Abstract
Continuous epidural block (CEB) is a popular clinical method for controlling postherpetic neuralgia (PHN). However, the long-term effects of CEB on PHN have not yet been established. This study aimed to confirm the clinical efficacy of epidural electrical stimulation catheters in CEB to manage PHN.
Patients were classified into 2 groups: those with subacute PHN, between 30 and 180 days after the onset of the rash; and those with chronic PHN, over 180 days after the onset of the rash. On the basis of the type of catheter used, the patients were further divided into the following 2 groups: the esopocan group, in which the site of herpes zoster infection was confirmed using a contrast medium alone; and the epistim group, in which an additional method of electrical stimulation through a guide-wire in the catheter was used for detecting the site affected by herpes. Clinical efficacy was assessed with a numerical rating scale immediately 1, 3, and 6 months after the procedure. We also investigated whether additional interventional treatment was necessitated because of insufficient pain control during the 6-month follow-up.
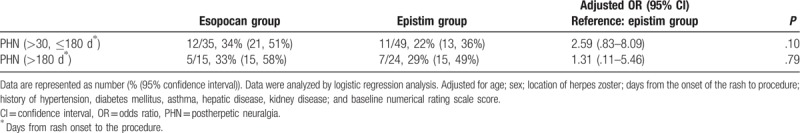
We examined 88 patients. In the subacute PHN period, the numerical rating scale score was significantly lower in the epistim group than in the esopocan group until 6 months. In the chronic PHN period, no significant differences in the numerical rating scale scores were observed between the 2 groups until 6 months. In the subacute PHN period, the adjusted odds ratio for other interventional procedures within 6 months in the esopocan group versus the epistim group was 2.59 (95% confidence interval [CI] 0.83–8.09, P = .10), and in the chronic PHN period, it was 1.31 (95% CI 0.11–5.46, P = .79).
Epidural drug administration to specific segments using electrical stimulation catheters may be more useful in mitigating zoster-associated pain in subacute PHN.
Keywords: continuous epidural block, electrical stimulation, postherpetic neuralgia
1. Introduction
Herpes zoster is caused by reactivation of dormant varicella zoster virus in the sensory ganglia of the spinal cord. Patients with reduced T-cell-mediated immunity due to stress, aging, or immunosuppression are at increased risk.[1–3] Postherpetic neuralgia (PHN), which is the most common complication of herpes zoster, is variously defined as pain that lasts for 30 days or more than 3 months after the onset of a skin rash.[4–6] The pathophysiology of PHN is not yet fully understood, but it can be explained on the basis of 2 mechanisms. One is the mechanism of sensitization, wherein inflammatory mediators such as substance P, histamine, and cytokines reduce the stimulation threshold of the nociceptors, and the other is the mechanism of deafferentation, wherein swelling with inflammation compresses the sensory ganglion of the intervertebral column causing ischemia and nerve tissue damage.[1] In particular, if the pain persists for more than 180 days after the onset of rash, it is considered to be well-established PHN, and the possibility of pain reduction has been reported to be very low.[4–6]
The severity of PHN varies from mild to extreme. However, in the elderly, severe unmanageable zoster-associated pain (ZAP) can cause depression, fatigue, and sleep disturbances.[7,8] The socioeconomic consequences of long-term severe pain include reduced socialization and decreased quality of life.[9]
The goal of PHN treatment is to improve quality of life by relieving pain.[1] Pharmacological agents, including anticonvulsants, tricyclic antidepressants, lidocaine patches, 8% capsaicin patches, and analgesics, and interventional therapies, such as the epidural nerve block, pulsed radiofrequency (pRF), sympathetic block, and spinal cord stimulation, have been reported to reduce the severity of PHN.[1,5,10–13] Continuous epidural block (CEB) is used in clinical practice when ZAP is refractory to conservative treatment.[14,15] Administered of CEB in the acute phase of herpes zoster reduces pain and prevents PHN.[14–16] In addition, CEB has been reportedly effective in managing PHN.[5,17] To maximize the therapeutic effect of CEB, it is important to identify the precise target site of herpes zoster and accurately insert the epidural catheter in the affected site of the epidural space.[18,19]
Conventionally, CEB administration mainly included evaluating the epidural levels associated with herpes zoster based on the site of the rash and the area of pain, and thereafter, examining the position of the catheter by using a diffusing contrast agent injected via the epidural catheter. However, we performed CEB using an electrical stimulation epidural catheter. This was an additional method of verifying whether the epidural catheter was correctly placed at the area of pain related to herpes zoster by providing electrical stimulation through the epidural catheter. In this retrospective study, we aimed to compare the use of a conventional epidural catheter and an epidural electrical stimulation catheter in CEB to manage PHN.
2. Methods
2.1. Study design
Our retrospective observational study adhered to the STROBE checklist (S1 checklist). This study was approved by the Institutional Review Board of the Korea University Medical Center, Guro Hospital, Seoul, Republic of Korea (2019GR0073) on March 11, 2019. We analyzed the medical records of patients who underwent CEB for ZAP between June, 2010 and October, 2017. Of these, only the patients who received procedures 30 days after the onset of the rash were investigated in our study. Patients who underwent the procedure between 30 and 180 days after the onset of the rash were classified as subacute PHN and those after 180 days of the onset of ZAP were classified as chronic PHN. At each time point, patients who underwent CEB were classified into 2 groups according to the type of catheter used. In the esopocan group, the site affected by herpes zoster was confirmed using only contrast medium. In the epistim group, electrical stimulation through a guide-wire in the catheter identified the site affected by herpes zoster.
