Abstract
To investigate the cognitive and psychological outcomes of pediatric allogeneic HSCT survivors in China.
A total of 135 3 to 18 years old children and adolescents who underwent allo-HSCT and survived at least 3 months post-HSCT were recruited and completed the assessments. Cognitive and psychological functions were assessed via age-appropriate standardized measures. Clinical information was extracted from the medical records.
Forty one 3 to 6 years old children completed Psychological Questionnaires for 3 to 6 years Children. The scores of 21(51.2%) children in cognitive development dimension, 18(43.9%) in motor development dimension, 16(39.0%) in language development and social development dimension, 15(36.6%) in emotion and will dimension and 14(34.1%) in living habits dimension were less than the standard. Fifty six 8 to 16 years old children and adolescents completed the Depression Self-rating Scale for Children and 9 (16.1%) of these met the criteria of depression. Sixty nine 7 to 16 years old children and adolescents completed the screening for Child Anxiety Related Disorders and 7 (10.1%) of these met the criteria of anxiety, especially social phobia and school phobia. Eighty nine 6 to 18 years old children and adolescents completed the Symptom Checklist-90 and 43.8% to 77.5% of these experienced mild symptoms like obsession-compulsion (77.5%), hostility (64%), and interpersonal sensitivity (60.7%). Children treated with total body irradiation (TBI) showed more cognitive impairments like motor deficits than those without TBI. Also older children and adolescents had more symptoms like psychoticism.
These findings demonstrated cognitive and psychological late effects of pediatric allo-HSCT survivors in a single center in China and highlighted that the survivors conditioned with TBI had more cognitive impairments and older children and adolescents had more symptoms. Early intervention in these children and adolescents might minimize the cognitive losses and psychological effects.
Keywords: cognitive function, hematopoietic stem cell transplantation, pediatric patients, psychological function
1. Introduction
Haemopoietic stem cell transplantation (HSCT) is a life-saving but an intensive procedure that is associated with potentially severe adverse late effects. For children and adolescents with life-threatening pediatric hematologic malignancies, immunologic deficiency diseases and inherited metabolic disorders, HSCT may be considered as the best or the only viable treatment option after disease relapse and failure of more conventional treatments.[1] Advancements in HSCT procedure (medically, technologically and pharmacologically) led to an increase in the number of HSCT procedures performed each year and dramatic increase in the survival rates of pediatric cancer.[2,3]
Late adverse consequences of HSCT occur both as a result of original treatment of the disease for which HSCT was performed and secondary to toxicity associated with conditioning regimens. Additional damage occurs through the toxic effects of antibiotics, antifungals or immunosuppressive agents that are used to prevent or treat infections or graft-versus-host disease (GVHD). Chronic GVHD may itself cause late treatment-related toxicity.[1–3]
Children undergoing HSCT have already suffered years of aggressive treatment for life-threatening illnesses. These children and their families face a future that may vary from a cure and normality to chronic GVHD, relapse, and/or even death.[1] For children and families those who are over-burdened with wide range of medical, social-emotional, and financial stresses related to the illness, HSCT mayrepresenta severe additional stressor with a profound disturbance for the entire family. HSCT puts each family member at risk for psychological maladjustment. Thus, studies investigating various psychosocial issues in the HSCT setting has increased in recent years.[4]
Children suffer from different psychosocial disorders in different phases of HSCT.[5] Meyers et al[5] reported that 40% of children experienced significant levels of anxiety before HSCT, and this level was significantly decreased during the period of hospitalization and remained low till 8 months post-HSCT. In contrast, Pot-Mees[6] in one of the study on psychosocial reactions to HSCT notedthat 40% children had a marked increase in anxiety, depression, peer isolation and behavioral problems in 6 months after HSCT, while only 15% pre-HSCT. Researchers agreed that depression is increased during hospitalization, and often for prolonged months after HSCT.[5,6] Extended hospital stays are particularly associated with worsened depressive symptoms and withdrawal.[7] Researchers suggest that physical isolation increases depressive symptoms during hospitalization.[6]
Pot-Mees[6] witnessed a constellation of behavioral symptoms in patients 6-months after HSCT, which pointed out to be an ‘after-stress reaction’ and corresponded to the symptoms of post-traumatic stress disorder. Further, this post-traumatic stress reaction persisted in 35% of pediatric patients 12 months after HSCT, indicating that psychological symptoms may remain for many months after undergoing HSCT. Jeong et al[8] reported that children who survived at least 100 days after HSCT had significantly lower scores on the total scale for behavior problems and on most subscales than a normative sample. Compared with the Taiwanese sample, scores of physical and psychosocial quality of life (except bodily pains, mental health, and behavior) were significantly lower in children with HSCT.[8] Baker et al[9] revealed that survivors still suffered from psychological problems, including fears about their future, sense of loss of control, anxiety, and depression during the first year after HSCT. Ferry et al[10] analyzed long-term outcomes and psychosocial aspects in 112 children with malignancies surviving 1 year after HSCT. The results showed that half of the patients had psychological disturbances, like depression, sleep disturbance, anorexia and hyperphagia. Lahaye et al[11] pointed out the presence of wide range of psychosocial impacts, such as affected self-image, social withdrawal, sense of lack of choice, and need for specific attention in cancer children who were 15 years or older and had been cured for more than 3 years after HSCT.
