Abstract
Synaptotagmin12 (SYT12) has been well characterized as the regulator of transmitter release in the nervous system, however the relevance and molecular mechanisms of SYT12 in oral squamous cell carcinoma (OSCC) are not understood. In the current study, we investigated the expression of SYT12 and its molecular biological functions in OSCC by quantitative reverse transcriptase polymerase chain reaction, immunoblot analysis, and immunohistochemistry. SYT12 were up-regulated significantly in OSCC-derived cell lines and primary OSCC tissue compared with the normal counterparts (P<0.05) and the SYT12 expression levels were correlated significantly with clinical indicators, such as the primary tumoral size, lymph node metastasis, and TNM stage (P<0.05). SYT12 knockdown OSCC cells showed depressed cellular proliferation, migration, and invasion with cell cycle arrest at G1 phase. Surprisingly, we found increased calcium/calmodulin-dependent protein kinase 2 (CAMK2) inhibitor 1 (CAMK2N1) and decreased CAMK2-phosphorylation in the knockdown cells. Furthermore, treatment with L-3, 4-dihydroxyphenylalanine (L-dopa), a drug approved for Parkinson's disease, led to down-regulation of SYT12 and similar phenotypes to SYT12 knockdown cells. Taken together, we concluded that SYT12 plays a significant role in OSCC progression via CAMK2N1 and CAMK2, and that L-dopa would be a new drug for OSCC treatment through the SYT12 expression.
Keywords: Oral squamous cell carcinoma, SYT12, CAMK2N1, CAMK2, L-dopa
Introduction
Oral cancer is the sixth most prevalent form of cancer 1. Oral squamous cell carcinomas (OSCCs), comprising about 90% of all oral cancer 2, are the highest morbidity of all head and neck cancers 3. More than 500,000 new cases and 300,000 deaths annually were reported 4. Although great progress has been made for the treatment and diagnosis, the 5-year survival rates for patients with OSCCs remain unchanged at 50% to 55% from nearly 5 decades ago 5. Therefore, understanding the molecular mechanisms underlying OSCC development and progression is necessary for improving the prognoses.
Synaptotagmins (SYTs) are membrane proteins comprised of a short N-terminal noncytoplasmic sequence, a single transmembrane region, a linker sequence of varying lengths, and two C2 domains. The SYTs were discovered because they are the Ca2+ sensor for neurotransmitter release in presynaptic nerve terminals, and SYT1 was identified first 6. Eight of these SYTs have C2 domains that bind to Ca2+ (SYT1-3, 5-7, 9, and 10) while the other eight do not (Syt4, 8, and 11-16) 7. The SYT12 gene encodes a family of proteins involved in regulating transmitter release in the nervous system 8,9.
A recent study reported that SYT12 overexpression was correlated with metastasis and SYT12 was a biomarker that tended to predict greater disease progression in patients with papillary thyroid cancer 10. However, the molecular biologic role of SYT12 in cancer remains unknown. In the current study, we explain for the first time the novel molecular mechanism of SYT12 in OSCCs and evaluate a new candidate for medical treatment of OSCCs.
Materials and Methods
Ethics statement
The ethical committee of the Graduate School of Medicine, Chiba University, approved the research protocol (protocol number 680). All patients provided written informed consent before inclusion in the study.
Cells and tissue samples
Eleven human OSCC-derived cell lines (HSC-2, HSC-3, HSC-3-M3, HSC-4, Sa3, Ca9-22, KOSC-2, SAS, SAS-H1, Ho-1-u-1, and Ho-1-N-1) were purchased from RIKEN BioResource Center (Tsukuba, Ibaraki, Japan) and the Japanese Collection of Research Bioresources Cell Bank (Ibaraki, Osaka, Japan) 11-13. We obtained human normal oral keratinocyte (HNOKs) from young healthy patients and cultured the cells as described previously 14-17. The cells used in this study were within 10 passages from thawing. They also were routinely tested using an EZ-PCR Mycoplasma Test Kit (Biological Industries, Kibbutz Beit Haemek, Israel).
mRNA expression analysis
Total RNA was prepared using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA). Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was carried out as described previously 11,18 with the following primers. The sequences of the gene-specific primers were as follows: SYT12 (5′-CGAAGCCATGATCTTCTCG-3' and 5'-GCTCTCAGCCACCGTCAC-3') and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (5′-AGCCACATCGCTCAGACAC-3′ and 5′-GCCCAATACGACCAAATCC-3′).
Reagents
L-dopa was purchased from Cayman Chemical (Ann Arbor, MI, USA). The following antibodies were used: anti-SYT12 (M519) (Merck & Co., Inc., Kenilworth, NJ, USA); anti-CAMK2N1 (PA5-23740) (Invitrogen, CA, USA); anti-CAMK2 (ab52476) and anti-CAMK2 (Phospho T286) (ab32678) (abcam, Tokyo, Japan); anti-p27Kip1 (#3686) and anti-CDK2 (#2546) (Cell Signaling Technology, Danvers, MA, USA); anti-cyclin E (sc-377100), and GAPDH (sc-3223) (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Immunoblotting
Immunoblotting was performed as previously reported 15,19 with each appropriate antibody mentioned previously.
