Abstract
Introduction
The aim of the study was to determine the prevalence of cardio‐metabolic risk factors in men under 30 in the United Arab Emirates.
Methods
This cross‐sectional observational study included 33 327 Emirati men aged 18‐29 attending an obligatory standardized medical examination between May 2015 and February 2017. Body mass index, fasting blood glucose, total cholesterol, triglycerides and blood pressure were assessed.
Results
Overall, 7720 subjects (24.4%) were overweight and 8835 (28.0%) obese. The age‐adjusted prevalence was 4.7% [95% CI: 4.4‐5.0] for diabetes, 41.3% [40.6‐41.9] for impaired fasting glucose, 5.5% [5.2‐5.8] for hypercholesterolaemia (total cholesterol ≥ 240 mg/dL), 11.5% [11.1%‐12.0%] for hypertriglyceridaemia (≥150 mg/dL) and 10.4% [10.0%‐10.8%] for hypertension (diastolic or systolic blood pressure—or both—above upper limit of normal). These conditions were already present in the youngest age groups and rise progressively and rapidly with age. Of the 26 648 subjects with valid data for all cardio‐metabolic risk factors, 16 563 subjects (62.2%) presented ≥ 1 factor, 6392 subjects presented ≥2 factors (24.0%) and 63 (0.2%) presented all five. Patients who were obese were more likely to present multiple cardio‐metabolic risk factors and to have hypertension (P < 0.0001). All cardio‐metabolic risk factors were highly correlated with each other.
Conclusions
This national cohort study in the UAE revealed that obesity, diabetes, impaired fasting glucose, hypercholesterolaemia, triglyceridaemia and hypertension are already highly prevalent in young adulthood. Public health initiatives are required to address these and to anticipate the future burden of diabetes and major cardiovascular disease for which these men are at high risk.
Keywords: age‐specific prevalence, diabetes, metabolic disorders, UAE
1. INTRODUCTION
The Middle East in general, and peninsular Arabia in particular, has one of the highest prevalence rates of diabetes and obesity in the world.1, 2, 3 According to the 2017 report of the International Diabetes Federation (IDF) on the worldwide burden of diabetes,1 Saudi Arabia, United Arab Emirates (UAE) and Egypt have the highest adult diabetes prevalence rates in the Middle East region. The only countries which have a higher prevalence worldwide are a number of small island nations in the Pacific and Indian Oceans. However, the data from the UAE are relatively old, dating from a survey of 4000 randomly selected householders conducted in 1999‐2000.1 In this study, the age‐adjusted prevalence of diabetes was 20.4% and of impaired fasting glucose 4.5%. In addition, 41% of respondents were overweight, 33% were obese and 25.9% had uncontrolled hypertension. Other studies have also indicated a high prevalence of metabolic syndrome, obesity and dyslipidaemia in the UAE.4, 5 For example, in a random sample of long‐standing adult residents in the UAE investigated in 1999‐2000, one third of all subjects were obese (BMI ≥ 30) and a further 40% were overweight (BMI 25‐29), 6and in the same sample, 40% fulfilled diagnostic criteria for metabolic syndrome.4 A later retrospective study of people consulting a private hospital for lipid profiling in 2005‐7, of whom 12% were the UAE nationals and 63% men, reported that the proportion of subjects with elevated LDL cholesterol rose with age from 55% in subjects under 30 to 64% in those aged >50.5
The prevalence of these disorders also appears to be rising fast. According to data collected by the IDF, the number of people with diabetes in the Middle East and North Africa region rose from 24.5 million in 2006 to 39 million in 2017 and is predicted to rise to 67 million in 2045. In the UAE, self‐reported prevalence rates in adults doubled between 1995 and 20006, 7 to reach 20% for diabetes and 17% for prediabetes.7 Rising obesity rates over the last 20 years have also been reported in Saudi Arabia and Kuwait.8 The high and rising prevalence of diabetes and other cardio‐metabolic conditions in Arabian countries may be associated with the major social changes in these countries over the past 50 years, which have evolved from a traditional semi‐rural lifestyle to a sedentarized, urban, high‐income society.3, 9 A national survey of Emirati adults and children performed in 2009/10 reported a high proportion of respondents with a caloric intake above requirements, a high caloric input from snacking and sodas and low levels of physical activity,9 with these lifestyle patterns being associated with urban residence and female gender.