Several researchers illustrated that age at transplantation, conditioning regimen and the length of isolation are considered as effects of HSCT on psychosocial functioning.[12] Age, specifically <7 year olds, and severity of illness influence the level of emotional reactions. Children below 5 years are likely to withdraw, lose their self-help skills and some might even lose their mobility and speech skills.[12] Age at transplantation and conditioning regimen affects the neurocognitive function of HSCT survivors.[13–15] The youngest patients who received total body irradiation (TBI) had a significantly lower IQ and more persistent neuropsychological impairments, which included motor deficits and varying degrees of perceptual, executive and cognitive functions than those who did not receive TBI.[13–15] Psychological morbidity in adolescent and young adult (AYA) patients remained high. The results of AYA hematologic cancer patients showed that 69% of patients reported fear of recurrence, 46% demonstrated symptoms of post-traumatic stress, 28% met the criteria for depression, and 23% met the criteria for anxiety.[16–19] There are more worries regarding the side-effects of treatment, relapse, and even death among AYA patients than younger children.[20] In many children, the social development associated with the length of hospital stay, the length of isolation and absence of school.[21] A majority of HSCT survivors returned to school within 1-year post-HSCT. These children, however, had increased behavioral problems and social isolation.[6]
Research in cognitive and psychological function of pediatric allo-HSCT survivors is rare in China. Hence, in this study, the cognitive and psychological outcomes of 3 to 18 years old children and adolescent post-HSCT in China who survived at least 3 months after undergoing allo-HSCT were investigated.
2. Methods
2.1. Patients and procedures
Patients who underwent allo-HSCT at Hematology/oncology Center of Beijing Children's Hospital affiliated to Capital Medical University between June 2006 and March 2017 were enrolled. Children and adolescents of 3 to 18 years who have survived at least 3 months post-HSCT were asked to participate in the study. Exclusion criteria included diagnosis of brain tumor and psychiatric illness history.
Children and adolescents were subsequently recruited through a preliminary phone contact that aimed at illustrating the objectives of the study. Children and adolescents who agreed to participate in the research were provided with a brief description of the study, informed consent and age-appropriate standardized measures. After receiving the informed consent, the age-appropriate standardized measures were completed online. Clinical information like diagnosis, donor type, conditioning regimen, age at transplantation and so on was extracted from the medical records.