Inclusion criteria were: patients older than 50 years who underwent a 6-month follow-up after CEB and were treated using standard medication, without any interventional treatments before CEB. The exclusion criteria were: follow-up loss within 6 months after CEB; insufficient medical records; patients receiving other epidural drugs, such as opioids; patients with an immunosuppressed status; patients who did not maintain the catheter for more than 10 days after CEB; patients who did not receive antiviral therapy at the beginning of herpes zoster infection; patients who did not receive standard medication before the procedure; patients who stopped medication (gabapentin or pregabalin, and opioids) because of side effects; and patients who could not communicate due to neurological deficits or dementia. In addition, patients who underwent other interventional procedures because of the aggravation of ZAP within 6 months after CEB were excluded from the study, and these patients were analyzed separately.
2.2. Procedures
2.2.1. Continuous epidural block: esopocan group
With the patient in the prone position, an 18-gauge Tuohy needle was inserted into the interlaminar space at the second or third vertebral level below the target level under fluoroscopic guidance. The epidural space was identified using the loss of resistance technique, and an esopocan catheter (Perifix Soft-Tip catheter: 20-gauge, closed-tip, and multi-orifice epidural anesthesia catheter; B. Braun, Germany) was inserted through the Tuohy needle and placed at the target level (Fig. 1). The position of the epidural catheter tip was confirmed under fluoroscopy using a contrast dye. After confirmation, 0.187% ropivacaine and 1 mg dexamethasone (8 mL total) were administered via the epidural catheter.
Figure 1.
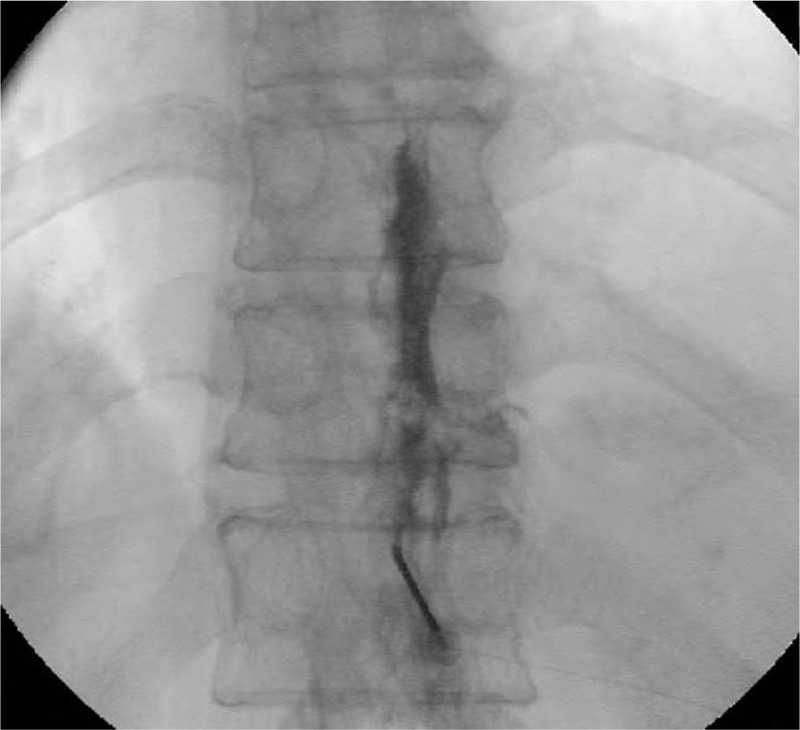
Fluoroscopic images of conventional continuous epidural block. The positioning of the catheter tip is confirmed using a contrast agent.
2.2.2. Continuous epidural block: epistim group
After the epidural space was confirmed using the loss of resistance technique, an epistim catheter (EpiStim catheter: 20-gauge, open-tip, 800-mm long epidural anesthesia catheter; Sewoon Medical Co., Ltd., Seoul, Korea) was inserted at the target vertebral level through the Tuohy needle. This epidural catheter was confirmed radiographically, and has a built-in conductive guide-wire (Nitinol; length: 1100 mm) with an 800-mm section inside the catheter and a 300-mm section exposed for connection to an electrical nerve stimulator. The cathode of the electrical nerve stimulator (EZstim; Life-Tech, Inc., Stafford, TX) was connected to the exposed guide-wire, and the anode was attached to an electrode on the patient's calf. Electrical stimulation of 0 to 5 mA was applied using the electrical nerve stimulator through the guide-wire. To place the epidural catheter precisely in the herpes zoster ganglion, the catheter was placed at the anticipated ganglion and electrical stimulation was performed. If the electrical stimulation was applied to a region other than the appropriate ganglion and skin segment, the catheter was re-adjusted under fluoroscopy and electrical stimulation repeated. After confirming that electrical stimulation was applied to the herpes zoster ganglion and herpes zoster skin segment, the guide-wire was removed and the epidural catheter was placed (Fig. 2). The position of the epidural catheter tip was confirmed using a contrast dye under fluoroscopy, and then 0.187% ropivacaine and 1 mg dexamethasone (8 mL total) were administered via the epidural catheter.