In order for health authorities to meet the challenges of the increase in diabetes and cardio‐metabolic risk factors in Arabian countries, continued surveillance of these conditions is needed in nationally representative samples. In this way, reliable and up‐to‐date prevalence data can be generated regularly.8 In the UAE, all men are obliged to perform a standardized medical examination at some time between the ages of 18 and 30 years. This examination provides an opportunity to collect information on health variables in participants. Using data collected at this medical examination, this study aimed to determine the prevalence of diabetes, obesity, hyperlipidaemia and hypertension in an exhaustive cross‐sectional sample of young Emirati men.
2. MATERIALS AND METHODS
This was a cross‐sectional analysis of medical data of a sample of young Emirati men aged between 18 and 29 years inclusive undergoing a standardized medical examination between May 2015 and February 2017.
2.1. Subjects
In UAE, all young Emirati men attend a compulsory medical examination when they leave high school (usually at the age of 18 or 19). Exceptions are only made for men entering university education, who have to attend the examination when they finish their studies and, in all cases, before their thirtieth birthday. The study included a cohort of all the young men attending their obligatory medical examination over the 18‐month recruitment period. The age distribution of the sample reflects the requirements, and consists of all young Emirati men aged 18 and 19 years not going to university, who make up the majority of the subjects, and a “tail” of older subjects aged 20‐29 years who had not undergone the medical examination when they left high school.
The obligatory standard medical examination was performed as a 1‐day outpatient visit at one of the three licensed hospital medical centres in the UAE. All staff working at these centres received training to ensure consistency in following the same procedures. Subjects received instructions to report in the morning, following fasting overnight prior to undergoing the medical fitness examination.
2.2. Procedures
For each subject, a standard electronic questionnaire was completed to document medical history. Subjects were asked to wear light clothing without shoes. A portable digital scale and stadiometer were used to measure weight and height. Subjects were asked to stand straight with their heads, backs and buttocks vertically aligned to the height gauge; their heights were then taken and rounded to the nearest 0.5 cm. Blood pressure (systolic and phase‐V diastolic) measurements were recorded as the mean of three separate readings in a sitting position after 10 minutes of rest, using a standard clinical mercury sphygmomanometer with a cuff of appropriate size. Blood pressure was measured on the right arm, to the nearest 2 mm Hg. Fasting venous samples were obtained for biochemical and haematological testing. Appropriate standard technical measures were taken for the processing and transportation of these samples to one of three central laboratories in Abu Dhabi, Al Ain and Sharjah. Biochemical assays were performed using a Dimension EXL 200 Integrated Chemistry System (Siemens Healthineers). For the purposes of this study, glucose, total cholesterol and total triglycerides were measured.
2.3. Outcome variables
The definitions of obesity, diabetes, dyslipidaemia and hypertension used in this study are listed in Table S1.
2.4. Statistical analysis
The presentation of the data is purely descriptive. Quantitative variables are presented as mean values with their standard deviations (SD) or median values with range. Categorical values are provided as frequency counts and percentage values. Missing data were not replaced. The chi‐square test was used to determine the statistical significance of differences between groups. Age‐adjusted prevalence rates with their 95% confidence intervals (CI) were estimated using standardized weights for the age groups (18‐19, 20‐24, 25‐29) based on mid‐2016 population estimates of the Emirate of Abu Dhabi. Associations between the different risk factors were evaluated using multivariate logistic regression analysis. Odds ratios with their 95% confidence intervals for presenting each metabolic risk factor, taken as the dependent variable, were determined for each 1‐year increase in age and for each one standard deviation increase in the value of the remaining risk factors, as independent variables. Stata 15 software (StataCorp. 2017. Stata Statistical Software: Release 15: StataCorp LLC) was used to perform the analysis. The significance level of the statistical tests was set at 5%.
2.5. Ethics
The study was conducted in accordance with relevant international and national legislation for medical research. All data were anonymized before entry into the study database, where each subject is identified by a number. The study was submitted to and approved by the Ethics Committee of Zayed Military Hospital.
3. RESULTS
3.1. Subjects evaluated
Overall, 33 327 young men aged between 18 and 29 years were available for analysis. However, valid blood biochemistry data were missing for up to 6295 subjects (18.9%), depending on the variable measured. The mean age was 21.6 years, with a median of 20 years Table 1. The age distribution was highly skewed, with a long tail of older subjects Figure S1.
Table 1.
