Despite their fungal morphology, oomycetes constitute a distinct group of protists related to brown algae and diatoms. Many oomycetes are pathogens and cause diseases of plants, insects, mammals, and humans. Extensive efforts have been made to understand the molecular basis of oomycete infection, but durable protection against these pathogens is yet to be achieved. We use a plant-pathogenic oomycete to decipher a key physiological aspect of oomycete growth and infection. We show that oomycete nuclei travel actively and over long distances within hyphae and during infection. Such movements require microtubules anchored on the centrosome. Nuclei hydrodynamically adapt their shape to travel in or against the flow. In contrast, fungi lack a centrosome and have much less flexible nuclei. Our findings provide a basis for modeling of flexible nuclear shapes in branched hyphal networks and may help in finding hard-to-evade targets to develop specific antioomycete strategies and achieve durable crop disease protection.
KEYWORDS: oomycetes, Phytophthora palmivora, nucleus movement, centrosome, hydrodynamics
ABSTRACT
Multinucleate fungi and oomycetes are phylogenetically distant but structurally similar. To address whether they share similar nuclear dynamics, we carried out time-lapse imaging of fluorescently labeled Phytophthora palmivora nuclei. Nuclei underwent coordinated bidirectional movements during plant infection. Within hyphal networks growing in planta or in axenic culture, nuclei either are dragged passively with the cytoplasm or actively become rerouted toward nucleus-depleted hyphal sections and often display a very stretched shape. Benomyl-induced depolymerization of microtubules reduced active movements and the occurrence of stretched nuclei. A centrosome protein localized at the leading end of stretched nuclei, suggesting that, as in fungi, astral microtubule-guided movements contribute to nuclear distribution within oomycete hyphae. The remarkable hydrodynamic shape adaptations of Phytophthora nuclei contrast with those in fungi and likely enable them to migrate over longer distances. Therefore, our work summarizes mechanisms which enable a near-equal nuclear distribution in an oomycete. We provide a basis for computational modeling of hydrodynamic nuclear deformation within branched tubular networks.
INTRODUCTION
Although nuclei are usually depicted as immobile, their movement is essential for the growth and development of all eukaryotes, including those with filamentous morphology (1). The nuclear dynamics of fungi have been studied during development in axenic culture as well as during infection of susceptible hosts (2). Nuclear movements may also contribute to infection success (3–6). Recent work in Magnaporthe oryzae documented the migration of nuclei into the appressorium and, later, into the primary infectious hypha upon rice infection (4).
Fungal nuclei often distribute equally within hyphae, such as in Aspergillus nidulans germ tubes upon asexual spore germination (7). This involves microtubules, cytoplasmic dynein, and components of the dynactin complex. Indeed, treatment with the microtubule-depolymerizing drug Benomyl or mutations in beta-tubulin alter nuclear distribution in A. nidulans (8). Similar nuclear distribution defects are observed when Neurospora crassa cytoplasmic dynein genes (9) or the dynactin component Arp1 (10) is mutated. Nuclear distribution is also affected in the Ashbya gossypii dynein null mutant, but nuclei accumulate at hyphal tips rather than at the spore end (11).
Studies in budding yeast (Saccharomyces cerevisiae) showed that proper orientation of the mitotic spindle during anaphase is crucial for the mother cell and the bud to receive a single nucleus after nuclear division (12). Such orientation is achieved through astral microtubules (12). Assembly of a functional mitotic spindle also requires precise coordination between the nuclear cycle and the major microtubule organizing center, also known as the centrosome (13). A canonical centrosome is composed of two centrioles surrounded by an electron-dense protein-containing matrix (pericentriolar matrix), which nucleates microtubules (14). All fungi except Chytridiomycota possess an acentriolar centrosome termed the spindle pole body (SPB) (14, 15). The SPB is embedded in the nuclear envelope and nucleates spindle microtubules inside the nucleus as well as astral microtubules toward the cytoplasmic side (14). The growing ends of microtubules are occupied by dynein. In A. gossypii, dynein capture by the cortical nuclear migration protein Num1 initiates the pulling of cytoplasmic microtubules, thereby moving the attached nucleus (16). Orthologs of Num1 include the AND1 gene from M. oryzae (5) and ApsA/ApsB from A. nidulans (17).
Oomycetes are a group of microorganisms with filamentous morphologies that are phylogenetically distant from fungi. They differ from fungi by structural, biochemical, and genetic features such as genome size, ploidy, cell wall composition, pigmentation, secondary metabolites, and the chemical nature of mating hormones and reserve compounds (18). Plant-pathogenic oomycetes cause devastating diseases worldwide that impact crop yield, threaten food security, and damage natural ecosystems (19, 20). For instance, blight, canker, and rot diseases caused by plant-infecting oomycetes from the genus Phytophthora, such as the Irish Famine pathogen Phytophthora infestans and its tropical relative Phytophthora palmivora, cause multimillion-dollar losses yearly (21–23). Whether oomycetes display similar nuclear dynamics as filamentous fungi remains to be addressed. Pioneering work based on staining methods revealed elongated nuclei, suggesting a potential movement in cysts of the diatom-infecting oomycete Lagenisma coscinodisci (24) as well as in hyphae and germinating cysts of P. infestans (25, 26). Similarly to fungi, oomycete nuclei are known to distribute equally within coenocytic (aseptate) hyphae, and this distribution is altered by the actin polymerization inhibitor latrunculin B (27–29) or by silencing the Phytophthora capsici cell cycle regulator homologue sda1 (30).
We used time-lapse imaging to investigate nuclear dynamics of P. palmivora during cyst germination and subsequent root and leaf infection in addition to axenically cultured hyphae. For this purpose, we generated a versatile toolbox for efficient dual labeling of oomycete hyphae and organelles that enabled the rapid testing of various promoter-reporter constructs. We found that P. palmivora nuclei undergo coordinated bidirectional movements during plant infection and actively or passively move during mycelial growth to achieve a near-equal distribution of nuclei within hyphae. Active movement of individual nuclei frequently resulted in dramatic alterations of near-globular nuclei into extensively stretched shapes. Shape changes often cooccurred with a rerouting of nuclei toward nucleus-depleted hyphal sections. The centrosome-labeling Centrin2 protein occupied the trailing end of near-globular nuclei and the leading end of stretched nuclei. Furthermore, the microtubule polymerization inhibitor Benomyl decreased nuclear stretching and rerouting. Hence, our results unravel commonalities and differences in the dynamics of oomycete and fungal nuclei. Together, this constitutes the basis for new models of nuclear migration and hydrodynamic deformability that may ultimately offer alternative strategies to tackle oomycete diseases.
RESULTS
Bidirectional nuclear movements occur during P. palmivora cyst germination.
To investigate nuclear dynamics during P. palmivora cyst germination, we generated the pTORKRm43GW Gateway vector, which allows for dual labeling of hyphae and organelles (see Fig. S1 and S2 and Table S1A in the supplemental material), and transformed the P. palmivora isolate LILI (P16830). The vector contains a cassette for the Ham34 promoter-driven constitutive expression of a cytoplasmic tdTomato fluorescent reporter in addition to a cyan nucleus-localized monomeric TFP1 (NLS:mTFP1) expressed under the P. palmivora ubiquitin-conjugating enzyme 2 (UBC2) native promoter (Fig. S3 and S4). Among seven independent transformants, two showed distinct hyphal populations expressing either tdTomato or mTFP1 reporters, while five showed both cytoplasmic tdTomato (td) fluorescence and nuclear mTFP1 (NT) fluorescence. The latter were used to obtain the transgenic P. palmivora line LILI-td-NT used in this study (Fig. S3).
Schematic representation of the pTOR-Gateway. A multisite attR4/attR3 Gateway insertion cassette allows for rapid testing of multiple promoter-reporter constructs. Adjacent is a cassette for constitutive expression of a cytoplasmic fluorescent reporter (either mTFP1, mWasabi, mCitrine, or tdTomato) under the control of Bremia lactucae promoter Ham34. Vectors carry neomycin phosphotransferase (nptII) selectable marker. Download FIG S1, PDF file, 0.2 MB (209.3KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Growth habit of transgenic P. palmivora strains carrying empty pTOR-Gateway vectors on N. benthamiana leaves. (A to D) Leaves from 4-week-old N. benthamiana plants were inoculated with mycelium plugs of transgenic P. palmivora strains carrying empty pTOR-Gateway vectors. Fluorescence was monitored within leaf tissues after 2 days. Representative images of areas infected with mycelium expressing mTFP1 (A), mWasabi (B), mCitrine (C), and tdTomato (D) are shown. Yellow asterisks indicate haustoria. Bar, 10 μm. Download FIG S2, PDF file, 1.5 MB (1.5MB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Dual labeling of P. palmivora hyphae and nuclei. (A) Schematic view of the construct used for dual labeling of nuclei and hyphae in P. palmivora strain LILI-td-NT. Backbone elements are not represented. (B) Representative pictures of a transformant expressing tdTomato as well as a nucleus-localized mTFP1 (NLS:mTFP1) driven by the P. palmivora ubiquitin-conjugating enzyme 2 (UBC2) native promoter. Representative pictures of transformants expressing both markers as well as rare cases of transformants expressing either tdTomato or mTFP1 in distinct hyphae. Bar, 10 μm. Download FIG S3, PDF file, 1.0 MB (1.1MB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
UBC2 transcript levels during N. benthamiana root infection. N. benthamiana roots were inoculated with zoospores from the transgenic P. palmivora strain ARI-tdTomato and harvested at different times corresponding to early infection (3 to 6 h), biotrophy (18 to 24 hai), and necrotrophy (30 to 48 hai). Expression data are given relative to P. palmivora WS21 and EF1α reference genes. Statistical significance was assessed using one-way analysis of variance and Tukey’s honestly significant difference test (P < 0.05). MZ, axenically grown mycelium with sporangia. Download FIG S4, PDF file, 0.2 MB (248.2KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) pTOR-Gateway vectors. pTOR-Gateway vectors follow naming conventions used for Gateway vectors. K indicates nptII, while C, F, Y, and R stand for cyan, green, yellow, and red fluorescence, respectively. The multisite Gateway cassette carries attR4 and attR3 sites and hence was arbitrarily named m43GW in the absence of a T-DNA left border to define cassette orientation. (B) Primers used in this study. Download Table S1, PDF file, 0.2 MB (188.1KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
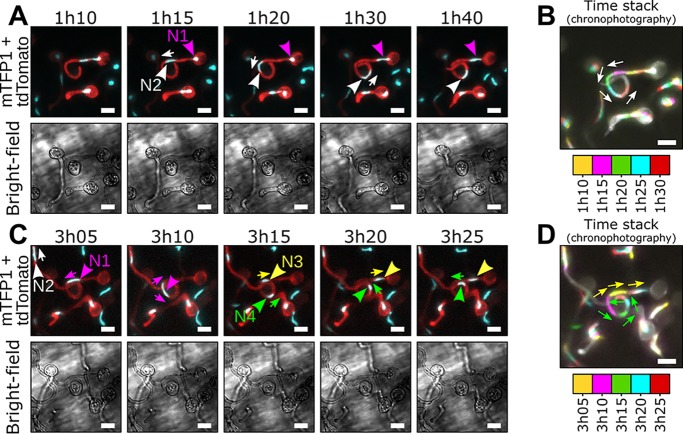
We then incubated encysted LILI-td-NT zoospores in a liquid compartment containing a Nicotiana benthamiana seedling (Fig. S5) and monitored nuclear movements during the growth of cyst germ tubes (Fig. 1A and B, video 1 [https://zenodo.org/record/3378706/files/Video1.mp4]). Germ tubes emerged from mononucleate cysts and were initially devoid of a nucleus. After 1 h when germ tubes were longer than 10 μm on average, the sole nucleus became stretched and moved from the cyst into the germ tube while the cyst remained filled with cytoplasm (Fig. 1A, video 1). The nucleus divided in the germ tube, but no leakage of mTFP1 fluorescence from the nucleus was detectable during division. The daughter nucleus N1 subsequently retracted to the cyst body, while the second daughter nucleus, N2, moved forward within the germ tube at an average speed of 0.02 μm/s (Fig. 1A, video 1). Upon further extension of the germ tube, the cyst-resident nucleus N1 reentered the germ tube, still leaving cytoplasm in the cyst behind (Fig. 1A, video 1) and divided 3 h after encystment into daughter nuclei N3 and N4 (Fig. 1B, video 1). N3 and N4 moved away from each other, with nucleus N3 moving toward the cyst body and nucleus N4 moving forward within the germ tube. Collectively, these findings demonstrate that nuclei of germinating P. palmivora cysts divide in a closed mitosis and are then distributed evenly along the germ tube through concomitant, bidirectional movements.