Immunohistochemistry (IHC)
IHC and calculation of the IHC score were performed as described previously 15,19-21, with appropriate antibody, anti-SYT12 (1:100 dilution), at 4°C. SYT12 was classified as positive and negative using the median score of all tumors as the cut-off points 22.
Transfection with shRNA plasmid
We established stable knockdown transfectants, and the cell lines (HSC-3, SAS) were transfected with shRNA targeting (shSYT12) (sc-96796) and control shRNA (shMock) (sc-108060) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) using Lipofectamine 3000 (Invitrogen, CA, USA) according to the manufacturer's protocol. The stable transfectants were isolated in a culture medium containing puromycin (2 µg/ml) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Four weeks after transfection, a small colony was viable. The cell colonies were picked, transferred to six-well plates, and expanded gradually to 10-cm dishes. To assess the efficiency of SYT12 knockdown, we performed RT-qPCR and immunoblotting.
Functional assays
A proliferation assay, invasion assay, and migration assay were performed as described previously 22-30.
Cellular viability assay
Cellular viability was measured using the MTS assay (Promega, Madison, WI, USA) 30. MTS assays with cells (HSC-3, SAS) (5 × 103) were performed in 96-well plates. After treatment with L-dopa (concentrations, 0, 50, 100, 200, 400, and 800 µM) for 24 h, we measured the 490-nm absorbance using a Benchmark Plus Microplate Reader (Bio-Rad, Hercules, CA, USA).
Cell cycle analysis
Cell cycle analyses were performed as described previously 25,27,31. shRNA transfected cells and L-dopa-treated cells were examined.
L-dopa treatment
To determine the appropriate concentration of L-dopa for OSCC cells, we performed the MTS assay, qRT-PCR, and immunoblotting. We then evaluate whether L-dopa affected the cellular proliferation and cell cycle. The cells were treated with L-dopa (50 µM) or dimethylsulfoxide as controls.
PCR array
To identify genes that potentially interact with SYT12, we used qRT-PCR to analyze the Epithelial to Mesenchymal Transition RT2 Profiler PCR Array (Qiagen, Hilden, Germany).
Statistical analysis
Each experiment was repeated at least three times, and all data are presented as the mean ± standard error of the mean. Statistical differences were analyzed using Fisher's exact test and a two-tailed, unpaired Student's t-test with Welch's correction for unequal variances using the BellCurve for Excel (Social Survey Research Information Co., Ltd.). P < 0.05 was considered statistically significant.
Results
Up-regulation of SYT12 in OSCC cell lines
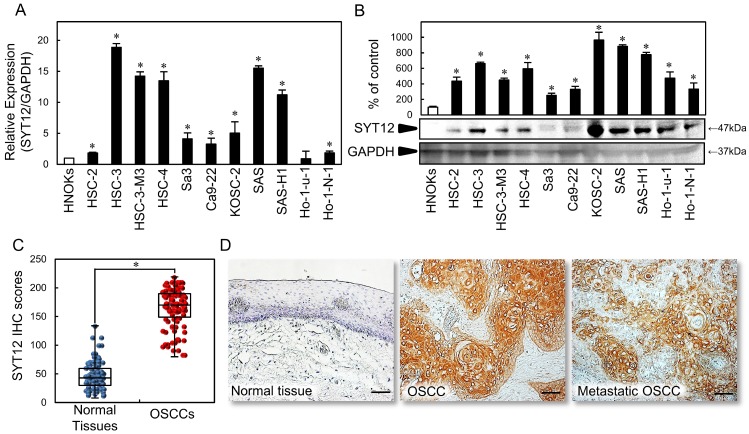
To investigate the status of the SYT12 expression as a cancer-related gene, we first conducted qRT-PCR and immunoblot analysis with 11 OSCC-derived cell lines and HNOKs. SYT12 mRNA expression was up-regulated significantly (P < 0.05) in OSCC-derived cell lines other than Ho-1-U-1 compared with the HNOKs (Fig. 1A). Fig. 1B shows representative results of immunoblot analysis. The SYT12 protein level also increased in all OSCC cell lines compared with the HNOKs (Fig. 1B).
Fig 1.
SYT12 expression in OSCC-derived cell lines and primary OSCCs. (A) Quantification of SYT12 mRNA expression in OSCC-derived cell lines by qRT-PCR analysis (N=3). (B) Representative immunoblot analysis of SYT12 protein expression. The densitometric SYT12 protein data are normalized to GAPDH protein levels. The values are expressed as a percentage of the HNOKs (N=3). (C) The SYT12 IHC scores of the normal oral tissues and OSCCs (N=112). (D) Representative IHC results for SYT12 protein in normal tissue, primary OSCCs, and metastatic regional lymph nodes. Original magnification, x200. Scale bars, 50 μm.
Evaluation of SYT12 status in primary OSCCs
We evaluated the SYT12 expression in primary OSCCs by IHC and the IHC scoring system 22,27,31. In oral normal tissues, the SYT12 IHC scores ranged from 8.1 to 134.2 (median, 43.0), while in OSCCs the scores ranged from 80.3 to 219.0 (median, 170.2). These IHC scores in primary OSCCs were significantly (P < 0.05) higher than in normal oral tissues (Fig. 1C). Intense cytoplasmic and cell membrane staining was seen for SYT12, whereas the normal oral tissues showed negative staining. Representative IHC figures for SYT12 staining are shown (×200 magnification) (Fig. 1D). In the clinical classifications, SYT12-positive OSCCs (IHC scores > median, 170.2) were associated significantly (P < 0.05) with tumor size, regional lymph node metastasis, and the TNM stage of the OSCCs (Table 1).
