Supplemental Digital Content is available in the text
Keywords: breast cancer, nonalcoholic fatty liver disease, prognosis, recurrence
Abstract
Breast cancer is the most common cancer among women worldwide, and it is a main cause of death in women. As with breast cancer, metabolic components are important risk factors for the development of nonalcoholic fatty liver disease (NAFLD). In this retrospective cohort study, we aimed to determine the prevalence of NAFLD in patients with breast cancer and the impact of NAFLD on the prognosis of breast cancer.
Patients with breast cancer were enrolled in the study from January 2007 to June 2017. Hepatic steatosis was evaluated through non-enhanced computed tomography scan by measuring Hounsfield Units in the liver and spleen, respectively; 123 healthy controls who underwent non-enhanced computed tomography scan were also analyzed.
The prevalence of NAFLD in patients with breast cancer was 15.8% (251/1587), which was significantly higher than in healthy controls (8.9%, 11/123) (P = .036). Overall survival did not significantly differ between the groups with and without NAFLD (P = .304). However, recurrence-free survival was significantly higher in patients without NAFLD than in those with NAFLD (P = .009). Among breast cancer patients receiving endocrine treatment, the NAFLD group showed a higher cumulative incidence of significant liver injury than the group without NAFLD (P < .001).
The prevalence of NAFLD in patients with breast cancer is significantly higher than in healthy controls. Moreover, breast cancer patients with NAFLD showed poorer prognosis in terms of recurrence. Therefore, diagnostic evaluation for NALFD is important in managing patients with breast cancer.
1. Introduction
Recently, the prevalence of nonalcoholic fatty liver disease (NAFLD) has continued to increase worldwide, currently affecting 20% to 30% of the adult population, as NAFLD has emerged as a main cause of chronic liver disease.[1] Approximately, 25% to 30% of patients with NAFLD may progress to nonalcoholic steatohepatitis (NASH), which is a progressive liver disease characterized by inflammation and ballooning, and that may further progress to cirrhosis and hepatocellular carcinoma.[2] In addition to advanced liver disease, NAFLD is associated with metabolic diseases including impaired fasting glucose, diabetes, and cardiovascular disease, resulting in decreased overall survival.[3,4] Moreover, NAFLD is also correlated with the development of extra hepatic cancers both of the gastrointestinal tract (esophagus, stomach, pancreas, and colon) and non-intestinal organs (kidney, prostate, and breast).[5]
Breast cancer is the most common cancer in women worldwide, and is a main cause of death in women.[6] Fortunately, mortality from breast cancer has been decreasing with time given the advances in screening strategies and adjuvant treatments.[7] It is well known that the incidence of breast cancer is correlated with age and other risk factors such as BRCA1 or BRCA2 mutation, family history of breast cancer, therapeutic radiation to the chest, hormonal factors, breast density, sedentary lifestyle, hyperlipidemia, and obesity in particular.[8,9] Similar to breast cancer, the importance of the association between NAFLD and metabolic syndrome is coming to be recognized as an important aspect of NAFLD development and the risk of cardiovascular disease.[10,11] Based on this shared association with these metabolic components, it is reasonable to hypothesize a potential relationship between NAFLD and breast cancer. Approximately 63% of newly diagnosed breast cancer patients were shown to have NAFLD,[12] and there was a significantly higher prevalence of NAFLD in patients with breast cancer than in healthy controls (45.2% vs 16.4%).[13] However, the exact prevalence of NAFLD in women with breast cancer is still unclear, and the influence of NAFLD on the prognosis of breast cancer has yet to be identified.
Many patients with breast cancer undergo long-term endocrine therapy with tamoxifen and other selective estrogen receptor modulators (SERMs) when the cancer expresses the estrogen receptor (ER).[14] Importantly, endocrine therapy is associated not only with the development of NAFLD but also with the aggravation of pre-existing NAFLD.[15] Endocrine treatment increases the risk of ALT elevation and the development of fatty liver, which are usually reversed upon discontinuation of the drug. Moreover, endocrine treatment-induced fatty liver disease and liver injury may interfere with scheduled anti-estrogen therapy. Therefore, identifying the association between NAFLD and endocrine treatment in breast cancer patients would be important.
In this retrospective cohort study, we analyzed the prevalence of NAFLD in patients with breast cancer in comparison to that in healthy controls. Furthermore, we evaluated the effects of NAFLD in patients with breast cancer on survival and recurrence.