FIG 1.
Repositioning of daughter nuclei follows division during P. palmivora cyst germination (video 1). (A to D) Time-lapse imaging of germinating cysts from a transgenic P. palmivora strain expressing cytoplasmic tdTomato as well as nucleus-localized mTFP1 (LILI-td-NT) during growth at the surface of N. benthamiana roots. (A) Following first nuclear division, daughter nucleus N1 (magenta arrowhead) migrates toward the tip of the germ tube (white arrow), while the second nucleus, N2 (white arrowhead), remains within the cyst body. (B) Chronophotographic view (time stack) of the previous sequence, showing the successive locations of nucleus N2. Time frames are color coded and overlaid. (C) Nuclear movements following N1 division. Daughter nuclei N3 (yellow arrowhead) and N4 (green arrowhead) move in opposite directions (yellow and green arrows, respectively) and distribute equally within the germ tube. (D) Chronophotographic view (time stack) of the sequence shown in panel C, showing opposite movement of nuclei N3 and N4. Bar, 10 μm.
Experimental setup used for time-lapse imaging of infected N. benthamiana roots. (A) Schematic representation of the experimental setup. A 1-week-old N. benthamiana seedling (2) is mounted in a liquid compartment (3) between a slide (1) and a coverslip (4). The edges of the coverslip are sealed with a 4:1 mix of paraffin and lanolin (5). The upper part of the coverslip is filled with water for use with a water-dipping objective. (B) Representative image of the experimental setup. Numbers refer to the same elements as described above. Download FIG S5, PDF file, 0.6 MB (607.8KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Appressorium differentiation alters nuclear dynamics in the germ tube.
We then investigated nuclear movements during the onset of N. benthamiana root infection, using a similar setup as previously described (Fig. 2 and Fig. S5, video 2 [https://zenodo.org/record/3378706/files/Video2.mp4]). Consistent with previous observations, the cyst-resident nucleus moved into the germ tube 1 h after encystment and reached the swollen tip of a differentiating appressorium. However, unlike cyst germination, we did not observe backward movement of a daughter nucleus toward the cyst after nuclear division. Instead, the cyst was progressively depleted from cytoplasm during appressorium differentiation (Fig. 2A, video 2A).
FIG 2.
Appressorium differentiation alters P. palmivora nuclear dynamics in the germ tube. (A and B) Time-lapse imaging of N. benthamiana root infection by a P. palmivora LILI-td-NT strain (video 2). (A) Representative sequence of a successful infection event (video 2A). Arrowheads indicate nuclei. Asterisks indicate migration of the cytoplasm out of the cyst. (B) Representative sequence of an unsuccessful infection event, leading to the differentiation of a second germ tube (indicated by asterisks) opposite the first one (video 2B). Arrowheads indicate nuclei. Bar, 10 μm.
In rare instances, appressorium differentiation did not occur (Fig. 2B, video 2B). Nuclear divisions and movements were then similar to those during cyst germination, where nucleus N2 moved toward the germ tube tip and N1 resided in the cyst. Upon infection failure, a second germ tube germinated from the cyst opposite the first after 3 h. Nucleus N1 then proceeded from the cyst into the new germ tube, while nucleus N2 simultaneously retreated into the cyst. A distance of 20 to 30 μm was maintained between the two nuclei through the entire process (Fig. 2B, video 2B). Taken together, these observations suggest that successful appressorium differentiation influences nuclear distribution dynamics and that nuclei may be recalled and rerouted into additional infection attempts.
Differential nuclear migration rates occur throughout the P. palmivora hyphal network both in planta and in axenic culture.
During the biotrophic infection stage in living N. benthamiana leaves, P. palmivora nuclei moved rapidly within intracellular hyphae (Fig. 3A and B, video 3 [https://zenodo.org/record/3378706/files/Video3.mp4]) but did not enter haustoria within plant cells (Fig. 3C and D, video 4 [https://zenodo.org/record/3378706/files/Video4.mp4]). Most nuclei adopted a round shape, likely due to the diameter of hyphae (up to 6 μm, n = 10) being greater than the diameter of nuclei (3.5 μm, n = 10). In contrast, in germ tubes (2 μm, n = 10) nuclei remained elongated even when not moving (Fig. 1). Interestingly, independent hyphae showed differential nuclear migration rates, as evidenced by chronophotographic display of the time-lapse image series (Fig. 3B). For instance, a continuous flow of nuclei was observed in the hyphal segment b, while no movement could be detected in the hyphal segment a (Fig. 3A). Most nuclei were dragged passively by the bulk nuclear flow (P-type nuclei). Conversely, 10% of the nuclei, such as N1, stretched and maintained a near-constant location over time within segment b despite a continuous flow of nuclei bypassing them (Fig. 3B), which suggests that they are actively anchored (A-type nuclei). These findings demonstrate that different motion speeds occur throughout the hyphal network during biotrophy and that some nuclei may behave independently of the surrounding mass nuclear flow.
FIG 3.
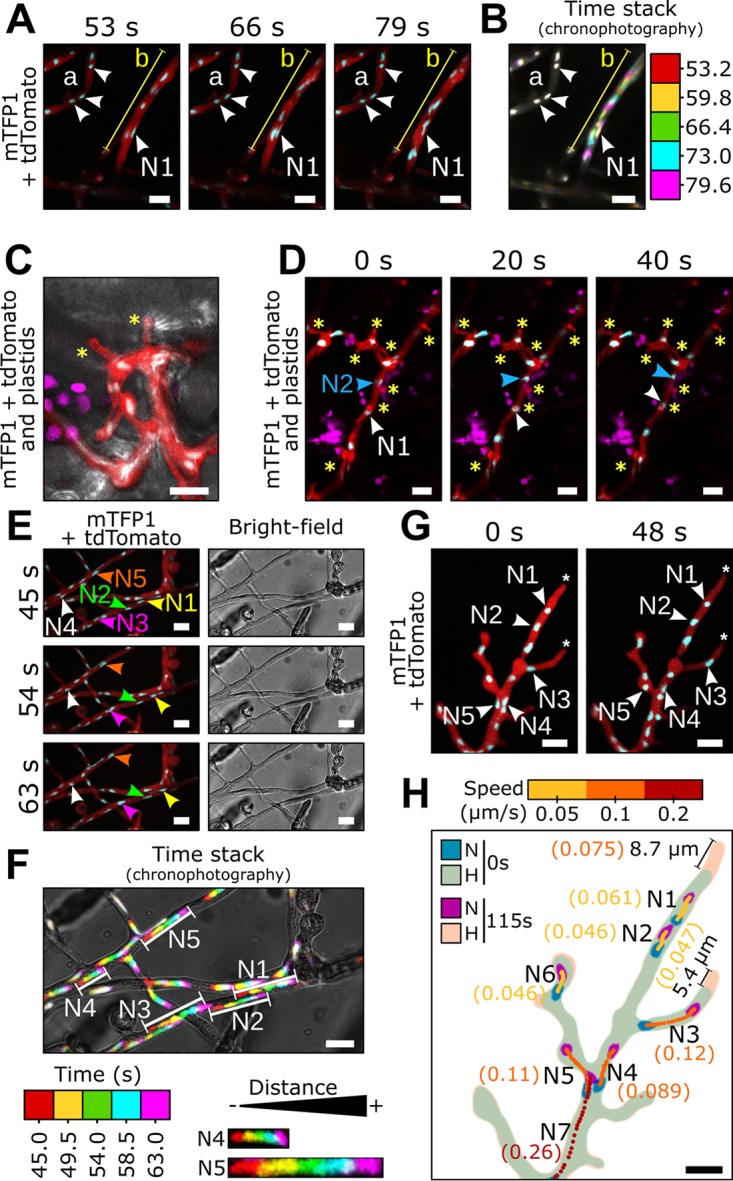
Differential nuclear migration rates occur throughout P. palmivora hyphal network. Time-lapse imaging of P. palmivora LILI-td-NT hyphae infecting an N. benthamiana leaf (A to D, videos 3 and 4) or growing axenically on V8 medium (E to H, videos 5 and 6). (A, video 3) Representative sequence of nuclear movements within infectious P. palmivora hyphae. (B, video 3) Chronophotographic display (time stack) of the sequence shown in panel A. The successive time frames are color coded and overlaid. (C) Representative sequence of P. palmivora haustoria (asterisks) on an N. benthamiana leaf. Arrowheads indicate nuclei. (D, video 4) Representative sequence of nuclear movements within haustoriated P. palmivora hyphae. (E, video 5) Representative sequence of nuclear migration on axenically grown mycelium. (F, video 5) Chronophotographic display of the sequence shown in panel E. (G, video 6) Representative sequence of nuclear movements near hyphal tips. (H) Nuclear trajectories for seven nuclei at the hyphal tip. Average speed is indicated in parentheses. Lines indicate hyphal segments. Arrowheads indicate individual nuclei. N, nuclei; H, hyphae. Bar, 10 μm.