Demographic and metabolic characteristics in the study population
| N | Mean ± SD | Median [range] | |
|---|---|---|---|
| Age (years) | 33 327 | 21.6 ± 3.3 | 20 [18‐29] |
| Height (cm) | 31 647 | 171.4 ± 6.4 | 171 [145‐199] |
| Weight (kg) | 31 728 | 79.0 ± 23.4 | 75 [37‐200] |
| Body mass index (kg/m2) | 31 610 | 26.8 ± 7.4 | 25 [12‐86] |
| Fasting blood glucose (mg/dL) | 27 339 | 100.7 ± 25.1 | 98 [40‐499] |
| Total cholesterol (mg/dL) | 27 089 | 169.0 ± 36.9 | 165 [59‐402 |
| Triglycerides (mg/dL) | 27 032 | 83.9 ± 58.1 | 68 [48‐101] |
| Systolic blood pressure (mm Hg) | 31 751 | 123.2 ± 12.0 | 69 [45‐120] |
| Diastolic blood pressure (mm Hg) | 31 590 | 69.9 ± 9.8 | 123 [80‐190] |
Data are presented as mean values with standard deviation (SD) or median values with range for all subjects in whom a valid measure was available (N).
3.1.1. Metabolic markers and blood pressure
Mean values for body mass index, fasting blood glucose, serum cholesterol, serum triglycerides and blood pressure are provided in Table 1. The distribution of values for each of these variables in the study population is presented in Figure [Link], [Link], [Link], [Link], [Link] and the proportion of subjects with abnormal values as a function of age is presented in Figure 1. Prevalence rates are displayed in Table 2.
Figure 1.
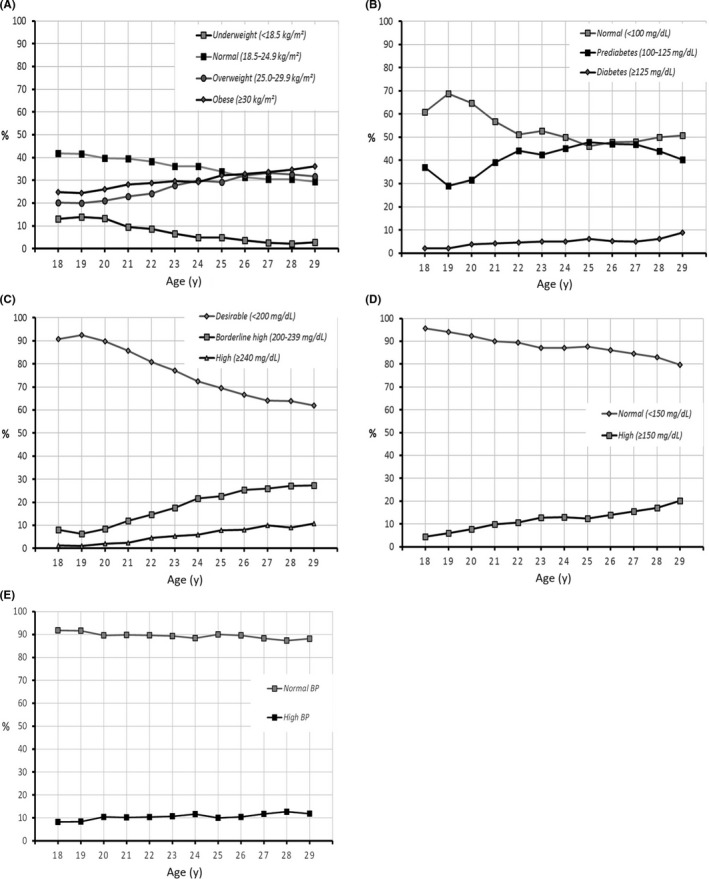
Body mass index, metabolic markers and blood pressure as a function of age. A, Body mass index (N = 31 610); B, fasting blood glucose (N = 27 339); C, total cholesterol (N = 27 089); D, triglycerides (N = 27 032); and E, blood pressure (N = 31 574). Data are presented as the proportion of subjects in each risk factor class as a function of age, for all subjects in whom a valid measure was available (N)
Table 2.