Table 1.
Correlation between SYT12 expression and clinical classification in OSCCs.
| Clinical classification | Results of immunostaining No. patients |
|||
|---|---|---|---|---|
| Total | SYT12 negative | SYT12 positive | P value | |
| Age at surgery (years) | ||||
| < 60 | 26 | 12 | 14 | 0.823 |
| ≧60 | 86 | 44 | 42 | |
| Gender | ||||
| Male | 66 | 32 | 34 | 0.706 |
| Female | 46 | 24 | 22 | |
| T-primary tumor | ||||
| T1+T2 | 64 | 38 | 26 | 0.035‡ |
| T3+T4 | 48 | 18 | 30 | |
| N-regional lymph node | ||||
| Negative | 74 | 43 | 31 | 0.028‡ |
| Positive | 38 | 13 | 25 | |
| TNM Stage | ||||
| I+II | 46 | 30 | 16 | 0.012‡ |
| III+IV | 66 | 26 | 40 | |
| Vascular invasion | ||||
| Negative | 88 | 48 | 40 | 0.106 |
| Positive | 24 | 8 | 16 | |
| Histopathologic type | ||||
| Well | 86 | 45 | 41 | 0.503 |
| Moderately+Poorly | 26 | 11 | 15 | |
Fisher's exact test, ‡P < 0.05.
Establishment of stable SYT12 knockdown OSCC cells
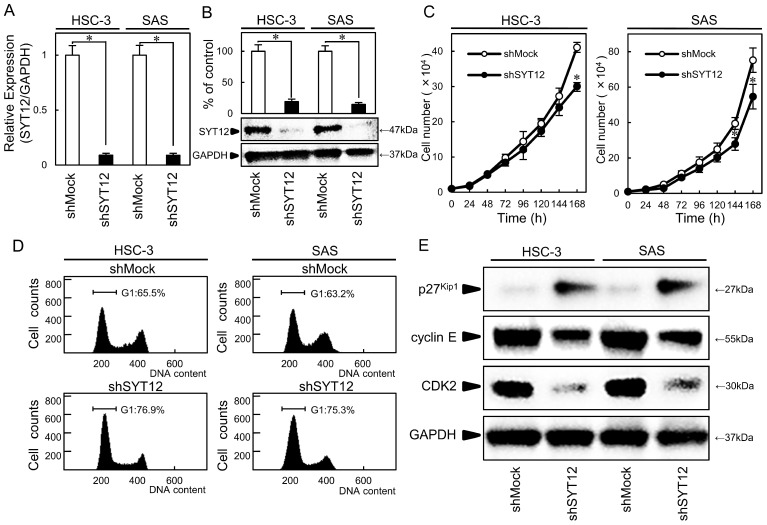
HSC-3 and SAS were subjected to knockdown experiments because they had higher expression levels among the OSCC cell lines. We transfected shSYT12 and shMock vectors into the cells. To investigate the efficiency of the transfection, we performed qRT-PCR and immunoblot analysis. The SYT12 mRNA and protein expression levels in the shSYT12 cells decreased significantly (P < 0.05) compared with the shMock cells (Fig. 2A, B).
Fig 2.
Establishment of SYT12 knockdown cells and decreased cell growth via cell cycle arrest at G1 phase. (A, B) Expression of SYT12 mRNA and protein in shMock and shSYT12 cells (HSC-3 and SAS-derived transfectants) (N=3). (C) The cellular growth of the shSYT12 cells are decreased significantly (P < 0.05) compared with the shMock cells after 7 days (168 h) (N=3). (D) A flow cytometric analysis shows that the percentage of the shSYT12 cells in the G1 phase is increased compared with the shMock cells (N=3). (E) Immunoblotting analysis shows up-regulation of p27Kip1 and down-regulation of cyclin E and CDK2 in the shSYT12 cells compared with the shMock cells (N=3).
Effect of shSYT12 cells on cellular growth and cell cycle
To evaluate the effect of SYT12 knockdown on cellular growth, a cellular proliferation assay and cell cycle analyses were performed. We found a significant (P < 0.05) decrease in cellular proliferation in the shSYT12 cells compared with the shMock cells (Fig. 2C). In the cell cycle analysis by flow cytometry, the percentage of the shSYT12 cells at the G1 phase was significantly (P < 0.05) higher than that of the shMock cells (Fig. 2D). Analysis of the G1 phase-related protein by immunoblotting showed increased p27Kip1 and subsequent decreased expression of cyclin E and CDK2 and in the shSYT12 cells compared with the shMock cells (Fig. 2E).