Nuclear movements during saprotrophic growth on a V8 agar plate (Fig. 3E to H, video 5 [https://zenodo.org/record/3378706/files/Video5.mp4]) were similar to those during plant infection, and again, differential nuclear motion speeds were observed within hyphal segments (Fig. 3E and F). For instance, nuclei N1 to N3 all moved with similar speed within the same hypha. In contrast, nucleus N5 moved faster than nuclei that moved passively with mass nuclear flow like N4. In addition, axenic hyphae allowed us to study hyphal tips (Fig. 3G and H, video 6 [https://zenodo.org/record/3378706/files/Video6.mp4]), where mass nucleus movement slowed and nucleus N7 (average speed, 0.26 μm/s) moved faster than downstream nuclei N1 to N6 (average speed ranging from 0.046 to 0.12 μm/s). Subapical zones were devoid of nuclei up to 9 μm (n = 10) from the hyphal tips (Fig. 3H). Again, some nuclei migrate at speeds different from mass nuclear movement, likely due to active anchoring (A-type nuclei).
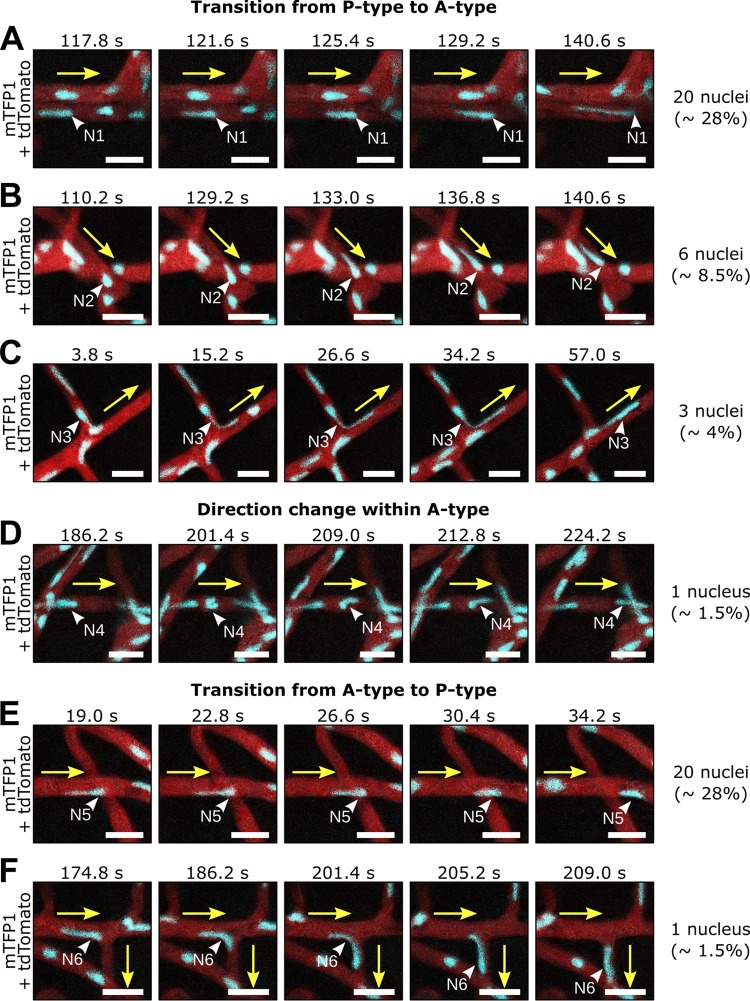
A-type nuclei stretch and move independently of surrounding nuclear flow.
To gain more insight on the dynamics of A-type P. palmivora nuclei, we tracked nuclear stretching and other trajectories that differed from the mass nuclear movement and assessed their frequency within the hyphal network (Fig. 4, video 5). We identified several different behaviors of A-type nuclei. Frequently (28%), P-type nuclei transition into the A-type state and stop within the flow to either stay at a given position and stretch out sternward (Fig. 4A), suggesting that they are anchored at one point, or move against the flow (Fig. 4B, 8.5%) and into branches (Fig. 4C, 4%). In some rare cases (Fig. 4D, 1.5%), A-type nuclei moving against the flow changed their direction of movement by tumbling, such that the stretched part always pointed to the direction of the migration. With a frequency similar to the P- to A-type transition (28%), A-type nuclei reverted to passive movement (P type) where they often shrank to adopt a round shape (Fig. 4E). In rare cases (1.5%), such passive flow then also dragged these nuclei into side branches (Fig. 4F). In summary, nuclei can undergo transitions between passive and active movements that allow them to position themselves within the hyphal network. Active movement correlates with changes in nuclear shape, where nuclei get remarkably stretched.
FIG 4.
Individual P. palmivora nuclei stretch and move independently of surrounding nuclear flow. (A to F, video 6) Time-lapse imaging of axenically grown P. palmivora LILI-td-NT hyphae containing nuclei transitioning from passive (P-type) to active (A-type) movements (A to D) or, conversely, from A type to P type (E and F). (A) Nucleus slows down and stretches sternward. (B) Nucleus shrinks and starts moving together with the surrounding nuclear flow. (C) Same as panel B, but nucleus enters a branch. (D) Nucleus stretches and moves opposite the surrounding nuclear flow. (E) Same as panel D but entering a branch. (F) Nucleus stretches and moves opposite the surrounding nuclear flow and then tumbles and initiates a similar movement in the opposite direction. Yellow arrows indicate mass nuclear flow. White arrowheads indicate nuclei. Frequencies are given based on a total of 70 nuclei. Bar, 10 μm.
Nuclear shape flexibility is often associated with the absence of lamin-encoding genes (2), but laminA has been identified in several Phytophthora species, including P. palmivora (31, 32). To test whether P. palmivora encodes a functional lamin A protein that controls nuclear shape, we generated transgenic strains expressing an mCitrine:laminA translational fusion under the control of either the constitutive Ham34 promoter or the native laminA promoter (Fig. S6). We found that expression of either construct resulted in a nucleoplasmic labeling with mCitrine. Notably, mCitrine:laminA overexpression triggered nuclear blebbing (Fig. S6A) and arrested hyphal growth at an early stage, while expression under the native promoter did not alter nuclear shape (Fig. S6B) and hyphal growth was indiscernible from the wild type. Therefore, P. palmivora lamin A may contribute to nuclear shape and integrity.
Generation of a P. palmivora lamin A reporter. Transformation of P. palmivora LILI with a construct for constitutive (A and B) or native (C and D) expression of an mCitrine:LamA-Cter reporter. (A) Schematic view of the construct used for constitutive or native expression of the lamin reporter together with a nucleus-localized mTFP1. Backbone elements are not represented. (B) Representative pictures of the bubbling phenotype observed upon constitutive expression of the lamin reporter. Arrowheads indicate bubbling nuclei. Bar, 10 μm. (C) Representative pictures of a hyphal segment upon native expression of the lamin reporter, showing absence of nuclear bubbling. Arrowheads indicate bubbling nuclei. Bar, 10 μm. Download FIG S6, PDF file, 2.3 MB (2.3MB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Nuclear shape is altered upon acceleration and deceleration.
To better understand how P. palmivora nuclei transition into A-type trajectories, we monitored the speed, shape, and location of nuclei neighboring individual nuclei at a hyphal branch point where the transition of P to A type may occur (Fig. 5, video 7 [https://zenodo.org/record/3378706/files/Video7.mp4]). We found that transitions between active and passive movement were frequently associated with change in nuclear shape (Fig. 5B to D) and facilitated a more equal nuclear distribution to fill empty areas (Fig. 5E to G). For instance, nucleus N1 initially followed a P-type behavior and then stretched and adopted an A-type behavior, eventually moving backward into the branch point (Fig. 5B) toward a 34-μm-long hyphal segment devoid of nuclei (Fig. 5E). Similarly, the stretched, A-type nucleus N2 moved backward into another branch point (Fig. 5C), reaching a 35-μm-long nucleus-depleted hyphal segment (Fig. 5F). In contrast, the P-type nucleus N3 maintained a round shape while moving (Fig. 5D), and an equal distance of 18 μm was maintained from the surrounding nuclei (Fig. 5G). Whether nucleus N3 is part of the same hypha as nuclei N1 and N2 could not be determined from our data set (Fig. 5A, video 7).
FIG 5.
P. palmivora nuclear stretching correlates with rerouting toward nucleus-depleted hyphal segments. (A, video 7) Representative sequence of nuclear movements at a hyphal branch point, highlighting both A-type (nuclei N1 and N2) and P-type (nucleus N3) movements. Arrowheads indicate nuclei. (B to J) Analysis of nuclear speed and trajectories for nuclei N1 to N3. (B, E, and H) Maps of nuclear content in the vicinity of nuclei N1 (B), N2 (E), and N3 (H). Black arrows indicate nuclear movement. (C, F, and I) Nuclear trajectories of N1 (C), N2 (F), and N3 (I). Locations of nuclei N1 to N3 at first (0 s), intermediate (135 s), and last (210 s) time points are shown in blue, red, and magenta, respectively. Nuclear centroids are represented as dots. Arrows indicate instantaneous speed and direction of the movement. Average nucleus speed is indicated on the graph. (D, G, and J) Variation of instantaneous speed with time for nuclei N1 (D), N2 (G), and N3 (J). Average speed in shown as a red dotted line. Representative pictures of nucleus shape over time are shown above the graphs with arrows indicating direction of the movement. Bar, 10 μm.