2. Materials and methods
2.1. Patients and diagnosis
In this study, a total of 1949 newly diagnosed breast cancer patients were consecutively enrolled at the Guro Hospital of the Korea University Medical Center between January 2007 and September 2017. Male patients with breast cancer as well as women with concurrent viable tumors, unverifiable steatosis, and those lost to follow-up without evaluation and treatment were excluded from analysis. Patients who had other chronic liver diseases such as chronic viral hepatitis B/C, autoimmune hepatitis, and primary biliary cholangitis, and who reported significant levels of alcohol consumption (140 g/week) were also excluded. All breast cancer patients were diagnosed by pathological confirmation using biopsy tissue or surgical specimens. The stage was determined by the TNM staging system using the AJCC 7th Edition because most patients were diagnosed before 2017 and treated using the AJCC 7th Edition criteria.[16] Significant liver injury was defined as ALT greater than three times the upper limit of normal (ULN), bilirubin greater than three times the ULN, or any hepatic decompensation. In patients with abnormal baseline ALT, significant liver injury was defined as having more than three times the baseline level of ALT. This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review board of Korea University Guro hospital (KUGH17210).
2.2. Evaluation of hepatic steatosis
Hepatic steatosis was evaluated through computed tomography (CT) scan. In our center, all patients with breast cancer underwent an abdominal CT scan for staging work-up; the abdominal CT scan included a non-enhancement phase. We measured the attenuation of the liver and spleen three times, respectively. NAFLD was diagnosed when the mean attenuation of the liver was lower than 40 Hounsfield Units (HU) or 10 HU lower than that of the spleen.[17] Overall, 123 healthy controls who underwent a non-enhanced CT scan were also analyzed.
2.3. Data collection
All data were collected retrospectively from medical records. The demographic characteristics that were extracted included age, past medical history of diabetes and hypertension (HTN), alcohol consumption, height, body weight, and laboratory data at time of diagnosis. The treatment modalities that patients received were also obtained, including surgery, radiotherapy, chemotherapy, and endocrine therapy. Recurrences were determined through CT scan, magnetic resonance imaging (MRI), or biopsy. The last follow-up date was determined by the patient's final visit to the hospital. Mortality status and date of death were assessed by chart review, issuance of a death certificate, or from the database of the MicroData Integrated Service provided by Statistics Korea.
2.4. Statistical analysis
The baseline characteristics of the patients were presented as medians with interquartile ranges or frequencies (percentages), as appropriate. The prevalence of diabetes, HTN, and NAFLD were compared using Pearson's chi-square test, and the Mann–Whitney U test was used for age, body mass index (BMI), and laboratory data. For the analyses of overall survival, recurrence-free survival, and cumulative incidence, Kaplan–Meier curves were used and the results were compared by log-rank test. To identify the significant prognostic factors for overall survival and recurrence-free survival, we used univariate and multivariate Cox's regression analyses.
Propensity score matching (PSM) was used in the analysis of the prevalence of NAFLD in the breast cancer patient group and the control group. Several variables showing a significant difference between breast cancer patients and controls were assigned to PSM with logistic regression.
All statistical analyses were performed using SPSS version 24 (SPSS, Inc., Chicago, IL, USA). All reported p values were two-tailed, and P value < .05 was considered to be statistically significant.
3. Results
3.1. Baseline characteristics and prevalence of NAFLD
After excluding patients with other accompanying malignancies, unverifiable liver or spleen HU, and chronic liver diseases including chronic hepatitis B, chronic hepatitis C, autoimmune hepatitis, excessive alcohol consumption, and liver cirrhosis, we examined a total of 1587 patients with breast cancer were who diagnosed and treated at the Korea University Guro hospital between January 2007 and June 2017 (Supplemental Fig. 1). We compared the prevalence of NAFLD in breast cancer patients with that in 123 controls who underwent an abdominal CT scan as part of a routine health checkup at the Korea University Guro Hospital over the same period. Baseline characteristics are shown Table 1. Age, HTN prevalence, and levels of hemoglobin, AST, ALT, total bilirubin, albumin, BUN, glucose, and cholesterol significantly differed between the control group and the breast cancer group. In breast cancer patients, the prevalence of NAFLD was 15.8% (251 of 1587) and it was significantly higher than in the control group (8.9%, 11 of 123) (P = .036) (Fig. 1A).