Cellular migration and invasion assays
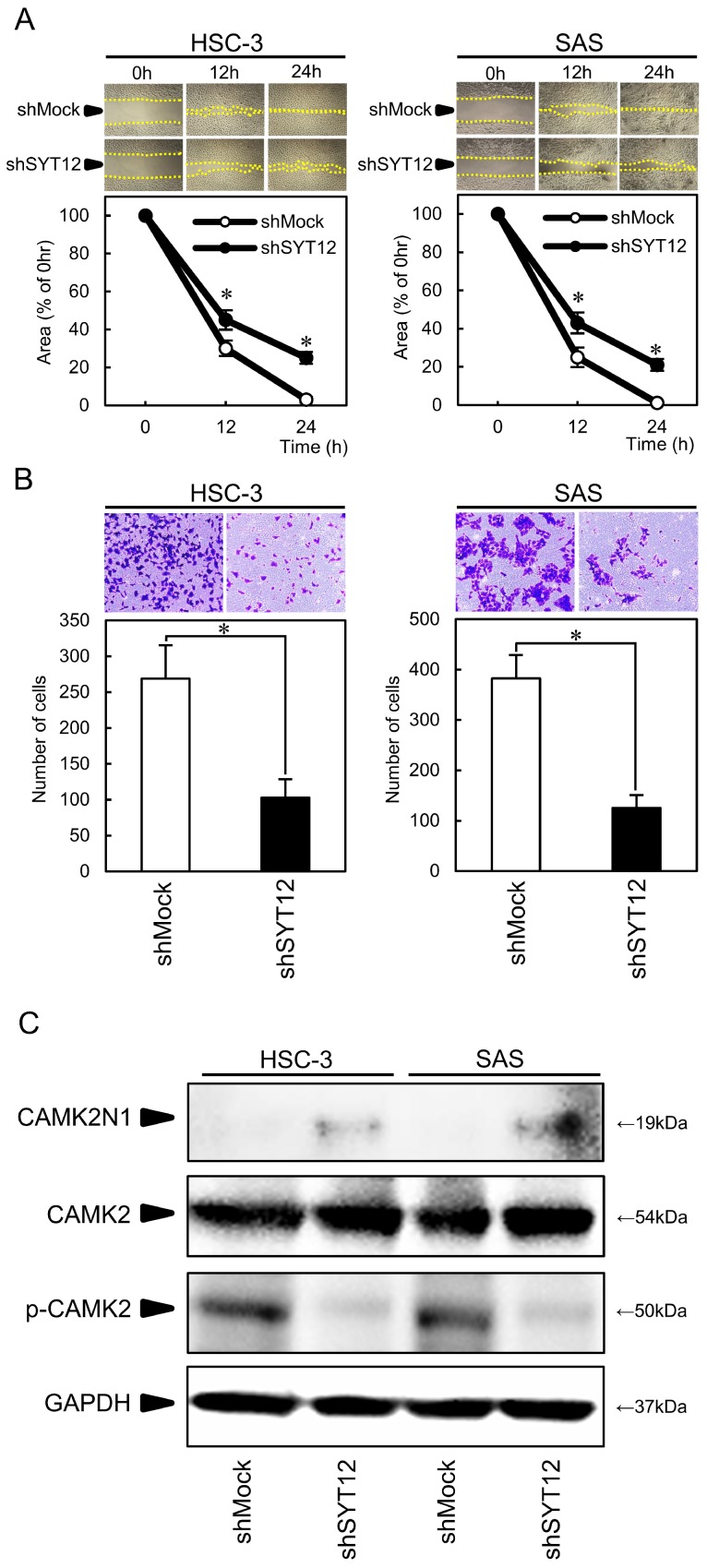
The migration assay showed that the area of micropipette wounds decreased significantly (P < 0.05) in shSYT12 cells after 12 h compared with the shMock cells (Fig. 3A). Analysis of the invasion assay showed significantly lower (P < 0.05) invasiveness of the shSYT12 cells compared with the shMock cells at 24 h (Fig. 3B).
Fig 3.
Involvement of the SYT12 signaling pathways that mediate the migratory and invasiveness phenotype of OSCCs. (A) Migration assay of shMock cells and shSYT12 cells (N=3). (B) Invasion assay of shMock cells and shSYT12 cells at 24h (N=3). (C) Immunoblot analysis shows CAMK2N1 expression is increased in the shSYT12 cells, CAMK2 expression is similar in the shMock cells and shSYT12, but phosphorylation of CAMK2 (p-CAMK2) is decreased in the shSYT12 cells compared with the shMock cells (N=3).
Involvement of SYT12 signaling pathways that mediated the malignant phenotype of OSCCs
To obtain the data to support the involvement of SYT12 in cancer progression, we performed PCR arrays in the shMock and shSYT12 cells. CAMK2N1 had a significantly (P<0.001) higher score (fold change, 12.4). CAMK2N1 is the endogenous inhibitor of CAMK2, which is activated by an increased intracellular Ca2+ concentration. CAMK2N1 expression was increased in the shSYT12 cells compared with the shMock cells. Furthermore, CAMK2 expression was similar in the shMock cells and shSYT12, but phosphorylation of CAMK2 decreased in the shSYT12 cells compared with the shMock cells (Fig. 3C).
