Abstract
Objectives
The long head of the biceps (LHB) is often resected in shoulder surgery and could therefore serve as a cell source for tissue engineering approaches in the shoulder. However, whether it represents a suitable cell source for regenerative approaches, both in the inflamed and non-inflamed states, remains unclear. In the present study, inflamed and native human LHBs were comparatively characterized for features of regeneration.
Methods
In total, 22 resected LHB tendons were classified into inflamed samples (n = 11) and non-inflamed samples (n = 11). Proliferation potential and specific marker gene expression of primary LHB-derived cell cultures were analyzed. Multipotentiality, including osteogenic, adipogenic, chondrogenic, and tenogenic differentiation potential of both groups were compared under respective lineage-specific culture conditions.
Results
Inflammation does not seem to affect the proliferation rate of the isolated tendon-derived stem cells (TDSCs) and the tenogenic marker gene expression. Cells from both groups showed an equivalent osteogenic, adipogenic, chondrogenic and tenogenic differentiation potential in histology and real-time polymerase chain reaction (RT-PCR) analysis.
Conclusion
These results suggest that the LHB tendon might be a suitable cell source for regenerative approaches, both in inflamed and non-inflamed states. The LHB with and without tendinitis has been characterized as a novel source of TDSCs, which might facilitate treatment of degeneration and induction of regeneration in shoulder surgery.
Cite this article: J. Schmalzl, P. Plumhoff, F. Gilbert, F. Gohlke, C. Konrads, U. Brunner, F. Jakob, R. Ebert, A. F. Steinert. Tendon-derived stem cells from the long head of the biceps tendon: Inflammation does not affect the regenerative potential. Bone Joint Res 2019;8:414–424. DOI: 10.1302/2046-3758.89.BJR-2018-0214.R2.
Keywords: Biceps tendon; Tendon-derived stem cell; Mesenchymal stem cell; Tissue engineering, Shoulder
Article focus
The long head of the biceps (LHB) tendon in the inflamed and non-inflamed state as a stem cell source for regenerative approaches.
Key messages
The LHB tendon might be a suitable cell source for regenerative approaches, both in inflamed and non-inflamed states.
Inflammation does not seem to affect the proliferation rate of the isolated tendon-derived stem cells (TDSCs).
Cells from both groups showed an equivalent osteogenic, adipogenic, chondrogenic, and tenogenic differentiation potential in histology and real-time-polymerase chain reaction (RT-PCR) analysis.
Strengths and limitations
Differences between TDSCs isolated from inflamed and non-inflamed LHB tendons were studied.
The patient population was not matched regarding sex and age, whereas it has been demonstrated that the sex of the donors, as well as age, can affect stem cell proliferation.
Introduction
The long head of the biceps (LHB) tendon originates from the superior labrum and the supraglenoid tubercle of the shoulder.1 While crossing the rotator cuff (RC) interval, the LHB tendon is stabilized in the bicipital groove by the capsuloligamentous biceps pulley complex.2 Before the transition to the upright gait, the LBH served as an important anterior stabilizer of the shoulder,3 however, today its role in shoulder biomechanics is controversial. Some shoulder surgeons even call it the “appendix” of the shoulder joint, so frequently is it affected by inflammation. The LHB tendon may be further affected by degenerative tendinopathy, dislocation, or partial or complete tears, and intra-articular resection of the tendon is frequently performed and has consistently provided appropriate pain relief under maintained biceps muscle function.4
Interestingly, the LHB has recently been identified as a tissue source for multipotent cells characterized as tendon-derived stem cells (TDSCs).5 The LHB might, therefore, be an attractive cell source to augment regenerative approaches within the shoulder.5 Despite significant recent progress in the development of RC repair techniques, failure rates after RC repair still remain highly variable mainly due to biological variability of the torn RC tendon and muscle with associated fatty atrophy.6-8 Histological studies have shown that tendon does not heal by rebuilding a normal fibrocartilage enthesis but by forming scar tissue with a high content of type III collagen.9,10 Therefore, TDSCs might be capable of promoting repair in the tendon bone junction and may influence the tendon structure after RC repair.11
Due to the current lack of information in the literature about whether inflammation impairs the regenerative capacities of LHB tendons, in this work we comparatively analyzed TDSCs isolated from resected intra-articular portions of the LHB with and without inflammation, and analyzed the respective cultures in the context of regeneration on a tissue, cellular, and molecular level.
Materials and Methods
Clinical classification in inflamed and non-inflamed tendons
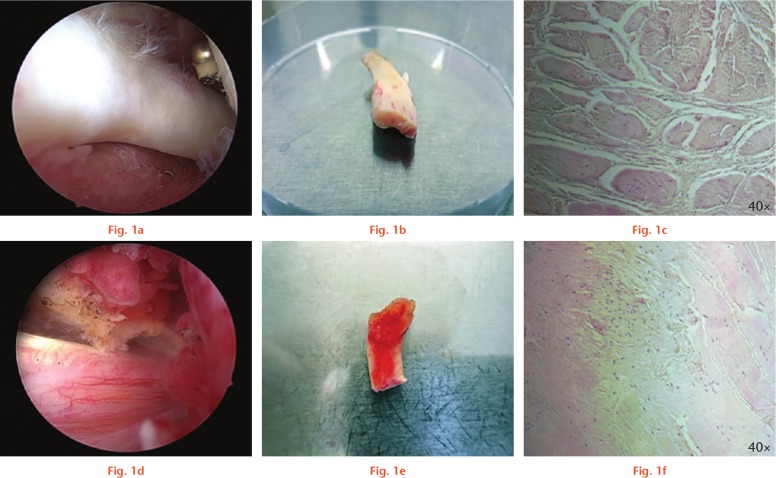
Classification in inflamed and non-inflamed tendons was realized during surgery upon clinical judgement. Tendons with clear macroscopic signs of inflammation were assigned to the ‘tendinitis’ group, whereas native tendons were assigned to the ‘no tendinitis’ group. Classification was performed by two experienced shoulder surgeons (CK, PP). If the two surgeons disagreed, the samples were not included, thus, there were no borderline tendons. Macroscopic and microscopic images were taken from all samples, and representative photographs are shown in Figure 1.
Fig. 1.
Macroscopic and microscopic view of inflamed and non-inflamed tendon samples. Long head of the biceps (LHB) tendon samples with (d, e, f) and without (a, b, c) tendinitis are shown. a) and d) Intraoperative arthroscopic view. b) and e) Tendon samples before processing in the laboratory in a 10 cm petri dish. c) and f) Microscopic appearance with haematoxylin and eosin (H&E) stain. Images are taken at 40× as indicated.