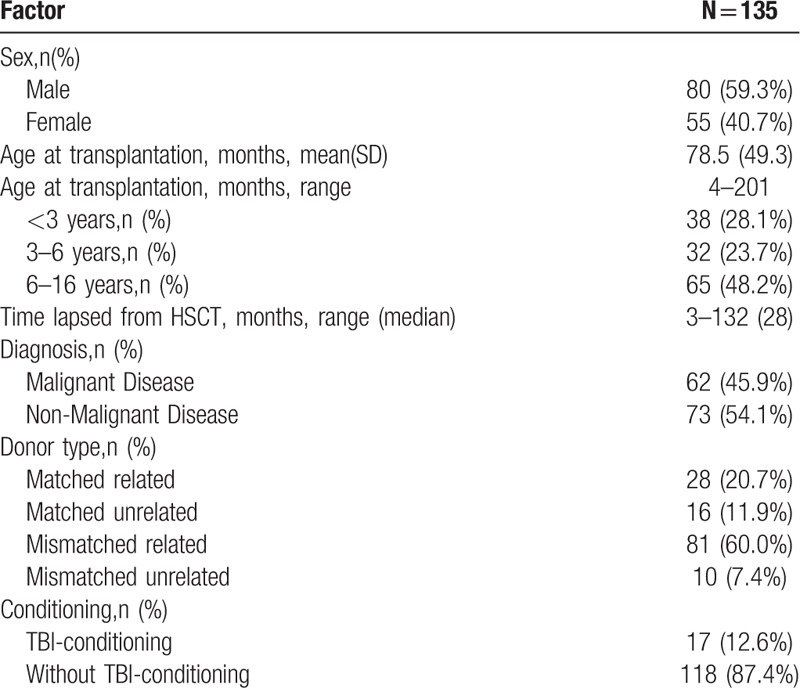
A total of 175 eligible children and adolescents were asked to participate in the study. Of these 175 children and adolescents, 27 were lost to follow-up and 13 refused to participate. Finally, a total of 135 children and adolescents completed the measures. Of these, 80 (59.3%) were male and 55 (40.7%) were female. The mean age at transplantation was 78.5 months (s.d. = 49.3, range 4–201), and the median time lapsed from HSCT was 28 months (range 3–132).
With regard to diagnosis, 45.9% were malignant diseases, including leukemia (42.2%) and lymphoma (3.7%), and 54.1% were non-malignant diseases, including aplastic anemia (31.9%), histocytic diseases (7.4%), and inherited metabolic disorders (14.8%). The type of transplant in majority of the patients was mismatched related (60.0%), followed by matched related (20.7%), matched unrelated (11.9%) and mismatched unrelated (7.4%). 12.6% survivors received TBI. Table 1 presented the demographic and clinical characteristics of the survivors.
Table 1.
Demographic and clinical characteristics of the survivors.
3. Measures
Psychological Questionnaires for 3 to 6 Years Old Children[22,23]: The questionnaires were developed by the researchers of Guangzhou Association of Children Development Center based on the Child Development Questionnaire developed by Levant. The questionnaires consist of 3 age groups: Psychological Questionnaire for 3 to 4 years old children, Psychological Questionnaire for 4 to 5 years old Children and Psychological Questionnaire for 5 to 6 years old Children. Each questionnaire comprises of 38 to 40 items, and consists of six dimensions (motor development, cognitive development, language development, the development of emotion and will, social development and living habits). Each item is rated as 1 (Yes) and 0 (No). The total score for each dimension can be obtained by summing up of all items included in the dimension. If the score of 1 dimension is less than the standard, then the child does not reach the level of health in the dimension.
Depression Self-rating Scale for Children (DSRSC)[24,25]: It was developed by Birleson et al in 1981.[24] It consists of 18 items related to depression in children and adolescents. Subjects are asked to rate their own condition in the most recent 1-week period on a 3-point scale. The scores for the scale range from 0 (never) to 2 (often). The possible score ranged from 0 to 36 and evidence suggested that scores ≥15 effectively discriminated between depressive and non-depressive youth.[25] It has been proved that DSRSC is a reliable and valid depression screening instrument in Chinese children and adolescents.[25]
Screen for Child Anxiety Related Disorders (SCARED)[26–28]: It was developed by Birmaher et al[26] for screening DSM-IV childhood anxiety disorders. The scale included five factors: panic/somatic symptoms (PN); generalized anxiety (GD); separation anxiety (SP); social phobia (SOC), and school phobia (SCH). The respondent is required to rate each item on a 3-point scale (0 = not true, 1 = sometimes true, and 2 = often true.) The possible score ranged from 0 to 82 and evidence suggested that scores ≥23 effectively discriminated between anxious and non-anxious youth.[28] It has been proved that SCARED is a reliable and valid anxiety screening instrument in Chinese children and adolescents.[27,28]
Symptom Checklist-90, Revised (SCL-90-R)[29]: This questionnaire is a 90-item self-report symptom inventory, which is primarily designed to collect the psychological symptom patterns of psychiatric and medical patients. It is a measure of perceived, current psychological symptoms during the previous week. Based on the subjective distress, each item is rated as 1 (no distress), 2 (a little bit distress), 3 (moderately distressed), 4 (quite a bit of distress), and 5 (extremely distressed). The SCL-90-R consists of nine primary symptom dimensions (somatization, obsession-compulsion, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation and psychoticism), and a group of additional items like sleep disorders. Each of the nine symptom dimensions comprises of 6 to 13 items, and the score on each dimension is the mean score derived from all items of such dimension.
