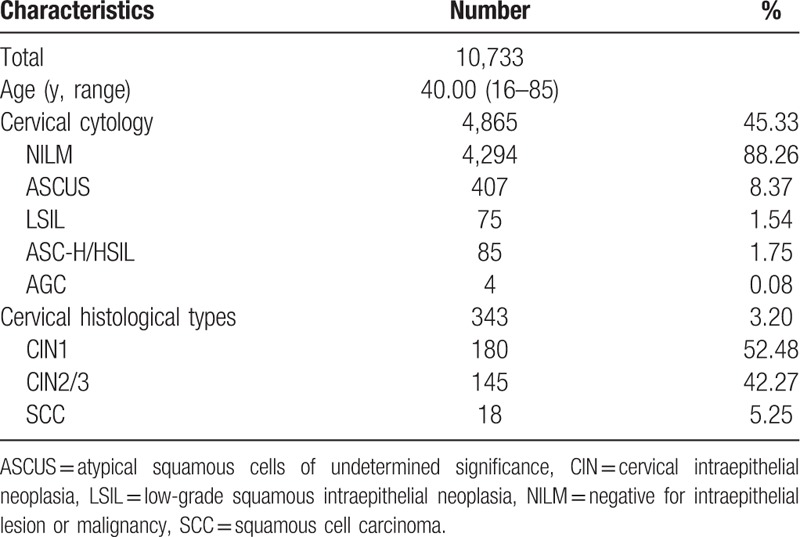
A total of 10,733 women who received medical treatment and physical examination in Taizhou People's Hospital from November 2016 to October 2018 were retrospectively analyzed. The characteristics of the study population were summarized in Table 1. Among 4865 women who simultaneously or subsequently underwent cervical cytology, 4294 had normal cytology, 407 had ASCUS, 75 had LSIL, and 85 had ASC-H/HSIL. Furthermore, 343 women were diagnosed with CIN or cervical cancer, 180 with CIN1, 145 with CIN2/3, and 18 with SCC.
Table 1.
Characteristics of subjects.
3.2. Prevalence of HPV types
Of 10,733 subjects, 3711 were positive for HPV infection, with the infection rate of 34.58%. The prevalence of HR-HPV and LR-HPV infections was 29.92% (3211/10,733) and 10.12% (1086/10,733), respectively. HPV-52 was the most prevalent genotype (6.21%), followed by HPV-16 (5.33%), HPV-53 (4.03%), HPV-58 (3.89%), and HPV-81 (3.75%) (Fig. 1). The most common HR-HPV types after HPV-52, HPV-16, HPV-53, and HPV-58 were HPV-51 (3.25%), HPV-68 (2.59%), HPV-59 (2.47%), and HPV-56 (2.33%). HPV-18 was found in 2.09% of women (n = 224), ranking the 13th most common HPV genotype. The most common LR-HPV types after HPV-81 were HPV-42 (2.17%), and HPV-43 (2.16%).
Figure 1.
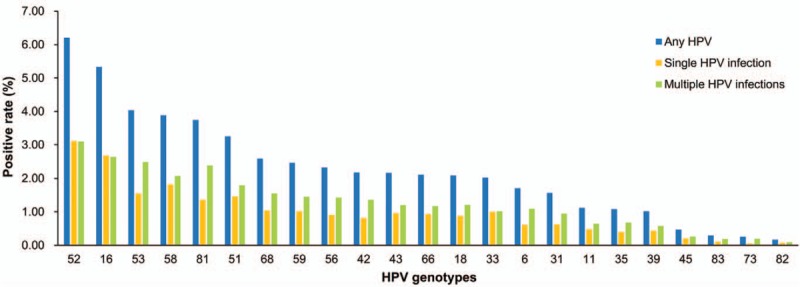
The prevalence and genotype distribution of HPV among 10,733 women. HPV = human papillomavirus.