L-dopa treatment
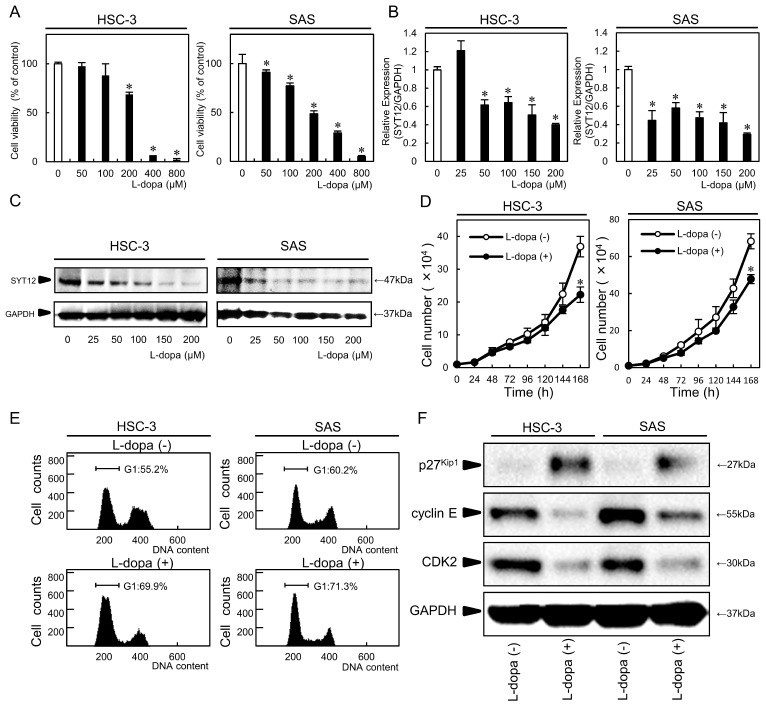
We detected the SYT12 inhibitor, L-dopa, using Ingenuity Pathway Analysis. To investigate the efficiency of L-dopa for OSCC cells (HSC-3, SAS), we performed an MTS assay, which showed significantly (P<0.05) decreased cellular viability above L-dopa concentrations of 200 µM in a concentration-dependent manner (Fig. 4A). The inhibitory concentration 50s (IC50) for L-dopa in the HSC-3 and SAS cell lines were 259 nM and 196 nM, respectively. To verify the inhibitory effect of L-dopa on SYT12, the SYT12 expression was examined after treatment which various concentrations of L-dopa, by qRT-PCR and immunoblot analysis. L-dopa significantly (P<0.05) inhibited the SYT12 mRNA and protein expression levels above concentrations of 50 µM in both cells compared with the control cells (Fig. 4B, C). Cellular growth of the L-dopa-treated cells were significantly (P < 0.05) lower than the control cells at 144 h (Fig. 4D). Cell cycle analysis showed that the percentage of the L-dopa-treated cells at the G1 phase was significantly (P < 0.05) higher than the control cells (Fig. 4E). The addition, L-dopa-treated cells showed that the p27Kip1 protein expression levels were up-regulated and the cyclin E and CDK2 levels were down-regulated compared with the control cells (Fig. 4F). These results indicated that L-dopa may regulate critical functions associated with tumoral growth through down-regulation of SYT12.
Fig 4.
L-dopa treatment. (A) An MTS assay shows a significant decrease in cellular viability above L-dopa concentrations of 200 µM in a concentration-dependent manner (N=3). (B, C) L-dopa concentrations above 50 µM inhibit SYT12 mRNA and protein expression levels in the L-dopa-treated cells compared with controls (N=3). (D) The cellular growth of the L-dopa-treated cells is significantly (P < 0.05) lower than that of the control cells after 7 days (168 h) (N=3). (E) A flow cytometric analysis shows the percentage of L-dopa-treated cells in the G1 phase is increased compared with the control cells (N=3). (F) Immunoblot analysis shows up-regulation of p27Kip1 and down-regulation of CDK2 and cyclin E in the L-dopa-treated cells compared with the control cells (N=3).
Discussion
The current results showed that SYT12 knockdown was characterized by decreased cellular growth, invasiveness, and migratory activities with CAMK2N1 overexpression and decreased CAMK2-phospholyration. In addition, L-dopa, a drug for Parkinson's disease, controlled SYT12 expression, leading to similar phenotypes of SYT12 knockdown cells.
Previous studies have shown that the SYT family genes regulate proliferation, invasion, and metastasis in several cancer cells in addition to their roles in neuronal cells 32-35. Of SYT family, SYT12 is detected as a biomarker of papillary thyroid cancer 10, but the molecular biologic function of SYT12 in other cancer cells and the interactions between SYT12 and cancer-associated signaling pathways remain unknown. To identify the SYT12-related genes, we conducted PCR array analysis using SYT12 knockdown cells and found that SYT12 affects the expression of CAMK2N1, inhibitor type1 of CAMK2, which is a known potent modulator of tumorigenesis 36. CAMK2N1 expression has tumor-suppressing roles, such as depressed cell proliferation and cell cycle arrest in certain cancers 37-39. Actually, SYT12 knockdown cells showed significant overexpression of CAMK2N1 (Fig. 3C), suggesting that down-regulation of SYT12 suppressed tumoral growth with cell cycle arrest by CAMK2N1 overexpression. CAMK2 is involved in controlling a range of cellular processes, including synaptic plasticity and memory consolidation 40,41, vascular smooth muscle polarization 42, and cellular proliferation 43. Moreover, several studies also suggest that CAMK2 regulates metastasis in several cancers 44-46. The biologic properties of CAMK2 are controlled by multisite phosphorylations; when intracellular calcium levels rise, calcium binds to calmodulin, which activates CAMK2 and leads to self-phosphorylation of CAMK2 at T286 47,48. Therefore, SYT12 may activate CAMK2 by decreased CAMK2N1 and increasing the intracellular calcium concentration in OSCCs, as a result, they promote the OSCC progression.