3.1. Statistical methods
Continuous variables are presented as means (SD) or medians. Categorical variables are presented as percentages. Logistic regression was used to screen the risk factors of cognitive and psychological late effects. A 2-sided P value threshold of <0.05 was considered as statistical significance. Statistical analyses were performed by using SPSS version 19.0 program package.
4. Results
A total of 41 3 to 6 years old children who were 0 to 6 years old at transplantation completed Psychological Questionnaires for 3 to 6 Year-Old Children. The scores of 21 (51.2%) children in cognitive development dimension, 18 (43.9%) in motor development dimension, 16 (39.0%) in language development and social development dimension, 15 (36.6%) in emotion and will dimension and 14 (34.1%) in living habits dimension were less than the standard. 34.1% to 51.2% post-HSCT children did not reach the level of health in each field of development.
A total of 56 8 to 16 years old children and adolescents who were 0 to 16 years old at the time of transplantation completed the Depression Self-rating Scale for Children and 9 (16.1%) met the criteria of depression. Sixty nine 7 to 16 years old children and adolescents who were 0 to 16 years old at transplantation completed the Screen for Child Anxiety Related Disorders and 7 (10.1%) met the criteria of anxiety, especially social phobia and school phobia. Eighty nine 6 to 18 years old children and adolescents who were 0 to 18 years old at transplantation completed Symptom Checklist-90 and 43.8% to 77.5% experienced symptoms like obsession-compulsion (77.5%), hostility (64%), interpersonal sensitivity (60.7%), depression (60.7%), a group of additional items like sleep disorders (58.4%), anxiety (55.1%), paranoid (47.2%), somatization (47.2%), psychoticism (44.9%), and phobic anxiety (43.8%).
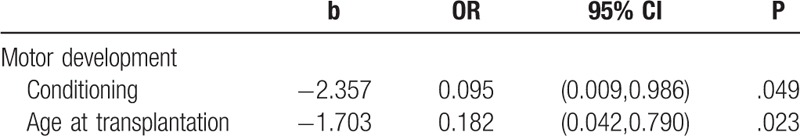
Univariate and multivariate logistic regression were used to analyze the factors of motor development, cognitive development, language development, the development of emotion and will, social development and living habits among 3 to 6 years old children who were 0 to 6 years old at transplantation. The outcomes were divided to 0 and 1, where 0 represents unfavorable outcomes and the score of the dimension was less than the standard, while 1 represents favorable outcomes. The independent variables included sex, diagnosis, donor type, conditioning regimen (TBI-conditioning group and without TBI-conditioning group), and age at transplantation (0–3 years old group and 3–6 years old group). The significant variables of univariate logistic regression included conditioning regimen (OR = 0.091, 95% CI = [0.010,0.846], P = .035) and age at transplantation (OR = 0.177, 95% CI = [0.045,0.696], P = .013) in motor development. The significant variables of multivariate logistic regression included conditioning regimen (OR = 0.095, 95% CI = [0.009,0.986], P = .049) and age at transplantation (OR = 0.182, 95% CI = [0.042,0.790], P = .023).
These results suggested that children who were 3 to 6 years old at transplantation and treated with TBI demonstrated more cognitive impairments like motor deficits than those who were 0 to 3 years old and without TBI. Tables 2 and 3 presented significant variables of univariate and multivariate logistic regression analyses.
Table 2.
The significant variable of univariate logistic regression.
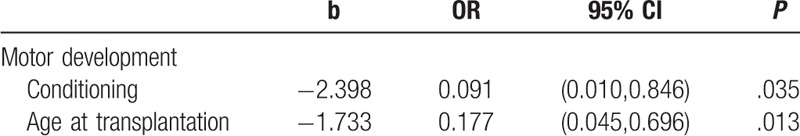
Table 3.