The infection rates of single and multiple HPV genotypes were 22.52% (2417/10,733) and 12.06% (1294/10,733), respectively. The prevalence of single HPV genotype in both LR-HPV and HR-HPV groups were higher than that of multiple HPV genotypes. For the single HPV infection, the most common genotype of HR-HPV was HPV-52 (3.11%), followed by HPV-16 (2.68%), HPV-58 (1.82%), HPV-53 (1.55%), and HPV-51 (1.46%). For multiple HPV infection, HPV-52 (3.09%) and HPV-16 (2.65%) were also the first and second leading common genotypes, respectively, followed by HPV-53 (2.49%), HPV-58 (2.07%), and HPV-51 (1.79%). As for the LR-HPV genotypes, HPV-81 was the most common genotypes, followed by HPV-42 or HPV-43 in both single and multiple HPV infections. HPV-83, HPV-82, and HPV-73 were consistently the third least frequent HPV genotypes in single and multiple HPV-infected subjects.
3.3. Age-specific HPV prevalence
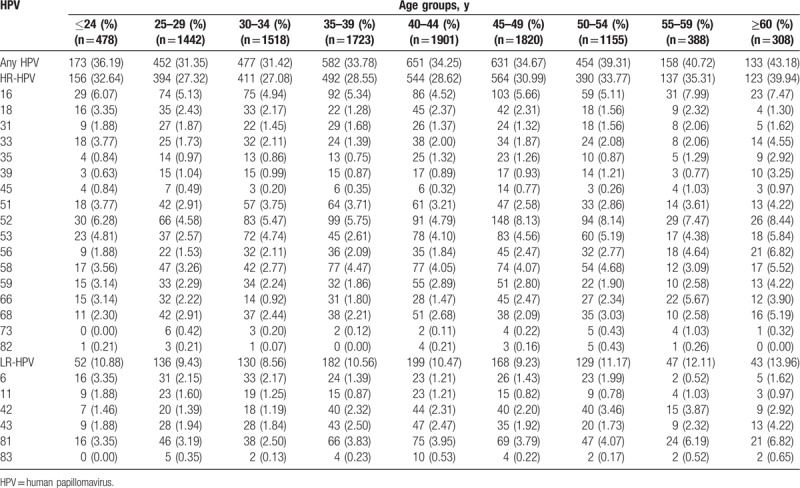
All women were divided into 9 age groups: ≤24 years old, 25 to 29 years old, 30 to 34 years old, 35 to 39 years old, 40 to 44 years old, 45 to 49 years old, 50 to 54 years old, 55 to 59 years old, and ≥60 years old. As shown in Table 2, the HPV infection prevalence in women ≤24 years old, 25 to 29 years old, 30 to 34 years old, 35 to 39 years old, 40 to 44 years old, 45 to 49 years old, 50 to 54 years old, 55 to 59 years old, and ≥60 years old was 36.19%, 12.07%, 1.53%, 10.21%, 9.31%, 9.78%, 15.50%, 46.39%, and 58.77%, respectively. A different age specific prevalence was observed in both overall and HR-HPV infection (P for trend < .001). The distribution of both overall and HR-HPV infection showed a bimodal pattern in different age groups. The fourth highest infection rate was observed in women aged ≤24 years old, which declined to its lowest point in women aged 25 to 29 years old and increased gradually and reached its highest point in women aged ≥60 years old. For LR-HPV infection, there was a weak but significant increase trend with age (P for trend = .030). In addition, HPV-16 and HPV-52 were consistently the 2 most common HPV genotypes, whereas HPV 83 was the least common HPV genotype in all 9 age groups. HPV-56 prevalence was increased with age (P for trend < .001), which was the fifth and fourth most common genotypes in women aged 55 to 59 years old (4.64%) and ≥60 years old (6.82%), respectively.
Table 2.
The prevalence and genotype distribution and of HPV according to age.