Age‐specific and age‐adjusted prevalence (95% CI) of cardio‐metabolic abnormalities
| Prevalence (95% CI) | ||||
|---|---|---|---|---|
| Overall age‐adjusteda | Age groups | |||
| 18‐19 y | 20‐24 y | 25‐29 y | ||
| Body mass index | ||||
| Underweight | 7.3 (7.0‐7.6) | 13.3 (12.6‐13.9) | 8.5 (8.0‐9.1) | 3.3 (2.9‐3.8) |
| Normal weight | 35.7 (35.0‐36.3) | 41.4 (40.4‐42.3) | 37.6 (36.7‐38.6) | 31.2 (30.1‐32.3) |
| Overweight | 27.0 (26.5‐27.6) | 20.7 (19.9‐21.5) | 25.3 (24.4‐26.1) | 31.7 (30.6‐32.8) |
| Obese | 30.0 (29.4‐30.6) | 24.7 (23.8‐25.5) | 28.6 (27.7‐29.5) | 33.8 (32.7‐34.9) |
| Fasting blood glucose | ||||
| Normal | 54.0 (53.4‐54.7) | 64.6 (63.7‐65.6) | 55.2 (54.2‐56.2) | 48.1 (46.9‐49.3) |
| IFG | 41.3 (40.6‐41.9) | 33.2 (32.3‐34.1) | 40.2 (39.2‐41.2) | 45.9 (44.8‐47.1) |
| Diabetes | 4.7 (4.4‐5.0) | 2.2 (1.9‐2.5) | 4.5 (4.1‐5) | 6.0 (5.4‐6.6) |
| Total cholesterol | ||||
| Desirable | 76.9 (76.3‐77.4) | 91.7 (91.1‐92.2) | 81.4 (80.6‐82.2) | 65.7 (64.6‐66.8) |
| Borderline high | 17.7 (17.2‐18.2) | 7.2 (6.7‐7.7) | 14.7 (14.0‐15.4) | 25.3 (24.3‐26.4) |
| High | 5.5 (5.2‐5.8) | 1.1 (0.9‐1.4) | 3.9 (3.6‐4.3) | 8.9 (8.3‐9.6) |
| Triglycerides | ||||
| Normal | 88.5 (88.0‐88.9) | 94.8 (94.3‐95.2) | 89.3 (88.7‐89.9) | 84.8 (84.0‐85.7) |
| High | 11.5 (11.1‐12.0) | 5.2 (4.8‐5.7) | 10.7 (10.1‐11.3) | 15.2 (14.3‐16) |
| Blood pressure | ||||
| Normal | 89.6 (89.2‐90.0) | 91.9 (91.3‐92.4) | 89.3 (88.7‐89.9) | 89 (88.2‐89.7) |
| High (broad definition) | 10.4 (10.0‐10.8) | 0.9 (0.7‐1.1) | 1.9 (1.6‐2.2) | 3.4 (3‐3.8) |
Abbreviation: IFG, impaired fasting glucose.
Estimated using standardization weights for the age groups (18‐19, 20‐24, 25‐29) based on mid‐2016 population estimates of the Emirate of Abu Dhabi.
Body mass index
The mean body mass index was 26.8 kg/m2, which is already in the overweight range. Overall, 7720 subjects were overweight (24.4%) and 8835 (28.0%) were obese. The BMI was ≥ 40 kg/m2 (morbid obesity Grade III) in 6.0% of subjects. The proportion of subjects who were obese rose progressively from 25% in subjects aged 18 years to 36.2% in those aged 29 years, while the proportion in the normal BMI range (20‐25 kg/m2) decreased from 42% to 29%. The age‐adjusted prevalence of obesity was 30.0% and of being overweight was 27.0%.
Fasting blood glucose (FBG)
The mean FBG was 101 mg/dL, above the normal range. Overall, 10 618 subjects (38.8%) fulfilled criteria for IFG and 1094 subjects (4.0%) criteria for diabetes. The proportion with diabetes increased with age, from 2.2% in 18‐year‐olds to 9% in 29‐year‐olds. The proportion of subjects with IFG rose from 37% to 40% and the proportion with FBG in the normal range declined from 61% to 51%. The age‐adjusted prevalence of diabetes was 4.7% [4.4‐5.0]. Fifty‐five men reported that they had been diagnosed with diabetes all of whom had FBG levels above the normal range.
Lipids
Mean serum total cholesterol was 169 mg/dL. Total cholesterol was ≥ 240 mg/dL in 4.2% of subjects (N = 1138), with an age‐adjusted prevalence of 5.5% [5.2‐5.8]. Total cholesterol was in the borderline high range in a further 14.6% (N = 3955). Again, a strong association with age was observed Figure 1, with the proportion of subjects with total cholesterol in the normal range falling from 91% in 18‐year‐olds to 62% in 29‐year‐olds.
Mean serum triglyceride levels were 83.9 mg/dL. Overall, levels were higher than the normal range in 9.8% of subjects (N = 2649). The relationship between triglyceride levels and age was less pronounced that for the other markers evaluated, although the proportion of subjects with normal triglycerides fell from 98% in 18‐year‐olds to 80% in 29‐year‐olds. The age‐adjusted prevalence of hypertriglyceridaemia was 11.5% [11.1‐12.0].
