Abstract
Background:
Shenqi Fuzheng injection (SFI) is a commonly used anti-cancer Chinese patent medicine and has long been prescribed as adjunctive treatment to platinum-based chemotherapy (PBC) in patients with stage III/IV non-small cell lung cancer (NSCLC). However, the efficacy and safety of this combination therapy remain unclear.
Methods:
A systematic review and meta-analysis will be conducted following the Preferred Reported Items for Systematic Review and Meta-analysis (PRISMA) guidelines. Seven databases will be searched for relevant studies from their inception to the present date: PubMed, Web of Science, Cochrane Library, EMBASE, ClinicalTrials.gov, China National Knowledge Infrastructure (CNKI), and Wanfang Databases. All randomized clinical trials comparing SFI in combination with PBC versus PBC alone will be retrieved and assessed for inclusion. Two researchers will independently perform the selection of the studies, data extraction, and synthesis. The Cochrane Risk of Bias Tool will be used to evaluate the risk of bias of the RCTs. The primary endpoint is the disease control rate (DCR), the secondary outcomes are the objective response rate (ORR), survival rate, quality of life (QOL), cellular immune function, and toxicities. Review Manager 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, 2014 Copenhagen, Denmark) will be used to analyze the outcomes.
Results:
This study will systematically evaluate the efficacy and safety of SFI combined with platinum-based chemotherapy in the treatment of stage III/IV NSCLC. The results will be published in a peer-reviewed journal.
Conclusion:
This systematic review will evaluate the effects of SFI as adjunctive treatment to platinum-based chemotherapy in the patients with stage III/IV non-small cell lung cancer, thus providing evidence to the clinical application of this combination therapy.
PROSPERO registration number:
CRD42019137196
Keywords: efficacy, meta-analysis, non-small cell lung cancer, platinum-based chemotherapy, randomized controlled trial, safety, Shenqi Fuzheng injection, systematic review
1. Introduction
Lung cancer is the most common cancer and the leading cause of cancer-related deaths in the world and in China.[1–4] More than 2.1 million people each year are diagnosed with lung cancer, 85% of them are non-small-cell lung cancers (NSCLC)[5,6] and approximately two-thirds of lung cancers are diagnosed at stage III/IV.[7] Although targeted therapy and immunotherapy have significantly improved the clinical outcomes of advanced lung cancer patients, many patients cannot benefit from these precision therapies because they lack an actionable biomarker or have no access to the precision therapies, for those patients, platinum-based chemotherapy (PBC) is still a commonly recommended treatment choice.[8–10] Unfortunately, compared with the patients treated with precision therapies, those treated with PBC usually have lower objective tumor response, worse prognosis, poor quality of life (QOL), and increased risk of chemotherapy-induced toxic effects.[11–15] Therefore, it is very important to find more optimal treatment regimens that can help to improve efficacy and alleviate the toxic effects of PBC treatment for advanced lung cancer patients.
In China, traditional Chinese medicine has been extensively used in the treatment of advanced NSCLC. Shenqi Fuzheng injection (SFI) is an important Chinese patent medicine (Drug Approval Number: Z19990065, China Food and Drug Administration) which is composed of Dangshen (Codonopsis pilosula) and Huangqi (Astragalus membranaceus) and has been widely used as an adjunctive therapy to chemotherapy in the treatment of various cancers, including lung cancer, breast cancer, gastric cancer, and colorectal cancer, etc.[16–22]
The bioactive components of SFI include syringin, calycosin-7-O-β-D-glucopyranoside, lobetyolin, ononin, and astragaloside IV, etc.[23,24] Increasing studies have shown that http://www.theplantlist.org/tpl1.1/record/kew-235193 SFI has antineoplastic properties, including inhibiting cancer growth, promoting apoptosis, increasing chemotherapy sensitivity, and improving immune functions, etc.[25–29]
Some clinical trials and a meta-analysis have already evaluated the effects of SFI combined with chemotherapy, indicating that SFI plus chemotherapy might improve the efficacy, immune function, and reduce adverse events in NSCLC.[30–33] However, the efficacy and safety of SFI plus PBC for patients with stage III/IV NSCLC have never been systematically evaluated. The objective of this systematic review is to assess the effects of SFI plus PBC for stage III/IV NSCLC.