3.4. HPV prevalence according to cervical cytology
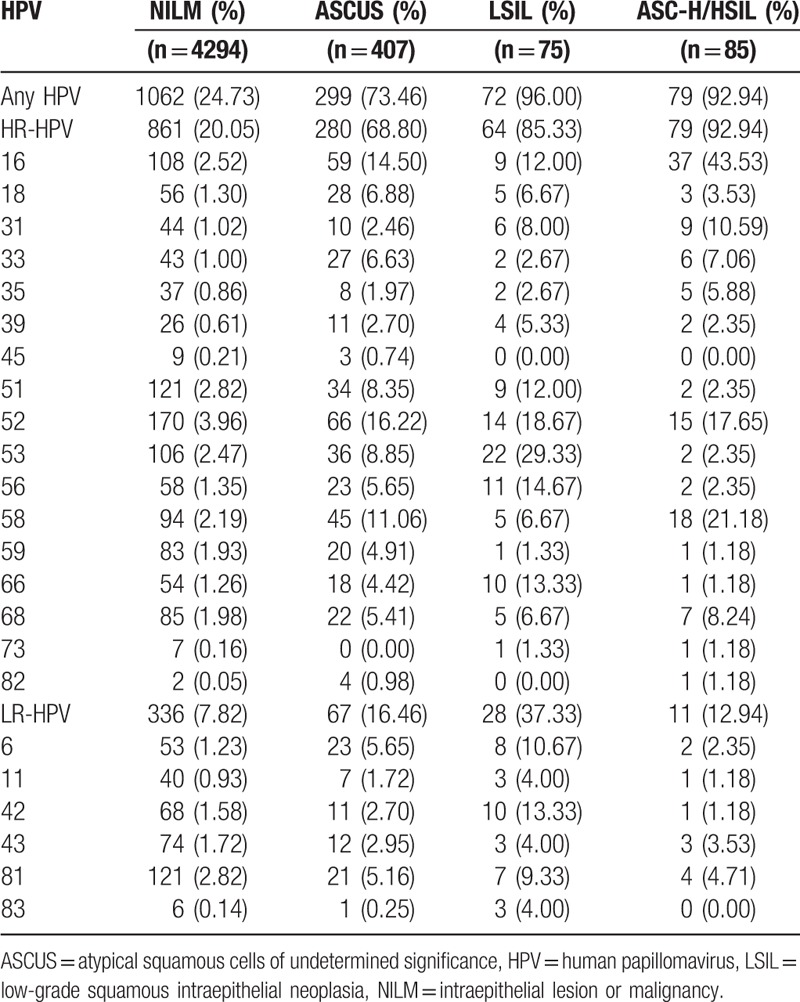
Among 4865 women with concurrent cytological results, the prevalence of overall HPV, HR-HPV, and LR-HPV were 24.73%, 20.05%, and 7.82% in women with NILM, respectively, which were significantly lower than those in women with ASCUS, LSIL, and ASC-H/HSIL (P < .001, Table 3). Furthermore, the prevalence of overall HPV and HR-HPV increased with the severity of cervical cytology (P < .001). Women with LSIL had the highest prevalence of overall HPV (96.00%) and LR-HPV (37.33%), whereas those with ASC-H/HSIL had the highest prevalence of HR-HPV (85.33%). HPV-52 was the most common genotype in women with NILM (3.96%) and ASCUS (16.22%) but ranked the second and third most common genotype in those with LSIL (18.67%) and ASC-H/HSIL (17.65%), respectively. HPV-53 and HPV-16 were the most common genotype in women with LSIL (29.33%) and ASC-H/HSIL (45.53%), respectively. As for 4 AGC cases, only 1 was infected with HPV.
Table 3.
The prevalence and genotype distribution and of HPV according to cervical cytology.
3.5. HPV prevalence according to cervical lesions
Among the 343 women with cervical intraepithelial neoplasia or SCC, the prevalence of overall HPV, HR-HPV, and LR-HPV infections was 85.56%, 83.33%, and 22.78% in CIN1 cases, 93.10%, 92.41%, and 14.48 in CIN2+ cases, and 94.44%, 94.44%, and 22.22% in SCC cases, respectively. The prevalence of overall HPV and HR-HPV increased with the severity of cervical lesions (P for trend = .028 and .011, respectively). The top 5 most prevalent HPV types in CIN1 and CIN2+ cases were HR-HPV, but LR-HPV-81 was the second common type in SCC cases (Fig. 2). The prevalence of HPV-16 increased with the severity of cervical lesions (P for trend < .001) and reached its highest point in SCC with the infection rate of 61.11%.
Figure 2.