Arterial blood pressure
The mean diastolic blood pressure was 69.9 mm Hg, and the mean systolic blood pressure was 123.2 mm Hg. Isolated diastolic hypertension was identified in 368 patients (1.2%), isolated systolic hypertension in 2153 (6.8%) and both elevated diastolic and systolic blood pressure (hypertension—narrow definition) in 558 (1.8%). Overall, 3079 subjects (9.8%) fulfilled the broad definition of hypertension, with an age‐adjusted prevalence of 10.4% [10.0%‐10.8%]. The proportion of patients fulfilling the broad definition of hypertension rose with age from 8% in 18‐year‐olds to 12% in 29‐year‐olds. Thirty‐eight men reported that they had received a diagnosis of hypertension, although measured blood pressure was in the normal range for nine of these.
3.2. Multiple cardio‐metabolic abnormalities
Of the 26 648 subjects with valid data for all cardio‐metabolic risk factors (BMI ≥ 30 kg/m2, triglycerides ≥ 150 mg/dL, total cholesterol ≥ 240 mg/dL, hypertension—broad definition, FPG ≥ 100 mg/dL), 16 563 subjects (62.2%) presented at least one such factor, 6392 subjects presented at least two factors (24.0%) and 63 (0.2%) presented all five. The presence of multiple risk factors (among the remaining four) was higher in subjects who were obese than in those who were not (P < 0.0001; χ2 test; Figure 2).
Figure 2.
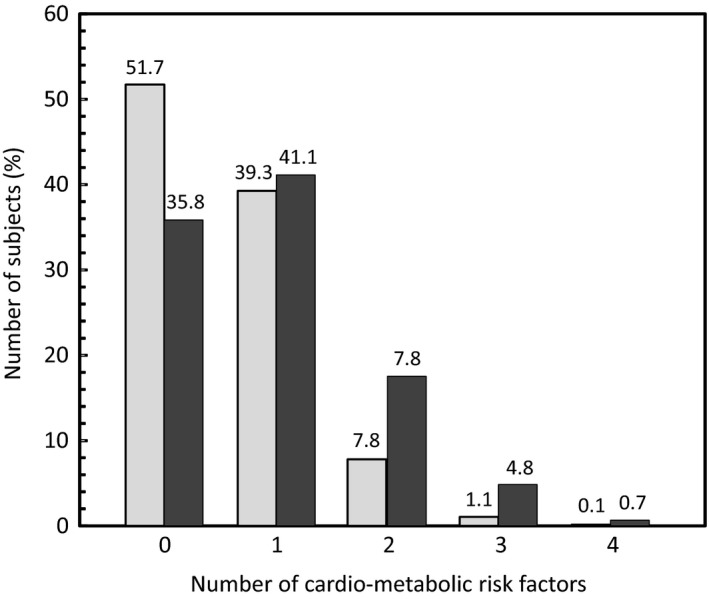
Relationship between obesity and the presence of multiple cardio‐metabolic risk factors. The number of cardio‐metabolic risk factors (FPG ≥ 100 mg/dL, triglycerides ≥ 150 mg/dL, total cholesterol ≥ 240 mg/dL or hypertension—broad definition) is presented as a function of obesity. Pale columns: BMI < 30 kg/m2 (no obesity); N = 19 133. Dark columns: BMI ≥ 30 kg/m2 (obesity); N = 7515. Data are presented for the 26 648 subjects for whom data were available for all risk factors
3.3. Relationships between different cardio‐metabolic risk factors
In multivariate logistic analysis, significant associations were observed between age, on the one hand, and having diabetes, hypercholesterolaemia and hypertriglyceridaemia, on the other. Significant associations were also observed between each pair of risk factors. Increased BMI was the risk factor with the strongest association with hypertension. Elevated triglycerides and elevated blood pressure had the strongest associations with having diabetes. Elevated cholesterol and triglycerides were strongly associated with each other. Increased BMI was strongly associated with having hypertriglyceridaemia Table 3.
Table 3.
Associations between cardio‐metabolic risk factors
| Risk factor | Risk of presenting a metabolic disorder or hypertension, OR (95% CI)a | ||||
|---|---|---|---|---|---|
| Obesity | Diabetes mellitus | Hypercholesterolaemia | Hypertriglyceridaemia | Hypertension | |
| Age | 0.99 (0.98‐1.00) | 1.06 (1.04‐1.08) | 1.20 (1.18‐1.22) | 1.06 (1.04‐1.07) | 0.99 (0.97‐1.00) |
| Body mass index | 1.14 (1.07‐1.21) | 1.26 (1.19‐1.34) | 1.51 (1.45‐1.57) | 1.87 (1.80‐1.94) | |
| Fasting blood glucose | 1.02 (0.99‐1.05) | 1.11 (1.07‐1.16) | 1.22 (1.18‐1.26) | 1.11 (1.08‐1.15) | |
| Total cholesterol | 1.35 (1.31‐1.39) | 1.14 (1.07‐1.22) | 1.91 (1.83‐1.99) | 1.09 (1.05‐1.14) | |
| Triglycerides | 1.56 (1.51‐1.61) | 1.47 (1.40‐1.53) | 1.55 (1.48‐1.62) | 1.20 (1.15‐1.26) | |
| Blood pressure | 3.04 (2.79‐3.31) | 1.74 (1.48‐2.06) | 1.24 (1.04‐1.48) | 1.29 (1.15‐1.46) | |
Odds ratio from multivariate models for having each of these disorders associated with a 1‐y increase in age and a one standard deviation increase in each cardio‐metabolic risk factor.