The significant variable of multivariate logistic regression.
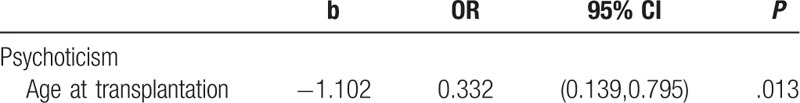
Univariate and multivariate logistic regression was used to analyze the factors of depression, anxiety and psychological symptoms among 6 to 18 years old children and adolescents who were 0 to 18 years at transplantation. The outcomes were divided to 0 and 1, where 0 represents depression, anxiety or with psychological symptoms and 1 represents non-depression, non-anxiety or without psychological symptoms. The independent variables included sex, diagnosis, donor type, conditioning regimen (TBI-conditioning group and without TBI-conditioning group) and age at transplantation (0–8 years old group and 8–16 years old group). The results showed no significant variables for depression or anxiety. Univariate logistic regression analysis showed that age at transplantation (OR = 0.332, 95% CI = [0.139,0.795], P = .013) was a significant variable in psychoticism dimension. This analysis suggested that older children and adolescents had more symptoms like psychoticism. Table 4 presented significant variables of univariate logistic regression.
Table 4.
The significant variable of univariate logistic regression.
5. Discussion
From animal model studies as well as human cancer studies, it is well known that chemotherapy and irradiation constitute potential neuro toxic treatment modalities, and particularly when combined in intensified protocols.[30] As the immature brain is particularly vulnerable to generalized and diffuse insult, pre-transplant conditioning may pose a risk for central nervous system (CNS) functioning and long-term development of the child.[15] Researches on survivors of HSCT have been conducted with the assumption that patients are at great risk for cognitive late effects.[13] This assumption was based on the prevalence of late effects observed in the survivors of leukemia and brain tumors who received similar, although more intense, rates of CNS-directed therapies.[31,32] However, there was minimal evidence regarding cognitive declination over time in HSCT population at large,[12–15,21,33–37] although there are some studies that contradict these findings.[12–15,21,33–35,37] Some studies indicated that the neurocognitive function of HSCT children remained stable over time.[21,33–35] Other studies revealed a significant declination in neurocognitive functioning after SCT, including intelligence quotient (IQ), motor development, language development, memory and so on,[12–14,37] which was in accordance with our findings.
Our research found that about half of 3 to 6 years old post-HSCT children did not reach the standard level of health in motor development, cognitive development, language development, the development of emotion and will, social development and living habits. The mean age of them at transplantation was 35.2 months (range 7–70), which was a critical period for motor development, language development, cognitive development and social development. About half of the children had malignant diseases and 12.6% received TBI-conditioning. The neurotoxicity of repeated chemotherapy, TBI and conditioning might affect the neurocognitive function of the children. Long-term hospitalization, limited activities, and usually 1 to 2 months of isolation during transplantation might have negative impact on their cognitive development. In addition, the children in our center came from different cities of China. They came to Beijing from their hometown, leaving their familiar environment and playmates, and lived in crowded rental rooms near the hospital. Their parents paid attention to the treatment and prognosis of their diseases, but neglected their cognitive development, which might in turn lead to backwardness in their cognitive development.
There are also several controversies about the risk factors of cognitive function in pediatric HSCT survivors. Some researches[38] reported that age at transplantation and treatment with TBI did not influence the cognitive function, while most of the studies clarified[13–15] the relationship between TBI and age on cognitive outcomes in pediatric HSCT survivors. TBI and younger age at transplantation (<6 years old, especially <3 years old) were considered as significant predictors of cognitive declination.[13–15] In our research, children treated with TBI had more cognitive impairments, which was consistent with most of the studies.[13–15] But our findings were surprising given that the children who were 3 to 6 years old at transplantation had more motor declination than those who were 0 to 3 years old, which was in contrast to most other studies.[13–15] The mean time lapsed from HSCT in 0 to 3 years group (M = 24.64, s.d. = 11.19) was significantly longer (P < .001) than the mean time in 3 to 6 years group (M = 6.25, s.d. = 3.22). Previous studies showed that there was a significant improvement in the cognitive function over time after HSCT and would return to baseline levels.[13] In our study, the motor function may be recovered in 0 to 3 years group and this may be the reason why the motor function of 0–3 years group was better than 3 to 6 years group. However, this finding needs further verification in a prospective longitudinal study with larger sample size.