Table 1.
Baseline characteristics.
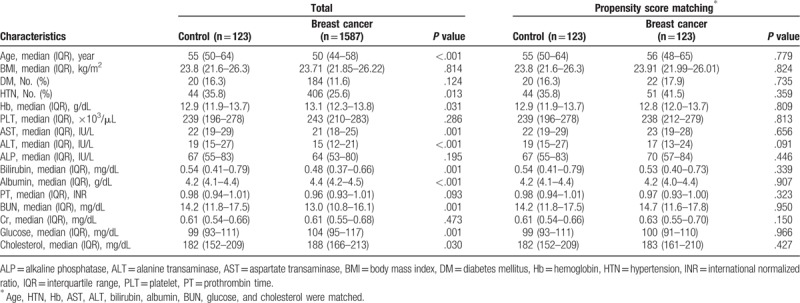
Figure 1.
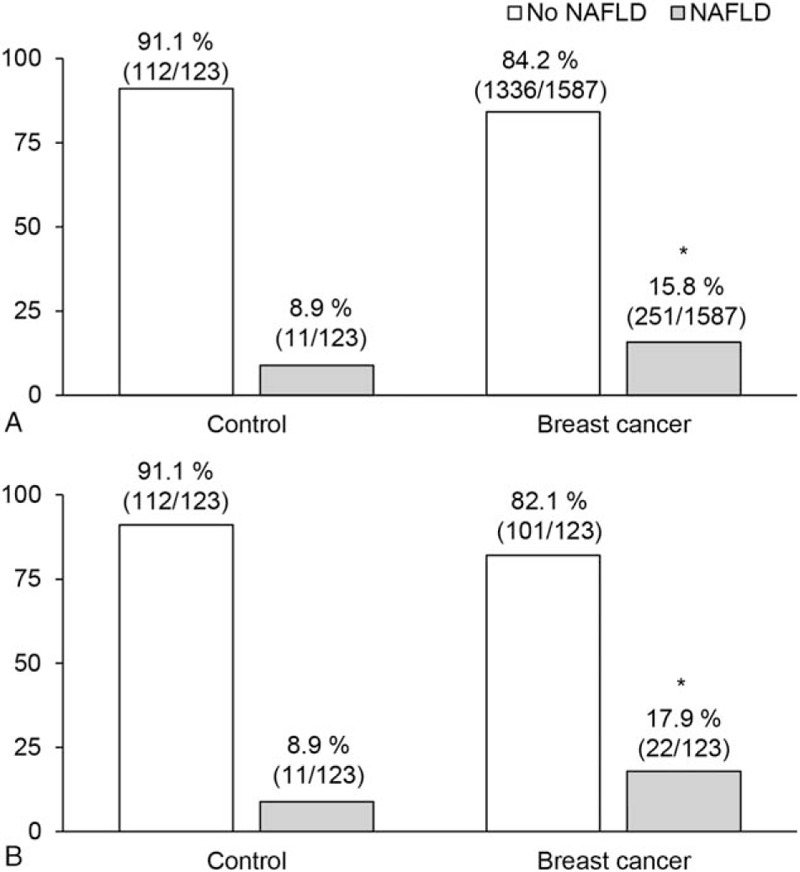
Prevalence of NAFLD in breast cancer patients and controls. NAFLD prevalence (A) before and, (B) after propensity score matching (PSM). ∗ indicates that the there is a significant difference in prevalence of NAFLD between patients of breast cancer and control group (P < .05).
Because there were significant differences in several factors between the control group and the breast cancer patients, we matched the two groups using PSM in order to adjust for age, HTN, hemoglobin, AST, ALT, total bilirubin, albumin, BUN, glucose, and cholesterol. According to PSM, 123 breast cancer patients and 123 controls were matched (Table 1). Following PSM, there was no remaining significant difference in any of the variables. The difference in NAFLD prevalence was still significant between the control group (8.9%, 11/123) and the breast cancer patients (17.9%, 22/123) (P = .040) (Fig. 1B).