Figure 2.
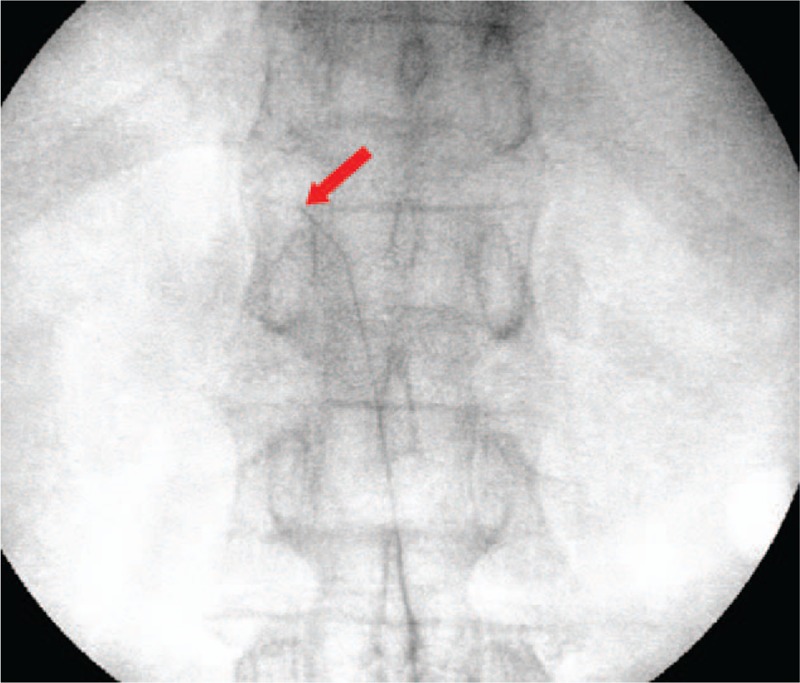
Fluoroscopic images of continuous epidural block with the epistim catheter. This catheter has a built-in conductive guide-wire that allows the detection of catheter tip location using radiography along with electrical stimulation. Arrow indicates the guide-wire in the epistim catheter.
In both groups, the catheter was fixed using subcutaneous tunneling to minimize the risk for infection and catheter migration. For continuous drug administration, the catheter was maintained for 10 days to 2 weeks. For both in-patients and outpatients, a physician observed the procedure site and performed daily dressing changes.
After an initial drug injection all patients received a continuous epidural infusion of 4 mL/h (275 mL of 0.11%–15% ropivacaine) using a portable balloon infusion device (AutoFuser pump; ACE Medical Co., Ltd., Seoul, Korea). The ropivacaine concentration was adjusted according to the pain relief and side effects. In addition to CEB, anticonvulsant agents (pregabalin or gabapentin) and analgesics, which are standard treatments for PHN, were administered to patients in both the groups.
2.3. Data collection
The following data were collected for demographic analysis: age, sex, involved dermatome, days from the onset of the rash to the CEB, and history of hypertension, diabetes, liver disease, kidney disease, and asthma. The collected ZAP data were recorded using the numerical rating scale (NRS) from 0 to 10, with 0 indicating “no pain” and 10 indicating the “maximum amount of pain imaginable.” On this scale, the patients were asked to indicate the number that best represents the average pain over the past 24 hours. We collected the NRS records before the procedure (baseline NRS score), NRS score immediately after the procedure, and NRS score 1 to 6 months after the procedure. We also investigated whether additional interventional treatments were performed because of insufficient pain control during the 6-month follow-up period after each procedure.
2.4. Outcome measurements
The analgesic effect in each group was assessed using the NRS. The subacute and chronic PHN periods were analyzed. For each period, we evaluated whether the NRS score was significantly reduced at 6 months compared with baseline in the esopocan and epistim groups. To compare the analgesic effects between the 2 groups, the NRS scores were compared immediately before the procedure, immediately after the procedure, and at 1, 3, and 6 months after the procedure.
2.5. Statistical analysis
Demographic data were analyzed using the Kolmogorov–Smirnov test to assess the normality of data distribution. Demographic data that followed a normal distribution were compared between the 2 groups using the independent t test. Data that were not normally distributed were compared using the Mann–Whitney U test. A repeated-measures analysis of variance was used to analyze whether the difference of NRS score trends between the 2 groups were statistically significant. We also evaluated whether the NRS scores decreased significantly after each procedure from the baseline using the post hoc Bonferroni test. After the correction of confounding variables (age, sex, location of herpes zoster, days from the onset of the rash to the procedure, and history of hypertension, diabetes mellitus, asthma, hepatic disease, and kidney disease) using the covariance analysis, we analyzed whether a significant difference existed in the NRS scores between the 2 groups. Logistic regression analysis was used to compare the percentages of patients who underwent other procedures within 6 months after the CEB in each group. A 2-sided P value <.05 was considered statistically significant. Data are presented as mean ± standard deviation or median [interquartile range]. Data were analyzed using the Statistical Package for the Social Sciences (SPSS) Windows software, version 17.0 (SPSS Inc., Chicago, IL).