We then measured instantaneous speed and direction of nuclei undergoing significant shape changes (Fig. 5H to J). We found that the onset of shape change in A-type nuclei correlated with changes in their instantaneous speed and that shape was affected by the direction of movement. For instance, nucleus N1 stretched while undergoing a 10-fold speed decrease from 0.8 μm/s down to 0.08 μm/s and reverted to a round shape after speed increased back to 0.4 μm/s (Fig. 5H). Similarly, stretched nucleus N2 had an initial speed of 0.1 μm/s and later became more round after speed increased (Fig. 5I). In contrast, the P-type nucleus N3 that maintained a steady speed did not change its shape (Fig. 5J). Taken together, these results suggest that trajectories of A-type nuclei lead to their reallocation of nuclei into nucleus-depleted hypha sections. The cooccurrence of nuclear stretching during these reallocations suggests that forces act most prominently at a single nuclear envelope pole.
A-type nuclear movement is impaired by the microtubule polymerization inhibitor Benomyl.
Since microtubules are required for nuclear movements in fungi (2, 33), we assessed the effect of the microtubule polymerization inhibitor Benomyl on P. palmivora nuclei (Fig. 6, video 8 [https://zenodo.org/record/3378706/files/Video8.mp4]). Benomyl restricted P. palmivora growth in a dose-dependent manner. While 50 mg/liter Benomyl arrested hyphal growth on a V8 agar plate and resulted in abnormally shaped hyphae (Fig. S7), 10 mg/liter Benomyl barely reduced P. palmivora growth (Fig. 6A and B) and did not alter hyphal shape (Fig. 6C and D). Thus, 10 mg/liter Benomyl can be used to assess P. palmivora nuclear movements independently of changes in overall growth rate and hyphal anatomy. A near-equal distribution of nuclei was maintained along hyphae, suggesting that such a concentration did not have a dramatic effect on P. palmivora physiology. Time-lapse imaging showed nuclear movements in Benomyl-treated hyphae (Fig. 6C to E, video 8B). However, only 14% of nuclei displayed A-type behavior upon Benomyl treatment, compared to 42% in the control (n = 23, 400 nuclei) (Fig. 6E, video 8A). Instead, most nuclei (86%) were round and followed P-type trajectories, compared to 58% in the control (Fig. 6E). Taken together, these findings suggest that microtubules are required for A-type nuclear movements in P. palmivora.
FIG 6.
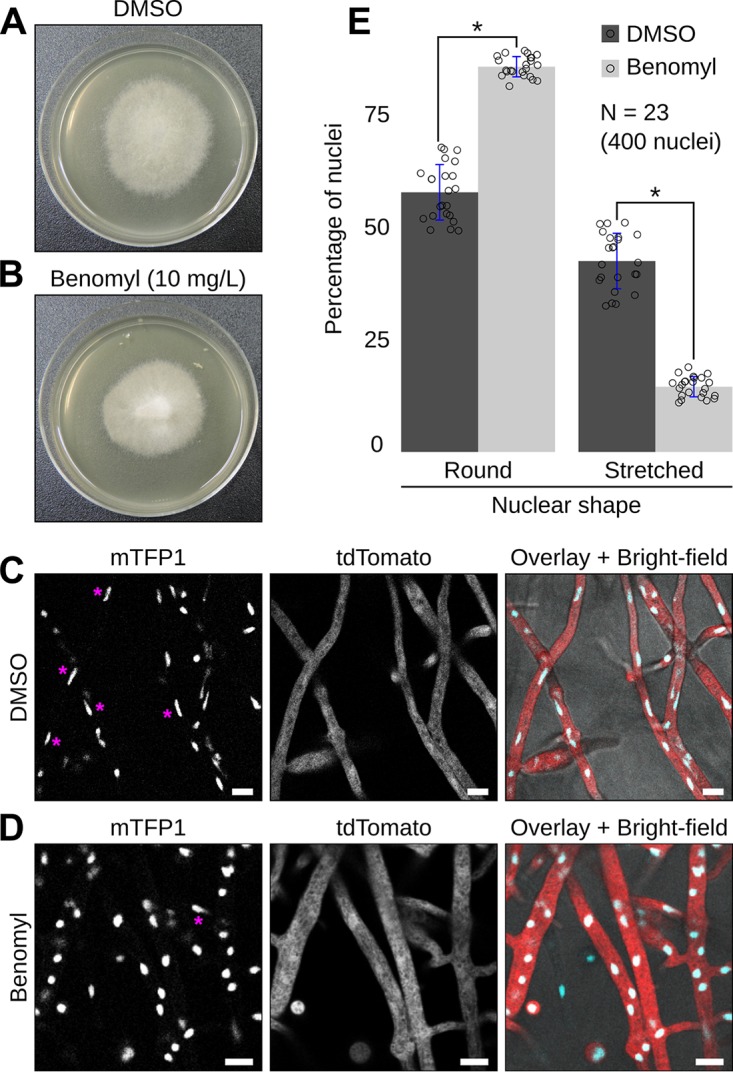
Antimicrotubule drug Benomyl impairs P. palmivora A-type nuclear movements. (A and B) Growth habit of transgenic P. palmivora LILI-td-NT mycelium growing on V8 agar plates without (dimethyl sulfoxide [DMSO] control) (A) or with (B) 10 mg/liter Benomyl. (C and D) Representative pictures of P. palmivora LILI-td-NT hyphae and nuclei in absence (C, video 8A) or presence (D, video 8B) of 10 mg/liter Benomyl. Bar, 10 μm. (E) Quantification of round (P-type) and stretched (A-type) nuclei within P. palmivora LILI-td-NT hyphae (n = 23, 400 nuclei). Statistical significance was assessed using the Wilcoxon test for paired samples (P < 0.05).
Effect of antimicrotubule drug Benomyl on P. palmivora growth. (A) Representative pictures of P. palmivora LILI-td-NT mycelium growing on V8 agar plates supplemented or not with 10, 50, or 100 mg/liter Benomyl. (B) Confocal imaging of P. palmivora LILI-td-NT hyphae grown on V8 agar plates supplemented with 50 mg/liter Benomyl. Bar, 10 μm. Download FIG S7, PDF file, 0.9 MB (936.1KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The centrosome-associated protein Centrin2 localizes at the stretched extremities of nuclei.
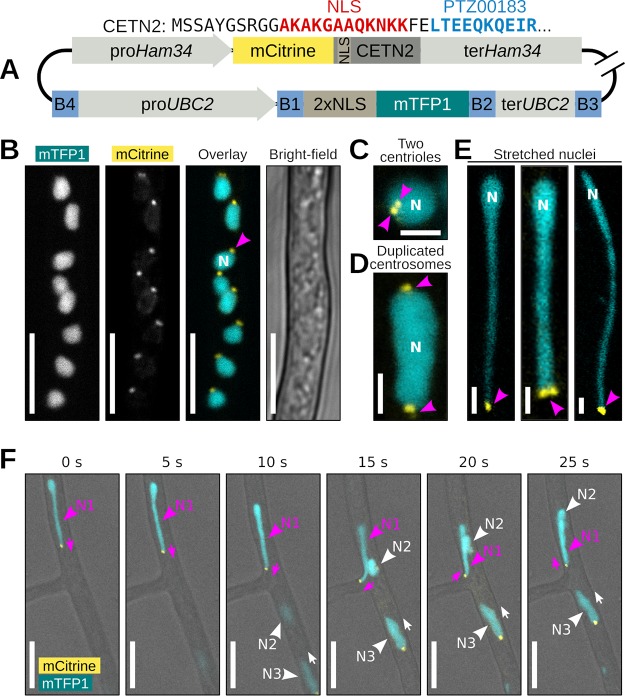
Fungal research has demonstrated that cell cortex-anchored dynein pulls the astral microtubules nucleated from the SPBs to move nuclei (2, 33). Centrosomes and centrioles which could serve as SPB equivalents in oomycetes have been reported for several Phytophthora species (34). We therefore labeled P. palmivora centrosomes using a fluorescently tagged centriolar lumen protein, Centrin2 (CETN2). To that end, we generated a transgenic P. palmivora LILI-NT-Ce strain by replacing the cytoplasmic tdTomato cassette from the previous dual reporter construct with an mCitrine fluorescent reporter fused N terminally to P. palmivora CETN2 coding sequence (Ce) and monitored fluorescence over time at branching hyphae (Fig. 7, video 9 [https://zenodo.org/record/3378706/files/Video9.mp4]). CETN2 is composed of a nuclear localization signal followed by a Ca2+-binding protein/EF-hand superfamily protein domain (PTZ00183) (Fig. 7A). Expression of the CETN2/nucleus dual reporter led to labeling of nuclei with two adjacent punctate structures at the nuclear periphery (Fig. 7B), likely corresponding to the two centrioles of the P. palmivora centrosome (Fig. 7C). Similar labeling was obtained with the constitutive Ham34 promoter as well as the native P. palmivora CETN2 promoter (Fig. S8). In addition, up to 15% of observed nuclei within a given region showed two sets of punctate structures located opposite each other, presumably as a result of centrosome duplication prior to nuclear division (Fig. 7D and Fig. S9).
FIG 7.
Centrin2 localizes to P. palmivora nuclear stretches. (A) Schematic view of the construct used for dual labeling of nuclei and centrosomes in P. palmivora strain LILI-NT-Ce. Backbone elements are not represented. (B, video 9) Representative picture of mCitrine-labeled Centrin2 (CETN2) within P. palmivora LILI-NT-Ce hyphae. (C) CETN2 localizes in two adjacent dots at the periphery of the nucleus. (D) Centrosome duplication in dividing nuclei. (E) Representative pictures of stretched P. palmivora nuclei (up to 30 μm long), with CETN2 localizing at the tip of the stretched areas. (F) Time-lapse imaging of nuclear movements at hyphal branch point. Nucleus N1 (magenta) follows an A-type trajectory backward to the branch point, while nuclei N2 and N3 follow a P-type trajectory. CETN2 localizes at the tip of the stretched part of nucleus N1, while CETN2 localizes at the back of nuclei N2 and N3. Bar, 2 μm (C to E) or 10 μm (B and F).