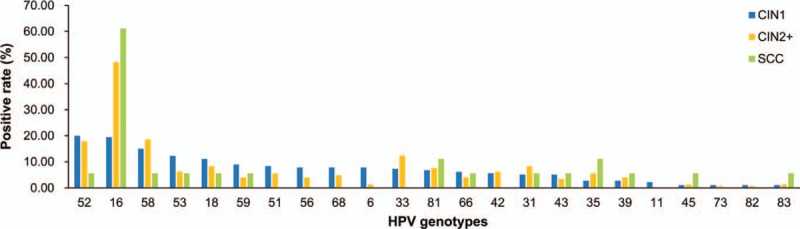
HPV prevalence according to cervical lesions. HPV = human papillomavirus.
4. Discussion
The persistent infection of HR-HPV is the key and necessary pathogenic factor for cervical cancer.[9,10] Therefore, HPV testing reduces the incidence and mortality of cervical cancer.[16] However, it is difficult to establish a comprehensive database of the prevalence and genotype distribution of HPV in China due to the large geographical area and the largest population in the world, which is important to determine the best prevention approach for cervical cancer and assess the effect of HPV vaccines. There is still lack of comprehensive data on the prevalence and genotype distribution of HPV in Taizhou. In the present study, we investigated the prevalence and genotype distribution of HPV in women who sought a medical examination and treatment. The overall prevalence of HPV (34.58%) in our study was higher than that in other regions in China, such as Shandong (28.4%),[17] Zhejiang (22.8%),[18] Shanghai (31.84%),[19] Nanhai area of Foshan (13.5%),[20] Jiangxi (22.49%),[21] and Jiangsu (15.5%).[22] The prevalence of HR-HPV (29.92%) was also higher than that in most regions in China but lower than that in Haikou (31.94%) and Shanghai (34.68%).[19] The findings reveal that Taizhou has a higher prevalence of HPV infection than other regions in China. High infection rate of HR-HPV in our study implies the inadequacy of routine screening for cervical cancer in Taizhou.
Previous studies have indicated that the 5 most common HPV types were HPV-16, HPV-18, HPV-31, HPV-33, and HPV-58 in Europe, HPV-16, HPV-53, HPV-52, HPV-18, and HPV-39 in northern America, HPV-16, HPV-52, HPV-18, HPV-58, and HPV-31 in Africa, and HPV-16, HPV-52, HPV-58, HPV-18, and HPV-56 in Asia.[23] HPV-16 and HPV-18 play a predominant role in above regions. In the present study, the 5 most common types of HPV in Taizhou were HPV-52, HPV-16, HPV-53, HPV-58, and HPV-81, whereas HPV-18 had a relatively low prevalence, which was in agreement with that in Shandong Province[17] but was slightly different from most previous studies in other regions of China, such as Zhejiang Province,[18] Shanghai,[19] and even Jiangsu Province.[22] The reason for the inconsistencies may be that hospital population-based surveys are opportunistic screenings with a selection bias and the levels of economic development in different regions of Jiangsu Province varies greatly. On the other hand, HPV-16 plays a predominant role in all regions while other subtypes vary in different regions in China. Taken together, HPV-52, HPV-16, HPV-53, and HPV-58 seem to be the most common HPV genotypes in most regions in China.
Age is an important factor associated with HPV infection. The prevalence and genotype distribution of HPV and HR-HPV in different age groups are varied. The highest prevalence of overall HPV, HR-HPV, and LR-HPV was observed in women aged ≥60 years old. Furthermore, overall HPV and HR-HPV infection showed higher rates in younger women but lower rates in middle-age women. Previous studies have revealed 2 peaks of age-specific prevalence of HPV, which varied in different studies.[17,20–22] However, some studies showed that HPV prevalence decreased with the increasing age in other regions worldwide.[24,25] The peak age of HPV infection may be affected by the immature immune system of young women and the physiological and immunologic disorders caused by hormone fluctuations of older women.