3. Results
Medical records of 189 patients were reviewed, 88 patients met the inclusion criteria. Patients (n = 101) were excluded for the following reasons: did not undergo 6-month follow-up or had insufficient medical records (n = 13); underwent additional interventional procedures within 6 months of CEB (n = 35); failed to maintain the catheter for more than 10 days (n = 2); developed another pain-causing disease (n = 1); received immunosuppressive treatment (n = 2); did not receive standard medication before the procedure or did not receive treatment with anticonvulsants or analgesics after the procedure (n = 9); and received additional drugs, such as opioids, through the epidural catheter (n = 39). Of the 88 patients included in the study, 33 underwent CEB with the esopocan catheter and 55 with the epistim catheter. In the esopocan group, 23 patients had subacute PHN and 10 had chronic PHN. In the epistim group, 38 patients had subacute PHN and 17 had chronic PHN (Fig. 3).
Figure 3.
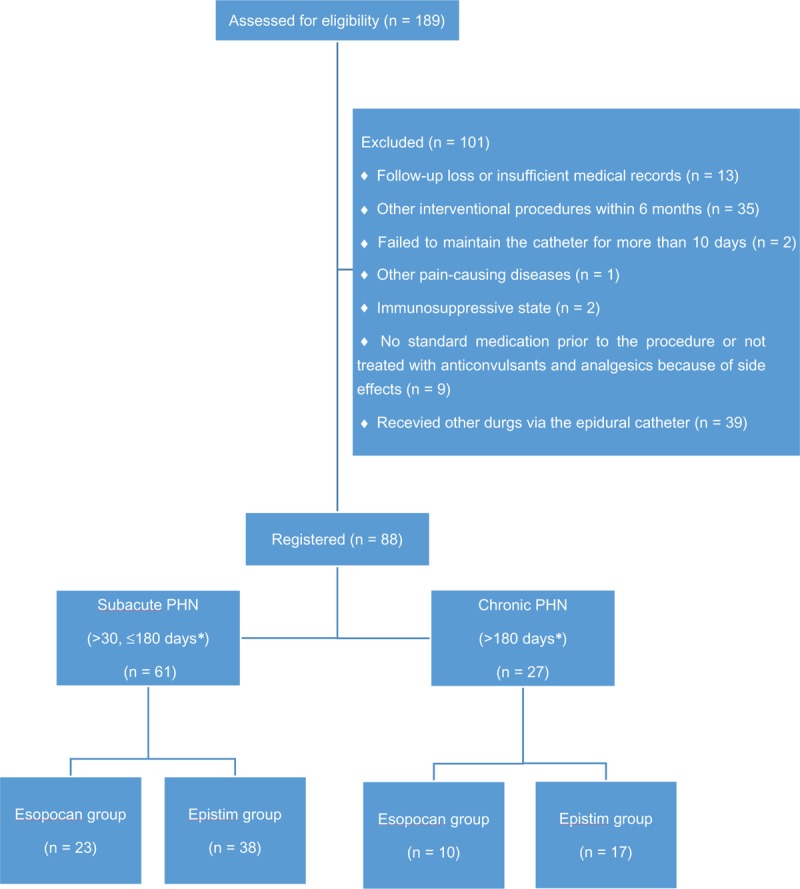
Flow diagram showing patient selection. PHN = postherpetic neuralgia; ∗days from rash onset to the procedure.
No significant differences in baseline characteristics were observed between groups during the subacute or chronic PHN periods (Table 1). In the subacute PHN period, with time and group interactions corrected, a significant difference between the 2 groups was observed in the trend of the NRS score to decline (Huynh-Feldt measure P = .03). In the chronic PHN period, no significant difference was observed in the trend of the NRS score to decline between the 2 groups (Huynh-Feldt measure P = .59). After post hoc Bonferroni test, we found a significant reduction in the NRS scores at all points in time when compared with baseline (Tables 2 and 3). In the subacute PHN period, after correction of confounding variables, the NRS scores were significantly lower in the epistim group when compared to the esopocan group (Table 4). In the chronic PHN period, no significant differences were observed between the 2 groups at any time point (Table 5). In the subacute PHN period, the fraction of patients undergoing other interventional procedures within 6 months after CEB was 2.59 times higher in the esopocan group than in the epistim group (adjusted odds ratio [OR] 2.59, 95% confidence interval [CI] 0.83–8.09, P = .10). In the chronic PHN period, the requirement of additional nerve block was 1.31 times higher in the esopocan group than in the epistim group (adjusted OR 1.31, 95% CI 0.11–5.46, P = .79) (Table 6).
Table 1.
Baseline characteristics of patients.
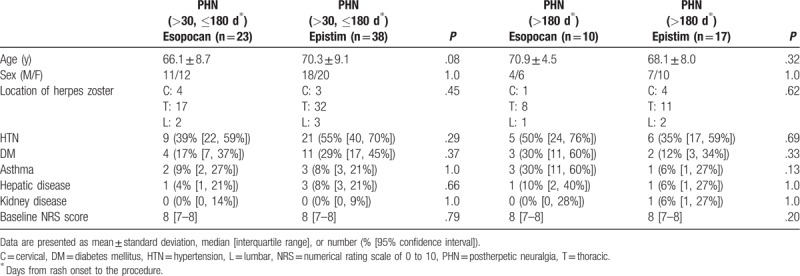
Table 2.