Generation of a P. palmivora Centrin 2 (CETN2) reporter. (A) Schematic view of the construct for Ham34-promoter-driven expression of an mCitrine:CETN2 reporter together with a nucleus-localized mTFP1. Backbone elements are not represented. (B) Confocal imaging of a sporangium from a P. palmivora LILI-NT-Ce transgenic strain expressing the construct shown in panel A. Bar, 10 μm. (C) Schematic view of the construct for CETN2-promoter-driven expression of an mScarlet:CETN2 reporter together with a constitutively expressed nucleus-localized mTFP1. (D) Confocal imaging of a sporangium from a transgenic P. palmivora strain expressing the construct shown in panel C. Bar, 10 μm. Download FIG S8, PDF file, 0.8 MB (849.7KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Frequency of centrosome duplication within P. palmivora hyphae. (A and B) Representative pictures of axenically grown hyphae from the transgenic P. palmivora LILI-NT-Ce strain growing on V8 medium. (A) Distribution of nuclei and Centrin2 (CETN2)-labeled centrosomes within a hyphal segment. Asterisks indicate nuclei with duplicated centrosomes. (B) Magnified views of nuclei 13 and 14. Bar, 10 μm. Download FIG S9, PDF file, 0.8 MB (886.8KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We then specifically looked for elongated nuclei within P. palmivora hyphae and found several instances of P. palmivora nuclei stretching up to 30 μm in length. In all cases, mCitrine:CETN2-labeled punctate structures occurred at the very tip of the nuclear stretch (Fig. 7E). We then investigated the relationships between nuclear movements and positioning of mCitrine:CETN2-labeled punctate structures. We found that migrating P-type nuclei had their punctate structures oriented sternward, that is, opposite the direction of movement (nuclei N2 and N3) (Fig. 7F, video 9). In contrast, stretched nuclei moving against the mass nuclear flow had their punctate structures oriented in front (nucleus N1) (Fig. 7F). Our results suggest that P. palmivora nuclei are highly deformable and that mCitrine:CETN2-labeled punctate structures may be involved in microtubule-aided nuclear anchorage within hyphae and their movement against cytoplasmic flow.
DISCUSSION
We studied the broad-host-range plant pathogen Phytophthora palmivora as a system to elucidate oomycete nuclear dynamics. By combining live imaging and a dual reporter expression system, we provide evidence for both active and passive nuclear movements within multinucleate, coenocytic hyphae. Different nuclear migration rates occur within the same hyphal segments, and nuclei move at a reduced rate in subapical regions compared to other hyphal segments, suggesting that the overall nuclear movement is influenced by cytoplasmic flow toward the mycelium periphery. Consistent with this hypothesis, nuclear migration in the fungus Neurospora crassa involves cytoplasmic flow (35, 36) and can be reversed by application of osmotic gradients across the colony (37). However, in contrast to observations made on Aspergillus nidulans (38), we did not observe a saltatory movement of nuclei, i.e., periodic inversion of the mass nuclear flow.
We describe the active, long-distance rerouting of individual nuclei toward nucleus-depleted areas, and our data suggest that such A-type movements contribute to the near-equal spacing of nuclei within germ tubes, infectious hyphae, and axenic mycelium (Fig. 8). We found that A-type movements are impaired by Benomyl, suggesting that they require microtubules. Consistent with our observations, a proper nuclear distribution within A. nidulans and N. crassa hyphae requires microtubules (7, 8), and an additional role of cytoplasmic dynein has been reported (9, 10). Future work will investigate the role of P. palmivora dynein in nuclear movement.
FIG 8.
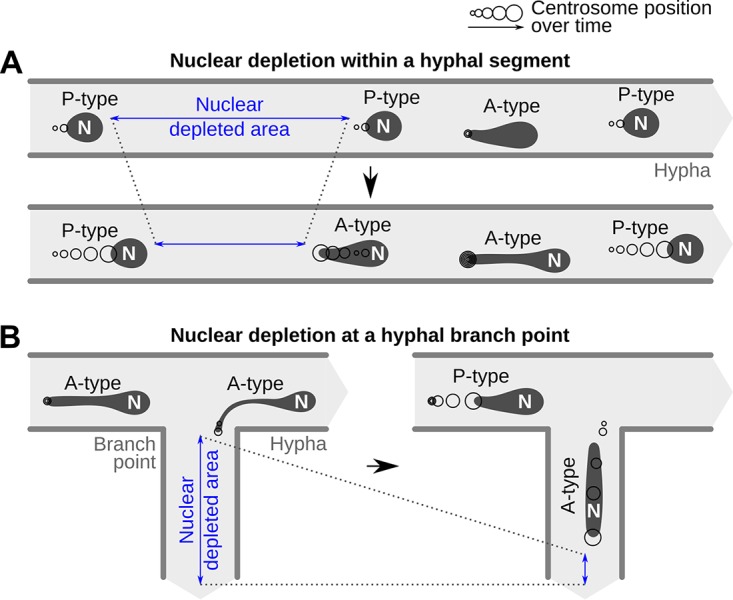
Proposed behavior change from passive (P-type) to active (A-type) movements within P. palmivora hyphae. (A) Decrease in nuclear density within a hyphal segment (blue) causes downstream nuclei to anchor until populated by P-type nuclei or to initiate retrograde movement to fill it directly. Anchorage is mediated by the centrosome and results in nuclear stretching. (B) Decrease in nuclear density in a branching hypha (blue) causes nuclei to anchor at the branch point and initiate active movement to enter the branch.
Several reports suggest that a local variation in tubulin concentration may affect microtubule polymerization in different model systems. For instance, a local increase in tubulin concentration in neurons may favor nucleation of noncentrosomal microtubule bundles (39). Besides, the onset of mitosis in the fungus A. nidulans triggers a rapid influx of tubulin into the nucleoplasm (40), and local accumulation of tubulin monomers in the nascent spindle region of mitotic nuclei has been reported from Caenorhabditis elegans embryos (41). The tempting speculation that local changes in tubulin concentration favor transition from P- to A-type nuclear trajectories in P. palmivora hyphae will require future experimentation.
Equidistant nuclear distribution mechanisms and nuclear migration are likely to occur in other oomycete species, including those not belonging to the family Peronosporaceae. For instance, indirect evidence suggests that nuclear migration occurs in germinating cysts of Lagenisma coscinodisci, a diatom parasite from an early-diverging Saprolegniomycetes lineage (42). In this species, a near-globular nucleus migrates from the cyst to the germ tube and concomitantly stretches, reaching a length of up to 10 μm, presumably due to the narrowness of the germ tube (24). Therefore, P. palmivora nuclear dynamics may be similar to other oomycetes.
We showed a correlation between active movement of P. palmivora nuclei and nuclear stretching. A-type nuclei were stretched up to 30 μm in length upon speed change, irrespective of the diameter of the surrounding hyphae. They revert back to round shape within seconds when returning to P-type trajectory. Nuclear deformability has also been reported in fungi. For instance, during rice infection by Magnaporthe oryzae, migration of the appressorial nucleus within the narrow penetration peg causes nuclear constriction up to 13 μm (43). While extreme constrictions of fungal nuclei were triggered by the narrowness of the surrounding hyphal structures (43), P. palmivora nuclei also deformed in hyphae with diameters much larger than their nuclei. Deformability has been associated with a specific nuclear lamina composition and chromatin organization (44). Variation in lamin gene expression regulates nuclear stiffness and its deformability in a variety of metazoan cells, including hematopoietic and immune cells (45). Lamin overexpression increases nuclear stiffness and reduces deformability (45), while loss of lamin gene expression in metazoans resulted in increased nuclear deformability but also in increased fragility (44, 46). Moreover, defects in lamina assembly are responsible for increased cell death and cause a large array of life-threatening laminopathies (44), and lamin null mutants show decreased nuclear stiffness. Fungal nuclei are devoid of lamina (47) and lack lamin-encoding genes (31), presumably explaining their deformability. In contrast to fungi, lamin-encoding genes are present in several Phytophthora species, including P. infestans, P. ramorum, and P. palmivora (31, 32). Furthermore, we showed that overexpression of the P. palmivora laminA gene alters the nuclear periphery and affects nuclear shape formation in Phytophthora.
The centrosome of stretched A-type nuclei always occupies the leading tip, suggesting that nuclear stretching is a result of direct or indirect forces exerted on the centrosome. In agreement with this hypothesis, we observed nuclear tumbling, indicative that A-type movement is polarized. Finally, the astern orientation of centrosomes in P-type nuclei suggests that passive movement may be a transient state that prepares for possible conversions to A type for nuclear rerouting. Taken together, P. palmivora is an accessible model organism to study the relationships between nuclear movements, deformability, and rerouting.
Models of lipid bilayer vesicles flowing through circular tubes (48–50) do not predict shapes like those observed in A-type nuclei but primarily reflect red blood cell microcirculation in capillaries and liposome flow through microfluidic compartments. These models therefore do not take into account nucleus-specific parameters such as interactions with the cytoskeleton (51). Besides, simple lipid bilayers may not reflect the biophysical properties of nuclear envelope. For instance, biomechanical models of nuclear shape changes during micropipette aspiration showed that the overall response of an isolated nucleus is highly sensitive to the apparent stiffness of the nuclear lamina (52). We speculate that dramatic stretching minimizes the constraints exerted on nuclei, allowing them to migrate greater distances with limited energy cost. Future work should aim at developing models for hydrodynamic shape adaptations of nuclei to sustain long-distance, flow-independent movements within branched filamentous hyphae.
We documented bidirectional movements of nuclei during cyst germination which were altered by appressorium differentiation. Failure to differentiate an appressorium triggered rerouting of nuclei into additional infection attempts. This suggests that P. palmivora nuclear dynamics and developmental transitions are coordinated. Similarly, during rice infection by the fungus M. oryzae, one postmitotic nucleus migrates into the developing appressorium, while other nuclei are degraded after entering the conidial cell (3). Later on, the appressorial nucleus migrates down into the primary hypha. Such migration requires a functional transketolase, TKL1, acting as a metabolic checkpoint. Indeed, loss of transketolase function results in depletion of ATP content and slowing down of the TOR signaling pathway (4). Whether an expressed transketolase ortholog of TKL1 in P. palmivora (PLTG_04838) (53) is also involved in early infection processes remains to be addressed.