Tissue harvest
After informed consent, LHB tendon samples were obtained from patients undergoing open or arthroscopic surgery for RC repair, LHB tenodesis, or shoulder arthroplasty. The study was approved by the local ethics committee (approval number 82/08).
Biceps tendon samples after tenotomy were gathered from 22 shoulders in total (mean donor age 59.6 years (44 to 80), 13 male patients, nine female patients) of whom 11 showed clear macroscopic signs of tendinitis. The donors’ demographics and reason for surgery are shown in Table I. Any insertion sites (bony/capsular) were removed and tendons were rinsed with saline. For each outcome measure, cells and samples from three representative donors with tendinitis and three donors without tendinitis were used. For each test, three replicates for each donor and test group were used.
Table I.
Donors’ demographics and reason for surgery
| Feature | Tendinitis (n = 11) | No tendinitis (n = 11) |
|---|---|---|
| Sex, male:female, n | 9:2 | 4:7 |
| Mean age, yrs (range) | 66 (60 to 82) | 57 (46 to 67) |
| Type of surgery, n | ||
| Arthroplasty | 2 | 2 |
| Rotator cuff repair | 7 | 6 |
| Isolated biceps surgery | 2 | 3 |
Isolation of primary cells
The tendons were minced into small pieces and incubated with collagenase (0.175 U/ml; Sigma–Aldrich, Munich, Germany) in Dulbecco's Modified Eagle Medium (DMEM)/Ham’s F12 (1:1; Life Technologies GmbH, Thermo Fisher Scientific, Waltham, Massachusetts) for two hours at 37°C.12 After the digested tendon material was washed and the centrifugation was completed, the released cells could be collected and plated in 75 cm² tissue culture flasks (Greiner Bio-One, Frickenhausen, Germany) at a density of 3×10⁶ cells per flask. The cells were cultured in DMEM/Ham’s F12 (1:1), supplemented with 10% foetal calf serum (FCS) (Biochrom AG, Berlin, Germany), and 1% penicillin/streptomycin, in an incubator in a humidified atmosphere (37°C, 5% CO₂) with a change of medium every two to three days. As soon as cultured cells reached 90% confluence, they were harvested for RNA isolation or seeded in well plates. The cells were passaged twice and second-passage cells were used for each donor and for each outcome measure.
Cell proliferation assay
Cell proliferation was assessed as described previously by measuring adenosine 5’ triphosphate (ATP), using the sensitive luminometric CellTiter-Glo Luminescent Cell Viability Assay (Promega GmbH, Mannheim, Germany), according to the manufacturer’s instructions.13 A total of three inflamed and three non-inflamed LHB samples were included.
RNA isolation, RT-PCR, and qRT-PCR
Total RNA was harvested from cells at passage two after reaching confluence in the culture flasks. Samples were isolated and real-time polymerase chain reaction (RT-PCR), as well as quantitative real-time polymerase chain reaction (qRT-PCR), were performed as described previously.13,14 Elongation factor 1α (EF1A) served as the housekeeping gene; target-specific sense and antisense primers used for RT-PCR analysis are listed in Table II, which also provides a summary of the primer details. Sequence-specific primers for qRT-PCR were obtained from biomers.net GmbH (Ulm, Germany) or Qiagen GmbH (Hilden, Germany) and are listed in Table III, which also shows the primer-specific details. Dissociation curve analysis for qRT-PCR results was carried out to verify the absence of primer dimers and/or non-specific PCR products. The expression of the genes of interest was normalized against the ribosomal protein S27a (RPS27A) housekeeping gene using the delta-delta Ct method.15
Table II.
Real-time-polymerase chain reaction (RT-PCR) primer sequences and polymerase chain reaction (PCR) conditions
| Gene | Oligonucleotide primer sequence | Cycles, n | Annealing temperature, °C |
|---|---|---|---|
| Tenogenic marker gene | |||
| SCX | S: 5′—CCTGAACATCTGGGAAATTTAATTTTAC—3′ | 36 | 58 |
| A: 5′—CGCCAAGGCACCTCCTT—3′ | |||
| TNMD | S: 5′—CCATGCTGGATGAGAGAGGT—3′ | 35 | 56 |
| A: 5′—CTCGTCCTCCTTGGTAGCAG—3′ | |||
| Osteogenic differentiation | |||
| ALPL | S: 5′—TGGAGCTTCAGAAGCTCAACACCA—3′ | 25 | 51 |
| A: 5′—ATCTCGTTGTCTGAGTACCAGTCC—3′ | |||
| COL1A2 | S: 5′—GGACACAATGGATTGCAAGG—3′ | 30 | 55 |
| A: 5′—TAACCACTGCTCCACTCTGG—3′ | |||
| RUNX2 | S: 5′—CCCCACGACAACCGCACCAT—3′ | 30 | 64 |
| A: 5′—CACTCCGGCCCACAAATC—3′ | |||
| Adipogenic differentiation | |||
| LPL | S: 5′—GAGATTTCTCTGTATGGCACC—3′ | 30 | 51 |
| A: 5′—CTGCAAATGAGACACTTTCTC—3′ | |||
| PPARG2 | S: 5′—GCTGTTATGGGTGAAACTCTG—3′ | 33 | 61 |
| A: 5′—ATAAGGTGGAGATGCAGGCTC—3′ | |||
| Chondrogenic differentiation | |||
| COL2A1 | S: 5′—TGGTGACAAAGGTGAAAAAGG—3′ | 35 | 51 |
| A: 5′—CATCAAATCCTCCAGCCATC—3′ | |||
| ACAN | S: 5′—GCCTTGAGCAGTTCACCTTC—3′ | 30 | 54 |
| A: 5′—CTCTTCTACGGGGACAGCAG—3′ | |||
| COMP | S: 5′—AGGATGGAGACGGACATCAG—3′ | 30 | 53 |
| A: 5′—TCTGCATCAAAGTCGTCCTG—3′ | |||
| Tenogenic differentiation | |||
| COL1A2 | S: 5′—GGACACAATGGATTGCAAGG—3′ | 30 | 55 |
| A: 5′—TAACCACTGCTCCACTCTGG—3′ | |||
| TNMD | S: 5′—CCATGCTGGATGAGAGAGGT—3′ | 30 | 56 |
| A: 5′—CTCGTCCTCCTTGGTAGCAG—3′ | |||
| FOS | S: 5′—CTGGCGTTGTGAAGACCATG—3′ | 30 | 55 |
| A: 5′—CTTCTCCTTCAGCAGGTTGG—3′ | |||
| VIM | S: 5′—TGCCCTTAAAGGAACCAATG—3′ | 38 | 52 |
| A: 5′—CTCAATGTCAAGGGCCATCT—3′ | |||
| SCX | S: 5′—CCTGAACATCTGGGAAATTTAATTTTAC—3′ | 36 | 58 |
| A: 5′—CGCCAAGGCACCTCCTT—3′ | |||
| PTGS2 | S: 5′—GCTGTCCCTTTACTTCATTC—3′ | 35 | 55 |
| A: 5′—TGGCATCTTGTGATAGTGTT—3′ | |||
| Internal control | |||
| EF1A | S: 5′—AGGTGATTATCCTGAACCATCC-—3′ | 24 | 54 |
| A: 5′—AAAGGTGGATAGTCTGAGAAGC—3′ |
SCX, scleraxis; S, sense; A, antisense; TNMD, tenomodulin; ALPL, alkaline phosphatase; COL1A2, collagen type I alpha 2; RUNX2, runt-related transcription factor 2; LPL, lipoprotein lipase; PPARG2, proliferator-activator receptor γ2; COL2A1, collagen type 2α1; ACAN, aggrecan core protein; COMP, cartilage oligomeric matrix protein; FOS, c-FOS activator protein 1; VIM, vimentin; PTGS2, prostaglandin-endoperoxide synthase 2; EF1A, elongation factor 1α
Table III.