Comparison of baseline numerical rating scale scores to scores in the subacute postherpetic neuralgia period.
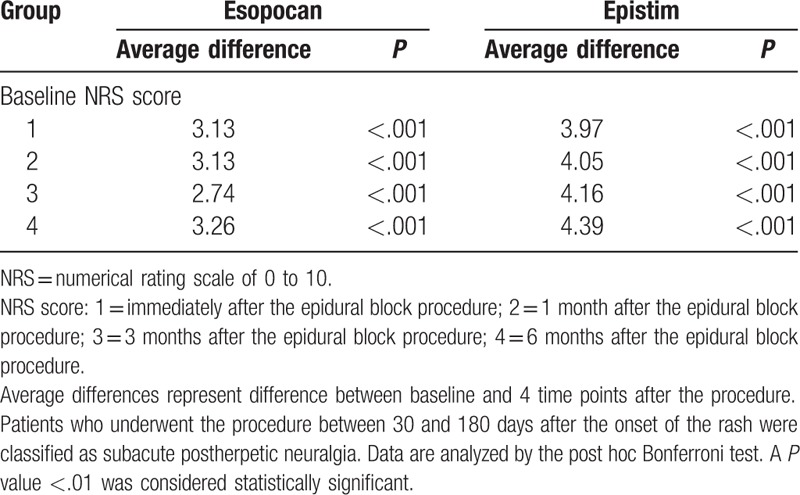
Table 3.
Comparison of baseline numerical rating scale scores to scores in the chronic postherpetic neuralgia period.
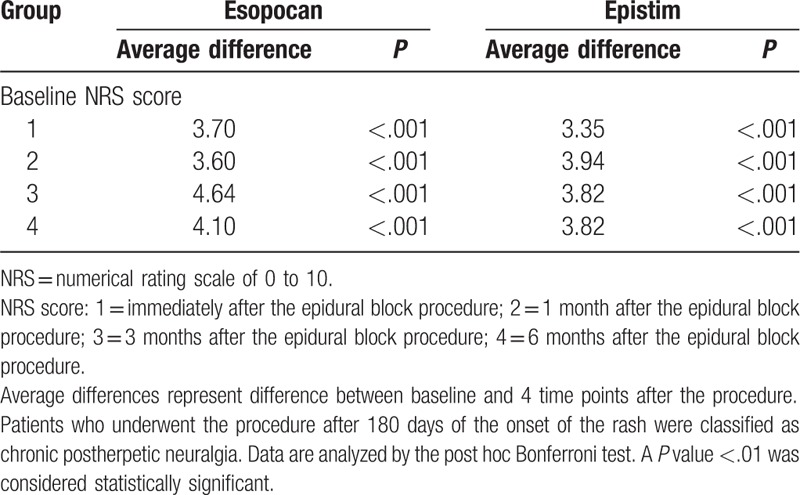
Table 4.
Comparison of the numerical rating scale scores in the subacute postherpetic neuralgia period between the 2 groups after correction of confounding variables.
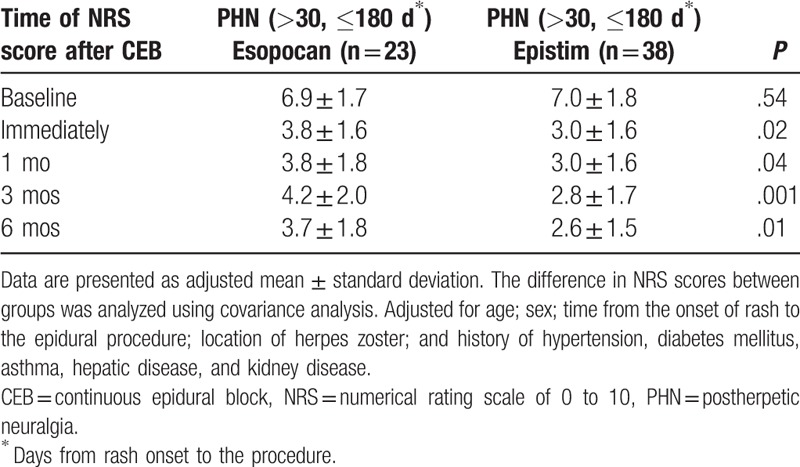
Table 5.
Comparison of the numerical rating scale scores in the chronic postherpetic neuralgia period between the 2 groups after correction of confounding variables.
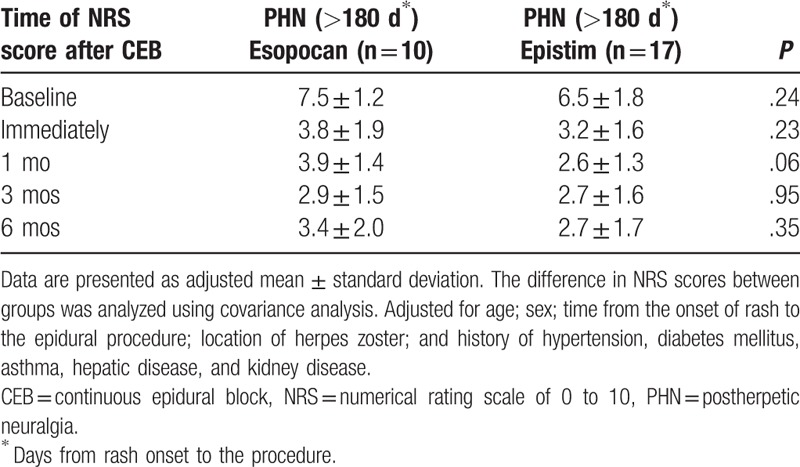
Table 6.
