ABSTRACT
More than 15% of the US population is currently >65 y old. As populations age there is a concomitant increase in age-related chronic diseases. One such disease is chronic kidney disease (CKD), which becomes more prevalent with age, especially over age 70 y. Individuals with CKD are at increased risk of cardiovascular disease, in part because arterial calcification increases as kidney function declines. Vitamin K is a shortfall nutrient among older adults that has been implicated in arterial calcification. Evidence suggests CKD patients have low vitamin K status, but data are equivocal because the biomarkers of vitamin K status can be influenced by CKD. Animal studies provide more compelling data on the underlying role of vitamin K in arterial calcification associated with CKD. The purpose of this review is to evaluate the strengths and limitations of the available evidence regarding the role of vitamin K in CKD.
Keywords: vitamin K, phylloquinone, chronic kidney disease, coronary artery calcium, cardiovascular disease
An Aging Population at Greater Risk of Chronic Kidney Disease
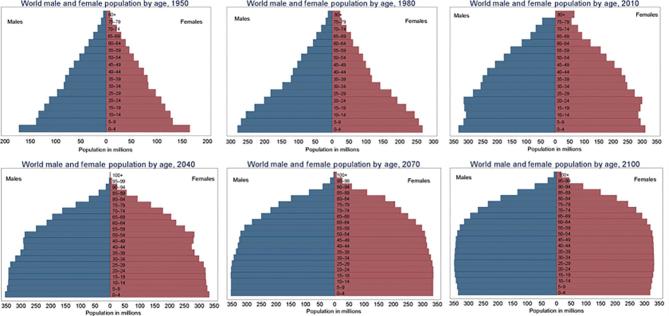
In the 1950s, the largest demographic of the world population was children under age 5 y. Adults over the age of 65 y represented <6% of the global population (Figure 1). With advances in medical therapies, there has been a radical shift in the global population and predictions are now that by the year 2050, there will be more adults over the age of 65 y than there are children below the age of 18 y (1). Age is recognized as the number 1 risk factor for multiple chronic diseases, including chronic kidney disease (CKD) (2, 3).
FIGURE 1.
World population by age from 1950 to 2100 (projected). Reproduced from reference 1 with permission.
Declines in renal function are related to the aging process and to age-related comorbidities that can also lead to renal impairment (3). To understand the impact of a growing aging population on the number of individuals afflicted with CKD, one must first consider how CKD is defined. Chronic kidney disease is evaluated using the glomerular filtration rate (GFR), which is a measure of the rate at which blood is filtered through glomeruli in the kidney. A normal GFR is defined as ≥60 mL · min−1 · 1.73 m−2. Individuals with an estimated GFR (eGFR) <60 mL · min−1 · 1.73 m−2 for ≥3 mo or an eGFR >60 with kidney damage (as indicated by elevated urine albumin) are considered to have CKD (4). There are 5 stages of CKD with stage 1 indicating some kidney damage but normal kidney function and stage 5 indicating end-stage kidney disease (ESKD, corresponding to a GFR ≤ 15 mL · min−1 · 1.73 m−2). Individuals with ESKD usually require dialysis or kidney transplant shortly after diagnosis.
The overall prevalence of CKD worldwide ranges from 8% to 16% (5). Much of this variation in prevalence is attributed to measurement differences in serum creatinine and albumin that are used to calculate GFR. This measurement variation notwithstanding, when considered by stages of life (Figure 2), there is a marked increase in CKD above age 70 y, especially among females, non-Hispanic blacks, and persons with diabetes or hypertension (6). As the global population ages, the impact of this marked increase in CKD on the health care system is staggering. In the United States alone, ∼14% of all adults have CKD, costing almost $80 billion/y in Medicare expenditures in 2016 (7).
FIGURE 2.
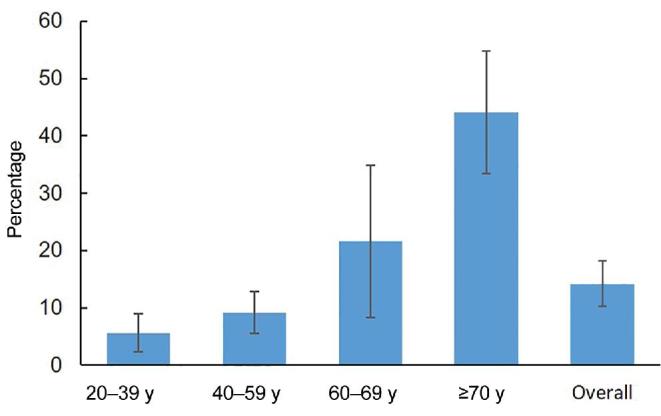
Prevalence of chronic kidney disease stages 1–4 by age, NHANES 2015–2016 (6).