3.2. Overall survival was not influenced by the presence of NAFLD in patients with breast cancer
Next, we compared the overall survival of breast cancer patients with NAFLD and those without NAFLD. During the 120-month follow-up period, overall survival did not significantly differ between the NAFLD group (251 patients) and the non-NAFLD group (1336 patients) (P = .304 by log-rank test) (Fig. 2A). The median follow-up durations were 39.8 months in the non-NAFLD group and 34.5 months in the NAFLD group. There were 77 deaths in the non-NAFLD group and 18 deaths in the NAFLD group during the follow-up period. The 5-year survival rate was similar between the NAFLD group (90.2%) and the non-NAFLD group (93.0%). The NAFLD group showed a significantly higher age, BMI, and prevalence of diabetes and HTN than did the non-NAFLD group (Supplemental Table 1). The levels of hemoglobin, platelets, AST, ALT, ALP, PT, and glucose also significantly differed between the two groups. In terms of cancer characteristics, the distributions of cancer stages also showed significant differences. The expression of ER, progesterone receptor (PR), and HER2 did not show significant difference between the NAFLD group and the non-NAFLD group. Non-NAFLD patients received more tamoxifen treatment than NAFLD patients.
Figure 2.
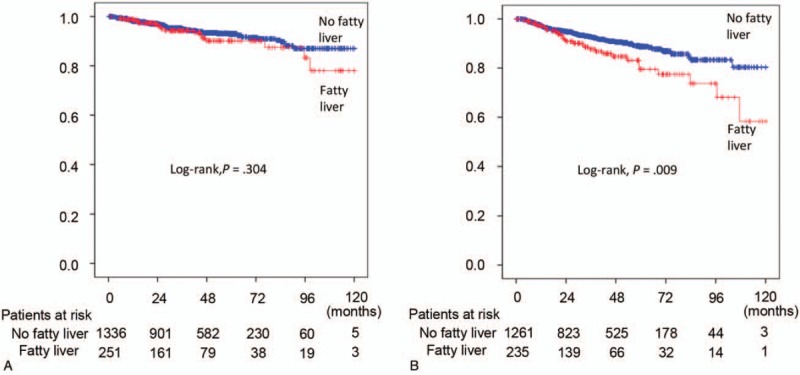
Kaplan–Meier curves for overall survival (A) and recurrence-free survival (B).
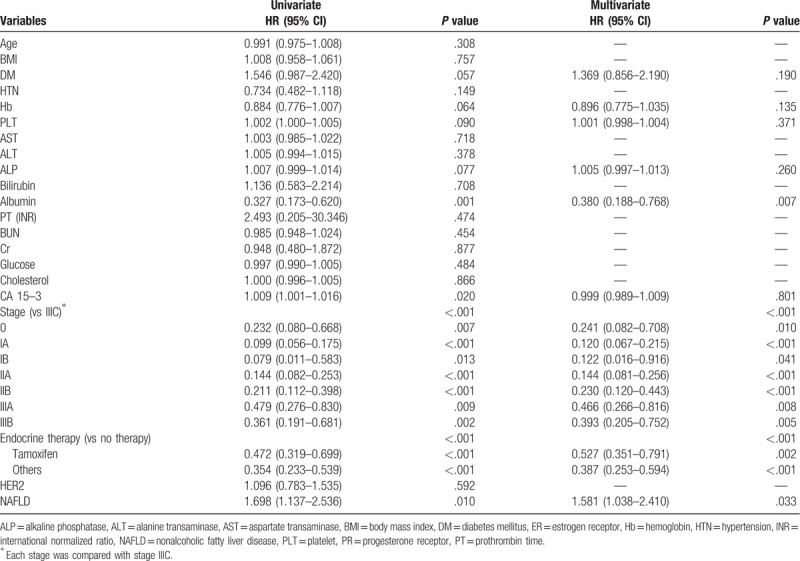
In univariate analysis, age, presence of diabetes and HTN, levels of hemoglobin, platelet, AST, ALP, albumin, PT, BUN, glucose, CA 15-3, tumor stage, and endocrine therapy had higher hazard ratios (HR) for survival (Supplemental Table 2). In multivariate analysis, age (HR 1.035, 95% CI 1.010–1.061; P = .005), presence of DM (HR 1.917, 95% CI 1.018–3.609; P = .044), platelets (HR 1.003, 95% CI 1.000–1.006; P = .039), cancer stage (P < .001), and endocrine therapy (P < .001) were all related to survival.