Over-burdened with a wide range of medical, social-emotional, and financial stresses related to the illness, HSCT puts each family member at risk for psychological maladjustment.[1,2,9,10] Studies pointed out that children who undergo HSCT often experience acute psychological effects before HSCT, and the level was markedly increased during the period of hospitalization and remained for a long time post-HSCT.[5,6] We found that 16.1% of 6 to 18 years old post-HSCT children and adolescents experienced depression, 10.1% experienced anxiety and 43.8% to 77.5% experienced different levels of symptoms like obsession-compulsion, hostility and interpersonal sensitivity. Older children and adolescents had more symptoms and required more psychological support.[17–20,39,40] However, there are few psychologists or counselors in the transplantation treatment team in China. The children and adolescents in this study did not receive any other psychological assessment, counseling or psychotherapy except psychological assessment. Post-HSCT children and adolescents in China with psychological maladjustment cannot be detected and treated early. It is imperative for psychologists to involve in the transplantation treatment team in China.
6. Limitations
The current study was a single center design, although our patients were from different cities all over China. Due to the retrospective nature of the study, multicenter and prospective longitudinal studies with larger sample sizes are needed to further verify these findings. Limited measures of Chinese version also restricted the assessment of cognitive and psychological function of children and adolescents in China.
7. Clinical implications
This study provided new insights into the cognitive and psychological outcomes of pediatric allogeneic HSCT survivors in China, which was rarely reported. Survivors conditioned with TBI had more cognitive impairments, and older children and adolescents had more symptoms. These findings highlighted the continuous concerns of the unmet cognitive and psychological needs among pediatric allogeneic HSCT survivors and demonstrated the importance of developing targeted interventions across pediatric allogeneic HSCT care in China, especially the survivors conditioned with TBI or older children and adolescents. It is necessary to involve psychologists in transplantation treatment team in China.
Author contributions
Conceptualization: Yanhui Luo.
Data curation: Yanhui Luo, Peiyi Yang, Yuting Yang, Peiling He, Bin Wang, Guanghua Zhu, Chenguang Jia, Yan Yan.
Formal analysis: Peiyi Yang, Yuting Yang, Peiling He, Bin Wang, Chenguang Jia, Yan Yan, Xuan Zhou.
Methodology: Maoquan Qin.
Resources: Guanghua Zhu.
Writing – original draft: Yuchen Zhou, Xu Peng.
Writing – review & editing: Ruixin Wang, Aihua Wang.
Footnotes
Abbreviations: AYA = adolescent and young adult, CNS = central nervous system, DSRSC = Depression Self-rating Scale for Children, GVHD = graft-versus-host disease, HSCT = Haemopoietic stem cell transplantation, IQ = Intelligence quotient, SCARED = Screen for Child Anxiety Related Disorders, SCL-90-R = Symptom Checklist-90, Revised, TBI = total body irradiation.
How to cite this article: Luo Y, Yang P, Yang Y, He P, Qin M, Wang B, Zhu G, Jia C, Yan Y, Zhou Y, Wang R, Wang A, Zhou X, Peng X. Cognitive and psychological outcomes of pediatric allogeneic hematopoietic stem cell transplantation survivors in a single center in China. Medicine. 2019;98:39(e17307).
The study was approved by the Ethics Committees of the Beijing Children's Hospital, Capital Medical University, and all participants provided written informed consent.
The study supported by the Beijing Natural Science Foundation (7164258).
All authors declare that they have no any conflict of interests.