CKD Patients Are at Greater Risk of Cardiovascular Disease
There is an emerging realization that many chronic diseases coexist owing to common pathological factors that disrupt the biology of individual organs, including the kidney. Such factors include inflammation, molecular damage, and metabolic alterations (2, 8). Therefore, it is not unexpected that individuals with CKD are at increased risk of other chronic diseases, such as cardiovascular disease (CVD). In 1 study, it was estimated that the age-adjusted rate of cardiovascular events increases from 2.11 events per 100 person-years for individuals with normal GFR (i.e., ≥60 mL · min−1 · 1.73 m−2) to 3.65 for those with CKD stage 1, to 36.60 for individuals with ESKD (9). Indeed, at all stages of CKD, CVD mortality is disproportionately high, compared to the general population (10). Patients with CKD are also far more likely to die from CVD than progress to ESKD (11). One proposed mechanism by which these patients have increased CVD is through altered mineral metabolism that affects the vasculature (12, 13), resulting in vascular calcium deposition. CKD patients frequently develop calcification in the coronary arteries [coronary artery calcium (CAC)], which reflects atherosclerotic plaque, as well as calcification in peripheral arteries, indicative of more systemic mineral imbalances, that can lead to arterial stiffening (14, 15). Both CAC and arterial stiffening are strong risk factors for CVD events and mortality (16–24). In a meta-analysis of community-dwelling adults with normal kidney function, arterial stiffness was associated with an 8–16% higher risk of developing CKD (24) and in patients with moderate to severe CKD, arterial stiffness was associated with further declines in kidney function (25). This suggests arterial calcification and stiffening may contribute to or exacerbate CKD as well. Vascular calcification is apparent in coronary arteries in CKD patients before ESKD (22, 26). Pathways that inhibit calcification and which normally protect against vascular calcification appear to be less functional in CKD, particularly in later stages (27). In the Chronic Renal Insufficiency Cohort (CRIC, a prospective observational study of adults with CKD), participants without a history of CVD and an Agatston score (a quantitative measure of CAC) of >0 to 100 (indicative of some calcification) had a 60% higher risk of CVD, defined as myocardial infarction, stroke, and heart failure, over 6 y of follow-up (HR: 1.60; 95% CI: 1.01, 2.53). For those with a moderate to severe burden of calcification (i.e., an Agatston score >100), the HR (95% CI) for CVD only modestly increased to 1.81 (1.16, 2.82) (28). This suggests that even modest amounts of CAC increase CVD in CKD patients. Accordingly, interventions targeted at reducing vascular calcification could offer tremendous therapeutic benefits to CKD patients. One potential intervention is vitamin K. A critical research gap in our knowledge regarding vitamin K and CKD has been identified by the international Kidney Disease: Improving Global Outcomes (KDIGO) initiative, which recommended “experimental and clinical research … to evaluate and focus on vitamin K-dependent proteins” with respect to vascular calcification in CKD (29).
Vitamin K Is an Essential Fat-Soluble Vitamin
The fat-soluble vitamin K has 11 known forms that share a napthoquinone ring, but vary in the length and saturation of their side chain. The form best characterized in terms of diet and human health is phylloquinone, also known as vitamin K-1. Phylloquinone is the plant-based form of vitamin K and is abundant in leafy vegetables and plant oils. Emerging data indicate that the menaquinones (10 known forms that are also referred to as vitamin K-2) are also abundant in the food supply, particularly in animal meats and fermented foods. However, knowledge of their content in foods is still very limited and there are no validated questionnaires from which to estimate usual intakes. Moreover, phylloquinone is the primary form of vitamin K in the circulation, and circulating phylloquinone responds to changes in intake (30). Although menaquinones are more abundant in the food supply than previously thought, menaquinone forms are not typically detected in the circulation (31).
The current dietary recommendations for vitamin K were developed in 2001 based on estimated mean intakes of phylloquinone as estimated from NHANES III (1988–1994) (32). At the time, there were insufficient data from which to develop an estimated average requirement, so median phylloquinone intakes were used to develop an adequate intake (AI) of 90 and 120 µg/d for women and men, respectively, regardless of age. In a recent re-evaluation of phylloquinone intakes in the 2012 NHANES, the percentage of men consuming intakes of phylloquinone that meet the AIs declines with age such that only one-third of men aged ≥70 y are considered to meet the AI of this vitamin. Whereas women consume more phylloquinone than men there is also a similar decline with age, such that ∼56% of all women over the age of 70 y consume intakes that meet the AI (Figure 3) (33). The mean phylloquinone intake of NHANES participants who were 20 y or older with CKD [defined as having an eGFR <60 mL · min−1 · 1.73 m−2 or albumin/creatinine ratio ≥30 mg/g (albuminuria)] was 97.5 µg/d, but >72% did not meet the recommended AI (34). The median phylloquinone intake of hemodialysis patients from Italy was 71.6 µg/d. Over 70% did not meet the US recommended AI and >80% did not meet the Italian RDA (which is 140 µg/d for adults <60 y old and 170 µg/d for adults ≥60 y old) (35). This may be related to CKD patients reducing their vegetable intake owing to being advised to restrict dietary potassium (36), which is found in many vegetables that are also sources of phylloquinone.
FIGURE 3.
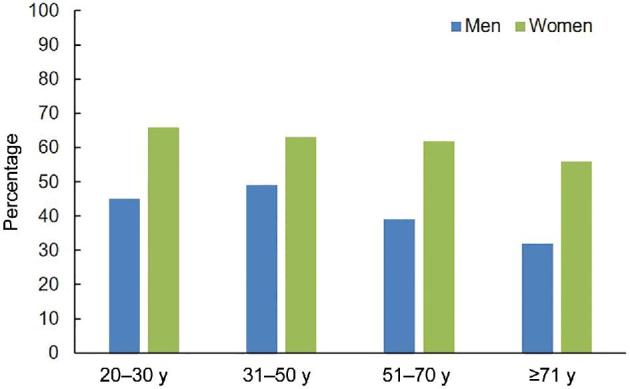
Percentage of men and women who meet the recommended Adequate Intakes for vitamin K, by age group, according to NHANES 2011–2012 (33).