4. DISCUSSION
This cross‐sectional study of a large sample of young men in the UAE has revealed high prevalence rates of diabetes (4.7%), impaired fasting glucose (41.3%), overweight (27.0%), obesity (29.4%), hypercholesterolaemia (5.5%), triglyceridaemia (11.5%) and hypertension (broad definition: 10.4%). Overall, 62% presented at least one of these risk factors. Less than 0.2% reported a diagnosis of diabetes or hypertension. These conditions were already present in significant proportions of men in the youngest age groups and rise progressively and rapidly with age.
The principal strengths of this study include the large sample size and the standardized medical examination performed. All blood biochemistry measurements were performed in three regional centres using identical procedures. In addition, the study focussed on young adults who are often excluded or under‐represented in studies of these medical conditions. The principal weakness of the study is that the standard medical examination was a general one and not designed to focus on diabetes and cardio‐metabolic conditions. For this reason, data on other variables of potential interest such as abdominal obesity and levels of HDL cholesterol are not available, and it was not possible to determine the prevalence of metabolic syndrome. In addition, dietary history, fast food consumption and physical activity were not assessed. Moreover, medication is not documented and subjects with diagnosed, treated and controlled hypertension or diabetes may thus have been missed. The amount of missing data for the biochemical variables was also relatively high (up to 19% for triglycerides). In addition, although subjects were requested to come to the medical examination after an overnight fast, it was not possible to verify that this was indeed the case. Failure to comply with this by certain individuals may have resulted in some overestimation of the number of cases with elevated blood glucose or lipids. It should also be noted that this study only evaluated men, and the prevalence of the conditions of interest in young women is not addressed.
The prevalence rates observed in the UAE were around twofold higher than those observed in men under thirty in recent general population studies from Western Europe, which are <2% for diabetes,10, 11, 12 <15% for obesity,13, 14 <8% for hypertension (broad definition)13, 14, 15 and <1% for dyslipidaemia.13 Similarly, in the most recent NHANES study from the United States (2012), the prevalence of diabetes in individuals aged between 20 and 44 was 3.3%.16 Compared with previous studies in the UAE, the prevalence of diabetes in young men has risen from <1% in the 19‐39 age group in the 1995 survey (diagnosed diabetes only) 7and from 3.7% in the 20‐24 age group in the 2000 survey. 6In addition, the proportion of young men with IFG rose from 3.2%‐3.7% in 2000 to 39.3% in the present study. The nearly ten‐fold difference between the prevalence of IFG and that of diabetes in the present study is unexpected compared with data from other countries. This large number of young men with IFG may represent a reservoir of individuals at high risk for developing type 2 diabetes as they age. Nonetheless, it should be emphasized that the relative prevalence of IFG and diabetes will depend on the cut‐off threshold used in the definition. Although our study only focussed on Emirati nationals, who are the only ones obliged to undergo the compulsory medical examination, other studies conducted over the last decade have reported high prevalence rates of obesity and of diabetes in expatriates residing in the UAE, particularly in those who were ethnic Arabs or South Asians.17, 18 Our study only investigated men, but a recent (2013‐2014) study of 555 young female university students in the UAE aged 17‐25 years reported that 23.1% of these young women were overweight and 10.4% as obese, with 6.8% already fulfilling criteria for metabolic syndrome.19 The prevalence of obesity is thus lower than in the young men of the same age range in our study. In contrast, other studies in the UAE and elsewhere in the region have shown the prevalence of diabetes, obesity and metabolic syndrome to be higher in women than men.3, 4, 6, 8 It is possible that the university students recruited into this study may not be representative of all young women in the UAE, being better educated and probably more physically active. Accessing a fully representative sample of young women in the UAE would be challenging for cultural reasons.