Comparisons of other procedures performed because of insufficient pain control during the 6-month follow-up period after each procedure.
4. Discussion
This study aimed to retrospectively confirm whether a procedure to identify the site of herpes zoster by using epidural electrical stimulation was more effective in reducing ZAP than was a procedure to determine the location of epidural catheters by using a contrast agent alone, when CEB was performed beyond the acute period of herpes zoster. In the present study, 2 groups of patients showed a significant decrease in their NRS scores over the 6-month follow-up period compared with their baseline scores. In the subacute PHN period, the NRS score significantly decreased 6 months after the follow-up period from immediately after the procedure in the epistim group compared with that in the esopocan group. However, in the chronic PHN period, no significant differences were observed in the NRS score reduction between the 2 groups during the 6-month follow-up period. This suggested that drug administration after epidural electrical stimulation to the correct site of herpes zoster might be more effective than the conventional CEB in the subacute PHN phase, but not in the well-established PHN phase. We think this is due to the irreversible changes in the herpes zoster ganglia in patients with chronic PHN. Previous studies have shown that the possibility of pain reduction when PHN persisted for more than 180 days is very low.[4–6] This may be due to an irreversible change of the ganglion damaged by herpes zoster (massive expansion and accumulation of immune-mediating cells, mostly lymphocytes).[20] In patients with subacute PHN (not in an irreversible state), pain relieved by a drug would have had a greater therapeutic effect if the drug was administered to the correct nerve injury site. However, in patients with chronic PHN (mean 550 days after the onset of the rash), the nerve damage may be irreversible, and the effect does not appear to be significant. Therefore, the difference in treatment effect between the 2 groups in the chronic PHN period is small.
In both the periods, the fraction of patients receiving other interventional treatments within 6 months after the procedure was higher in the esopocan group when compared with the epistim group. In the subacute PHN period, the ratio was 2.59-fold higher in the esopocan group. In the chronic PHN period the ratio was 1.31-fold higher in the esopocan group. This suggested that drug administration after confirming the correct location of herpes zoster using epidural electrical stimulation may be more effective in reducing herpes zoster pain before ZAP is well-established.
There is a difference in the shape of the injection port of the 2 catheters. The catheter used in the epistim group is an open-tip, end-hole catheter, whereas the catheter used in the esopocan group is a closed-tip, multiorifice catheter. Previous reports[21,22] have documented that closed-tip, multiorifice catheters are more effective than open-tip, end-hole catheters for sensory blocks. CEB for shingles is to administer the drug to the sensory ganglia damaged by shingles. Previous studies[21,22] have shown that the closed-tip, multiorifice catheter can provide improved pain relief because it is more effective in sensory blocks than open-tip, end-hole catheters. However, the subacute PHN period had a greater pain relief effect in the epistim group, and there was no significant difference between the 2 groups in the chronic PHN period. Based on these results, we conclude that the administration of drugs after epidural electrical stimulation to identify the site of the herpes zoster results in greater pain relief than that seen with the differences in catheter shape.
Previous studies have reported that CEB in the subacute PHN period could reduce ZAP.[5,13,14,17] Local anesthetics and steroids used in epidural blockade are known to play a major role in this phenomenon.[23] After herpes zoster infection, a state of central sensitization is maintained by continuous input from damaged primary afferent nociceptors generated by inflammation and peripheral neural damage.[24] Therefore, it is necessary to stop the central sensitization process by preventing abnormal and ectopic impulses from propagating to the central nervous system.[25]
Epidural blocks allow the direct administration of drugs to the nervous tissue via the epidural space. Nerve blocks to treat herpes zoster decrease the transmission of invasive afferent stimuli to the central nervous system and to improve blood flow to the nervous system, minimizing nerve damage and weakening the central sensitization. Epidural administration of steroids reduces inflammation and deafferentation by reducing the neural ischemia resulting from inflammatory swelling.[26] Epidural administration of local anesthetics interferes with the sensitization process by blocking the sympathetic nerves with their analgesic action. This action can reduce the occurrence of nerve damage and the resulting neuropathic pain.[27] However, for this action to take place, it is important that the drug reaches its target structure. Effective target epidural block requires that the correct location be determined and that the analgesic be administered to the specific spinal segment. To ensure this, we used an epidural catheter with an electrically stimulating guide-wire. After the electrical stimulation through the guide-wire, the specific segment affected by herpes zoster was identified, the catheter placed, and the drug administered, resulting in administration of the drug precisely to the specific segments affected by herpes zoster, unlike the conventional epidural block.