2. Method
2.1. Study registration
This study has been registered as PROSPERO CRD42019137196 (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=137196). Ethical approval is not required because all the research materials are published studies.
2.2. Criteria for considering studies for this review
2.2.1. Types of studies
All relevant prospective clinical trials such as randomized clinical trials, controlled trials will be included in this study. Retrospective studies and non-RCTs will be excluded.
2.2.2. Types of participants
Patients with a clear diagnosis of stage III/IV NSCLC, the age, sex, and ethnicity of the patients are not limited.
2.2.3. Types of interventions
The intervention in the experimental groups is SFI combined with PBC. The intervention in the control groups is PBC alone.
2.2.4. Types of outcome measures
Primary outcomes will be disease control rate (DCR), and secondary outcomes will be the objective response rate (ORR), QOL, cellular immune function, and toxicities.
2.3. Search methods for the identification of studies
2.3.1. Search strategy
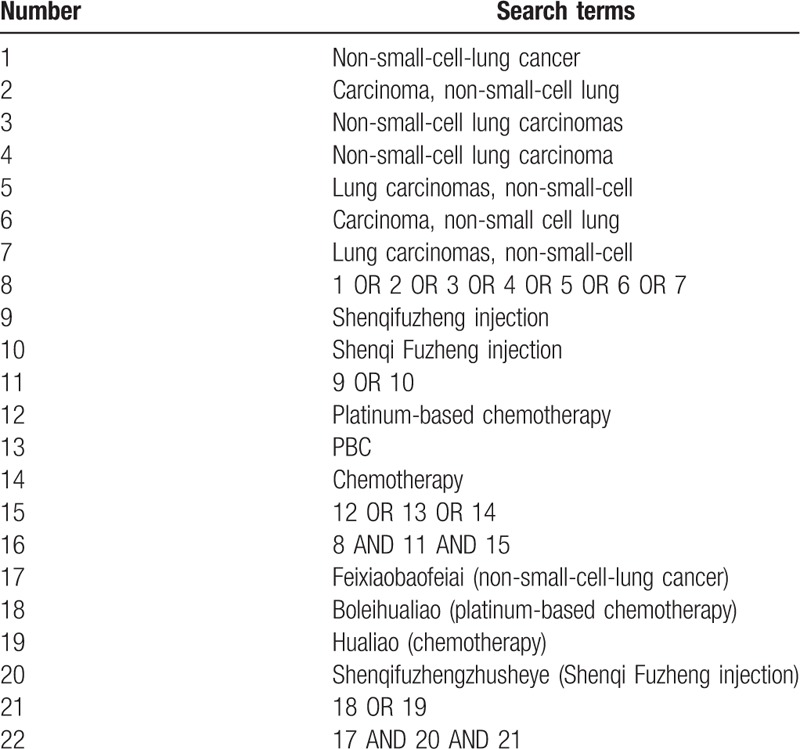
Two independent reviewers (HWC and XJY) will carry out a comprehensive search of the PubMed, Medline, Web of Science, Cochrane Library, EMBASE, China National Knowledge Infrastructure (CNKI), Wanfang Databases. The last search date will be Sept. 30, 2019. The strategy for searching in English databases and Chinese databases has been presented as an example in Table 1. Besides, we also searched and assessed the relevant systematic reviews and meta-analyses, aimed at finding the potential studies from their references.
Table 1.
Search strategy applied in English databases (PubMed, Medline, EMBASE, etc) and search strategy applied in Chinese databases (China National Knowledge Infrastructure [CNKI], Wanfang Databases).
2.3.2. Study selection
Two reviewers (HWC and XJY) will independently select relevant and inclusive articles by screening article titles and abstracts. Any disagreements will be resolved by consensus.
2.3.3. Data collection and management
Two reviewers (HWC and ZTL) will independently evaluate the included RCTs and extract the data. We will use the intention-to-treat (ITT) analysis to analyze the results whenever available. We will extract all information from each report such as the authors, date of publication, countries, participants, interventions, outcomes, and results, study methods, etc. Any disagreement will be resolved by discussing with a third reviewer (QBW).