L-dopa is used widely to increase dopamine concentrations in the treatment of Parkinson's disease with a dopa decarboxylase inhibitor, such as carbidopa and benserazide, which inhibit aromatic amino acid decarboxylase, thus preventing the conversion of L-dopa into dopamine 49. L-dopa was recently reported to suppress cellular growth via down-regulation of prolactin-mediated JAK2/STAT5A in breast cancer cells 50, and carbidopa was reported to be an aryl hydrocarbon receptor agonist and to suppress the growth of pancreatic cancer 51. However, the role of L-dopa in oral cancer remains unknown. In the current study, we revealed for the first time that L-dopa led to down-regulation of SYT12 and decreased cellular proliferation by the cell cycle arrest at the G1 phase via p27Kip1.
In conclusion, we present here that SYT12 plays a significant role in OSCC progression via CAMK2N1 and CAMK2, and that L-dopa would be a new drug for OSCC treatment through the SYT12 expression.
Supplementary Material
Supplementary figures.
Acknowledgments
We thank Lynda C. Charters for editing this manuscript. The authors received no financial support.
Abbreviations
- SYT12
Synaptotagmin12
- OSCC
oral squamous cell carcinoma
- L-dopa
L-3, 4-dihydroxyphenylalanine
- HNOKS
human normal oral keratinocytes
- CAMK2N1
calcium/calmodulin-dependent protein kinase 2 inhibitor 1
- CAMK2
calcium/calmodulin-dependent protein kinase 2
References
- 1.Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Choi S, Myers JN. Molecular Pathogenesis of Oral Squamous Cell Carcinoma: Implications for Therapy. J Dent Res. 2008;87:14–32. doi: 10.1177/154405910808700104. [DOI] [PubMed] [Google Scholar]
- 3.Liao L, Wang J, Ouyang S, Zhang P, Wang J, Zhang M. Expression and clinical significance of microRNA-1246 in human oral squamous cell carcinoma. Med Sci Monit. 2015;21:776–81. doi: 10.12659/MSM.892508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Fuller CD, Wang SJ, Thomas CR, Hoffman HT, Weber RS, Rosenthal DI. Conditional survival in head and neck squamous cell carcinoma. Cancer. 2007;109:1331–43. doi: 10.1002/cncr.22563. [DOI] [PubMed] [Google Scholar]
- 6.Perin MS, Fried VA, Mignery GA, Jahn R, Südhof TC. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345:260–3. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 7.Pang ZP, Südhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maximov A, Shin O-H, Liu X, Südhof TC. Synaptotagmin-12, a synaptic vesicle phosphoprotein that modulates spontaneous neurotransmitter release. J Cell Biol. 2007;176:113–24. doi: 10.1083/jcb.200607021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaeser-Woo YJ, Younts TJ, Yang X, Zhou P, Wu D, Castillo PE. et al. Synaptotagmin-12 Phosphorylation by cAMP-Dependent Protein Kinase Is Essential for Hippocampal Mossy Fiber LTP. J Neurosci. 2013;33:9769–80. doi: 10.1523/JNEUROSCI.5814-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonklaas J, Murthy S, Liu D, Klubo-Gwiezdzinska J, Krishnan J, Burman K. et al. Novel biomarker SYT12 may contribute to predicting papillary thyroid cancer outcomes. Futur Sci OA. 2018;4:FSO249. doi: 10.4155/fsoa-2017-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endo Y, Uzawa K, Mochida Y, Shiiba M, Bukawa H, Yokoe H. et al. Sarcoendoplasmic reticulum Ca2+ ATPase type 2 downregulated in human oral squamous cell carcinoma. Int J Cancer. 2004;110:225–31. doi: 10.1002/ijc.20118. [DOI] [PubMed] [Google Scholar]
- 12.Kasamatsu A, Iyoda M, Usukura K, Sakamoto Y, Ogawara K, Shiba M. et al. Gibberellic acid induces α-amylase expression in adipose-derived stem cells. Int J Mol Med. 2012;30:243–7. doi: 10.3892/ijmm.2012.1007. [DOI] [PubMed] [Google Scholar]
- 13.Koyama T, Ogawara K, Kasamatsu A, Okamoto A, Kasama H, Minakawa Y. et al. ANGPTL3 is a novel biomarker as it activates ERK/MAPK pathway in oral cancer. Cancer Med. 2015;4:759–69. doi: 10.1002/cam4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto I, Kasamatsu A, Yamatoji M, Nakashima D, Saito K, Higo M. et al. Kinesin family member 14 in human oral cancer: A potential biomarker for tumoral growth. Biochem Biophys Reports. 2015;3:26–31. doi: 10.1016/j.bbrep.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Usukura K, Kasamatsu A, Okamoto A, Kouzu Y, Higo M, Koike H. et al. Tripeptidyl peptidase II in human oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2013;139:123–30. doi: 10.1007/s00432-012-1307-y. [DOI] [PubMed] [Google Scholar]