This study excluded patients who did not maintain the epidural catheter for more than 10 days. One-time epidural block does not significantly reduce ZAP.[20,28] After the acute phase had elapsed, if the local anesthetic was continuously injected before the establishment of PHN, blocking the signal transmission to the central nervous system may have prevented further changes in the neuropathy. In previous studies, continuous drug administration via epidural catheters was effective in ZAP; therefore, in this study, we included only patients in whom the epidural catheter was inserted for at least 10 days.[5,13,14,17]
Postherpetic neuralgia is a well-known type of neuropathic pain. Patients included in this study with CEB were also prescribed neuropathic pain medications, such as pregabalin or gabapentin, and analgesics. To avoid any drug-induced bias, patients who had discontinued oral medication because of drug side effects and patients requiring drugs other than local anesthetics and steroids during the maintenance of the epidural catheter, were excluded from the analysis.
Kim et al[13] reported that pRF was more effective for PHN than was CEB. However, they used only local anesthetics and not steroids, and the location of the herpes zoster ganglion was identified using a contrast agent. Therefore, it would be useful to re-examine the effects of pRF and continuous epidural nerve block using steroids and electrical stimulation on ZAP reduction.
Epidural infection during CEB is a possible complication. However, no epidural infections were observed in this study likely due to patient education and daily dressing changes performed by a physician. The risk for hematoma with epidural block is low.[1] In this study, no complications such as hematomas were reported during continuous epidural catheterization. Two patients discontinued epidural catheterization because of dysuria, and control of ZAP with oral drugs and a single epidural block.
This study has limitations. First, our research was retrospective, and unmeasured variables may have influenced or complicated the results. However, to control the potential confounding factors, we conducted a covariance analysis with the baseline demographics of the patients and underlying diseases as the covariates. In addition, only patients who took both anticonvulsants and opioids together with the procedure were included in this study to ensure uniform drug use.
Second, our research data were derived from electronic medical records, which might have underestimated the actual incidence of side effects. In this study, only 2 patients discontinued CEB because of side effects. However, data on patients who had side effects such as dysuria and motor weakness, but underwent CEB because the symptoms were not severe, might not have been added to the medical records.
Third, those who underwent other interventional procedures because the pain was not controlled within 6 months after epidural catheterization were excluded from the analysis in this study. The results of this study might have been different if data on the extent of pain before other interventions were included in the present study. However, a greater fraction of patients received other interventional procedures in the esopocan group than in the epistim group because of the lack of pain control. Therefore, even if the excluded patient group was included in the present study, the results of this study would not have been significantly different.
5. Conclusions
Continuous epidural block combined with standard drug therapy may be effective in treating ZAP beyond the acute phase of herpes zoster. Epidural drug administration to specific segments using electrical stimulation catheters appears to improve ZAP in subacute PHN before well-established PHN. Nevertheless, the results of this study should be validated using well-planned prospective studies.
Acknowledgments
Statistical analysis was conducted after consulting Soon Young Hwang (Korea University Medical Center, Guro Hospital), a statistical expert.
The authors thank all the participants in the study.
We would like to thank Editage (www.editage.co.kr) for English language editing.
Author contributions
Conceptualization: Chung Hun Lee, Sang Sik Choi, Mi Kyoung Lee.
Data curation: Chung Hun Lee, Yeon Joo Lee, Mido Lee, Jong Sun Park.
Formal analysis: Chung Hun Lee, Jong Sun Park.
Investigation: Chung Hun Lee, Mi Kyoung Lee, Yeon Joo Lee, Mido Lee, Jong Sun Park.
Methodology: Chung Hun Lee, Sang Sik Choi, Yeon Joo Lee, Mido Lee, Jong Sun Park.
Project administration: Chung Hun Lee, Mi Kyoung Lee, Yeon Joo Lee.
Resources: Chung Hun Lee, Yeon Joo Lee, Mido Lee, Jong Sun Park.
Software: Chung Hun Lee.
Supervision: Chung Hun Lee, Sang Sik Choi, Mi Kyoung Lee.
Validation: Chung Hun Lee, Sang Sik Choi, Jong Sun Park.
Visualization: Chung Hun Lee.
Writing – original draft: Chung Hun Lee, Yeon Joo Lee, Mido Lee.
Writing – review & editing: Chung Hun Lee, Sang Sik Choi, Mi Kyoung Lee.
Footnotes
Abbreviations: CEB = continuous epidural block, NRS = numerical rating scale, PHN = postherpetic neuralgia, pRF = pulsed radiofrequency, SPSS = Statistical Package for the Social Sciences, ZAP = zoster-associated pain.
How to cite this article: Lee CH, Choi SS, Lee MK, Lee YJ, Lee M, Park JS. Comparison of the efficacy of continuous epidural block with epidural electrical stimulation and conventional continuous epidural block for management of zoster-associated pain beyond the acute phase. Medicine. 2019;98:39(e17026).
The authors have no funding and conflicts of interest to disclose.