2.4. Assessment of risk of bias in included studies
Following the “Risk of Bias Assessment Tool” of the Cochrane Handbook for Randomized Controlled Trials,[34] 2 researchers will independently assess the methodological treatment of the included literature. The risk of biases will be evaluated by some contents such as sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting, etc.
2.4.1. Measurement of treatment effect
For dichotomous data, risk ratio (RR), odds ratio (OR), or hazard ratio (HR) with their 95% confidence intervals (95% CIs) will be showed. For continuous data, weighted mean difference (WMD) or standardized mean difference (SMD) with their 95% confidence intervals (95% CIs) will be presented.
2.4.2. Assessment of heterogeneity
The I2 statistic will be used to determine the heterogeneity. If I2 < 50%, heterogeneity is regarded as minor. If I2 > 50%, heterogeneity is regard as substantial. When heterogeneity is observed, subgroup analysis will be performed to identify the possible causes.[34,35]
2.4.3. Data synthesis
If heterogeneity is minor, a fixed-effects model will be used to estimate the summary RR (OR or RD), WMD (or SMD) and their 95% CIs, and meta-analysis will be carried out; if heterogeneity is substantial, a random-effects model will be used for data pooling, and meta-analysis will be conducted. If quantitative synthesis is not appropriate, a systematic narrative synthesis will be provided with the information presented to summarize and explain the characteristics and findings of the included studies.[36] The strength of the body of evidence will be judged using the Grading of Recommendations Assessment, Development and Evaluation working group methodology (GRADE).[37]
2.4.4. Risk of bias across trials
Funnel plots will be used to detect reporting biases. When the number of the included trials is >10, Begg tests, Egger test, and funnel plots will be used to examine the potential bias in the RCTs included in the meta-analysis.[38,39]
2.4.5. Additional analyses
When heterogeneity is significant, subgroup analysis will be used to determine the possible causes such as sample size, age, sex, drug dose, dosage form, and course of treatment, etc. Sensitivity analysis, subgroup analysis will be used to determine the robustness of results. A meta-regression analysis will be performed to test the potential heterogeneity.
3. Discussion
Currently, there is no published systematic review and meta-analysis evaluating the efficacy and safety of SFI plus PBC for stage III/IV NSCLC, this review will provide valuable evidence to this combination therapy for the patients with advanced NSCLC.
Author contributions
QBW, WZ, and LHL conceived and designed the study, revised the manuscript, HWC, XJY, and ZTL developed the criteria and performed literature research, and wrote the protocol, TL, CX, and JW advised on protocol design and revised the manuscript. All authors read and approved the final manuscript. QBW is the guarantor of the review.
Hongwei Chen orcid: 0000-0001-9010-9728.
Xiaojun Yao orcid: 0000-0002-6972-2971.
Zhengtang Liu orcid: 0000-0002-1155-8195.
Ting Li orcid: 0000-0003-4990-5997.
Cong Xu orcid: 0000-0002-9110-4600.
Jue Wang orcid: 0000-0002-6151-1117.
Xinbing Sui orcid: 0000-0001-7330-0467.
Elaine Lai-Han Leung orcid: 0000-0002-3705-8084.
Qibiao Wu orcid: 0000-0002-1670-1050.
Footnotes
Abbreviations: CI = confidence interval, CNKI = China National Knowledge Infrastructure, DCR = disease control rate, EMBASE = Excerpt Medica Database, HR = hazard ratio, ITT = intention-to-treat, NSCLC = non-small cell lung cancer, OR = odds ratio, ORR = objective response rate, PBC = platinum-based chemotherapy, QOL = quality of life, RCT = randomized controlled trial, RR = risk ratio, SMD = standardized mean difference, SQZFI = Shenqi Fuzheng injection, WMD = weighted mean difference.
How to cite this article: Chen H, Yao X, Liu Z, Li T, Xu C, Wang J, Sui X, Leung EL-H, Wu Q. Efficacy and safety of Shenqi Fuzheng injection combined with platinum-based chemotherapy for stage III/IV non-small cell lung cancer. Medicine. 2019;98:39(e17350).
HC, XY, and ZL are equal contributors and co-first authors.