HPV infection, especially HR-HPV, is responsive for the majority of CIN lesions. Our findings revealed that the prevalence of HR-HPV positivity increased with the severity of cervical lesion, which was in agreement with those found in other regions or countries.[26,27] A worldwide meta-analysis indicated that HPV-16 and HPV-18 were the most prevalent types of HPV associated with cervical lesions worldwide, while HPV-52 and HPV-58 were more prevalent in East Asian women.[28] Previous studies have suggested that HPV-52 and HPV-58 are 2 important HPV types associated with cervical cancer in China.[29] Our study showed that HPV-16, HPV-52, HPV-58, and HPV-18 were 4 of the 5 most common HPV genotypes in CIN1 and CIN2+ cases. Furthermore, HPV-53 was the fourth, fifth, and third common genotypes in CNI1, NILM, and SCC cases, respectively. The result is required to validated in larger sample studies due to small size of SCC. A recent study by Qian et al[30] showed that HPV-53 was one of the most common genotypes in different cervical diseases including cervical cancer. These findings indicate that HPV-53 may be the major risk factor for cervical cancer in China. HPV vaccines against HPV including HPV-53 should be developed for Chinese women specifically.
In summary, this study represents the most comprehensive assessment of the prevalence and genotype distribution of HPV in women in Taizhou. The findings revealed a high HPV prevalence and implied a high incidence of cervical cancer in Taizhou. HPV-52, HPV-16, HPV-53, HPV-58 were the 4 most common HPV genotypes, which was consistent with those in other regions of China but was obviously different from those found in developed countries. It is necessary to develop the next-generation HPV vaccines targeting a broader spectrum of HR-HPV types such as HPV-53 for Chinese women.
Author contributions
Conceptualization: Rongrong Jin, Hua Qian, Hong Yu.
Data curation: Rongrong Jin.
Formal analysis: Hua Qian, Yongsheng Zhang, Jingjing Bao, Junxing Huang.
Funding acquisition: Hua Qian, Hong Yu.
Investigation: Hong Yu.
Methodology: Rongrong Jin, Yongsheng Zhang, Donglan Yuan, Jingjing Bao, Huilin Zhou.
Resources: Rongrong Jin, Donglan Yuan, Min Chen.
Writing – original draft: Rongrong Jin, Hua Qian, Hong Yu.
Writing – review & editing: Hua Qian, Junxing Huang, Hong Yu.
Footnotes
Abbreviations: AGC = atypical glandular cells, ASC-H = atypical squamous cells-cannot exclude high-grade squamous intraepithelial neoplasia, ASCUS = atypical squamous cells of undetermined significance, CIN = cervical intraepithelial neoplasia, HPV = human papillomavirus, HR = high risk, HSIL = high-grade squamous intraepithelial neoplasia, IARC = International Agency for Research on Cancer, LR = low risk, LSIL = low-grade squamous intraepithelial neoplasia, NILM = negative for intraepithelial lesion or malignancy, SCC = squamous cell carcinoma.
How to cite this article: Jin R, Qian H, Zhang Y, Yuan D, Bao J, Zhou H, Chen M, Huang J, Yu H. The prevalence and genotype distribution of human papillomaviruses among female in Taizhou, China. Medicine. 2019;98:39(e17293).
RJ and HQ are equal contributors.
This study was supported by the Scientific Research Project Foundation of Health Department, Jiangsu, China (grant No. H2017075), the Foundation of Jiangsu Provincial Medical Innovation Team (grant No. CXTDA2017042), and the Scientific Research Project Foundation of Taizhou People's Hospital (grant No. ZL201815).