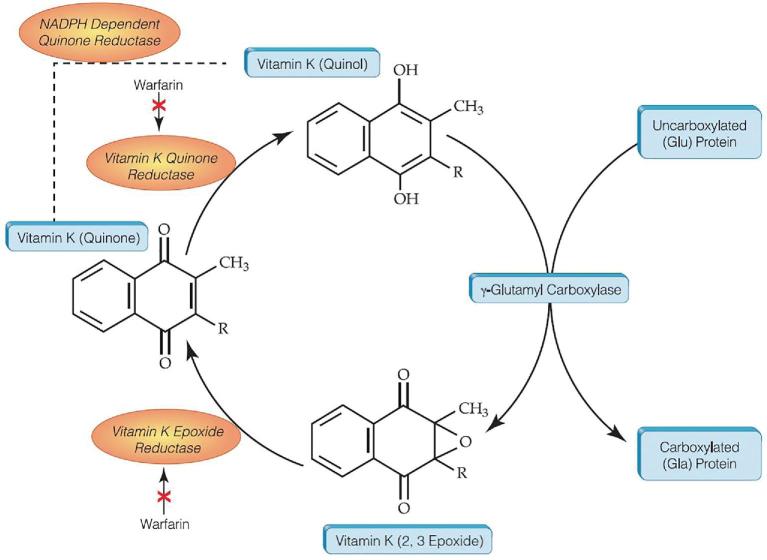
Vitamin K functions as an enzyme cofactor for the carboxylation of vitamin K–dependent proteins involved in various physiological processes (Figure 4), including vascular calcification. Although several vitamin K–dependent proteins have been identified in vascular tissue and have also been implicated in arterial calcification and CVD (37–39), the most studied is matrix gla protein (MGP). This is primarily due to the availability of animal models and biochemical assays that directly measure the protein in tissue and circulation.
FIGURE 4.
The vitamin K cycle: carboxylation of vitamin K–dependent proteins. The vitamin K hydroquinone is reduced to the vitamin K quinol, which serves as a cofactor to the γ-glutamyl carboxylase enzyme that carboxylates vitamin K–dependent proteins. As a result, the quinol is oxidized to vitamin K epoxide, which is reduced back to the quinone form.
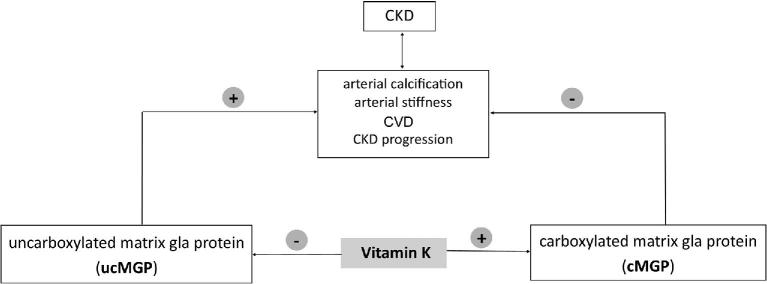
Like all vitamin K–dependent proteins, MGP is synthesized in its uncarboxylated form (ucMGP) (40). In the presence of vitamin K, it is posttranslationally carboxylated (cMGP) (Figure 4). It is only the cMGP form that inhibits vascular calcification (41). When the gene encoding MGP was deleted in a mouse model, of those that were viable, the animals survived <8 wk owing to rupture of the aorta, which was completely calcified (42). Given that MGP is a critical protein in inhibiting abnormal calcification in the vasculature and that vitamin K is critical for MGP function, it has been proposed that vitamin K has a role in the complications of CKD that are associated with abnormal calcification, including arterial calcification, arterial stiffening, and CVD (Figure 5). Because arterial calcification and stiffening have been associated with incident CKD and declines in kidney function (24, 25), it is also possible vitamin K influences CKD development and progression indirectly through mechanisms linked to abnormal calcification.
FIGURE 5.
A working model depicting the role of vitamin K in CKD and CVD. CKD, chronic kidney disease; CVD, cardiovascular disease.
Evidence of Vitamin K’s role in Vascular Calcification and CKD
Animal studies
Rodent studies using warfarin provide indirect evidence that vitamin K–dependent pathways are involved in vascular calcification. Warfarin is used as an anticoagulant because it antagonizes the carboxylation of vitamin K–dependent proteins. In addition to interfering with the carboxylation of clotting proteins, warfarin also renders MGP and other vitamin K–dependent proteins in extrahepatic tissues uncarboxylated and thus less functional. In rodents, high doses of warfarin induce vascular calcification (41, 43), which has been shown to be attenuated with high intakes of vitamin K (44). In rats with CKD, warfarin increased renal artery, carotid artery, and thoracic and abdominal aortic calcium deposition, whereas high vitamin K intake attenuated calcium accumulation in these same tissues (45). In a subsequent experiment conducted by the same research group, rats with mild and severe CKD had lower vitamin K concentrations in the kidney, liver, spleen, and heart than rats without CKD. The enzymes involved in vitamin K metabolism were also altered in the rats with CKD. More specifically, vitamin K epoxide reductase (VKOR) expression in the kidney and thoracic aorta was decreased and MGP expression in the kidney and thoracic aorta was increased. In contrast, γ-glutamyl carboxylase (GGCX) expression did not differ in any tissue and there were no differences in the expression of any enzyme in the liver (46). In a similar experiment, aortic GGCX expression also was not different between rats with and without CKD, but aortic GGCX activity was reduced in those with CKD (47). Although VKOR expression was not measured, renal VKOR activity did not differ in the rats with CKD. Collectively, these experiments suggest CKD can lead to decreases in tissue vitamin K and increases in arterial calcification, as well as potential alterations in vitamin K enzyme expression and/or activity. However, the precise mechanisms underlying these metabolic disturbances related to calcification and vitamin K in CKD need further clarification. It is also uncertain whether CKD causes vitamin K deficiency or if the disease is exacerbated by a vitamin K deficiency created by other mechanisms.
Observational and clinical studies
Warfarin studies
Similar to animal models, studies of patients using warfarin have been proposed as indirect evidence that vitamin K–dependent pathways are involved in vascular calcification. Patients with CKD are frequently treated with warfarin because they are at increased risk of atrial fibrillation, which is associated with an increased risk of thromboembolism (48). In humans, it is currently uncertain whether therapeutic doses of warfarin cause vascular calcification. In an analysis based on retrospective chart review, lower-extremity arterial calcification was 44% greater in warfarin users than in controls matched on age, sex, and diabetes status (49). In patients with aortic valve disease, those treated with warfarin had more CAC and valvular calcium than those not treated with warfarin (50). In a study of hemodialysis patients, those treated with warfarin had >2-fold higher odds of iliac artery and aortic calcification than patients not treated with warfarin (51). However, not all studies have found arterial calcification to be higher in warfarin users (52, 53). Moreover, because the available studies are cross-sectional, the temporal relation between warfarin therapy and vascular calcification in humans remains unclear (54). Because people treated with warfarin are typically at increased risk of CVD, it is possible the vascular calcification existed before warfarin initiation. Therefore, although suggestive, the evidence from clinical studies of warfarin patients is limited in its utility to understand if low vitamin K increases risk of vascular calcification in CKD patients or if the CKD patient has underlying pathologies that result in treatment strategies which reduce vitamin K status.