This study was funded by the Science and Technology Development Fund, Macau SAR (file No. 130/2017/A3, 0099/2018/A3, 0052/2018/A2, and 0096/2018/A3), the National Natural Science Foundation of China (grant No. 81874380, 81672932, and 81730108), Zhejiang Provincial Natural Science Foundation of China for Distinguished Young Scholars (grant No. LR18H160001), Zhejiang province science and technology project of TCM (grant No. 2019ZZ016) and Key Project of Hangzhou Ministry of Science and Technology (grant No. 20162013A07, 20142013A63). The funders did not play any role in developing the protocol.
Amendments: If we need to amend this protocol, we will describe the change, the date of each amendment, and give the rationale in this section. Changes will not be incorporated into the protocol.
The authors have no conflicts of interest to disclose.
References
- [1].AHRQ. Agency for Healthcare Research and Quality, Effective Health Care Program. Research Protocol–Jul 21, 2014: treatment of non-metastatic muscle-invasive bladdercancer; 2014. Available at: http://effectivehealthcare.ahrq.gov/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=1940#9004 Accessed August 15, 2019. [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [4].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [5].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [6].American Cancer Society. Cancer Facts and Figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- [7].Morgensztern D, Ng SH, Gao F, et al. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol 2010;5:29–33. [DOI] [PubMed] [Google Scholar]
- [8].Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest 2003;123:2096–103. [DOI] [PubMed] [Google Scholar]
- [9].Goffin J, Lacchetti C, Ellis PM, et al. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol 2010;5:260–74. [DOI] [PubMed] [Google Scholar]
- [10].de Castro J, Tagliaferri P, de Lima VCC, et al. Systemic therapy treatment patterns in patients with advanced non-small cell lung cancer (NSCLC): PIvOTAL study. Eur J Cancer Care (Engl) 2017;26: doi: 10.1111/ecc.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mannion E, Gilmartin JJ, Donnellan P, et al. Effect of chemotherapy on quality of life in patients with non-small cell lung cancer. Support Care Cancer 2014;22:1417–28. [DOI] [PubMed] [Google Scholar]
- [12].Takimoto T, Nakabori T, Osa A, et al. Tubular nephrotoxicity induced by docetaxel in non-small-cell lung cancer patients. Int J Clin Oncol 2012;17:395–8. [DOI] [PubMed] [Google Scholar]
- [13].De Marinis F, Barberis M, Barbieri V, et al. Diagnosis and first-line treatment of non-small cell lung cancer in the era of novel immunotherapy: recommendations for clinical practice. Expert Rev Respir Med 2019;13:217–28. [DOI] [PubMed] [Google Scholar]
- [14].Wang XW, Liu ZT, Sui XB, et al. Elemene injection as adjunctive treatment to platinum-based chemotherapy in patients with stage III/IV non-small cell lung cancer: a meta-analysis following the PRISMA guidelines. Phytomedicine 2018;59:152787. [DOI] [PubMed] [Google Scholar]
- [15].Leung EL, Wu QB. Concurrent use of herbal products with prescription drugs is a double-edged sword and evidence-based medicine contributes to reshaping the practice. Pharmacol Res 2019;141:609–10. [DOI] [PubMed] [Google Scholar]
- [16].Yang Y, Ting W, Xiao L, et al. Immunoregulation of Shenqi Fuzheng injection combined with chemotherapy in cancer patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2017;2017:5121538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li PW, Bi GW, Liu BK. Shenqi Fuzheng injection combined with chemotherapy in the treatment of malignant tumors. China J Chin Materia Medica 2000;25:115–7. [Google Scholar]
- [18].Liu S, Zhang D, Wu J, et al. Shenqi Fuzheng injection in the treatment of breast cancer: a meta-analysis of randomized controlled trials. Integr Cancer Ther 2019;18:1534735418816824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yao K, Ma Y, Ma W, et al. Shenqifuzheng injection combined with chemotherapy in the treatment of advanced gastric cancer: a systematic review and meta-analysis. J Cancer Res Ther 2014;10suppl:70–4. [DOI] [PubMed] [Google Scholar]