Quantitative real-time-polymerase chain reaction (qRT-PCR) primer sequences and polymerase chain reaction (PCR) conditions
| Gene | Primer | Sequence | Length, bp | Annealing temperature, °C | Efficiency |
|---|---|---|---|---|---|
| Tenogenic marker gene | |||||
| SCX | Forward | CCTGAACATCTGGGAAATTTTAC | 111 | 60 | 1.68 |
| SCX | Reverse | CGCCAAGGCACCTCCTT | |||
| Housekeeping gene | |||||
| RPS27A | Forward | TCGTGGTGGTGCTAAGAAAA | 141 | 60 | 2.01 |
| RPS27A | Reverse | TCTCGACGAAGGCGACTAAT |
bp, base pairs; SCX, scleraxis; RPS27A, ribosomal protein S27a
Osteogenesis
The LHB-derived stem cells were seeded at a density of 3000 cells/cm² into six-well polystyrene tissue culture plates (Greiner Bio-One). After reaching confluence, mineralization was induced over a period of 28 days, as described previously.14 Controls were cultured in normal culture medium. Cultures were stained for matrix mineralization using Alizarin Red, as described previously.14 Additionally, markers of osteogenesis were analyzed by RT-PCR, using the protocol described above. Expression of the following human messenger RNAs (mRNAs) was examined: alkaline phosphatase (ALPL), collagen type I alpha 2 (COL1A2), and runt-related transcription factor 2 (RUNX2). Primer sequences are provided in Table II. Expression was normalized to the housekeeping gene EF1A.
Adipogenesis
Cells were grown to confluence in six-well polystyrene tissue culture plates. Afterwards, adipogenesis was induced as described previously.13 Control cultures without adipogenic supplements were also maintained. After four weeks, cultures were examined for evidence of adipogenesis staining with freshly prepared Oil red O solution, as previously reported.16 In addition, RT-PCR was realized, using the protocol described above, to detect expression of the following human genes: lipoprotein lipase (LPL); and peroxisome proliferator-activator receptor gamma 2 (PPARG2). Primer sequences are provided in Table II. Expression was normalized to the housekeeping gene EF1A.
Chondrogenesis
Chondrogenic differentiation was assessed by the pellet culture method as modified recently.17 Pellets were incubated with chondrogenic medium as reported previously.16 Aggregates were collected at three or four weeks for analysis. For detection of matrix proteoglycan, representative sections were stained with 1% (w/v) Alcian Blue (Sigma–Aldrich), as described previously.13 For gene expression analyses, RNA was isolated and markers of chondrogenesis were amplified by RT-PCR, using the protocol described above. Expression of the following human mRNAs was examined: collagen type II alpha 1 (COL2A1); aggrecan core protein (ACAN); and cartilage oligomeric matrix protein (COMP). Primer sequences are provided in Table II. Expression was normalized to the housekeeping gene EF1A.
Tendogenesis
For tenogenic induction, cyclic stretching was performed on cultures with 5×10⁵ cells per well, being seeded on four-well polyurethane plates and cultivated for one week in complete culture medium, supplemented with 10% FCS (Biochrom AG) and 1% penicillin/streptomycin. Cyclic stretching was performed for 30 minutes at 1 Hz and 1% extension by using a bioreactor, as described previously.18 After stretching and cultivating the cells for 15 minutes, four hours, and for 24 hours, cells were harvested and total RNA was isolated as described above. Known mechanoresponsive genes such as Fos proto-oncogene, AP-1 transcription factor subunit (FOS), and prostaglandin-endoperoxide synthase 2 (PTGS2), as well as the tenogenic markers scleraxis (SCX), vimentin (VIM), and tenomodulin (TNMD), were analyzed by RT-PCR.
Statistical analysis
Statistical analysis was performed using IBM SPSS 22.0 (IBM, Armonk, New York) and p-values < 0.05 were considered significant. Determination of the statistical significance between groups was performed using Student’s t-test, or the Mann–Whitney U test as indicated.
Results
There were important differences regarding the sex and age of donors. There were nine male donors (82%) in the tendinitis group and seven female donors (64%) in the no-tendinitis group. The mean donor age in the tendinitis group was 66 years (60 to 82), and 57 years in the no-tendinitis group (46 to 67). The mean cell yield per tendon sample was 6.8×10⁶ (3×10⁶ to 15×10⁶), however there was no statistical difference between the two groups.
Cell morphology
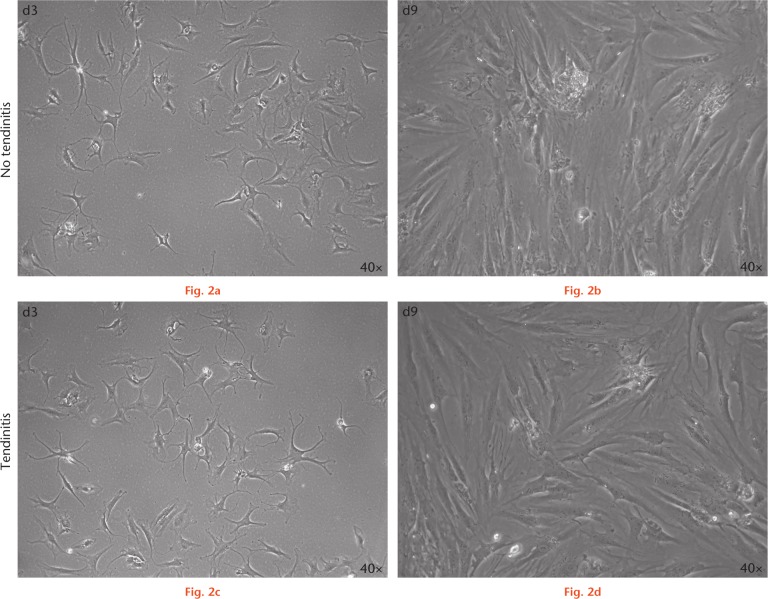
Cells isolated from both inflamed and non-inflamed human LHB had a spindle-shaped, fibroblast-like morphology (Fig. 2) and formed colonies upon adherent culture typical for mesenchymal cell types.19
Fig. 2.