CKD patients have subclinical vitamin K deficiency
To evaluate subclinical deficiency in the CKD patient, whether they are on warfarin or not, one needs robust biomarkers of status. In contrast to other micronutrients, there is no single circulating biomarker that is considered the gold standard for evaluating vitamin K status. Rather, multiple biomarkers that reflect different aspects of vitamin K metabolism are used to provide a more thorough estimation of vitamin K status, as reviewed elsewhere (55).
Circulating phylloquinone, a global indicator of vitamin K status, has been inversely associated with CAC progression in community-dwelling adults, most notably in those treated for hypertension, which is common in people with CKD (56). However, circulating phylloquinone has not been studied in relation to vascular calcification or CVD in a CKD population. This is unfortunate because plasma phylloquinone would capture variance in dietary intakes of vitamin K, which is critical in a patient population that follows a wide range of dietary recommendations/restrictions. Moreover, circulating phylloquinone also reflects absorption, which may be suboptimal given the high prevalence of vitamin K subclinical deficiency in CKD patients (57–59). Like any single biomarker, plasma phylloquinone has its limitations, primarily its dependence on circulating lipids. In CKD patients, lipid abnormalities, especially elevated triglycerides, are common (60). This may influence the interpretation of the plasma phylloquinone results because phylloquinone is transported on triglyceride-rich lipoproteins. Hypertriglyceridemia is associated with an increased risk of CVD (61). Therefore, triglycerides should be considered in studies evaluating plasma phylloquinone in relation to CVD, especially in CKD patients.
To capture vitamin K function in vascular tissue, the uncarboxylated (inactive) fractions of MGP can also be measured in circulation. Although there are 4 isoforms of MGP, only the dephosphorylated uncarboxylated MGP [(dp)ucMGP] form reflects vitamin K status (62). Several studies have reported that higher (dp)ucMGP, reflecting lower vitamin K status, was associated with more vascular calcification in CKD patients (63–68). Because these studies are all cross-sectional, we do not know whether elevated (dp)ucMGP is an independent risk factor for vascular calcification and CVD [as some suggest (69)] or whether vascular calcification leads to higher plasma (dp)ucMGP. Because the synthesis of MGP can be upregulated in response to calcium (70), and circulating (dp)ucMGP and total MGP are highly correlated (55), the latter is also plausible. In this case, the positive association between (dp)ucMGP and vascular calcification could reflect an increase in MGP synthesis in response to vascular calcification, and not only vitamin K insufficiency. Unfortunately, there is no commercial assay to measure total MGP in circulation, so one is unable to correct for changes in total MGP irrespective of vitamin K status, which is the practice with other vitamin K–dependent proteins (71). In a post hoc analysis of a randomized controlled trial (RCT) in healthy community-dwelling adults, in which vitamin K supplementation reduced CAC progression [among those adherent to the intervention (72)] and reduced circulating (dp)ucMGP, the change in (dp)ucMGP was not correlated with the change in Agatston score in the vitamin K–supplemented group. In the placebo group, the baseline (dp)ucMGP did not predict the change in Agatston score (73). The generalizability of these findings to the CKD patient population is uncertain. Larger prospective studies of CKD patients are needed to clarify the temporal relation between (dp)ucMGP and vascular calcification in CKD.
An additional limitation in interpreting the (dp)ucMGP data involves assay changes. The original data for (dp)ucMGP were generated using a noncommercial assay that was only available in the laboratory of origin. This assay is now automated and commercially available. The lower limit of detection (300 pM) for the commercial assay suggests lower sensitivity than originally reported (62). Differences in assay sensitivity challenge the ability of investigators to directly compare data generated from the commercial assay with data generated from the original assay.
In the context of these strengths and limitations of vitamin K biomarkers, CKD patients appear to have a subclinical vitamin K deficiency. In a series of 3 studies in CKD patients (57–59), those with end stage renal disease (defined as stage 4 CKD, hemodialysis, or peritoneal dialysis) had a higher prevalence of subclinical vitamin K deficiency (as defined by serum phylloquinone concentrations <0.4 nmol/L) than those patients with earlier stages of CKD. A similar finding was noted with measurements of the percentage of the vitamin K-dependent protein (VKDP) osteocalcin that was undercarboxylated, which is also an indication of vitamin K status (57–59). To capture vitamin K function once in the tissues, the undercarboxylated (inactive) fractions of certain vitamin K–dependent proteins are also measurable in serum or plasma, and higher concentrations reflect lower vitamin K status (55). The caveat to the interpretation of the percentage of undercarboxylated osteocalcin (%ucOC data) is that osteocalcin is cleared by the kidney, and impaired kidney function is associated with increased concentrations of total OC and ucOC (74, 75), which challenges the use of ucOC as a vitamin K biomarker in CKD patients. In contrast, less is known about the renal clearance of MGP but evidence suggests the kidney is able to extract some MGP from the circulation, and this appears to be independent of renal function (75). However, additional studies are needed to clarify the renal handling of MGP and (dp)ucMGP. Finally, 2 studies used a third biomarker, PIVKA-II (protein induced in vitamin K absence or antagonism - II), which measures the undercarboxylation of the coagulation protein, prothrombin (also referred to as factor II). When CKD patients were compared with peritoneal dialysis patients, there were no differences in PIVKA-II (57, 58). In contrast, others have reported that PIVKA-II is higher (indicative of poorer vitamin K status) among CKD patients receiving warfarin therapy and who have calciphylaxis (76).