References
- [1].Barrera M, Boyd-Pringle LA, Sumbler K, et al. Quality of life and behavioral adjustment after pediatric bone marrow transplantation. Bone Marrow Transplant 2000;26:427–35. [DOI] [PubMed] [Google Scholar]
- [2].Clarke SA, Eiser C, Skinner R. Health-related quality of life in survivors of BMT for paediatric malignancy: a systematic review of the literature. Bone Marrow Transplant 2008;42:73–82. [DOI] [PubMed] [Google Scholar]
- [3].Clarke SA, Skinner R, Guest J, et al. Clinical outcomes and health-related quality of life (HRQOL) following haemopoietic stem cell transplantation (HSCT) for paediatric leukaemia. Child Care Health Dev 2011;37:571–80. [DOI] [PubMed] [Google Scholar]
- [4].Andrykowski MA. Psychosocial factors in bone marrow transplantation: a review and recommendations for research. Bone Marrow Transplant 1994;13:357–75. [PubMed] [Google Scholar]
- [5].Meyers CA, Weitzner M, Byrne K, et al. Evaluation of the neurobehavioral functioning of patients before, during, and after bone marrow transplantation. J Clin Oncol 1994;12:820–6. [DOI] [PubMed] [Google Scholar]
- [6].C P-M The Psychological Effects of Bone Marrow Transplantation in Children. Delft, The Netherlands: Eburon Delf; 1989. [Google Scholar]
- [7].Patenaude AF. Psychological impact of bone marrow transplantation: current perspectives. Yale J Biol Med 1990;63:515–9. [PMC free article] [PubMed] [Google Scholar]
- [8].Jeong MS, Choi JY, Chung HI, et al. Psychosocial adjustment and quality of life of children after hematopoietic stem cell transplantation in South Korea. J Pediatr Oncol Nurs 2013;30:218–26. [DOI] [PubMed] [Google Scholar]
- [9].Baker F, Zabora J, Polland A, et al. Reintegration after bone marrow transplantation. Cancer Pract 1999;7:190–7. [DOI] [PubMed] [Google Scholar]
- [10].Ferry C, Gemayel G, Rocha V, et al. Long-term outcomes after allogeneic stem cell transplantation for children with hematological malignancies. Bone Marrow Transplant 2007;40:219–24. [DOI] [PubMed] [Google Scholar]
- [11].Lahaye M, Aujoulat I, Vermylen C, et al. Long-term effects of haematopoietic stem cell transplantation after pediatric cancer: a qualitative analysis of life experiences and adaptation strategies. Front Psychol 2017;8:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pot-Mees C, Zeitlin H. Psychosocial consequences of bone marrow transplantation in children: a preliminary communication. J Psychosoc Oncol 1987;52:73–81. [Google Scholar]
- [13].Willard VW, Leung W, Huang Q, et al. Cognitive outcome after pediatric stem-cell transplantation: impact of age and total-body irradiation. J Clin Oncol 2014;32:3982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mulcahy Levy JM, Tello T, Giller R, et al. Late effects of total body irradiation and hematopoietic stem cell transplant in children under 3 years of age. Pediatr Blood Cancer 2013;60:700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Smedler AC, Winiarski J. Neuropsychological outcome in very young hematopoietic SCT recipients in relation to pretransplant conditioning. Bone Marrow Transplant 2008;42:515–22. [DOI] [PubMed] [Google Scholar]
- [16].Barata A, Wood WA, Choi SW, et al. Unmet needs for psychosocial care in hematologic malignancies and hematopoietic cell transplant. Curr Hematol Malig Rep 2016;11:280–7. [DOI] [PubMed] [Google Scholar]
- [17].Keegan TH, Lichtensztajn DY, Kato I, et al. Unmet adolescent and young adult cancer survivors information and service needs: a population-based cancer registry study. J Cancer Surviv 2012;6:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Muffly LS, Hlubocky FJ, Khan N, et al. Psychological morbidities in adolescent and young adult blood cancer patients during curative-intent therapy and early survivorship. Cancer 2016;122:954–61. [DOI] [PubMed] [Google Scholar]
- [19].Mattson MR, Demshar RK, Daly BJ. Quality of life of young adult survivors of hematologic malignancies. Cancer Nurs 2013;36:E1–7. [DOI] [PubMed] [Google Scholar]