Microscopic appearance of human long head of the biceps (LHB) tendon-derived cells with and without inflammation in culture. Human LHB tendon-derived cell cultures without tendinitis at a) day 3 (d3) and b) day 9 (d9) of culture, and c) and d) with tendinitis at the same timepoints. Images are taken as 40× as indicated.
Cell proliferation
Cells isolated from inflamed LHB samples proliferated well when compared with native non-inflamed LHB tendon-derived cells, and no significant differences were observed between groups (Fig. 3). Nevertheless, the mean proliferation rate was slightly decreased in the tendinitis group. Notably, there was a high interindividual variability without clear-cut differences between groups.
Fig. 3.
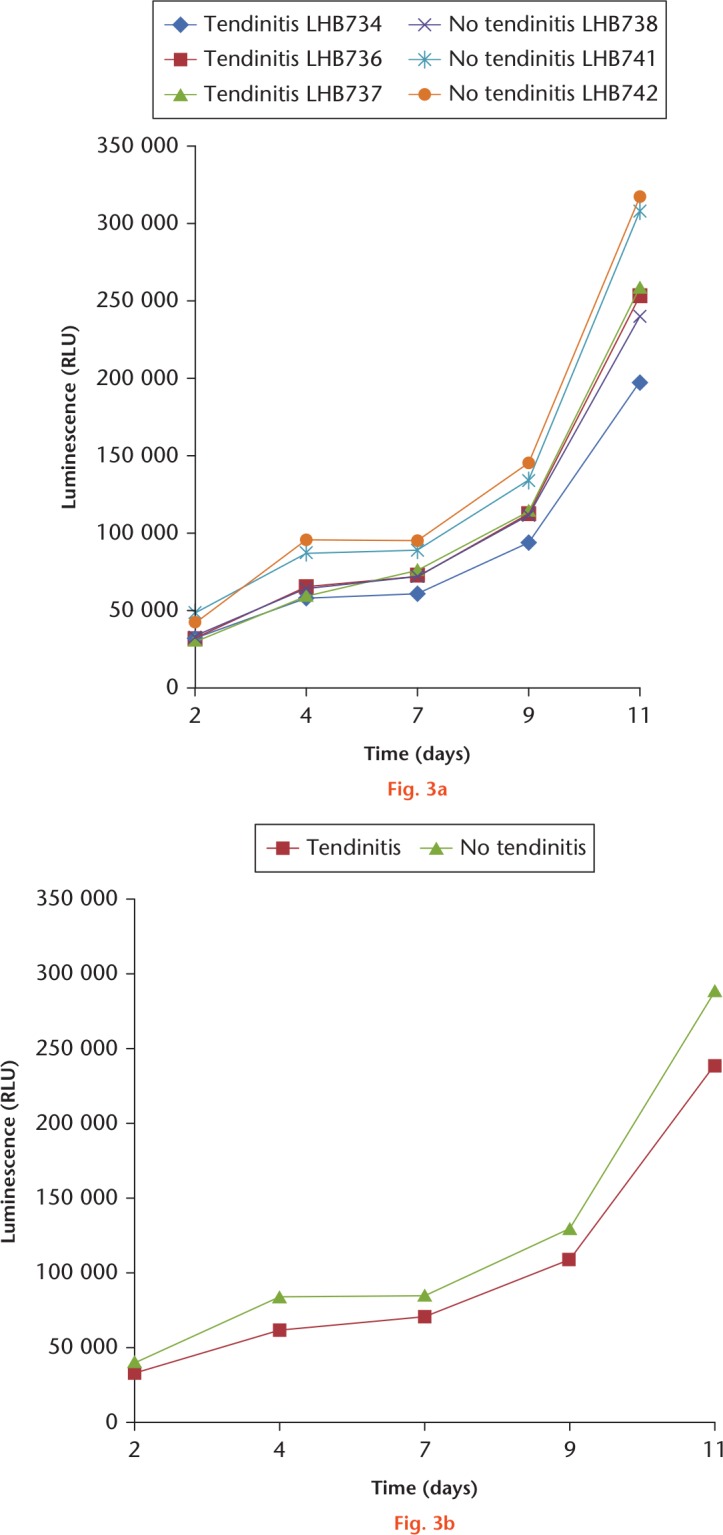
Cell proliferation behaviour of long head of the biceps (LHB) cells isolated from samples with and without tendinitis. a) Illustration of the values and sds of inflamed (LHB734/736/737) and non-inflamed (LHB738/741/742) tissue samples. b) Illustration of the means with sd. Proliferation of both cell types increased over time, but differences were not statistically significant. A total of six donors were included, and measurements were made in triplicates for each donor and timepoint.
Qualitative and qRT-PCR analysis
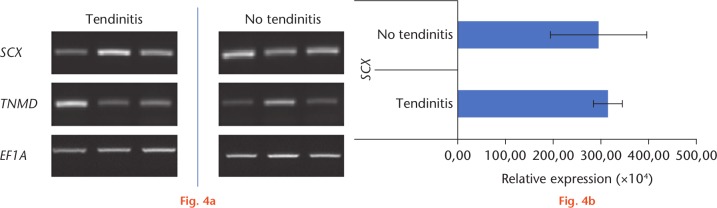
In RT-PCR analysis (Fig. 4a), similar expression of tenogenic marker genes, such as SCX and TNMD could be observed in the two groups. These findings were confirmed by qRT-PCR analysis (Fig. 4b), showing no significant differences regarding SCX expression between the two groups.
Fig. 4.
a) Real-time-polymerase chain reaction (RT-PCR) and b) quantitative real-time-polymerase chain reaction (qRT-PCR) analyses of inflamed and non-inflamed tendon samples. Expression patterns of scleraxis (SCX) and tenomodulin (TNMD) in long head of the biceps (LHB) samples with and without tendinitis. For RT-PCR, elongation factor 1α (EF1A), and for qRT-PCR, ribosomal protein S27a (RPS27A) served as housekeeping genes for normalization of the expression values. A total of six donors were included.