3.3. Breast cancer patients with NAFLD had more frequent recurrence than those with non-NAFLD after curative surgery
Among the 1496 patients with breast cancer that received curative surgery, 235 patients had NAFLD whereas 1261 patients did not. Demographic characteristics, laboratory data, and tumor characteristics showed some differences between the two groups (Supplemental Table 1). Recurrence-free survival was significantly higher in the non-NAFLD group compared than in the NAFLD group (P = .009 by log-rank test) (Fig. 2B). The median follow-up durations were 38.0 months in the non-NAFLD group and 31.8 months in the NAFLD group. During the follow-up period, there were 106 recurrences in the non-NAFLD group and 31 recurrences in the NAFLD group. Five-year recurrence rate was higher in the NAFLD group (20.4%) than in the non-NAFLD group (11.2%).
According to univariate analysis, albumin, CA 15-3, cancer stage, endocrine therapy, and presence of NAFLD were all significant factors for recurrence after curative surgery (Table 2). Multivariate analysis included diabetes, levels of hemoglobin, platelet, ALP, albumin, CA 15-3 expression, cancer stage, endocrine therapy, and presence of NAFLD. Of these, albumin (HR 0.380, 95% CI 0.188–0.768; P = .007), cancer stage (P < .001), endocrine therapy (P < .001), and presence of NAFLD (HR 1.581, 95% CI 1.038–2.410; P = .033) were significant factors for recurrence after curative surgery (Table 2).
Table 2.
Univariate and multivariate analyses for recurrence.
3.4. Endocrine therapy, especially tamoxifen, induced significant liver injury
Next, we analyzed the cumulative incidence of significant liver injury in breast cancer patients during endocrine treatment. The NAFLD group had an increased cumulative incidence of significant liver injury compared to the non-NAFLD group (P < .001 by log-rank test) (Fig. 3A). When we stratified patients into two groups as the tamoxifen-treated group and the other drug-treated group, NAFLD patients showed an increased incidence of significant liver injury in both groups (Fig. 3B and C). When we compared the incidence of significant liver injury between tamoxifen-treated patients with NAFLD and other drug-treated patients with NAFLD, the cumulative incidence of significant liver injury increased in the tamoxifen-treated group compared with the other drug-treated group (P < .001 by log-rank test) (Fig. 3D). Of the 62 total patients with significant liver injury during endocrine treatment, 37 patients suspended endocrine therapy and only seven discontinued endocrine therapy permanently. In the 37 patients who had their endocrine treatment interrupted, only three patients experienced recurrence of breast cancer.
Figure 3.
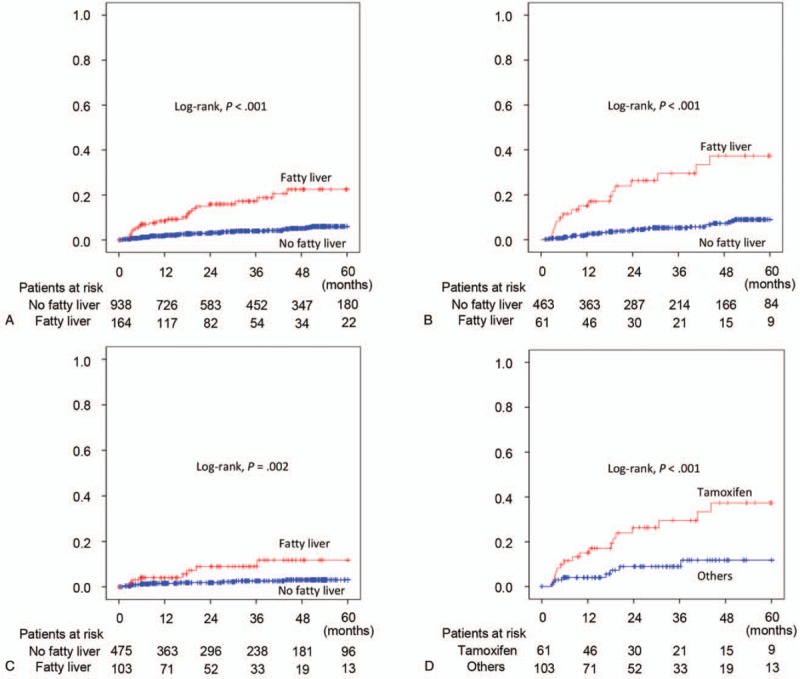
Kaplan–Meier curves for cumulative incidence of significant liver injury in patients receiving endocrine therapy after curative surgery. (A) All patients treated with endocrine therapy. (B) Patients treated with tamoxifen. (C) Patients treated with other endocrine drug. (D) NAFLD patients treated with endocrine therapy.