In conclusion, P. palmivora is an accessible system which allowed us to highlight commonalities as well as differences in nuclear dynamics between oomycetes and previously studied fungi. Our work sheds new light on nuclear deformability in filamentous microorganisms and uncovers the role of nuclear rerouting to dynamically maintain equal distribution within coenocytic hyphae as well as during plant infection. These findings provide a foundation for hydrodynamic modeling of nuclear shape adaptations in branched hyphal networks. Developing chemical inhibitors targeting the oomycete-specific nuclear movement machinery could stop cyst germination early and therefore represents robust, hard-to-evade targets for durable crop protection against oomycete diseases.
MATERIALS AND METHODS
Phytophthora strains and growth conditions.
P. palmivora Butler isolate LILI (accession no. P16830) was initially isolated from oil palm in Colombia (54) and maintained in the P. palmivora collection at the Sainsbury Laboratory (Cambridge, United Kingdom). P. palmivora strains were maintained on petri plates of V8 agar (1.5% agar) at 25°C. For maintenance of transformed strains, Geneticin (G418) was added to the medium at a concentration of 100 mg/liter. Mycelium was grown for 5 days in the dark followed by 2 days under constant light conditions. For the latter, plates were left unsealed to remove excess humidity. For production of zoospores, 7-day-old plates were incubated at 4°C for 30 min. Plates were then flooded with 5 ml sterile water and incubated at room temperature for another 30 min. N. benthamiana growth conditions were described previously (53).
Plasmid construction.
An attR3/attR4 MultiSite Gateway cassette was PCR amplified from pK7m34GW (55) using GoTaq DNA polymerase (Promega UK, Southampton, United Kingdom) with primers NdeI-R3 and NdeI-R4 (see Table S1B in the supplemental material), flanking each side of the cassette with NdeI restriction sites. The MultiSite Gateway cassette was then ligated into pGEM-T Easy vector (Promega UK, Southampton, United Kingdom), and the insert sequence was confirmed by sequencing (Source BioScience, Nottingham, United Kingdom). Thereafter, the cassette was ligated into pTOR vector (56) using the NdeI restriction sites replacing the vector’s Ham34 promoter, multiple cloning site, and Ham34 terminator, resulting in plasmid pTORKm43GW. The orientation of the attR3/attR4 cassette was selected so that promoters inserted using the Gateway cassette will not be in close proximity to the Hsp70 promoter driving the antibiotic selection marker.
pTOR vectors carrying a cassette for Ham34 promoter-driven expression of mTFP1 (cyan) (57), mWasabi (green) (58), mCitrine (yellow) (59), or tdTomato (red) (60) were derived from KpnI-linearized pTORKm43GW. Briefly, Ham34 promoter, Ham34 terminator, and the reporters were amplified individually using primer pairs listed in Table S1B. The final cassettes were assembled by overlap extension PCR and used for In-Fusion cloning (Clontech, Palo Alto, CA, USA) into linearized pTOR-Gateway vectors. An outline of the pTOR-Gateway backbone is shown in Fig. S1.
Genomic DNA extraction.
Genomic DNA was extracted from axenically grown P. palmivora mycelium using a protocol modified from the work of Möller et al. (61). Briefly, samples were incubated in a 1:1 mix of STES buffer (50 mM Tris-HCl, pH 8.0, 10 mM EDTA, 150 mM NaCl, 2% [vol/vol] SDS) and Tris-buffered phenol solution (pH 8.0) for 30 min at 65°C. After phenol-chloroform extraction, nucleic acids were treated with RNase T1 (Life Technologies Ltd., Paisley, United Kingdom) for 30 min at 37°C. RNase was removed by phenol-chloroform extraction prior to isopropanol precipitation of genomic DNA.
Cloning of Phytophthora promoters and terminators.
Primers for P. palmivora promoters and terminators used in this study were derived from publicly available genomic resources (32) (Table S1B). Constructs were generated using two-step Gateway PCR (Invitrogen, Carlsbad, CA, USA) and subsequently cloned into pDONR221 entry vectors carrying attP4-attP1R or attP2R-attP3 Gateway cassette, respectively. Sequences were confirmed by sequencing (Source BioScience, Nottingham, United Kingdom). The ubiquitin-conjugating enzyme 2 (UBC2) promoter was defined as 1,500 bp upstream of the start codon. Terminator consisted of 500 bp downstream of the UBC2 stop codon. LaminA and Centrin2 (CETN2) promoters were defined as 1,000 bp upstream of the start codon. The nuclear reporter used in this study was obtained by fusing a tandem repeat of Phytophthora sojae bZIP1 nuclear localization signal (29) to the 5′ end of mTFP1. The centrosome reporter was obtained by fusing mCitrine to the 5′ end of P. palmivora CETN2.
Generation of transgenic P. palmivora.
Transgenic P. palmivora was obtained by zoospore electrotransformation using the method from the work of Huitema et al. (62) with the following modifications: for electroporation, 680 μl of high-concentration (>106 zoospores/ml), high-mobility zoospore suspension was mixed with 80 μl of 10× modified Petri’s solution and 40 μl (20 to 80 μg) of plasmid DNA. Electroporation settings were as follows: voltage, 500 V; capacitance, 50 μF; resistance, 800 Ω. After electroporation, zoospore suspensions were diluted to 6 ml with clarified V8 medium and incubated at 25°C for 6 h on a rocking shaker. The encysted zoospore suspension was plated on a 15-cm-diameter plate with selective medium containing 100 mg/liter Geneticin. Transformants were transferred to fresh selective plates up to 10 days after transformation.
Quantitative reverse transcription-PCR (qRT-PCR) analyses.
Total RNA was extracted from axenically grown P. palmivora mycelium containing sporangia (sample MZ) and N. benthamiana roots harvested at 3, 6, 18, 24, 30, and 48 h after inoculation (hai) with P. palmivora ARI-td zoospores (63) using the RNeasy plant minikit (Qiagen, Germantown, MD, USA). One microgram was reverse transcribed to generate first-strand cDNA, using the Bio-Rad IScript cDNA synthesis kit according to the manufacturer’s instructions (Bio-Rad, Hercules, CA, USA). RNA quality was assessed by electrophoresis on an agarose gel. Conditions for quantitative PCR were described previously (53).
Confocal microscopy.
Confocal laser scanning microscopy images were acquired with a Leica SP8 laser-scanning confocal microscope equipped with a Leica HC Fluotar 25× 0.95-numerical-aperture (NA) objective (Leica, Wetzlar, Germany). A white-light laser was used for excitation at 477 nm for mTFP1 (excitation maximum, 462 nm; emission maximum, 492 nm) visualization, 488 nm for mWasabi visualization, 514 nm for mCitrine visualization, and 543 nm for the visualization of tdTomato. Fluorescence acquisition was done sequentially. For time-lapse imaging of infected N. benthamiana roots, seedlings were mounted between a slide and a coverslip in sterile water containing the zoospore suspension. The coverslip was sealed to the slide using a modified Valap sealing (64) composed of a 4:1 mix of paraffin (Sigma-Aldrich, United Kingdom) and lanolin (Sigma-Aldrich, United Kingdom) to prevent dehydration (Fig. S5). Time-lapse imaging of axenically grown mycelium was carried out on plates flooded with 10 ml sterile water. Pictures were analyzed with the ImageJ software (http://imagej.nih.gov/ij/) using the plugin Bio-Formats (https://imagej.net/Bio-Formats). Signal-to-noise ratio was optimized uniformly on time-lapse images series by adjusting minimum and maximum intensity levels. Then, Z-stacks were overlaid using maximum intensity projection and saved in false colors. Videos were generated from overlaid images with ffmeg (https://ffmpeg.org/).
Measurements of nuclear speed and shape.
Nuclear position was defined as the coordinates of its centroid, obtained from the ImageJ software. Instantaneous speed was plotted using the R software (https://www.r-project.org/) and ggplot2 package (https://ggplot2.tidyverse.org/). Nuclear shape was defined as the ratio (RF) between Feret’s maximum diameter (maximum caliper) and Feret’s minimum diameter (minimum caliper). Nuclei were considered round when RF was <2, while larger RF values indicated stretched nuclei.
ACKNOWLEDGMENTS
We are grateful to François Nédélec (SLCU, Cambridge) for helpful discussions. We thank Lothar Kalmbach (SLCU, Cambridge) for providing mScarlet fluorescent reporter and Raymond Wightman (SLCU, Cambridge) for assistance with confocal microscopy. We are grateful to Philip Carella (SLCU, Cambridge) for proofreading the manuscript.
The authors declare no competing financial interests.
E. Evangelisti conceived the experimental strategy, conducted experiments, acquired data, analyzed data, and wrote the manuscript. L. Shenhav, T. Yunusov, M. Le Naour–Vernet, and P. Rink conducted experiments, acquired data, and analyzed data. S. Schornack acquired funding, conceived the experimental strategy, analyzed data, and wrote the manuscript.
This work was supported by the Gatsby Charitable Foundation (GAT3395/GLD) and by the Royal Society (UF160413). Marie Le Naour–Vernet and Philipp Rink were funded by the ERASMUS program.
Footnotes
Citation Evangelisti E, Shenhav L, Yunusov T, Le Naour–Vernet M, Rink P, Schornack S. 2019. Hydrodynamic shape changes underpin nuclear rerouting in branched hyphae of an oomycete pathogen. mBio 10:e01516-19. https://doi.org/10.1128/mBio.01516-19.