Osteogenic and adipogenic differentiation
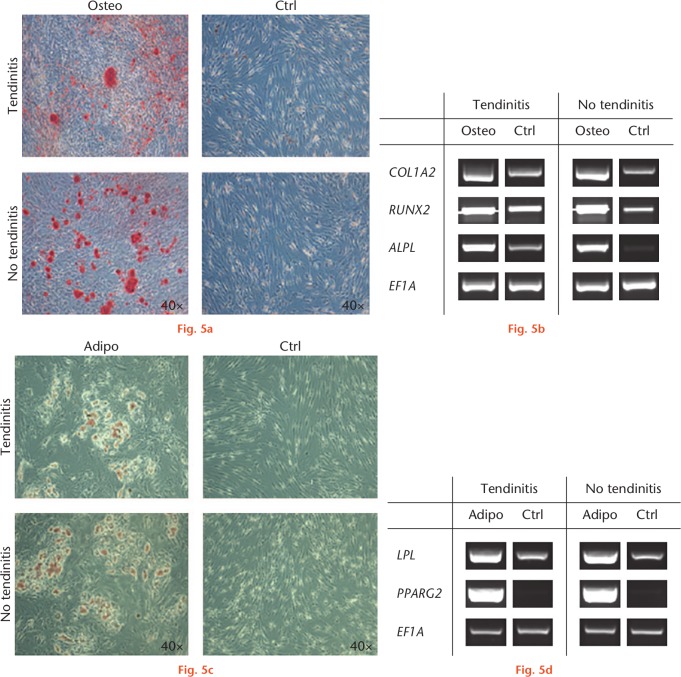
Monolayer cultures of inflamed and non-inflamed LHB tendon cells responded equivalently to osteogenic medium in terms of staining for mineralization with Alizarin Red (Fig. 5a), resulting in increased areas of positive staining (Fig. 5a) in the respective osteogenic cultures of the two cell types compared with controls. This corresponds to the induction of transcripts encoding for ALPL and RUNX2, as well as the increased expression of COL1A2, in all osteogenic cultures compared with controls (Fig. 5b). Monolayer cultures of both groups showed a similar response to adipogenic conditions, as assessed by staining with Oil red O (Fig. 5c). Equivalently, adipogenic culture conditions induced an overexpression of LPL and PPARG2 compared with controls within all groups (Fig. 5d).
Fig. 5.
Osteogenic and adipogenic differentiation of cells isolated from inflamed and non-inflamed long head of the biceps (LHB) samples at four weeks. a) Cultures treated with osteogenic supplements produced a mineralized extracellular matrix as shown by intense staining for Alizarin Red. Control cultures did not produce a mineralized matrix. b) Real-time polymerase chain reaction (RT-PCR) revealed that cultures of both groups expressed the runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALPL), and collagen type I alpha 2 (COL1A2) in response to osteogenic stimuli compared with controls. c) Cells cultivated in adipogenic medium showed formation of lipid droplets in both groups as determined by Oil red O staining. Cultivation in control medium, in contrast, did not result in droplet enrichment. d) Expression of the adipogenic marker genes lipoprotein lipase (LPL) and peroxisome proliferator-activated receptor gamma 2 (PPARG2) was increased in both groups treated with adipogenic medium, but was not detectable in cells cultivated with control medium. The housekeeping gene elongation factor 1α (EF1A) showed equal expression levels in all groups. Six donor samples were tested, and representative images from three different donors are shown. Osteo, osteogenic; Adipo, adipogenic; Ctrl, control.
Chondrogenic differentiation
When placed into pellet culture, both inflamed and non-inflamed LHB-derived stem cells underwent chondrogenic differentiation in the presence, but not absence, of transforming growth factor beta 1 (TGFB1) as judged by histology (Fig. 6a) and by the expression of transcripts encoding COMP, ACAN, and COL2A1 (Fig. 6b). Pellets formed by the two different groups showed no differences in staining for proteoglycans with Alcian Blue. However, chondrogenic pellets from both cell types produced more glycosaminoglycans (GAG) compared with non-chondrogenic controls, as can be seen in Figure 6a. Consistent with this, chondrogenic pellets from both cell types produced significantly more mRNA transcripts encoding COMP and induced an overexpression of ACAN as well as COL2A1 compared with respective non-chondrogenic controls (Fig. 6b).