Although the high prevalence of obesity and diabetes in the Middle East region is well documented,1, 2, 3 the present study has two novel aspects. Firstly, the study specifically evaluated young adults aged between 18 and 29 years and shows that, even at the age of 20, these young men already show a high prevalence of cardio‐metabolic risk factors, which then rises rapidly with age. This has important consequences for public health since prevention measures such as lifestyle and dietary changes need to be implemented at school‐leaving age, before cardiovascular and metabolic damage becomes irreversible. Secondly, previous epidemiological studies in peninsular Arabia have looked at selected subgroups of people, such as university students, urban populations or immigrants, whereas the present study enrolled an age cohort including all young men in the UAE. This allows national prevalence rates by age group to be estimated accurately and shows that cardio‐metabolic disorders in early adulthood are a national problem.
The high prevalence of these abnormalities in young adults is unlikely to be unique to the UAE, and a comparable situation no doubt prevails in other countries in peninsular Arabia, and possibly elsewhere in the Middle East. Although vulnerability to diabetes and obesity in Arabian countries may have a genetic component, 2 the fact that the prevalence of these conditions was much lower as little as 20 years ago implies that lifestyle factors are primarily responsible. 2The data obtained in this study paint a worrying picture of the state of health of young Emirati men. Over one in four of these are obese, and this is likely to contribute to the emergence of diabetes and hypertension in these individuals. High BMI was indeed the metabolic factor that was most robustly associated with hypertension and dyslipidaemia in this population. Two studies dating from 2009/10 evaluating children and adolescents in the UAE have indicated that 25.0% of boys and 40.7% of girls aged 6‐10 years9 , as well as 34% of adolescents aged 12‐18 years,20 were already overweight or obese and consistent findings have been reported in other studies.21, 22 The implications of these findings for health policymakers are clear. Educational measures directed at parents and schools to encourage children to eat healthily and to maintain their weight need to be established. Young adults need to be screened systematically for diabetes and cardio‐metabolic conditions, and when these are detected, lifestyle modifications or pharmacological management put in place. The systematic medical examination provides a first‐level mechanism for this in men, but screening programmes also need to be put in place for young women. Only a very small number of the subjects evaluated claimed to have received a diagnosis of hypertension or diabetes. Since these conditions are perceived as being diseases of maturity, community physicians may not consider checking for them in young people. The findings of our study suggest that their presence in young people under thirty cannot be neglected, particularly in individuals who are obese. People with identified cardio‐metabolic risk factors need to be monitored closely and managed appropriately to prevent the occurrence of major cardiovascular events at a relatively young age. Data collected over the last 30 years in the United States illustrate that the escalating prevalence of cardio‐metabolic risk factors is not inevitable and can be reversed.23
The present study could easily be reiterated on a regular basis in order to monitor the evolution of the prevalence of diabetes and cardio‐metabolic risk factors in young adult men in the UAE. In general, it will be important to collect longitudinal data on the incidence of cardiovascular disease and diabetes in such populations of young adults who are obese or present elevated blood glucose or lipids.
In conclusion, this exhaustive survey of all men under thirty in the UAE reports that the prevalence of diabetes, IFG and other cardio‐metabolic risk factors is already worryingly high in young adulthood. Public health initiatives are required to address these and to anticipate the future burden of diabetes and major cardiovascular disease for which young Emirati men are at high risk.
CONFLICT OF INTEREST
Nothing to declare.
AUTHOR CONTRIBUTION
AA initiated and co‐ordinated the study. AA, LAA, FAM, AFF and JAK conceptualized and planned the analyses. LAA performed the statistical analysis. AA, LAA, FAM, AFF and JAK contributed to the preparation of the manuscript and data interpretation. AA, LAA, FAM, AFF and JAK validated and approved the final version.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank the IT department of Zayed Military Hospital and all the medical team involved in gathering the medical data for their participation in the study.
Alzaabi A, Al‐Kaabi J, Al‐Maskari F, Farhood AF, Ahmed LA. Prevalence of diabetes and cardio‐metabolic risk factors in young men in the United Arab Emirates: A cross‐sectional national survey. Endocrinol Diab Metab. 2019;2:e00081 10.1002/edm2.81
Funding information
The study was funded by Zayed Military Hospital and Astra Zeneca.
DATA AVAILABILITY
The data are owned by a third party (Zayed Military Hospital). Access to the data will require special permission. Reasonable requests can be made to the corresponding author, who will transfer the request to the database manager.