The authors have no conflicts of interest to disclose.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Zheng RS, Sun KX, Zhang SW, et al. [Report of cancer epidemiology in China, 2015]. Zhonghua Zhong Liu Za Zhi 2019;41:19–28. [DOI] [PubMed] [Google Scholar]
- [3].Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018;30:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012;13:607–15. [DOI] [PubMed] [Google Scholar]
- [5].Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012;30suppl:F12–23. [DOI] [PubMed] [Google Scholar]
- [6].Bzhalava D, Eklund C, Dillner J. International standardization and classification of human papillomavirus types. Virology 2015;476:341–4. [DOI] [PubMed] [Google Scholar]
- [7].Burd EM. Human papillomavirus laboratory testing: the changing paradigm. Clin Microbiol Rev 2016;29:291–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McGraw SL, Ferrante JM. Update on prevention and screening of cervical cancer. World J Clin Oncol 2014;5:744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010;11:1048–56. [DOI] [PubMed] [Google Scholar]
- [10].Radley D, Saah A, Stanley M. Persistent infection with human papillomavirus 16 or 18 is strongly linked with high-grade cervical disease. Hum Vaccin Immunother 2016;12:768–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol 2009;10:321–2. [DOI] [PubMed] [Google Scholar]
- [12].Arbyn M, Tommasino M, Depuydt C, et al. Are 20 human papillomavirus types causing cervical cancer? J Pathol 2014;234:431–5. [DOI] [PubMed] [Google Scholar]
- [13].Moosa K, Alsayyad AS, Quint W, et al. An epidemiological study assessing the prevalence of human papillomavirus types in women in the Kingdom of Bahrain. BMC Cancer 2014;14:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fonseca AJ, Taeko D, Chaves TA, et al. HPV infection and cervical screening in socially isolated indigenous women inhabitants of the Amazonian rainforest. PLoS One 2015;10:e0133635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grunberg MG, Chan M, Adhin MR. Distinctive distribution of HPV genotypes in cervical cancers in multi-ethnic suriname: implications for prevention and vaccination. Epidemiol Infect 2017;145:245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Goodman A. HPV testing as a screen for cervical cancer. BMJ 2015;350:h2372. [DOI] [PubMed] [Google Scholar]
- [17].Jiang L, Tian X, Peng D, et al. HPV prevalence and genotype distribution among women in Shandong Province, China: analysis of 94,489 HPV genotyping results from Shandong's largest independent pathology laboratory. PLoS One 2019;14:e0210311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu XX, Fan XL, Yu YP, et al. Human papillomavirus prevalence and type-distribution among women in Zhejiang Province, Southeast China: a cross-sectional study. BMC Infect Dis 2014;14:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Singh S, Zhou Q, Yu Y, et al. Distribution of HPV genotypes in Shanghai women. Int J Clin Exp Pathol 2015;8:11901–8. [PMC free article] [PubMed] [Google Scholar]
- [20].Yuan XW, Li YJ, Qiu Q, et al. Prevalence and genotype distribution of human papillomavirus among 9945 women from the Nanhai area of Foshan. BMC Infect Dis 2019;19:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhong TY, Zhou JC, Hu R, et al. Prevalence of human papillomavirus infection among 71,435 women in Jiangxi Province, China. J Infect Public Health 2017;10:783–8. [DOI] [PubMed] [Google Scholar]
- [22].Ge Y, Zhong S, Ren M, et al. Prevalence of human papillomavirus infection of 65,613 women in East China. BMC Public Health 2019;19:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Crow JM. HPV: the global burden. Nature 2012;488:S2–3. [DOI] [PubMed] [Google Scholar]
- [24].Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003-2006. J Infect Dis 2011;204:566–73. [DOI] [PubMed] [Google Scholar]
- [25].Tabrizi SN, Brotherton JM, Stevens MP, et al. HPV genotype prevalence in Australian women undergoing routine cervical screening by cytology status prior to implementation of an HPV vaccination program. J Clin Virol 2014;60:250–6. [DOI] [PubMed] [Google Scholar]
- [26].Wheeler CM, Hunt WC, Cuzick J, et al. A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer 2013;132:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shen Y, Gong JM, Li YQ, et al. Epidemiology and genotype distribution of human papillomavirus (HPV) in women of Henan Province, China. Clin Chim Acta 2013;415:297–301. [DOI] [PubMed] [Google Scholar]
- [28].Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349–59. [DOI] [PubMed] [Google Scholar]
- [29].Li Y, Huang K, Ji PL, et al. Cervical infection of oncogenic human papillomavirus (hpv) types in Beijing, China. Biomed Environ Sci 2016;29:734–41. [DOI] [PubMed] [Google Scholar]
- [30].Qian L, Zhang Y, Cui D, et al. Analysis of epidemiological trends in human papillomavirus infection among gynaecological outpatients in Hangzhou, China, 2011-2015. BMC Infect Dis 2017;17:393. [DOI] [PMC free article] [PubMed] [Google Scholar]