Fig. 6.
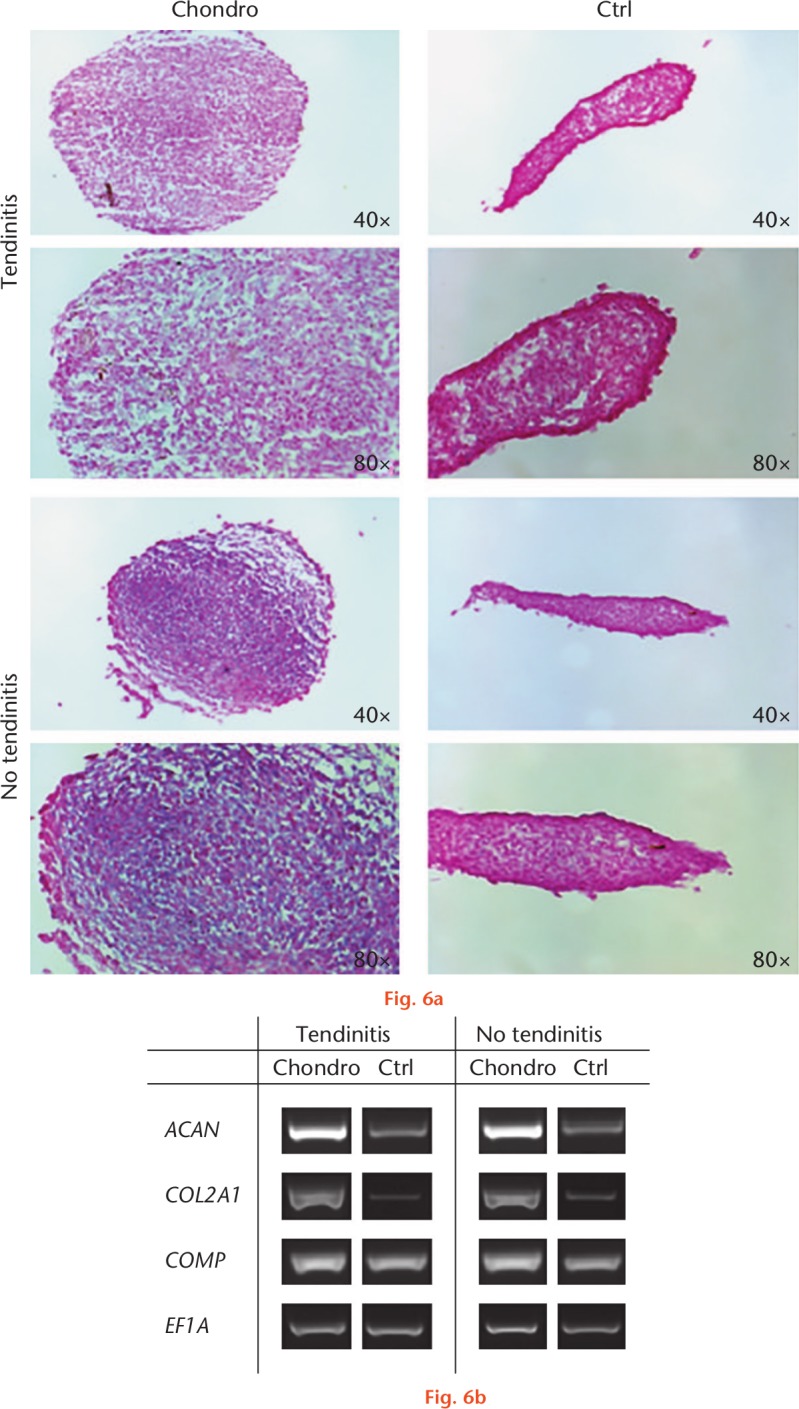
Chondrogenic differentiation by cell cultures isolated from inflamed and non-inflamed tendon samples. After four weeks in chondrogenic medium, aggregates derived from both groups were stained with Alcian Blue. a) Unlike controls, aggregates treated with transforming growth factor beta 1 (TGFB1) populations produced an extracellular matrix rich in sulphate. b) Aggregate cultures of the two cell types collected at three weeks expressed the cartilage-specific genes encoding collagen type 2 (COL2A1) and aggrecan core protein (ACAN) in response to TGFB1 treatment, in contrast with controls lacking TGFB1. Control aggregates expressed cartilage oligomeric matrix protein (COMP) mRNA at low levels, but expression was markedly increased in the presence of TGFB1. The expression of elongation factor 1α (EF1A) was included as an internal control for RNA loading. Results are presented using representative patient populations from at least three independent experiments at four weeks. Six donor samples were tested, and representative images from three different donors are shown. Chondro, chondrogenic; Ctrl, control.
Tenogenic differentiation
Undergoing cyclic stretching for 15 minutes, four hours, and for 24 hours, cells from the two different groups showed a time-dependent overexpression of tendogenesis-related genes such as SCX, VIM, and TNMD, as well as mechanoresponsive mRNA transcripts such as PTGS2 and FOS (Fig. 7).20,21 Both the tendinitis and no tendinitis groups showed an increasing expression of the examined genes at the three different timepoints after cyclic loading.
Fig. 7.
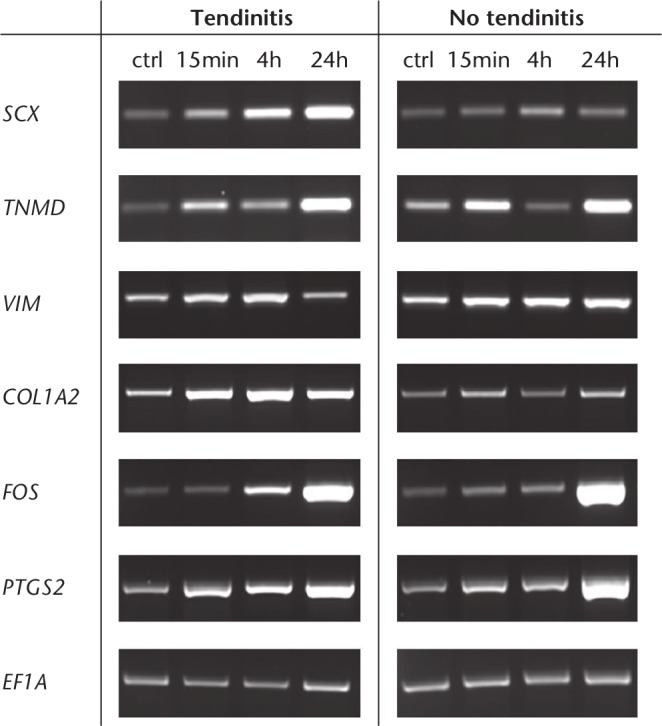
Tenogenic differentiation of cells isolated from inflamed and non-inflamed tendon samples. Real-time polymerase chain reaction (RT-PCR) analysis after cyclic stretching of cells isolated from inflamed and non-inflamed long head of the biceps (LHB) samples for 15 minutes, four hours, and 24 hours. After cyclic stretching, cells of the two groups showed a time-dependent increase in the expression of both tenogenic and mechanoresponsive genes. Tendogenesis-related genes including scleraxis (SCX), tenomodulin (TNMD), vimentin (VIM), and collagen type 1 alpha 2 (COL1A2) were examined, while prostaglandin-endoperoxide synthase 2 (PTGS2) and Fos proto-oncogene, AP-1 transcription factor subunit (FOS) served for the analysis of mechanosensitivity. The expression of elongation factor 1α (EF1A) was included as an internal control for RNA loading. A total of six donors were included.