4. Discussion
Advances in imaging technology and treatment modalities for breast cancer have led to reductions in mortality in patients with breast cancer. However, breast cancer is still the most frequent cancer and a main cause of mortality in women.[6] NAFLD is also a disease in which prevalence is rapidly increasing worldwide.[18] Therefore, the prevalence of breast cancer and NAFLD are likely closely related, and NAFLD may represent an important factor in the development or outcomes of breast cancer. In this large-scale, retrospective cohort study, we found that NAFLD is very frequent in patients with breast cancer and is related to a higher recurrence after curative surgery.
Some studies have previously reported a high prevalence of NAFLD in patients with breast cancer. However, the small number of patients enrolled in these studies limited the strength of these findings.[12,13] In this study, we evaluated 1587 newly diagnosed breast cancer patients and compared the prevalence of NAFLD between these patients and healthy controls over the same period. The prevalence of NAFLD was found significantly higher in patients with breast cancer than in healthy controls. Moreover, this significance was maintained after PSM. Several metabolic diseases are related to the development of NAFLD, such as obesity, diabetes, and HTN.[1] Because breast cancer is also known to be associated with obesity and diabetes, it is plausible that the prevalence of NAFLD is increased in patients with breast cancer.
Although liver biopsy is the gold standard for diagnosis of NAFLD, there are several limitations to perform liver biopsy in all patients with suspected NAFLD, such as cost, inconvenience, sampling error, inter- and intra-observer variability, and invasiveness.[19] There have been many attempts to evaluate hepatic steatosis through imaging studies including sonography, CT scan, and MRI.[20] Among them, CT scan evaluates hepatic steatosis using HU and is a more objective method for measuring hepatic steatosis than sonography, which is operator dependent.[21,22] Because all breast cancer patients routinely underwent CT scan for staging work-up, it is optimized for the evaluation of hepatic steatosis in patients with breast cancer. Herein, NAFLD was diagnosed when the mean attenuation of the liver was lower than 40 HU or 10 HU lower than that of the spleen.[23,24] The major limitation of this method is the low accuracy in the diagnosis of mild hepatic steatosis,[24] thus, the prevalence of NAFLD in our control group (8.9%) was lower than the global prevalence (25.24%).[1] Boyce et al reported a 6.9% prevalence of hepatic steatosis using CT, determined as liver attenuation ≤40 HU.[25] More accurate methods are needed in order to identify mild steatosis in further study.
NAFLD is associated with many other non-liver-related diseases as it is not only a risk factor for obesity, type 2 diabetes, dyslipidemia, metabolic syndrome, and polycystic ovary syndrome, but also associated with other adverse outcomes including cardiovascular disease and extra hepatic cancer.[26] Long-term outcomes of NAFLD patients have been reported to be poor, showing lower overall survival, and elevating liver-related mortality and other comorbidities.[3] Rosato et al reported that metabolic syndrome significantly increased the risk of breast cancer in postmenopausal women.[27] Although that study did not analyze the risk of breast cancer according to the presence of NAFLD, other metabolic components, including obesity, diabetes, hypertension, and waist circumference ≥88 cm, increased the risk of breast cancer, meaning that the correlation between breast cancer and NAFLD could be suggested from its findings. Cancer development in patients with NAFLD may be associated with a bidirectional interaction between NAFLD and metabolic syndrome, although the exact mechanism of this interaction remains unclear.[5] A more recent study reported that sonography-diagnosed NAFLD was associated with the development of breast cancer in women.[28] Our results showed a significantly increased prevalence of NALFD in patients with breast cancer compared to healthy controls. Therefore, NALFD is considered a risk factor for the development of breast cancer and one of the more common comorbidities in patients with breast cancer.
Berrino et al reported that metabolic syndrome is an important prognostic factor in patients with breast cancer.[29] A number of metabolic syndrome components have been associated with breast cancer recurrence, particularly hypertriglyceridemia and low HDL. Our study also found that NAFLD is a significant prognostic factor of the recurrence of breast cancer after curative surgery, although it was not a significant factor in overall survival. This inconsistency might be due to the close surveillance of breast cancer recurrence after surgery and the advancement of imaging modalities. Indeed, most patients with recurrence had undergone curative resection, and some patients had undergone surgery three times. Nevertheless, NAFLD patients showed a tendency of lower overall survival than non-NAFLD patients without statistical significance. Therefore, breast cancer patients with NAFLD might require more careful surveillance for recurrence after surgery than do those with no evidence of NAFLD.