References
- [1].van Wijck AJ, Wallace M, Mekhail N, et al. Evidence-based interventional pain medicine according to clinical diagnoses. 17. Herpes zoster and post-herpetic neuralgia. Pain Pract 2011;11:88–97. [DOI] [PubMed] [Google Scholar]
- [2].Arvin A. Aging, immunity, and the varicella-zoster virus. N Engl J Med 2005;352:2266–7. [DOI] [PubMed] [Google Scholar]
- [3].Levin MJ. Immune senescence and vaccines to prevent herpes zoster in older persons. Curr Opin Immunol 2012;24:494–500. [DOI] [PubMed] [Google Scholar]
- [4].Dworkin RH, Gnann JW, Jr, Oaklander AL, et al. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain 2008;9:S37–44. [DOI] [PubMed] [Google Scholar]
- [5].Jeon YH. Herpes zoster and postherpetic neuralgia: practical consideration for prevention and treatment. Korean J Pain 2015;28:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Choudhary S, Dhande S, Kharat S, et al. Safety and efficacy of different systemic treatment modalities for acute pain of herpes zoster: a pilot study. Indian Dermatol Online J 2018;9:101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sacks GM. Unmet need in the treatment of postherpetic neuralgia. Am J Manag Care 2013;19:S207–13. [PubMed] [Google Scholar]
- [8].Pickering G, Leplege A. Herpes zoster pain, postherpetic neuralgia and quality of life in the elderly. Pain Pract 2011;11:397–402. [DOI] [PubMed] [Google Scholar]
- [9].Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain 2002;18:350–4. [DOI] [PubMed] [Google Scholar]
- [10].Werner RN, Nikkels AF, Marinović B, et al. European consensus-based (S2k) Guideline on the Management of Herpes Zoster: guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), part 2: treatment. J Eur Acad Dermatol Venereol 2017;31:20–9. [DOI] [PubMed] [Google Scholar]
- [11].O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 2009;12210 suppl:S22–32. [DOI] [PubMed] [Google Scholar]
- [12].Opstelten W, Eekhof J, Neven AK, et al. Treatment of herpes zoster. Can Fam Physician 2008;54:373–7. [PMC free article] [PubMed] [Google Scholar]
- [13].Kim ED, Lee YI, Park HJ. Comparison of efficacy of continuous epidural block and pulsed radiofrequency to the dorsal root ganglion for management of pain persisting beyond the acute phase of herpes zoster. PLoS One 2017;12:e0183559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pasqualucci A, Pasqualucci V, Galla F, et al. Prevention of post-herpetic neuralgia: acyclovir and prednisolone versus epidural local anesthetic and methylprednisolone. Acta Anaesthesiol Scand 2000;44:910–8. [DOI] [PubMed] [Google Scholar]
- [15].Kim HJ, Ahn HS, Lee JY, et al. Effects of applying nerve blocks to prevent postherpetic neuralgia in patients with acute herpes zoster: a systematic review and meta-analysis. Korean J Pain 2017;30:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kumar V, Krone K, Mathieu A. Neuraxial and sympathetic blocks in herpes zoster and postherpetic neuralgia: an appraisal of current evidence. Reg Anesth Pain Med 2004;29:454–61. [DOI] [PubMed] [Google Scholar]
- [17].Seo YG, Kim SH, Choi SS, et al. Effectiveness of continuous epidural analgesia on acute herpes zoster and postherpetic neuralgia: a retrospective study. Medicine (Baltimore) 2018;97:e9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Manabe H, Dan K, Hirata K, et al. Optimum pain relief with continuous epidural infusion of local anesthetics shortens the duration of zoster associated pain. Clin J Pain 2004;20:302–8. [DOI] [PubMed] [Google Scholar]
- [19].Kim JE, Lee MK, Lee CH, et al. A novel treatment for herpes zoster pain using an electrical stimulating catheter with a steering guidewire. J Clin Anesth 2017;41:46–7. [DOI] [PubMed] [Google Scholar]
- [20].Kikuchi A, Kotani N, Sato T, et al. Comparative therapeutic evaluation of intrathecal versus epidural methylprednisolone for long-term analgesia in patients with intractable postherpetic neuralgia. Reg Anesth Pain Med 1999;24:287–93. [DOI] [PubMed] [Google Scholar]
- [21].Toledano RD, Tsen LC. Epidural catheter design: history, innovations, and clinical implications. Anesthesiology 2014;121:9–17. [DOI] [PubMed] [Google Scholar]
- [22].Michael S, Richmond MN, Birks RJ. A comparison between open-end (single hole) and closed-end (three lateral holes) epidural catheters. Complications and quality of sensory blockade. Anaesthesia 1989;44:578–80. [DOI] [PubMed] [Google Scholar]
- [23].Doran C, Yi X. The anti-inflammatory effect of local anesthetics. Pain Clin 2007;19:207–13. [Google Scholar]
- [24].Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis 1998;5:209–27. [DOI] [PubMed] [Google Scholar]
- [25].Devor M. Neuropathic pain and injured nerve. Br Med Bull 1991;47:619–30. [DOI] [PubMed] [Google Scholar]
- [26].Winnie AP, Hartwell PW. Relationship between time of treatment of acute herpes zoster with sympathetic blockade and prevention of post-herpetic neuralgia: clinical support for a new theory of the mechanism by which sympathetic blockade provides therapeutic benefit. Reg Anesth 1993;18:277–82. [PubMed] [Google Scholar]
- [27].Opstelten W, van Wijck AJ, Stolker RJ. Interventions to prevent postherpetic neuralgia: cutaneous and percutaneous techniques. Pain 2004;107:202–6. [DOI] [PubMed] [Google Scholar]
- [28].van Wijck AJ, Opstelten W, Moons KG, et al. The PINE study of epidural steroids and local anaesthetics to prevent postherpetic neuralgia: a randomised controlled trial. Lancet 2006;367:219–24. [DOI] [PubMed] [Google Scholar]