Vitamin K status, vascular calcification, and CVD in CKD patients
When it comes to relating vitamin K status to vascular calcification, CVD, or other clinical outcomes in CKD patients, the current literature is limited to the use of single biomarkers of vitamin K status. This presents a critical barrier to establishing the importance of vitamin K to CKD patients for all the aforementioned reasons. Indirect evidence of the role of VKDPs in coronary calcification and survival in CKD was provided in a genetic analysis of 86 CKD patients with data on CAC and 4-y survival rates (77). The gene of interest was VKOR, which is a critical enzyme in the recycling of vitamin K (see Figure 4). The CC/CG genotype at rs8050894 has been associated with a blunted response to warfarin requiring higher warfarin doses to maintain anticoagulation (78). In the study of CKD patients, the CG/GG genotype was associated with a 4-fold increase in CAC Agatston score > 50 units and a significant increase in mortality (77). This could be related to greater VKOR function, but this needs to be confirmed.
Randomized trials of vitamin K supplementation and cardiovascular outcomes
RCTs are considered the “gold standard” study design to establish causation between an exposure and a health outcome. At this time, to the best of our knowledge there are only a few complete RCTs that evaluated vitamin K supplementation and cardiovascular outcomes. Phylloquinone supplementation (500 μg/d) reduced CAC progression over 3 y, compared with placebo, in generally healthy community-dwelling adults who were adherent to the intervention (72). In a subsequent post hoc analysis, the effect of phylloquinone supplementation on CAC progression appeared to be more pronounced in those with hypertension, suggesting a beneficial effect in higher-risk groups (79). RCTs designed to test the effect of phylloquinone supplementation on CAC in renal patients have not been completed, but are underway (80, 81). The results will provide important evidence regarding the cardiovascular benefit of vitamin K supplementation in patients with kidney disease. There have been smaller studies using a different form of vitamin K, menaquinone-7 (MK7), to examine the role of vitamin K supplementation in improving vascular health. In an RCT of healthy postmenopausal women, 180 μg MK7/d supplementation for 3 y improved arterial stiffness in a subgroup of women with stiffer arteries at baseline (82). In a single-arm intervention in renal transplant patients, arterial stiffness improved after 8 wk of supplementation with 360 μg MK7/d (83). Given the small sample size and lack of control group, any effect of MK7 must be cautiously interpreted. Moreover, until trials are designed to compare the effects of phylloquinone and menaquinone on CAC and other cardiovascular outcomes, it is not known if one form of vitamin K is superior to any other form of the vitamin in terms of cardiovascular health.
Concluding Comments
With the current dramatic shift towards an aging population, the number of individuals afflicted with CKD has concomitant increases. The current literature consistently reports that 1) vitamin K–dependent mechanisms are implicated in vascular calcification and arterial stiffness in rodent CKD models (45–47); 2) vascular calcification and arterial stiffness are common in nondialysis CKD (22, 84); 3) low vitamin K status is associated with CVD, arterial calcification progression, and arterial stiffness in community-dwelling men and women (56, 72, 85); and 4) vitamin K insufficiency is prevalent in CKD (57–59). This suggests CKD patients could be a high-yield population likely to benefit from improved vitamin K status. This is a critical research area identified by the international KDIGO initiative because there are gaps in understanding the underlying mechanisms, hence causalities, that link this nutrient with 2 chronic diseases, i.e., CKD and CVD. Prospective cohort trials and RCTs are needed to determine if improving vitamin K status will reduce the risk of vascular calcification in CKD patients.
Acknowledgements
The authors’ responsibilities were as follows—both authors: conducted the literature review, wrote the manuscript, and read and approved the final manuscript.
Notes
Supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK111392 (to MKS) and the USDA Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707 (to SLB).
Author disclosures: MKS and SLB, no conflicts of interest.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA.
Abbreviations used: AI, Adequate Intake; CAC, coronary artery calcium; CKD, chronic kidney disease; cMGP, carboxylated matrix gla protein; CRIC, Chronic Renal Insufficiency Cohort; CVD, cardiovascular disease; (dp)ucMGP, dephosphorylated uncarboxylated matrix gla protein; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; GFR, glomerular filtration rate; GGCX, γ-glutamyl carboxylase; KDIGO, Kidney Disease: Improving Global Outcomes; MGP, matrix gla protein; MK, menaquinone; OC, osteocalcin; PIVKA-II, protein induced in vitamin K absence or antagonism-II; RCT, randomized controlled trial; ucOC, undercarboxylated osteocalcin; VKDP, vitamin K-dependent protein; VKOR, vitamin K epoxide reductase.