Endocrine therapy is a major treatment modality of ER-positive cancer.[14] In patients with ER-positive tumors, 5-year treatment of tamoxifen or an aromatase inhibitor significantly increased overall survival and reduced recurrence after surgery in patients with early stage breast cancer.[30] However, tamoxifen is associated with other side effects including endometrial cancer, pulmonary embolism, deep vein thrombosis, hot flushes, and hepatic steatosis.[15] Tamoxifen induces or aggravates hepatic fatty liver by elevating serum triglycerides, interfering with β-oxidation, and suppressing estrogen synthesis.[15] In this study, 1496 breast cancer patients were treated with surgery, and of these, 1102 patients received endocrine therapy after surgery, consisting of 524 patients treated with tamoxifen and 578 treated with other drugs. Among these 1102 patients, 62 patients experienced significant liver injury during endocrine treatment. Significant liver injury was more frequent in patients with NAFLD. Among patients with NAFLD, tamoxifen significantly increased the risk of transaminase elevation compared to other endocrine drugs. Therefore, more attention should be paid to transaminase abnormalities in patients with NAFLD undergoing endocrine treatment, especially in the case of tamoxifen treatment. However, most patients that experienced significant liver injury temporarily discontinued endocrine treatment in response. Of these 37 patients who discontinued endocrine therapy due to significant liver injury, breast cancer recurred in only three patients. This finding indicates that short term interruption of endocrine therapy is not a significant factor for breast cancer recurrence.
This study has several limitations. A major limitation is that this is retrospective study, which may lead to a lack of information regarding medical history and laboratory data. However, most breast cancer patients were admitted to the hospital repeatedly and their medical records were sufficiently detailed. We also conducted an extensive review of patient medical charts in order to ensure that there were no missing records. Secondly, there is a possibility that underling liver disease may have induced hepatic steatosis. To avoid this bias, we excluded patients with underlying liver disease, especially patients with a history of alcohol abuse, using records from both doctors and a nurses. Finally, hepatic steatosis was evaluated through CT scanning, which may be slightly inaccurate in the mild stage of steatosis. In future studies, more accurate assessment methods should be preferred, such as controlled attenuation parameters, MRI-estimated proton density fat fraction, and MR spectroscopy.
In conclusion, the prevalence of NAFLD is significantly higher in breast cancer patients than in healthy controls. Moreover, the co-existence of NALFD in patients with breast cancer may be an important prognostic factor for tumor recurrence after curative surgery. Although it is hard to reach a definitive conclusion on the basis of the present results, interdisciplinary expertise should be considered to determine the presence of NAFLD in patients with breast cancer. In addition, patients with NAFLD needed stricter evaluation of recurrence and management of metabolic syndrome. Further studies regarding therapeutic interventions are necessary to improve prognosis in breast cancer patients with NAFLD.
Acknowledgments
This study was supported by a National Research Foundation of Korea grant from the Korean government (the Ministry of Education, Science and Technology) (2018R1A1A1A05076977) and (2018R1A2B2006183).
Author contributions
Conceptualization: Young-Sun Lee, Sang Uk Woo, Jae Hong Seo, Jong Eun Yeon
Data curation: Young-Sun Lee, Ha Seok Lee, Sung Won Chang, Chan Uk Lee, Jung Sun Kim, Young Kul Jung,
Formal analysis: Young-Sun Lee, Ha Seok Lee, Jong Eun Yeon.
Funding acquisition: Young-Sun Lee, Jong Eun Yeon.
Methodology: Chang Hee Lee.
Supervision: Ji Hoon Hoon Kim, Yeon Seok Seo, Jong Eun Yeon.
Validation: Hyung Joon Yim, Sang Uk Woo, Jae Hong Seo, Soon Ho Um, Kwan Soo Byun.
Writing – original draft: Young-Sun Lee, Jong Eun Yeon.
Writing – review & editing: Young-Sun Lee, Ha Seok Lee, Jong Eun Yeon.
Young-Sun Lee orcid: 0000-0001-6396-0859.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CT = computed tomography, ER = estrogen receptor, HTN = hypertension, HU = Hounsfield units, MRI = magnetic resonance imaging, NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis, PR = progesterone receptor, PSM = propensity score matching, SERS = selective estrogen receptor modulators, ULN = upper limit of normal.