- 16.Kasamatsu A, Uzawa K, Usukura K, Koike K, Nakashima D, Ishigami T. et al. Loss of heterozygosity in oral cancer. Oral Sci Int. 2011;8:37–43. [Google Scholar]
- 17.Tanzawa H, Uzawa K, Kasamatsu A, Endo-Sakamoto Y, Saito K, Ogawara K. et al. Targeting gene therapies enhance sensitivity to chemo- and radiotherapy of human oral squamous cell carcinoma. Oral Sci Int. 2015;12:43–52. [Google Scholar]
- 18.Shiiba M, Ishige S, Saito Y, Shimizu T, Minakawa Y, Kasamatsu A. et al. Down-regulated expression of family with sequence similarity 3, member B (FAM3B), in oral squamous cell carcinoma. Oral Sci Int. 2012;9:9–16. [Google Scholar]
- 19.Saito T, Kasamatsu A, Ogawara K, Miyamoto I, Saito K, Iyoda M. et al. Semaphorin7A Promotion of Tumoral Growth and Metastasis in Human Oral Cancer by Regulation of G1 Cell Cycle and Matrix Metalloproteases: Possible Contribution to Tumoral Angiogenesis. Tang C-H, editor. PLoS One. 2015;10:e0137923. doi: 10.1371/journal.pone.0137923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minakawa Y, Kasamatsu A, Koike H, Higo M, Nakashima D, Kouzu Y. et al. Kinesin Family member 4A: A Potential Predictor for Progression of Human Oral Cancer. Guan X-Y, editor. PLoS One. 2013;8:e85951. doi: 10.1371/journal.pone.0085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uzawa K, Kasamatsu A, Saito T, Takahara T, Minakawa Y, Koike K. et al. Long-term culture of human odontoma-derived cells with a Rho kinase inhibitor. Exp Cell Res. 2016;347:232–240. doi: 10.1016/j.yexcr.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Toeda Y, Kasamatsu A, Koike K, Endo-Sakamoto Y, Fushimi K, Kasama H. et al. FBLIM1 enhances oral cancer malignancy via modulation of the epidermal growth factor receptor pathway. Mol Carcinog. 2018;57:1690–1697. doi: 10.1002/mc.22889. [DOI] [PubMed] [Google Scholar]
- 23.Koide N, Kasamatsu A, Endo-Sakamoto Y, Ishida S, Shimizu T, Kimura Y. et al. Evidence for Critical Role of Lymphocyte Cytosolic Protein 1 in Oral Cancer. Sci Rep. 2017;7:43379. doi: 10.1038/srep43379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukamachi M, Kasamatsu A, Endo-Sakamoto Y, Fushimi K, Kasama H, Iyoda M. et al. Multiple coagulation factor deficiency protein 2 as a crucial component in metastasis of human oral cancer. Exp Cell Res. 2018;368:119–25. doi: 10.1016/j.yexcr.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Ishida S, Kasamatsu A, Endo-Sakamoto Y, Nakashima D, Koide N, Takahara T. et al. Novel mechanism of aberrant ZIP4 expression with zinc supplementation in oral tumorigenesis. Biochem Biophys Res Commun. 2017;483:339–345. doi: 10.1016/j.bbrc.2016.12.142. [DOI] [PubMed] [Google Scholar]
- 26.Fukushima R, Kasamatsu A, Nakashima D, Higo M, Fushimi K, Kasama H. et al. Overexpression of Translocation Associated Membrane Protein 2 Leading to Cancer-Associated Matrix Metalloproteinase Activation as a Putative Metastatic Factor for Human Oral Cancer. J Cancer. 2018;9:3326–33. doi: 10.7150/jca.25666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okubo Y, Kasamatsu A, Yamatoji M, Fushimi K, Ishigami T, Shimizu T. et al. Diacylglycerol lipase alpha promotes tumorigenesis in oral cancer by cell-cycle progression. Exp Cell Res. 2018;367:112–8. doi: 10.1016/j.yexcr.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto J, Kasamatsu A, Okubo Y, Nakashima D, Fushimi K, Minakawa Y. et al. Evaluation of tryptophan-aspartic acid repeat-containing protein 34 as a novel tumor-suppressor molecule in human oral cancer. Biochem Biophys Res Commun. 2018;495:2469–74. doi: 10.1016/j.bbrc.2017.12.138. [DOI] [PubMed] [Google Scholar]
- 29.Kita A, Kasamatsu A, Nakashima D, Endo-Sakamoto Y, Ishida S, Shimizu T. et al. Activin B Regulates Adhesion, Invasiveness, and Migratory Activities in Oral Cancer: a Potential Biomarker for Metastasis. J Cancer. 2017;8:2033–41. doi: 10.7150/jca.18714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimura S, Kasamatsu A, Nakashima D, Iyoda M, Kasama H, Saito T. et al. UBE2S associated with OSCC proliferation by promotion of P21 degradation via the ubiquitin-proteasome system. Biochem Biophys Res Commun. 2017;485:820–5. doi: 10.1016/j.bbrc.2017.02.138. [DOI] [PubMed] [Google Scholar]
- 31.Sawai Y, Kasamatsu A, Nakashima D, Fushimi K, Kasama H, Iyoda M. et al. Critical role of deoxynucleotidyl transferase terminal interacting protein 1 in oral cancer. Lab Investig. 2018;98:980–8. doi: 10.1038/s41374-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 32.Kanda M, Tanaka H, Shimizu D, Miwa T, Umeda S, Tanaka C. et al. SYT7 acts as a driver of hepatic metastasis formation of gastric cancer cells. Oncogene. 2018;37:5355–66. doi: 10.1038/s41388-018-0335-8. [DOI] [PubMed] [Google Scholar]