References
- 1. Bloom DE, Luca DL. The global demography of aging: facts, explanations, future. In: Piggott J, Woodland A, editors. Handbook of the economics of population aging, vol. 1 Amsterdam: North Holland; 2016. pp. 3–56. [Google Scholar]
- 2. Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE et al.. Geroscience: linking aging to chronic disease. Cell 2014;159(4):709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sobamowo H, Prabhakar SS. The kidney in aging: physiological changes and pathological implications. Prog Mol Biol Transl Sci 2017;146:303–40. [DOI] [PubMed] [Google Scholar]
- 4. The National Kidney Foundation Estimated glomerular filtration rate (eGFR) [Internet] New York: The National Kidney Foundation; 2019[cited 15 April, 2019] Available from: https://www.kidney.org/atoz/content/gfr. [Google Scholar]
- 5. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382(9888):260–72. [DOI] [PubMed] [Google Scholar]
- 6. US Department of Health and Human Services, Centers for Disease Control and Prevention The Kidney Disease Initiative; 2019 [Internet] CDC; [cited 27 March, 2019] Available from: https://www.cdc.gov/kidneydisease/index.html. [Google Scholar]
- 7. United States Renal Data System Annual Data Report 2018: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [Google Scholar]
- 8. Burch JB, Augustine AD, Frieden LA, Hadley E, Howcroft TK, Johnson R, Khalsa PS, Kohanski RA, Li XL, Macchiarini F et al.. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci 2014;69(Suppl 1):S1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 10. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ et al.. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension 2003;42(5):1050–65. [DOI] [PubMed] [Google Scholar]
- 11. Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, Seliger S, Siscovick D, Newman AB, Fried L. Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med 2011;26(4):379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCullough PA, Chinnaiyan KM, Agrawal V, Danielewicz E, Abela GS. Amplification of atherosclerotic calcification and Monckeberg's sclerosis: a spectrum of the same disease process. Adv Chronic Kidney Dis 2008;15(4):396–412. [DOI] [PubMed] [Google Scholar]
- 13. Paloian NJ, Giachelli CM. A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol 2014;307(8):F891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv 2014;83(6):E212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 2000;15(7):1014–21. [DOI] [PubMed] [Google Scholar]
- 16. Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension 1998;32(3):570–4. [DOI] [PubMed] [Google Scholar]
- 17. Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R et al.. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 2010;3(12):1229–36. [DOI] [PubMed] [Google Scholar]
- 18. Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K et al.. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;61(12):1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010;121(4):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol 2004;43(9):1663–9. [DOI] [PubMed] [Google Scholar]
- 21. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55(13):1318–27. [DOI] [PubMed] [Google Scholar]
- 22. Budoff MJ, Rader DJ, Reilly MP, Mohler ER III, Lash J, Yang W, Rosen L, Glenn M, Teal V, Feldman HI. Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) study. Am J Kidney Dis 2011;58(4):519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Madero M, Peralta C, Katz R, Canada R, Fried L, Najjar S, Shlipak M, Simonsick E, Lakatta E, Patel K et al.. Association of arterial rigidity with incident kidney disease and kidney function decline: the Health ABC study. Clin J Am Soc Nephrol 2013;8(3):424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sedaghat S, Mattace-Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, Franco OH, Dehghan A. Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol 2015;10(12):2190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension 2010;55(5):1110–15. [DOI] [PubMed] [Google Scholar]
- 26. Nakano T, Ninomiya T, Sumiyoshi S, Fujii H, Doi Y, Hirakata H, Tsuruya K, Iida M, Kiyohara Y, Sueishi K. Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: the Hisayama study. Am J Kidney Dis 2010;55(1):21–30. [DOI] [PubMed] [Google Scholar]
- 27. Moe SM, Reslerova M, Ketteler M, O'Neill K, Duan D, Koczman J, Westenfeld R, Jahnen-Dechent W, Chen NX. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int 2005;67(6):2295–304. [DOI] [PubMed] [Google Scholar]
- 28. Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M, Zhang X, Roy JA, Lustigova E, Nessel L et al.. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol 2017;2(6):635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ketteler M, Elder GJ, Evenepoel P, Ix JH, Jamal SA, Lafage-Proust MH, Shroff R, Thadhani RI, Tonelli MA, Kasiske BL et al.. Revisiting KDIGO clinical practice guideline on chronic kidney disease—mineral and bone disorder: a commentary from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int 2015;87(3):502–28. [DOI] [PubMed] [Google Scholar]
- 30. Booth SL, Martini L, Peterson JW, Saltzman E, Dallal GE, Wood RJ. Dietary phylloquinone depletion and repletion in older women. J Nutr 2003;133(8):2565–9. [DOI] [PubMed] [Google Scholar]
- 31. Karl JP, Fu X, Dolnikowski GG, Saltzman E, Booth SL. Quantification of phylloquinone and menaquinones in feces, serum, and food by high-performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2014;963:128–33. [DOI] [PubMed] [Google Scholar]
- 32. Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 33. Harshman SG, Finnan EG, Barger KJ, Bailey RL, Haytowitz DB, Gilhooly CH, Booth SL. Vegetables and mixed dishes are top contributors to phylloquinone intake in US adults: data from the 2011–2012 NHANES. J Nutr 2017;147(7):1308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheung CL, Sahni S, Cheung BM, Sing CW, Wong IC. Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin Nutr 2015;34(2):235–40. [DOI] [PubMed] [Google Scholar]