Discussion
This study confirms that human LHB samples have multilineage potential in the inflamed, as well as non-inflamed state, and share several characteristics with mesenchymal stem cells (MSCs) as previously isolated from tendon, muscle, ligament, or bursa tissue.13,16,22-26 Up-to-date clinical outcomes after massive RC tears are still unsatisfying and the development of stem cell-based therapies could be an option in the future.27 In general, LHB- derived stem cells (LHBSCs) may be a new, endogenous, patient-specific tendon-derived stem cell source for tissue regeneration. With the present investigation, we report that cells from the LHB can be easily isolated, cultured, expanded, and differentiated in chondrocytes, adipocytes, and osteocytes in vitro. Interestingly, we showed that cells isolated from inflamed and non-inflamed LHBs significantly overexpressed both tenogenic and mechanoresponsive marker genes after cyclic loading for 24 hours. This endorses the in vivo results by Zumstein et al28 that tendon regeneration is facilitated by mechanical stimulation. Randelli et al5 showed the multipotency of the LHBSCs by inducing differentiation of these cells towards osteoblasts, adipocytes, and skeletal muscle, as well as MSC-specific surface antigen expression, thus defining them as a new MSC source. However, it is not clear whether inflamed samples were used in this study. We showed that inflamed samples do not present a significant difference in cell yield, differentiation potential, or proliferation rate compared with LHBSCs harvested from native LHB tendons, which means that both groups are a suitable stem cell source. In the future, tendon stem cells could be collected directly in situ at the same time as shoulder surgery, without requiring additional invasive procedures for cell harvesting. Recent human in vivo studies that applied MSCs harvested from the iliac crest showed promising results. Hernigou et al29 performed augmentation by injecting a mean 51 000 MSCs (sd 25 000) into the tendon-to-bone junction and in the bone at the site of the footprint after arthroscopic RC repair. At ten-year MRI follow-up, intact RCs were found in 39 of the 45 patients (87%) in the MSC treatment group, but in just 20 of the 45 patients (44%) in the control group.29 Ellera Gomes et al30 injected a mean of 5×10⁶ MSCs per tendon sample into the tendon-to-bone junction and the footprint of 14 patients undergoing open RC repair. The two-year MRI follow-up revealed intact RC muscles in 13 of the 14 patients (93%), however, the study did not include a control group.30 Taking into account the mean cell yield per tendon sample in our study (6.8×10⁶ cells per sample), the LHB seems to be a viable cell source for regenerative approaches. Nevertheless, randomized controlled trials and animal studies with histological analysis of the tendon-to-bone junction are still necessary to prove positive effects on RC healing of this procedure, and further studies are required to determine if cell-based strategies are sufficient. Another future study could be the use of matrix- or scaffold-based delivery of multipotent stem cells such as the LHBSCs for RC augmentation or even for irreparable RC tears. Chen et al31 and Funakoshi et al32 showed the effectiveness of tenocyte-seeded scaffolds regarding histological healing potential and biomechanical strength in rabbit RC models. To further increase regenerative potential, combination with growth factors could be an option. However, the transfer from bench to bedside is challenging and these promising findings should be implemented in clinical trials in the future.
At present, only a few genes have been reported as general tendon markers. Recently, SCX has been reported as an important marker of tendon progenitor populations and tenocytes, promoting tendon development and differentiation.33 Shukunami et al34 were able to show that SCX positively regulates the expression of TNMD, a differentiation marker of tenocytes. Mice lacking TNMD display a severe decrease in tenocyte proliferation in newborn tendons and a disrupted adult collagen fibril structure.22 In our study, we found no significant difference in the expression pattern of TNMD and SCX in the tendinitis and no tendinitis groups, therefore we conclude that the ongoing inflammatory processes do not negatively affect the tenogenic regeneration potential of cells isolated from inflamed LHBs. However, more samples are required in order to determine any subtle differences in expression between the test conditions.
Today, very little is known about the regenerative capacity of inflamed tissues in musculoskeletal pathologies. Therefore, in previous studies, we analyzed the regenerative capacity of cells derived from inflamed subacromial bursa tissue of the shoulder relative to bone marrow stromal cells, and found that both cell types revealed the main characteristics of MSCs, including their capacity to proliferate and differentiate into cartilage, fat, bone, and tendon under appropriate in vitro culture conditions.13 Notably, the tissue sources were different in this study, and it is currently unknown whether stem cells isolated from the LHB tendon can be used effectively to augment tissue repair in vivo; cells might behave differently under defined in vitro conditions as when they are incorporated in inflamed tissue i.e. in a potential catabolic environment. Similar results were found when investigating the MSC characteristics from anterior cruciate ligament (ACL) cells, which were either isolated from the traumatically ruptured ACLs of young donors (18 to 35 years) or from degeneratively altered ACLs from older patients (45 to 67 years) with knee osteoarthritis, with almost equipotent MSC capacities between both cell types.16 Although the tissue source was similar in this work (ACL), the influence of inflammation on the MSCs and presumably on their regenerative capacity, remains uncertain. As an inflammatory component is regularly present, both after trauma and in a degenerative setting as part of the respective disease processes, phenomena frequently overlap.35 Interestingly, an inflammatory phase is also seen during regeneration and wound healing in almost every tissue of the body. Therefore, the role of the inflammatory response in the repair processes of mesenchymal tissue is of great interest. However, it is not yet well characterized so the question of whether tissue inflammation during the regenerative process constitutes friend or foe is still a matter of controversy and of great debate among clinicians and basic scientists.36
A limitation of this study is the fact that the patient population was not matched regarding sex, whereas it has been demonstrated that the sex, as well as the age,37 of the donors38 can affect stem cell proliferation. Since only small central tissue pieces from each sample were used for histological confirmation of the treatment groups, there was not enough material to perform all of the tests on all samples, which is another limitation of this study.
In conclusion, being a source of anterior shoulder pain, the LHB tendon is often resected during shoulder surgery and could therefore be used as a possible cell source for tissue-engineering approaches. In this study, the LHB with and without tendinitis has been characterized as a suitable, novel source of multipotent tendon-derived cells. However, it remains unclear whether such cell types might be suitable in the treatment of degeneration and induction of regeneration in shoulder surgery.
Acknowledgments
The authors wish to thank Beate Geyer and Melanie Krug for their excellent technical assistance.
Footnotes
Author contributions: J. Schmalzl: Wrote the manuscript.
P. Plumhoff: Collected the specimens.
F. Gilbert: Helped analyze the data.
F. Gohlke: Supervised the writing of the manuscript.
C. Konrads: Collected the specimens.
U. Brunner: Supervised the writing of the manuscript.
F. Jakob: Supervised the writing of the manuscript.
R. Ebert: Helped to collect and analyze the data.
A. F. Steinert: Initiated and supervised the study
Conflicts of interest statement: All of the authors report an institutional grant (paid to Julius-Maximilians-University of Wuerzburg) from Novartis Pharma GmbH.
Ethical review statement: All procedures performed participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Follow us @BoneJointRes
Funding statement
All of the authors report an institutional grant (paid to Julius-Maximilians-University of Wuerzburg) from the Federal Ministry of Education and Research (FKZ 13N13454, “AutoLoop”).This publication was funded by the German Research Foundation (DFG) and the University of Wuerzburg in the funding programme Open Access Publishing.
Although none of the authors has received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article, benefits have been or will be received but will be directed solely to a research fund, foundation, educational institution, or other non- profit organization with which one or more of the authors are associated.