How to cite this article: Lee YS, Lee HS, Chang SW, Lee CU, Kim JS, Jung YK, Kim JH, Seo YS, Yim HJ, Lee CH, Woo SU, Seo JH, Yeon JE, Um SH, Byun KS. Underlying nonalcoholic fatty liver disease is a significant factor for breast cancer recurrence after curative surgery. Medicine. 2019;98:39(e17277).
Y-SL and HSL contributed equally to this work.
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
Supplemental Digital Content is available for this article.
References
- [1].Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- [2].Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263–73. [DOI] [PubMed] [Google Scholar]
- [3].Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865–73. [DOI] [PubMed] [Google Scholar]
- [4].Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011;43:617–49. [DOI] [PubMed] [Google Scholar]
- [5].Sanna C, Rosso C, Marietti M, et al. Non-alcoholic fatty liver disease and extra-hepatic cancers. Int J Mol Sci 2016;17:E717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].World Health Organization. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. 2015. Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx Accessed April 3, 2018. [Google Scholar]
- [7].Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784–92. [DOI] [PubMed] [Google Scholar]
- [8].Warner E. Clinical practice. Breast-cancer screening. N Engl J Med 2011;365:1025–32. [DOI] [PubMed] [Google Scholar]
- [9].Emaus MJ, van Gils CH, Bakker MF, et al. Weight change in middle adulthood and breast cancer risk in the EPIC-PANACEA study. Int J Cancer 2014;135:2887–99. [DOI] [PubMed] [Google Scholar]
- [10].Lonardo A, Ballestri S, Marchesini G, et al. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis 2015;47:181–90. [DOI] [PubMed] [Google Scholar]
- [11].Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med 2007;24:1–6. [DOI] [PubMed] [Google Scholar]
- [12].Bilici A, Ozguroglu M, Mihmanli I, et al. A case-control study of non-alcoholic fatty liver disease in breast cancer. Med Oncol 2007;24:367–71. [DOI] [PubMed] [Google Scholar]
- [13].Nseir W, Abu-Rahmeh Z, Tsipis A, et al. Relationship between non-alcoholic fatty liver disease and breast cancer. Isr Med Assoc J 2017;19:242–5. [PubMed] [Google Scholar]
- [14].Sledge GW, Mamounas EP, Hortobagyi GN, et al. Past, present, and future challenges in breast cancer treatment. J Clin Oncol 2014;32:1979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yang YJ, Kim KM, An JH, et al. Clinical significance of fatty liver disease induced by tamoxifen and toremifene in breast cancer patients. Breast 2016;28:67–72. [DOI] [PubMed] [Google Scholar]
- [16].Edge SB. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th edNew York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- [17].Hamer OW, Aguirre DA, Casola G, et al. Fatty liver: imaging patterns and pitfalls. Radiographics 2006;26:1637–53. [DOI] [PubMed] [Google Scholar]
- [18].Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686–90. [DOI] [PubMed] [Google Scholar]
- [19].Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2014;20:475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol 2014;20:7392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Oh H, Jun DW, Saeed WK, et al. Non-alcoholic fatty liver diseases: update on the challenge of diagnosis and treatment. Clin Mol Hepatol 2016;22:327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Strauss S, Gavish E, Gottlieb P, et al. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol 2007;189:W320–3. [DOI] [PubMed] [Google Scholar]
- [23].Limanond P, Raman SS, Lassman C, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology 2004;230:276–80. [DOI] [PubMed] [Google Scholar]
- [24].Lee DH. Imaging evaluation of non-alcoholic fatty liver disease: focused on quantification. Clin Mol Hepatol 2017;23:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Boyce CJ, Pickhardt PJ, Kim DH, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol 2010;194:623–8. [DOI] [PubMed] [Google Scholar]
- [26].Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- [27].Rosato V, Bosetti C, Talamini R, et al. Metabolic syndrome and the risk of breast cancer in postmenopausal women. Ann Oncol 2011;22:2687–92. [DOI] [PubMed] [Google Scholar]
- [28].Kim GA, Lee HC, Choe J, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol 2018;68:140–6. [DOI] [PubMed] [Google Scholar]
- [29].Berrino F, Villarini A, Traina A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat 2014;147:159–65. [DOI] [PubMed] [Google Scholar]
- [30].Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med 1998;339:1609–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