- 35. Fusaro M, D'Alessandro C, Noale M, Tripepi G, Plebani M, Veronese N, Iervasi G, Giannini S, Rossini M, Tarroni G et al.. Low vitamin K1 intake in haemodialysis patients. Clin Nutr 2017;36(2):601–7. [DOI] [PubMed] [Google Scholar]
- 36. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013(1):1–150. [DOI] [PubMed] [Google Scholar]
- 37. Tesfamariam B. Involvement of vitamin K-dependent proteins in vascular calcification. J Cardiovasc Pharmacol Ther 2019;24(4):323–33. [DOI] [PubMed] [Google Scholar]
- 38. Viegas CS, Rafael MS, Enriquez JL, Teixeira A, Vitorino R, Luis IM, Costa RM, Santos S, Cavaco S, Neves J et al.. Gla-rich protein acts as a calcification inhibitor in the human cardiovascular system. Arterioscler Thromb Vasc Biol 2015;35(2):399–408. [DOI] [PubMed] [Google Scholar]
- 39. Ken-Dror G, Cooper JA, Humphries SE, Drenos F, Ireland HA. Free protein S level as a risk factor for coronary heart disease and stroke in a prospective cohort study of healthy United Kingdom men. Am J Epidemiol 2011;174(8):958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barone LM, Owen TA, Tassinari MS, Bortell R, Stein GS, Lian JB. Developmental expression and hormonal regulation of the rat matrix GLA protein (MGP) gene in chondrogenesis and osteogenesis. J Cell Biochem 1991;46(4):351–65. [DOI] [PubMed] [Google Scholar]
- 41. Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol 1998;18(9):1400–7. [DOI] [PubMed] [Google Scholar]
- 42. Luo G, D'Souza R, Hogue D, Karsenty G. The matrix Gla protein gene is a marker of the chondrogenesis cell lineage during mouse development. J Bone Miner Res 1995;10(2):325–34. [DOI] [PubMed] [Google Scholar]
- 43. Kruger T, Oelenberg S, Kaesler N, Schurgers LJ, van de Sandt AM, Boor P, Schlieper G, Brandenburg VM, Fekete BC, Veulemans V et al.. Warfarin induces cardiovascular damage in mice. Arterioscler Thromb Vasc Biol 2013;33(11):2618–24. [DOI] [PubMed] [Google Scholar]
- 44. Schurgers LJ, Spronk HM, Soute BA, Schiffers PM, DeMey JG, Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood 2007;109(7):2823–31. [DOI] [PubMed] [Google Scholar]
- 45. McCabe KM, Booth SL, Fu X, Shobeiri N, Pang JJ, Adams MA, Holden RM. Dietary vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int 2013;83(5):835–44. [DOI] [PubMed] [Google Scholar]
- 46. McCabe KM, Booth SL, Fu X, Ward E, Adams MA, Holden RM. Vitamin K metabolism in a rat model of chronic kidney disease. Am J Nephrol 2017;45(1):4–13. [DOI] [PubMed] [Google Scholar]
- 47. Kaesler N, Magdeleyns E, Herfs M, Schettgen T, Brandenburg V, Fliser D, Vermeer C, Floege J, Schlieper G, Kruger T. Impaired vitamin K recycling in uremia is rescued by vitamin K supplementation. Kidney Int 2014;86(2):286–93. [DOI] [PubMed] [Google Scholar]
- 48. Nelson SE, Shroff GR, Li S, Herzog CA. Impact of chronic kidney disease on risk of incident atrial fibrillation and subsequent survival in Medicare patients. J Am Heart Assoc 2012;1(4):e002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Han KH, O'Neill WC. Increased peripheral arterial calcification in patients receiving warfarin. J Am Heart Assoc 2016;5(1):e002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koos R, Mahnken AH, Muhlenbruch G, Brandenburg V, Pflueger B, Wildberger JE, Kuhl HP. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol 2005;96(6):747–9. [DOI] [PubMed] [Google Scholar]
- 51. Fusaro M, Tripepi G, Noale M, Plebani M, Zaninotto M, Piccoli A, Naso A, Miozzo D, Giannini S, Avolio M et al.. Prevalence of vertebral fractures, vascular calcifications, and mortality in warfarin treated hemodialysis patients. Curr Vasc Pharmacol 2015;13(2):248–58. [DOI] [PubMed] [Google Scholar]
- 52. Palaniswamy C, Aronow WS, Sekhri A, Adapa S, Ahn C, Singh T, Malhotra B, Lerner R. Warfarin use and prevalence of coronary artery calcification assessed by multislice computed tomography. Am J Ther 2014;21(3):148–51. [DOI] [PubMed] [Google Scholar]
- 53. Villines TC, O'Malley PG, Feuerstein IM, Thomas S, Taylor AJ. Does prolonged warfarin exposure potentiate coronary calcification in humans? Results of the warfarin and coronary calcification study. Calcif Tissue Int 2009;85(6):494–500. [DOI] [PubMed] [Google Scholar]
- 54. Palaniswamy C, Sekhri A, Aronow WS, Kalra A, Peterson SJ. Association of warfarin use with valvular and vascular calcification: a review. Clin Cardiol 2011;34(2):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shea MK, Booth SL. Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients 2016;8(1):E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shea MK, Booth SL, Weiner DE, Brinkley TE, Kanaya AM, Murphy RA, Simonsick EM, Wassel CL, Vermeer C, Kritchevsky SB. Circulating vitamin K is inversely associated with incident cardiovascular disease risk among those treated for hypertension in the Health, Aging, and Body Composition Study (Health ABC). J Nutr 2017;147(5):888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Holden RM, Iliescu E, Morton AR, Booth SL. Vitamin K status of Canadian peritoneal dialysis patients. Perit Dial Int 2008;28(4):415–18. [PubMed] [Google Scholar]
- 58. Holden RM, Morton AR, Garland JS, Pavlov A, Day AG, Booth SL. Vitamins K and D status in stages 3–5 chronic kidney disease. Clin J Am Soc Nephrol 2010;5(4):590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pilkey RM, Morton AR, Boffa MB, Noordhof C, Day AG, Su Y, Miller LM, Koschinsky ML, Booth SL. Subclinical vitamin K deficiency in hemodialysis patients. Am J Kidney Dis 2007;49(3):432–9. [DOI] [PubMed] [Google Scholar]
- 60. Kaysen GA. New insights into lipid metabolism in chronic kidney disease. J Ren Nutr 2011;21(1):120–3. [DOI] [PubMed] [Google Scholar]
- 61. Reiner Z. Hypertriglyceridaemia and risk of coronary artery disease. Nat Rev Cardiol 2017;14(7):401–11. [DOI] [PubMed] [Google Scholar]
- 62. Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewe RB, Brandenburg VM, Bekers O, Vermeer C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost 2010;104(4):811–22. [DOI] [PubMed] [Google Scholar]