REFERENCES
- 1.Morris NR. 2000. Nuclear migration: from fungi to the mammalian brain. J Cell Biol 148:1097. doi: 10.1083/jcb.148.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiang X. 2018. Nuclear movement in fungi. Semin Cell Dev Biol 82:3–16. doi: 10.1016/j.semcdb.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veneault-Fourrey C, Barooah M, Egan M, Wakley G, Talbot NJ. 2006. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science 312:580–583. doi: 10.1126/science.1124550. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez J, Marroquin-Guzman M, Wilson RA. 2014. Evidence for a transketolase-mediated metabolic checkpoint governing biotrophic growth in rice cells by the blast fungus Magnaporthe oryzae. PLoS Pathog 10:e1004354. doi: 10.1371/journal.ppat.1004354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon J, Rho H, Kim S, Kim KS, Lee YH. 2014. Role of MoAND1-mediated nuclear positioning in morphogenesis and pathogenicity in the rice blast fungus, Magnaporthe oryzae. Fungal Genet Biol 69:43–51. doi: 10.1016/j.fgb.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Jones K, Jenkinson CB, Borges Araújo M, Zhu J, Kim RY, Kim DW, Khang CH. 2016. Mitotic stopwatch for the blast fungus Magnaporthe oryzae during invasion of rice cells. Fungal Genet Biol 93:46–49. doi: 10.1016/j.fgb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Morris NR. 1975. Mitotic mutants of Aspergillus nidulans. Genet Res 26:237–254. doi: 10.1017/S0016672300016049. [DOI] [PubMed] [Google Scholar]
- 8.Oakley BR, Morris NR. 1980. Nuclear movement is β-tubulin-dependent in Aspergillus nidulans. Cell 19:255–262. doi: 10.1016/0092-8674(80)90407-9. [DOI] [PubMed] [Google Scholar]
- 9.Xiang X, Beckwith SM, Morris NR. 1994. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc Natl Acad Sci U S A 91:2100–2104. doi: 10.1073/pnas.91.6.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plamann M, Minke PF, Tinsley JH, Bruno KS. 1994. Cytoplasmic dynein and actin-related protein Arp1 are required for normal nuclear distribution in filamentous fungi. J Cell Biol 127:139–149. doi: 10.1083/jcb.127.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberti-Segui C, Dietrich F, Altmann-Jöhl R, Hoepfner D, Philippsen P. 2001. Cytoplasmic dynein is required to oppose the force that moves nuclei towards the hyphal tip in the filamentous ascomycete Ashbya gossypii. J Cell Sci 114:975–986. [DOI] [PubMed] [Google Scholar]
- 12.Palmer RE, Sullivan DS, Huffaker T, Koshland D. 1992. Role of astral microtubules and actin in spindle orientation and migration in the budding yeast, Saccharomyces cerevisiae. J Cell Biol 119:583–594. doi: 10.1083/jcb.119.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leo M, Santino D, Tikhonenko I, Magidson V, Khodjakov A, Koonce MP. 2012. Rules of engagement: centrosome-nuclear connections in a closed mitotic system. Biol Open 1:1111–1117. doi: 10.1242/bio.20122188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito D, Bettencourt-Dias M. 2018. Centrosome remodelling in evolution. Cells 7:71. doi: 10.3390/cells7070071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell MJ. 1980. Mitosis in the aquatic fungus Rhizophydium spherotheca (Chytridiales). Am J Bot 67:839. doi: 10.2307/2442424. [DOI] [Google Scholar]
- 16.Gibeaux R, Politi AZ, Philippsen P, Nédélec F. 2017. Mechanism of nuclear movements in a multinucleated cell. Mol Biol Cell 28:645–660. doi: 10.1091/mbc.E16-11-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veith D, Scherr N, Efimov VP, Fischer R. 2005. Role of the spindle-pole-body protein ApsB and the cortex protein ApsA in microtubule organization and nuclear migration in Aspergillus nidulans. J Cell Sci 118:3705–3716. doi: 10.1242/jcs.02501. [DOI] [PubMed] [Google Scholar]
- 18.Judelson HS, Blanco FA. 2005. The spores of Phytophthora: weapons of the plant destroyer. Nat Rev Microbiol 3:47–58. doi: 10.1038/nrmicro1064. [DOI] [PubMed] [Google Scholar]
- 19.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186–194. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bebber DP, Gurr SJ. 2015. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genet Biol 74:62–64. doi: 10.1016/j.fgb.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Erwin DD, Ribeiro OK. 1996. Phytophthora diseases worldwide. American Phytopathological Society (APS Press), St. Paul, MN. [Google Scholar]
- 22.Guest D. 2007. Black pod: diverse pathogens with a global impact on cocoa yield. Phytopathology 97:1650–1653. doi: 10.1094/PHYTO-97-12-1650. [DOI] [PubMed] [Google Scholar]
- 23.Haverkort AJ, Struik PC, Visser RGF, Jacobsen E. 2009. Applied biotechnology to combat late blight in potato caused by Phytophthora infestans. Potato Res 52:249–264. doi: 10.1007/s11540-009-9136-3. [DOI] [Google Scholar]
- 24.Schnepf E, Heinzmann J. 1980. Nuclear movement, tip growth and colchicine effects in Lagenisma coscinodisci Drebes (Oomycetes, Lagenidiales). Biochem Physiol Pflanz 175:67–76. doi: 10.1016/S0015-3796(80)80092-8. [DOI] [Google Scholar]
- 25.Marks GE. 1965. The cytology of phytophthora infestans. Chromosoma 16:681–692. doi: 10.1007/bf00285117. [DOI] [PubMed] [Google Scholar]
- 26.Whittaker SL, Shattock RC, Shaw DS. 1991. Variation in DNA content of nuclei of Phytophthora infestans as measured by a microfluorimetric method using the fluorochrome DAPI. Mycol Res 95:602–610. doi: 10.1016/S0953-7562(09)80074-9. [DOI] [Google Scholar]
- 27.Ketelaar T, Meijer HJG, Spiekerman M, Weide R, Govers F. 2012. Effects of latrunculin B on the actin cytoskeleton and hyphal growth in Phytophthora infestans. Fungal Genet Biol 49:1014–1022. doi: 10.1016/j.fgb.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Meijer HJG, Hua C, Kots K, Ketelaar T, Govers F. 2014. Actin dynamics in Phytophthora infestans; rapidly reorganizing cables and immobile, long-lived plaques. Cell Microbiol 16:948–961. doi: 10.1111/cmi.12254. [DOI] [PubMed] [Google Scholar]
- 29.Fang Y, Tyler BM. 2017. Nuclear localization of a putative Phytophthora sojae bZIP1 transcription factor is mediated by multiple targeting motifs. Mol Microbiol 104:621–635. doi: 10.1111/mmi.13652. [DOI] [PubMed] [Google Scholar]
- 30.Zhu C, Yang X, Lv R, Li Z, Ding X, Tyler BM, Zhang X. 2016. Phytophthora capsici homologue of the cell cycle regulator SDA1 is required for sporangial morphology, mycelial growth and plant infection. Mol Plant Pathol 17:369–387. doi: 10.1111/mpp.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kollmar M. 2015. Polyphyly of nuclear lamin genes indicates an early eukaryotic origin of the metazoan-type intermediate filament proteins. Sci Rep 5:10652. doi: 10.1038/srep10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali SS, Shao J, Lary DJ, Kronmiller BA, Shen D, Strem MD, Amoako-Attah I, Akrofi AY, Begoude BAD, ten Hoopen GM, Coulibaly K, Kebe BI, Melnick RL, Guiltinan MJ, Tyler BM, Meinhardt LW, Bailey BA. 2017. Phytophthora megakarya and Phytophthora palmivora, closely related causal agents of cacao black pod rot, underwent increases in genome sizes and gene numbers by different mechanisms. Genome Biol Evol 9:536–557. doi: 10.1093/gbe/evx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris NR, Xiang X, Beckwith SM. 1995. Nuclear migration advances in fungi. Trends Cell Biol 5:278–282. doi: 10.1016/S0962-8924(00)89039-X. [DOI] [PubMed] [Google Scholar]
- 34.Heath IB. 1974. Centrioles and mitosis in some oomycetes. Mycologia 66:354. doi: 10.2307/3758372. [DOI] [Google Scholar]
- 35.Lew RR. 2005. Mass flow and pressure-driven hyphal extension in Neurospora crassa. Microbiology 151:2685–2692. doi: 10.1099/mic.0.27947-0. [DOI] [PubMed] [Google Scholar]
- 36.Ramos-García SL, Roberson RW, Freitag M, Bartnicki-García S, Mouriño-Pérez RR. 2009. Cytoplasmic bulk flow propels nuclei in mature hyphae of Neurospora crassa. Eukaryot Cell 8:1880–1890. doi: 10.1128/EC.00062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roper M, Simonin A, Hickey PC, Leeder A, Glass NL. 2013. Nuclear dynamics in a fungal chimera. Proc Natl Acad Sci U S A 110:12875–12880. doi: 10.1073/pnas.1220842110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer R. 1999. Nuclear movement in filamentous fungi. FEMS Microbiol Rev 23:39–68. doi: 10.1111/j.1574-6976.1999.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 39.Hernández-Vega A, Braun M, Scharrel L, Jahnel M, Wegmann S, Hyman BT, Alberti S, Diez S, Hyman AA. 2017. Local nucleation of microtubule bundles through tubulin concentration into a condensed tau phase. Cell Rep 20:2304–2312. doi: 10.1016/j.celrep.2017.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ovechkina Y, Maddox P, Oakley C, Xiang X, Osmani S, Salmon E, Oakley B. 2003. Spindle formation in Aspergillus is coupled to tubulin movement into the nucleus. Mol Biol Cell 14:2192–2200. doi: 10.1091/mbc.e02-10-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi H, Kimura K, Kimura A. 2012. Localized accumulation of tubulin during semi-open mitosis in the Caenorhabditis elegans embryo. Mol Biol Cell 23:1688–1699. doi: 10.1091/mbc.E11-09-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thines M, Nam B, Nigrelli L, Beakes G, Kraberg A. 2015. The diatom parasite Lagenisma coscinodisci (Lagenismatales, Oomycota) is an early diverging lineage of the Saprolegniomycetes. Mycol Prog 14:75. doi: 10.1007/s11557-015-1099-y. [DOI] [Google Scholar]
- 43.Jenkinson CB, Jones K, Zhu J, Dorhmi S, Khang CH. 2017. The appressorium of the rice blast fungus Magnaporthe oryzae remains mitotically active during post-penetration hyphal growth. Fungal Genet Biol 98:35–38. doi: 10.1016/j.fgb.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Davidson PM, Lammerding J. 2014. Broken nuclei—lamins, nuclear mechanics, and disease. Trends Cell Biol 24:247–256. doi: 10.1016/j.tcb.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bone CR, Starr DA. 2016. Nuclear migration events throughout development. J Cell Sci 129:1951–1961. doi: 10.1242/jcs.179788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rowat AC, Jaalouk DE, Zwerger M, Ung WL, Eydelnant IA, Olins DE, Olins AL, Herrmann H, Weitz DA, Lammerding J. 2013. Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions. J Biol Chem 288:8610–8618. doi: 10.1074/jbc.M112.441535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardenas ME, Laroche T, Gasser SM. 1990. The composition and morphology of yeast nuclear scaffolds. J Cell Sci 96:439–450. [DOI] [PubMed] [Google Scholar]