- [20].Luo YH, Sun Y, Zhou X, et al. Quality of life analysis of children with pediatric acute leukemia post hematopoietic stem cell transplantation. J Appl Clin Pediatr 2014;29:1149–53. [Google Scholar]
- [21].Phipps S, Brenner M, Heslop H, et al. Psychological effects of bone marrow transplantation on children and adolescents: preliminary report of a longitudinal study. Bone Marrow Transplant 1995;15:829–35. [PubMed] [Google Scholar]
- [22].Liu W. Psychological Health Education for Young Children. Beijing: Sino-Culture Press; 2004. [Google Scholar]
- [23].Song Z. Psychological Health Measurement. Guangzhou: JiNan University Press; 2002. [Google Scholar]
- [24].Birleson P. The validity of depressive disorder in childhood and the development of a self-rating scale: a research report. J Child Psychol Psychiatry 1981;22:73–88. [DOI] [PubMed] [Google Scholar]
- [25].Linyan SU, Wang K, Zhu Y. Norm of The Depression Self-rating Scale for Children in Chinese Urban Children. Chin Mental Health J 2003;17:547–9. [Google Scholar]
- [26].Birmaher B, Brent DA, Chiappetta L, et al. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry 1999;38:1230–6. [DOI] [PubMed] [Google Scholar]
- [27].Su L, Wang K, Fan F, et al. Reliability and validity of the screen for child anxiety related emotional disorders (SCARED) in Chinese children. J Anxiety Disord 2008;22:612–21. [DOI] [PubMed] [Google Scholar]
- [28].Wang K, Lin-Yan SU, Zhu Y. Norms of the screen for child anxiety related emotional disorders in chinese urban children. Chin J Clin Psychol 2002;10:270–2. [Google Scholar]
- [29].Jin H, Wu W, Zhang M. Analysis of assessment results among normal Chinese by using SCL-90 (in Chinese). J Psychosis Neuol Chin 1986;19:260–2. [Google Scholar]
- [30].Buizer AI, de Sonneville LM, van den Heuvel-Eibrink MM, et al. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: effect of treatment intensity. Pediatr Blood Cancer 2005;45:281–90. [DOI] [PubMed] [Google Scholar]
- [31].Robinson KE, Kuttesch JF, Champion JE, et al. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer 2010;55:525–31. [DOI] [PubMed] [Google Scholar]
- [32].Campbell LK, Scaduto M, Sharp W, et al. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr Blood Cancer 2007;49:65–73. [DOI] [PubMed] [Google Scholar]
- [33].Kupst MJ, Penati B, Debban B, et al. Cognitive and psychosocial functioning of pediatric hematopoietic stem cell transplant patients: a prospective longitudinal study. Bone Marrow Transplant 2002;30:609–17. [DOI] [PubMed] [Google Scholar]
- [34].Phipps S, Dunavant M, Srivastava DK, et al. Cognitive and academic functioning in survivors of pediatric bone marrow transplantation. J Clin Oncol 2000;18:1004–11. [DOI] [PubMed] [Google Scholar]
- [35].Phipps S, Rai SN, Leung WH, et al. Cognitive and academic consequences of stem-cell transplantation in children. J Clin Oncol 2008;26:2027–33. [DOI] [PubMed] [Google Scholar]
- [36].Barrera M, Atenafu E. Cognitive, educational, psychosocial adjustment and quality of life of children who survive hematopoietic SCT and their siblings. Bone Marrow Transplant 2008;42:15–21. [DOI] [PubMed] [Google Scholar]
- [37].Kunkele A, Engelhard M, Hauffa BP, et al. Long-term follow-up of pediatric patients receiving total body irradiation before hematopoietic stem cell transplantation and post-transplant survival of >2 years. Pediatr Blood Cancer 2013;60:1792–7. [DOI] [PubMed] [Google Scholar]
- [38].Perkins JL, Kunin-Batson AS, Youngren NM, et al. Long-term follow-up of children who underwent hematopoeitic cell transplant (HCT) for AML or ALL at less than 3 years of age. Pediatr Blood Cancer 2007;49:958–63. [DOI] [PubMed] [Google Scholar]
- [39].Hoffmeister PA, Storer BE, Syrjala KL, et al. Physician-diagnosed depression and suicides in pediatric hematopoietic cell transplant survivors with up to 40 years of follow-up. Bone Marrow Transplant 2016;51:153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kazak AE, Derosa BW, Schwartz LA, et al. Psychological outcomes and health beliefs in adolescent and young adult survivors of childhood cancer and controls. J Clin Oncol 2010;28:2002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