- 63. Schlieper G, Westenfeld R, Kruger T, Cranenburg EC, Magdeleyns EJ, Brandenburg VM, Djuric Z, Damjanovic T, Ketteler M, Vermeer C et al.. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol 2011;22(2):387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G, Massy ZA. The circulating inactive form of matrix Gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol 2010;5(4):568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Meuwese CL, Olauson H, Qureshi AR, Ripsweden J, Barany P, Vermeer C, Drummen N, Stenvinkel P. Associations between thyroid hormones, calcification inhibitor levels and vascular calcification in end-stage renal disease. PLoS One 2015;10(7):e0132353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Delanaye P, Krzesinski JM, Warling X, Moonen M, Smelten N, Medart L, Pottel H, Cavalier E. Dephosphorylated-uncarboxylated Matrix Gla protein concentration is predictive of vitamin K status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC Nephrol 2014;15:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Thamratnopkoon S, Susantitaphong P, Tumkosit M, Katavetin P, Tiranathanagul K, Praditpornsilpa K, Eiam-Ong S. Correlations of plasma desphosphorylated uncarboxylated matrix Gla protein with vascular calcification and vascular stiffness in chronic kidney disease. Nephron 2017;135(3):167–72. [DOI] [PubMed] [Google Scholar]
- 68. Aoun M, Makki M, Azar H, Matta H, Chelala DN. High dephosphorylated-uncarboxylated MGP in hemodialysis patients: risk factors and response to vitamin K2, a pre-post intervention clinical trial. BMC Nephrol 2017;18(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roumeliotis S, Dounousi E, Eleftheriadis T, Liakopoulos V. Association of the inactive circulating Matrix Gla protein with vitamin K intake, calcification, mortality, and cardiovascular disease: a review. Int J Mol Sci 2019;20(3):E628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mendoza FJ, Martinez-Moreno J, Almaden Y, Rodriguez-Ortiz ME, Lopez I, Estepa JC, Henley C, Rodriguez M, Aguilera-Tejero E. Effect of calcium and the calcimimetic AMG 641 on matrix-Gla protein in vascular smooth muscle cells. Calcif Tissue Int 2011;88(3):169–78. [DOI] [PubMed] [Google Scholar]
- 71. Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab 1998;83(9):3258–66. [DOI] [PubMed] [Google Scholar]
- 72. Shea MK, O'Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr 2009;89(6):1799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shea MK, O'Donnell CJ, Vermeer C, Magdeleyns EJ, Crosier MD, Gundberg CM, Ordovas JM, Kritchevsky SB, Booth SL. Circulating uncarboxylated matrix Gla protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. J Nutr 2011;141(8):1529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nagata Y, Inaba M, Imanishi Y, Okazaki H, Yamada S, Mori K, Shoji S, Koyama H, Okuno S. Increased undercarboxylated osteocalcin/intact osteocalcin ratio in patients undergoing hemodialysis. Osteoporos Int 2015;26(3):1053–61. [DOI] [PubMed] [Google Scholar]
- 75. Rennenberg RJ, Schurgers LJ, Vermeer C, Scholte JB, Houben AJ, de Leeuw PW, Kroon AA. Renal handling of matrix Gla-protein in humans with moderate to severe hypertension. Hypertens Res 2008;31(9):1745–51. [DOI] [PubMed] [Google Scholar]
- 76. Nigwekar SU, Bloch DB, Nazarian RM, Vermeer C, Booth SL, Xu D, Thadhani RI, Malhotra R. Vitamin K–dependent carboxylation of matrix Gla protein influences the risk of calciphylaxis. J Am Soc Nephrol 2017;28(6):1717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Holden RM, Booth SL, Tuttle A, James PD, Morton AR, Hopman WM, Nolan RL, Garland JS. Sequence variation in vitamin K epoxide reductase gene is associated with survival and progressive coronary calcification in chronic kidney disease. Arterioscler Thromb Vasc Biol 2014;34(7):1591–6. [DOI] [PubMed] [Google Scholar]
- 78. Wang D, Chen H, Momary KM, Cavallari LH, Johnson JA, Sadee W. Regulatory polymorphism in vitamin K epoxide reductase complex subunit 1 (VKORC1) affects gene expression and warfarin dose requirement. Blood 2008;112(4):1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shea MK, Booth SL, Miller ME, Burke GL, Chen H, Cushman M, Tracy RP, Kritchevsky SB. Association between circulating vitamin K1 and coronary calcium progression in community-dwelling adults: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2013;98(1):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Krueger T, Schlieper G, Schurgers L, Cornelis T, Cozzolino M, Jacobi J, Jadoul M, Ketteler M, Rump LC, Stenvinkel P et al.. Vitamin K1 to slow vascular calcification in haemodialysis patients (VitaVasK trial): a rationale and study protocol. Nephrol Dial Transplant 2014;29(9):1633–8. [DOI] [PubMed] [Google Scholar]
- 81. Holden RM, Booth SL, Day AG, Clase CM, Zimmerman D, Moist L, Shea MK, McCabe KM, Jamal SA, Tobe S et al.. Inhibiting the progression of arterial calcification with vitamin K in HemoDialysis patients (iPACK-HD) trial: rationale and study design for a randomized trial of vitamin K in patients with end stage kidney disease. Can J Kidney Health Dis 2015;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP, Vermeer C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women: double-blind randomised clinical trial. Thromb Haemost 2015;113(5):1135–44. [DOI] [PubMed] [Google Scholar]
- 83. Mansour AG, Hariri E, Daaboul Y, Korjian S, El Alam A, Protogerou AD, Kilany H, Karam A, Stephan A, Bahous SA. Vitamin K2 supplementation and arterial stiffness among renal transplant recipients—a single-arm, single-center clinical trial. J Am Soc Hypertens 2017;11(9):589–97. [DOI] [PubMed] [Google Scholar]
- 84. Townsend RR, Wimmer NJ, Chirinos JA, Parsa A, Weir M, Perumal K, Lash JP, Chen J, Steigerwalt SP, Flack J et al.. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens 2010;23(3):282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pivin E, Ponte B, Pruijm M, Ackermann D, Guessous I, Ehret G, Liu YP, Drummen NE, Knapen MH, Pechere-Bertschi A et al.. Inactive matrix Gla-protein is associated with arterial stiffness in an adult population–based study. Hypertension 2015;66(1):85–92. [DOI] [PubMed] [Google Scholar]