REFERENCES
- 1. International Diabetes Foundation . IDF Diabetes ATlas, 8th ed Brussels, Belgium: International Diabetes Foundation; 2017. [Google Scholar]
- 2. Kharroubi AT, Darwish HM. Diabetes mellitus: the epidemic of the century. World J Diabetes. 2015;6(6):850–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meo SA, Usmani AM, Qalbani E. Prevalence of type 2 diabetes in the Arab world: impact of GDP and energy consumption. Eur Rev Med Pharmacol Sci. 2017;21(6):1303‐1312. [PubMed] [Google Scholar]
- 4. Malik M, Razig SA. The prevalence of the metabolic syndrome among the multiethnic population of the United Arab Emirates: a report of a national survey. Metab Syndr Relat Disord. 2008;6(3):177‐186. [DOI] [PubMed] [Google Scholar]
- 5. Vela BK, Alhessi AY, Popovic M, Al‐Shaqra MA. Prevalence of unrecognized dyslipidaemia in Dubai and Northern Emirates: a cross‐sectional hospital based study. Coll Antropol. 2008;32(4):1087‐1092. [PubMed] [Google Scholar]
- 6. Malik M, Bakir A, Saab BA, Roglic G, King H. Glucose intolerance and associated factors in the multi‐ethnic population of the United Arab Emirates: results of a national survey. Diabetes Res Clin Pract. 2005;69(2):188‐195. https://doi:10.1016/j.diabres.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 7. Fikri M, Farid SM. United Arab Emirates Family Health Survey 1995. Abu Dhabi, United Arab Emirates: Ministry of Health; 2000. [Google Scholar]
- 8. Ng SW, Zaghloul S, Ali HI, Harrison G, Popkin BM. The prevalence and trends of overweight, obesity and nutrition‐related non‐communicable diseases in the Arabian Gulf States. Obes Rev. 2011;12(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 9. Ng SW, Zaghloul S, Ali H, et al. Nutrition transition in the United Arab Emirates. Eur J Clin Nutr. 2011;65(12):1328‐1337. https://doi:10.1038/ejcn.2011.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eschwege E, Basdevant A, Crine A, Moisan C, Charles MA. Type 2 diabetes mellitus in France in 2012: results from the ObEpi survey. Diabetes Metab. 2015;41(1):55‐61. [DOI] [PubMed] [Google Scholar]
- 11. Gatineau M, Hancock C, Naomi H, Outhwaite H, Lorraine O, Christie A, Ells L. Adult obesity and type 2 diabetes. 2014. Retrieved from London.
- 12. Tamayo T, Brinks R, Hoyer A, Kuss OS, Rathmann W. The prevalence and incidence of diabetes in Germany. Dtsch Arztebl Int. 2016;113(11):177‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eschwège E, Charles M‐A, Basdevant A. Obépi 2012. Enquête épidémiologiquenationale sur le surpoids et l'obésité; 2012. Retrieved from.
- 14. NHS Digital . Health Survey for England, 2015: Trend tables;2016. Retrieved from London.
- 15. Neuhauser H, Diederichs C, Boeing H, et al. Hypertension in Germany. Dtsch Arztebl Int. 2016;113(48):809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caspard H, Jabbour S, Hammar N, Fenici P, Sheehan JJ, Kosiborod M. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: an analysis of the NHANES surveys from 1999 to 2014. Diabetes Obes Metab. 2018;20(3):667‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah SM, Ali R, Loney T, et al. Prevalence of diabetes among migrant women and duration of residence in the United Arab Emirates: a cross sectional study. PLoS ONE. 2017;12(1):e0169949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sulaiman N, Elbadawi S, Hussein A, et al. Prevalence of overweight and obesity in United Arab Emirates Expatriates: the UAE National Diabetes and Lifestyle Study. Diabetol Metab Syndr. 2017;9:88 https://doi:10.1186/s13098-017-0287-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al Dhaheri AS, Mohamad MN, Jarrar AH, et al. A cross‐sectional study of the prevalence of metabolic syndrome among young female emirati adults. PLoS ONE. 2016;11(7):e0159378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mehairi AE, Khouri AA, Naqbi MM, et al. Metabolic syndrome among Emirati adolescents: a school‐based study. PLoS ONE. 2013;8(2):e56159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al Junaibi A, Abdulle A, Sabri S, Hag‐Ali M, Nagelkerke N. The prevalence and potential determinants of obesity among school children and adolescents in Abu Dhabi, United Arab Emirates. Int J Obes (Lond). 2013;37(1):68‐74. [DOI] [PubMed] [Google Scholar]
- 22. AlBlooshi A, Shaban S, AlTunaiji M, et al. Increasing obesity rates in school children in United Arab Emirates. Obes Sci Pract. 2016;2(2):196‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun X, Du T. Trends in cardiovascular risk factors among U.S. men and women with and without diabetes, 1988–2014. BMC Public Health. 2017;17(1):893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are owned by a third party (Zayed Military Hospital). Access to the data will require special permission. Reasonable requests can be made to the corresponding author, who will transfer the request to the database manager.