- 48.Barakat JM, Shaqfeh E. 2018. Stokes flow of vesicles in a circular tube. J Fluid Mech 851:606–635. doi: 10.1017/jfm.2018.533. [DOI] [Google Scholar]
- 49.Kaoui B, Tahiri N, Biben T, Ez-Zahraouy H, Benyoussef A, Biros G, Misbah C. 2011. Complexity of vesicle microcirculation. Phys Rev E Stat Nonlin Soft Matter Phys 84(4 Pt 1):041906. doi: 10.1103/PhysRevE.84.041906. [DOI] [PubMed] [Google Scholar]
- 50.Aouane O, Thiébaud M, Benyoussef A, Wagner C, Misbah C. 2014. Vesicle dynamics in a confined Poiseuille flow: from steady state to chaos. Phys Rev E Stat Nonlin Soft Matter Phys 90(3):033011. doi: 10.1103/PhysRevE.90.033011. [DOI] [PubMed] [Google Scholar]
- 51.Dickinson RB, Neelam S, Lele TP. 2015. Dynamic, mechanical integration between nucleus and cell—where physics meets biology. Nucleus 6:360–365. doi: 10.1080/19491034.2015.1090074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaziri A, Mofrad M. 2007. Mechanics and deformation of the nucleus in micropipette aspiration experiment. J Biomech 40:2053–2062. doi: 10.1016/j.jbiomech.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 53.Evangelisti E, Gogleva A, Hainaux T, Doumane M, Tulin F, Quan C, Yunusov T, Floch K, Schornack S. 2017. Time-resolved dual transcriptomics reveal early induced Nicotiana benthamiana root genes and conserved infection-promoting Phytophthora palmivora effectors. BMC Biol 15:39. doi: 10.1186/s12915-017-0379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torres GA, Sarria GA, Varon F, Coffey MD, Elliott ML, Martinez G. 2010. First report of bud rot caused by Phytophthora palmivora on African oil palm in Colombia. Plant Dis 94:1163–1163. doi: 10.1094/PDIS-94-9-1163A. [DOI] [PubMed] [Google Scholar]
- 55.Karimi M, De Meyer B, Hilson P. 2005. Modular cloning in plant cells. Trends Plant Sci 10:103–105. doi: 10.1016/j.tplants.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Judelson HS, Michelmore RW. 1991. Transient expression of genes in the oomycete Phytophthora infestans using Bremia lactucae regulatory sequences. Curr Genet 19:453–459. doi: 10.1007/BF00312736. [DOI] [Google Scholar]
- 57.Ai H, Henderson JN, Remington SJ, Campbell RE. 2006. Directed evolution of a monomeric, bright and photostable version of Clavularia cyan fluorescent protein: structural characterization and applications in fluorescence imaging. Biochem J 400:531–540. doi: 10.1042/BJ20060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ai HW, Olenych SG, Wong P, Davidson MW, Campbell RE. 2008. Hue-shifted monomeric variants of Clavularia cyan fluorescent protein: identification of the molecular determinants of color and applications in fluorescence imaging. BMC Biol 6:13. doi: 10.1186/1741-7007-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. 2001. Reducing the environmental sensitivity of yellow fluorescent protein. J Biol Chem 276:29188–29194. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- 60.Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 61.Möller EM, Bahnweg G, Sandermann H, Geiger HH. 1992. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res 20:6115–6116. . doi: 10.1093/nar/20.22.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huitema E, Smoker M, Kamoun S. 2011. A straightforward protocol for electro-transformation of Phytophthora capsici zoospores. Methods Mol Biol 712:129–135. doi: 10.1007/978-1-61737-998-7_11. [DOI] [PubMed] [Google Scholar]
- 63.Le Fevre R, O’Boyle B, Moscou MJ, Schornack S. 2016. Colonization of barley by the broad-host hemibiotrophic pathogen Phytophthora palmivora uncovers a leaf development dependent involvement of MLO. Mol Plant Microbe Interact 29:385–395. doi: 10.1094/MPMI-12-15-0276-R. [DOI] [PubMed] [Google Scholar]
- 64.Chazotte B. 2011. Mounting live cells onto microscope slides. Cold Spring Harb Protoc 2011:pdb.prot5554. doi: 10.1101/pdb.prot5554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic representation of the pTOR-Gateway. A multisite attR4/attR3 Gateway insertion cassette allows for rapid testing of multiple promoter-reporter constructs. Adjacent is a cassette for constitutive expression of a cytoplasmic fluorescent reporter (either mTFP1, mWasabi, mCitrine, or tdTomato) under the control of Bremia lactucae promoter Ham34. Vectors carry neomycin phosphotransferase (nptII) selectable marker. Download FIG S1, PDF file, 0.2 MB (209.3KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Growth habit of transgenic P. palmivora strains carrying empty pTOR-Gateway vectors on N. benthamiana leaves. (A to D) Leaves from 4-week-old N. benthamiana plants were inoculated with mycelium plugs of transgenic P. palmivora strains carrying empty pTOR-Gateway vectors. Fluorescence was monitored within leaf tissues after 2 days. Representative images of areas infected with mycelium expressing mTFP1 (A), mWasabi (B), mCitrine (C), and tdTomato (D) are shown. Yellow asterisks indicate haustoria. Bar, 10 μm. Download FIG S2, PDF file, 1.5 MB (1.5MB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Dual labeling of P. palmivora hyphae and nuclei. (A) Schematic view of the construct used for dual labeling of nuclei and hyphae in P. palmivora strain LILI-td-NT. Backbone elements are not represented. (B) Representative pictures of a transformant expressing tdTomato as well as a nucleus-localized mTFP1 (NLS:mTFP1) driven by the P. palmivora ubiquitin-conjugating enzyme 2 (UBC2) native promoter. Representative pictures of transformants expressing both markers as well as rare cases of transformants expressing either tdTomato or mTFP1 in distinct hyphae. Bar, 10 μm. Download FIG S3, PDF file, 1.0 MB (1.1MB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
UBC2 transcript levels during N. benthamiana root infection. N. benthamiana roots were inoculated with zoospores from the transgenic P. palmivora strain ARI-tdTomato and harvested at different times corresponding to early infection (3 to 6 h), biotrophy (18 to 24 hai), and necrotrophy (30 to 48 hai). Expression data are given relative to P. palmivora WS21 and EF1α reference genes. Statistical significance was assessed using one-way analysis of variance and Tukey’s honestly significant difference test (P < 0.05). MZ, axenically grown mycelium with sporangia. Download FIG S4, PDF file, 0.2 MB (248.2KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) pTOR-Gateway vectors. pTOR-Gateway vectors follow naming conventions used for Gateway vectors. K indicates nptII, while C, F, Y, and R stand for cyan, green, yellow, and red fluorescence, respectively. The multisite Gateway cassette carries attR4 and attR3 sites and hence was arbitrarily named m43GW in the absence of a T-DNA left border to define cassette orientation. (B) Primers used in this study. Download Table S1, PDF file, 0.2 MB (188.1KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Experimental setup used for time-lapse imaging of infected N. benthamiana roots. (A) Schematic representation of the experimental setup. A 1-week-old N. benthamiana seedling (2) is mounted in a liquid compartment (3) between a slide (1) and a coverslip (4). The edges of the coverslip are sealed with a 4:1 mix of paraffin and lanolin (5). The upper part of the coverslip is filled with water for use with a water-dipping objective. (B) Representative image of the experimental setup. Numbers refer to the same elements as described above. Download FIG S5, PDF file, 0.6 MB (607.8KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Generation of a P. palmivora lamin A reporter. Transformation of P. palmivora LILI with a construct for constitutive (A and B) or native (C and D) expression of an mCitrine:LamA-Cter reporter. (A) Schematic view of the construct used for constitutive or native expression of the lamin reporter together with a nucleus-localized mTFP1. Backbone elements are not represented. (B) Representative pictures of the bubbling phenotype observed upon constitutive expression of the lamin reporter. Arrowheads indicate bubbling nuclei. Bar, 10 μm. (C) Representative pictures of a hyphal segment upon native expression of the lamin reporter, showing absence of nuclear bubbling. Arrowheads indicate bubbling nuclei. Bar, 10 μm. Download FIG S6, PDF file, 2.3 MB (2.3MB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Effect of antimicrotubule drug Benomyl on P. palmivora growth. (A) Representative pictures of P. palmivora LILI-td-NT mycelium growing on V8 agar plates supplemented or not with 10, 50, or 100 mg/liter Benomyl. (B) Confocal imaging of P. palmivora LILI-td-NT hyphae grown on V8 agar plates supplemented with 50 mg/liter Benomyl. Bar, 10 μm. Download FIG S7, PDF file, 0.9 MB (936.1KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Generation of a P. palmivora Centrin 2 (CETN2) reporter. (A) Schematic view of the construct for Ham34-promoter-driven expression of an mCitrine:CETN2 reporter together with a nucleus-localized mTFP1. Backbone elements are not represented. (B) Confocal imaging of a sporangium from a P. palmivora LILI-NT-Ce transgenic strain expressing the construct shown in panel A. Bar, 10 μm. (C) Schematic view of the construct for CETN2-promoter-driven expression of an mScarlet:CETN2 reporter together with a constitutively expressed nucleus-localized mTFP1. (D) Confocal imaging of a sporangium from a transgenic P. palmivora strain expressing the construct shown in panel C. Bar, 10 μm. Download FIG S8, PDF file, 0.8 MB (849.7KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Frequency of centrosome duplication within P. palmivora hyphae. (A and B) Representative pictures of axenically grown hyphae from the transgenic P. palmivora LILI-NT-Ce strain growing on V8 medium. (A) Distribution of nuclei and Centrin2 (CETN2)-labeled centrosomes within a hyphal segment. Asterisks indicate nuclei with duplicated centrosomes. (B) Magnified views of nuclei 13 and 14. Bar, 10 μm. Download FIG S9, PDF file, 0.8 MB (886.8KB, pdf) .
Copyright © 2019 Evangelisti et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.