References
- 1. Werner A, Mueller T, Boehm D, Gohlke F. The stabilizing sling for the long head of the biceps tendon in the rotator cuff interval. A histoanatomic study. Am J Sports Med 2000;28:28-31. [DOI] [PubMed] [Google Scholar]
- 2. Habermeyer P, Magosch P, Pritsch M, Scheibel MT, Lichtenberg S. Anterosuperior impingement of the shoulder as a result of pulley lesions: a prospective arthroscopic study. J Shoulder Elbow Surg 2004;13:5-12. [DOI] [PubMed] [Google Scholar]
- 3. Bechtol CO. Biomechanics of the shoulder. Clin Orthop Relat Res 1980;146:37-41. [PubMed] [Google Scholar]
- 4. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, O’Brien SJ. Role of the long head of the biceps brachii in glenohumeral stability: a biomechanical study in cadavera. J Shoulder Elbow Surg 1996;5:255-262. [DOI] [PubMed] [Google Scholar]
- 5. Randelli P, Conforti E, Piccoli M, et al. Isolation and characterization of 2 new human rotator cuff and long head of biceps tendon cells possessing stem cell-like self-renewal and multipotential differentiation capacity. Am J Sports Med 2013;41:1653-1664. [DOI] [PubMed] [Google Scholar]
- 6. Gilbert F, Böhm D, Eden L, et al. Comparing the MRI-based Goutallier Classification to an experimental quantitative MR spectroscopic fat measurement of the supraspinatus muscle. BMC Musculoskelet Disord 2016;17:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gobezie R, Bruno T, Roman B, Lafosse L. The outcome and structural integrity of arthroscopic rotator cuff repair using the double-row suture anchor technique (SS-39). Arthrosc J Arthrosc Relat Surg 2007;23(6 Suppl):e20. [Google Scholar]
- 8. Takahashi N, Sugaya H, Matsuki K, et al. Hypertrophy of the extra-articular tendon of the long head of biceps correlates with the location and size of a rotator cuff tear. Bone Joint J 2017;99-B:806-811. [DOI] [PubMed] [Google Scholar]
- 9. Galatz LM, Sandell LJ, Rothermich SY, et al. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res 2006;24:541-550. [DOI] [PubMed] [Google Scholar]
- 10. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg [Am] 1993;75-A:1795-1803. [DOI] [PubMed] [Google Scholar]
- 11. Rodeo SA. Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg 2007;16(5 Suppl):S191-S197. [DOI] [PubMed] [Google Scholar]
- 12. Schulze-Tanzil G, Mobasheri A, Clegg PD, et al. Cultivation of human tenocytes in high-density culture. Histochem Cell Biol 2004;122:219-228. [DOI] [PubMed] [Google Scholar]
- 13. Steinert AF, Kunz M, Prager P, et al. Characterization of bursa subacromialis-derived mesenchymal stem cells. Stem Cell Res Ther 2015;6:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reichert JC, Schmalzl J, Prager P, et al. Synergistic effect of Indian hedgehog and bone morphogenetic protein-2 gene transfer to increase the osteogenic potential of human mesenchymal stem cells. Stem Cell Res Ther 2013;4:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steinert AF, Kunz M, Prager P, et al. Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells. Tissue Eng Part A 2011;17:1375-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Penick KJ, Solchaga LA, Welter JF. High-throughput aggregate culture system to assess the chondrogenic potential of mesenchymal stem cells. Biotechniques 2005;39:687-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seefried L, Mueller-Deubert S, Schwarz T, et al. A small scale cell culture system to analyze mechanobiology using reporter gene constructs and polyurethane dishes. Eur Cell Mater 2010;20:344-355. [DOI] [PubMed] [Google Scholar]
- 19. Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med 2004;8:301-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liedert A, Wagner L, Seefried L, et al. Estrogen receptor and Wnt signaling interact to regulate early gene expression in response to mechanical strain in osteoblastic cells. Biochem Biophys Res Commun 2010;394:755-759. [DOI] [PubMed] [Google Scholar]
- 21. Peake MA, El Haj AJ. Preliminary characterisation of mechanoresponsive regions of the c-fos promoter in bone cells. FEBS Lett 2003;537:117-120. [DOI] [PubMed] [Google Scholar]
- 22. Docheva D, Hunziker EB, Fässler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol 2005;25:699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Docheva D, Müller SA, Majewski M, Evans CH. Biologics for tendon repair. Adv Drug Deliv Rev 2015;84:222-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferreira E, Porter RM. Harnessing extracellular vesicles to direct endochondral repair of large bone defects. Bone Joint Res 2018;7:263-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kohler J, Popov C, Klotz B, et al. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell 2013;12:988-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoshikawa M, Nakasa T, Ishikawa M, Adachi N, Ochi M. Evaluation of autologous skeletal muscle-derived factors for regenerative medicine applications. Bone Joint Res 2017;6:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg 2015;24:1493-1505. [DOI] [PubMed] [Google Scholar]
- 28. Zumstein MA, Frey E, von Rechenberg B, et al. Device for lengthening of a musculotendinous unit by direct continuous traction in the sheep. BMC Vet Res 2012;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop 2014;38:1811-1818. [DOI] [PubMed] [Google Scholar]
- 30. Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc 2012;20:373-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen JM, Willers C, Xu J, Wang A, Zheng MH. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng 2007;13:1479-1491. [DOI] [PubMed] [Google Scholar]
- 32. Funakoshi T, Majima T, Iwasaki N, et al. Application of tissue engineering techniques for rotator cuff regeneration using a chitosan-based hyaluronan hybrid fiber scaffold. Am J Sports Med 2005;33:1193-1201. [DOI] [PubMed] [Google Scholar]
- 33. Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell 2003;113:235-248. [DOI] [PubMed] [Google Scholar]
- 34. Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol 2006;298:234-247. [DOI] [PubMed] [Google Scholar]
- 35. Thankam FG, Dilisio MF, Dietz NE, Agrawal DK. TREM-1, HMGB1 and RAGE in the shoulder tendon: dual mechanisms for inflammation based on the coincidence of glenohumeral arthritis. PLoS One 2016;11:e0165492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walden G, Liao X, Donell S, et al. A clinical, biological, and biomaterials perspective into tendon injuries and regeneration. Tissue Eng Part B Rev 2017;23:44-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prager P, Kunz M, Ebert R, et al. Mesenchymal stem cells isolated from the anterior cruciate ligament: characterization and comparison of cells from young and old donors. Knee Surg Relat Res 2018;30:193-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCann RM, Marsh DR, Horner A, Clarke SA. Body mass index is more predictive of progenitor number in bone marrow stromal cell population than age in men: expanding the predictors of the progenitor compartment. Tissue Eng Part A 2010;16:889-896. [DOI] [PubMed] [Google Scholar]