- [20].Ai Q, Zhang W, Xie Y, et al. Post-marketing safety monitoring of Shenqi Fuzheng injection: a solution made of dangshen (Radix Codonopsis) and huangqi (Radix Astragali Mongolici). J Tradit Chin Med 2014;34:498–503. [DOI] [PubMed] [Google Scholar]
- [21].Lv Y, Zhang G, Ma Y, et al. Shenqi Fuzheng injection combined with chemotherapy for breast cancer: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2015;2015:635858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xu R, Lin L, Li Y, et al. ShenQi FuZheng injection combined with chemotherapy in the treatment of colorectal cancer: a meta-analysis. PLoS One 2017;12:e0185254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang L, Qu H. Development and optimization of SPE-HPLC-UV/ELSD for simultaneous determination of nine bioactive components in Shenqi Fuzheng injection based on quality by design principles. Anal Bioanal Chem 2016;408:2133–45. [DOI] [PubMed] [Google Scholar]
- [24].Wang J, Tong X, Li P, et al. Bioactive components on immuno-enhancement effects in the traditional Chinese medicine Shenqi Fuzheng Injection based on relevance analysis between chemical HPLC fingerprints and in vivo biological effects. J Ethnopharmacol 2014;155:405–15. [DOI] [PubMed] [Google Scholar]
- [25].Li W, Xu Q, He YF, et al. Anti-tumor effect of steamed Codonopsis lanceolata in H22 tumor-bearing mice and its possible mechanism. Nutrients 2015;7:8294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dong BY, Wang C, Tan L, et al. Inhibitory effect of Shenqi Fuzheng injection combined with docetaxel on lung cancer cells. J Zhejiang Univ Sci B 2017;18:76–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xiong Y, Zhao Q, Gu L, et al. Shenqi Fuzheng Injection reverses cisplatin resistance through mitofusin-2-mediated cell cycle arrest and apoptosis in A549/DDP cells. Evid Based Complement Alternat Med 2018;2018:8258246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Du J, Cheng BC, Fu XQ, et al. In vitro assays suggest Shenqi Fuzheng injection has the potential to alter melanoma immune microenvironment. J Ethnopharmacol 2016;194:15–9. [DOI] [PubMed] [Google Scholar]
- [29].Ding ZG, Li NQ, Tao DS. Effects of Shenqi Fuzheng injection on gene expression profile of liver tissue with metastatic carcinoma in mice. Zhongguo Zhong Xi Yi Jie He Za Zhi 2008;28:135–8. [PubMed] [Google Scholar]
- [30].Ren L. Effect of Shenqi Fuzheng injection on the immunologic and hematopoietic function in patients with advanced non-small cell lung cancer treated with chemotherapy. Chin J Clin Oncol Rehabil 2014;21:463–5. [Google Scholar]
- [31].Luo BP, Zhou Y, Cao T, et al. Clinical trial of Shenqi Fuzheng injection on quality of life in patients with non-small cell lung cancer after chemotherapy. Chin J Clin Pharmacol 2018;34:2137–9. [Google Scholar]
- [32].Chen YF, Sun YN, Ou WH, et al. Clinical observation of Shenqi Fuzheng injection combined with chemotherapy in treating non-small cell lung cancer. Chin J Clin Rational Drug Use 2018;11:86–7. [Google Scholar]
- [33].Dedong C, Huilin X, Anbing H, et al. The effect of ShenQi FuZheng injection in combination with chemotherapy versus chemotherapy alone on the improvement of efficacy and immune function in patients with advanced non-small cell lung cancer: a meta-analysis. PLoS One 2016;11:e0152270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; 2011. Available at: http://www.cochrane-handbook.org Accessed on August 5, 2018. [Google Scholar]
- [35].Wu QB, Li GC, Lei WI, et al. The efficacy and safety of tiotropium in Chinese patients with stable chronic obstructive pulmonary disease: a meta-analysis. Respirology 2009;14:666–74. [DOI] [PubMed] [Google Scholar]
- [36].Whitehead PJ, Drummond AE, Walker MF, et al. Interventions to reduce dependency in personal activities of daily living in community-dwelling adults who use homecare services: protocol for a systematic review. Syst Rev 2013;2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines. 11: Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013;66:151–7. [DOI] [PubMed] [Google Scholar]
- [38].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [39].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