- 33.Wang K, Xiao H, Zhang J, Zhu D. Synaptotagmin7 Is Overexpressed In Colorectal Cancer And Regulates Colorectal Cancer Cell Proliferation. J Cancer. 2018;9:2349–56. doi: 10.7150/jca.25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jahn JE, Best DH, Coleman WB. Exogenous expression of synaptotagmin XIII suppresses the neoplastic phenotype of a rat liver tumor cell line through molecular pathways related to mesenchymal to epithelial transition. Exp Mol Pathol. 2010;89:209–16. doi: 10.1016/j.yexmp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Sung HY, Han J, Ju W, Ahn JH. Synaptotagmin-like protein 2 gene promotes the metastatic potential in ovarian cancer. Oncol Rep. 2016;36:535–41. doi: 10.3892/or.2016.4835. [DOI] [PubMed] [Google Scholar]
- 36.Häfner N, Steinbach D, Jansen L, Diebolder H, Dürst M, Runnebaum IB. RUNX3 and CAMK2N1 hypermethylation as prognostic marker for epithelial ovarian cancer. Int J Cancer. 2016;138:217–28. doi: 10.1002/ijc.29690. [DOI] [PubMed] [Google Scholar]
- 37.Wang T, Guo S, Liu Z, Wu L, Li M, Yang J. et al. CAMK2N1 inhibits prostate cancer progression through androgen receptor-dependent signaling. Oncotarget. 2014;5:10293–306. doi: 10.18632/oncotarget.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T, Liu Z, Guo S, Wu L, Li M, Yang J. et al. The tumor suppressive role of CAMK2N1 in castration-resistant prostate cancer. Oncotarget. 2014;5:3611–21. doi: 10.18632/oncotarget.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Li N, Liu X, Zheng Y, Cao X. A Novel Endogenous Human CaMKII Inhibitory Protein Suppresses Tumor Growth by Inducing Cell Cycle Arrest via p27 Stabilization. J Biol Chem. 2008;283:11565–74. doi: 10.1074/jbc.M800436200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–3. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 41.Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. Disruption of Dendritic Translation of CaMKII Impairs Stabilization of Synaptic Plasticity and Memory Consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- 42.Mercure MZ, Ginnan R, Singer HA. CaM kinase IIδ 2 -dependent regulation of vascular smooth muscle cell polarization and migration. Am J Physiol Physiol. 2008;294:C1465–75. doi: 10.1152/ajpcell.90638.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skelding KA, Rostas JAP, Verrills NM. Controlling the cell cycle: The role of calcium/calmodulin-stimulated protein kinases I and II. Cell Cycle. 2011;10:631–9. doi: 10.4161/cc.10.4.14798. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Zhao R, Zhe H. The emerging role of CaMKII in cancer. Oncotarget. 2015;6:11725–34. doi: 10.18632/oncotarget.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daft PG, Yuan K, Warram JM, Klein MJ, Siegal GP, Zayzafoon M. Alpha-CaMKII Plays a Critical Role in Determining the Aggressive Behavior of Human Osteosarcoma. Mol Cancer Res. 2013;11:349–59. doi: 10.1158/1541-7786.MCR-12-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Q, Symes AJ, Kane CA, Freeman A, Nariculam J, Munson P. et al. A Novel Role for Wnt/Ca2+ Signaling in Actin Cytoskeleton Remodeling and Cell Motility in Prostate Cancer. Hotchin NA, editor. PLoS One. 2010;5:e10456. doi: 10.1371/journal.pone.0010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skelding KA, Rostas JAP. Regulation of CaMKII In vivo: The Importance of Targeting and the Intracellular Microenvironment. Neurochem Res. 2009;34:1792–804. doi: 10.1007/s11064-009-9985-9. [DOI] [PubMed] [Google Scholar]
- 48.Britschgi A, Bill A, Brinkhaus H, Rothwell C, Clay I, Duss S. et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci USA. 2013;110:E1026–34. doi: 10.1073/pnas.1217072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. 2002;287:1653–61. doi: 10.1001/jama.287.13.1653. [DOI] [PubMed] [Google Scholar]
- 50.Sinha S, Sharma S, Vora J, Shah H, Srivastava A, Shrivastava N. Mucuna pruriens (L.) DC chemo sensitize human breast cancer cells via downregulation of prolactin-mediated JAK2/STAT5A signaling. J Ethnopharmacol. 2018;217:23–35. doi: 10.1016/j.jep.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Ogura J, Miyauchi S, Shimono K, Yang S, Gonchigar S, Ganapathy V. et al. Carbidopa is an activator of aryl hydrocarbon receptor with potential for cancer therapy. Biochem J. 2017;474:3391–402. doi: 10.1042/BCJ20170583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.