Short abstract
In the healthy human brain, the protein tau serves the essential function of stabilizing microtubules. However, in a diseased state, tau becomes destabilized and aggregates into a pathogenic form that ultimately creates one of the two major hallmarks of Alzheimer’s disease (AD), tau tangles. Multiple neurodegenerative diseases, termed tauopathies, such as Pick’s disease, and progressive supranuclear palsy, are also linked to mutations in tau. While AD does include a second hallmark in the form of amyloid beta (Aβ) plaques, to date all therapeutics aimed at these hallmark features have failed. The nonsteroidal anti-inflammatory drug tolfenamic acid (TA) has been shown to reduce the levels of multiple neurodegenerative endpoints viz amyloid precursor protein (APP), Aβ, tau, phosphorylated tau (p-tau) and improve cognitive function, in various murine models, via a new mechanism that targets specificity protein 1 (SP1). Sp1 is a zinc-finger transcription factor essential for the regulation of tau and CDK5 genes (among others). The impact of TA on these neurodegenerative endpoints occurred in animal models and systems in which both the tau and the APP genes were present. The experimental model utilized in this paper tested whether the same beneficial outcomes of TA can take place after the removal of endogenous murine tau. We found that the impact of TA, both molecular and behavioral, was no longer significant in the absence of the tau gene. This ability of TA occurred independently of its action on anti-inflammatory targets. Therefore, these findings suggest the essentiality of tau for the novel mechanism of action of TA.
Impact statement
The number of people suffering from Alzheimer’s disease (AD) is expected to increase exponentially in the coming decades. It is estimated to cost the economy about $200 billion annually. With the failure of standard therapeutic approaches, there is a need to develop new drugs in order to avoid an “epidemic crisis” in the future. We have discovered that tolfenamic acid (TA) lowers the levels of proteins associated with AD, by targeting common transcriptional mechanisms that regulate genes involved in common pathogenic pathways. Here, we investigated whether TA had effects on both the amyloid and tau pathways, or whether it selectively targets one of these pathways which impacted the other. Behavioral and molecular studies revealed that TA loses its AD therapeutic potential when tau gene is removed. This ability of TA occurred independently of its action on anti-inflammatory targets. These findings suggest that tau is essential for the new action of TA.
Keywords: Alzheimer’s disease, tau, tolfenamic acid, specificity protein 1, neurodegeneration
Introduction
Neurodegenerative diseases as a whole are defined as disorders with a selective loss of neurons and distinct involvement of functional systems defining a clinical presentation.1 Research has demonstrated that proteins with altered physicochemical properties may tend to accumulate in definitive regions of the brain, such as the frontal cortex. Among various neurodegenerative disorders, Alzheimer’s disease (AD) is perhaps the most widely studied. The deposition of proteins such as intracellular hyperphosphorylated tau and extracellular plaques of amyloid are the two major biological hallmarks of AD and the accepted markers of postmortem diagnosis of AD.2 Tau belongs to a family of microtubule-associated proteins that undergoes hyperphosphorylation in the AD state, and ultimately forms neurofibrillary tangles (NFTs). This improper phosphorylation of tau alters its conformation, allowing it to detach from microtubules, abnormally aggregate, and form the diagnostic insoluble NFTs.3–5
By the year 2050, the prevalence of AD is expected to quadruple, leaving approximately 1 in 85 people afflicted with the disease.6 Considering that the nature of the disease carries with it extreme phenotypic changes in the form of varying and progressive cognitive deficits, these numbers are particularly alarming. Trials of approved prescriptions for AD such as donepezil (Aricept) showed a positive impact on cognitive functions after usage of these drugs and others in their class.7 These drugs, among others, work to alleviate the symptomatic aspects of AD, rather than obstruct the progressive cellular death that underlies the phenotype. Consequently, there is no disease-modifying drug available for AD.
In spite of years of research on a wide spectrum of neurodegenerative disorders, the primary and decisive pathway under dysregulation in AD is unresolved. Previously established research has found that in AD, both amyloid and tau become insoluble and form plaques and NFTs, respectively.8 Though normal phosphorylation occurs through kinase and phosphatase mechanisms at a large variety of sites, NFT’s hyperphosphorylation is site-specific.9 These specific sites have been identified as serine or threonine residues, acting as substrates for kinase activity.10 The most notable family of kinases capable of tau phosphorylation are those of the mitogen-activated protein kinase family, specifically cyclin-dependent kinase-5 (CDK5).11 CDK5’s activation is supported largely by p35/25, a complex of regulatory subunits abundantly found in postmitotic neurons.12,13 Hyperphosphorylation of tau has shown to be responsible for the neurofibrillary lesions and thus any interference in this pathway would be expected to provide therapeutic benefits.14
Specificity protein 1 (Sp1) is a zinc-finger transcription factor essential for the regulation of tau and CDK5 genes, among others.15 CDK5 is responsible for the phosphorylation of tau on sites that are unusually hyperphosphorylated in tauopathies.16 Sp1 also regulates the expression of tau, thus, mutations on the Sp1 binding regions on the tau promoter may affect tau expression.17 Previous reports from our laboratory have provided convincing evidence that either silencing of the SP1 gene using small interfering RNA18 or treatment of animals with tolfenamic acid (TA) lowers the expression of Sp1 target genes.19 Sp1 binding motifs were also found on the promoter regions of p35 and p39.15,20–22 Therefore, targeting Sp1 is an ideal approach to reduce tau levels, and such reduction is likely to impact any post-translational modifications of tau, thereby providing a mechanistic approach to reduce the pathological features of neurodegenerative diseases while providing cognitive improvement.
The drug, TA also known as (Clotam® Rapid), possesses the ability to reduce Sp1, and has been used in Europe as a migraine medication for decades due to its anti-inflammatory properties associated with cyclo-oxygenase (COX) inhibition.23 However, TA is not approved for any human indication in the United States. The European Medicine Agency and the US Food and Drug Administration have designated TA as a potential treatment for frontotemporal dementia and progressive supranuclear palsy in response to our application.
Going forward, an ideal drug candidate for AD should possess the ability to attenuate widespread cell death, as well as the ability to halt or restore cognitive decline. As a potential drug candidate, TA shows the promise of arresting neuropathology in addition to improving cognitive functions in murine models in which it is applied. In addition, TA, unlike other failed therapeutics, possesses the ability to target an upstream transcription factor, Sp1, which controls APP, CDK5, and p25 gene expression, all of which are found upregulated in AD patients. TA has demonstrated its capability in attenuating the cognitive deficits in a transgenic mouse model of AD, R1.40, through a study published by Subaiea et al.24 Mnemonic improvements in cognitive tasks occurred in parallel to reductions in APP expression, and soluble and insoluble Aβ40–42 levels, which correlated with reductions in Sp1 protein expression. Seminal work by Adwan et al.25 used the identical R1.40 model, and after treatment with TA, the model showed a reduction in total murine tau protein levels by 46%, concordant with a 50% reduction in the levels of CDK5. Together, these results suggest that TA is capable of concurrent action on both the amyloid and tau pathways when both were present in these experimental models.
The present study was conducted to assess two specific aims which would aid in determining the ability of TA to modulate the overexpression of tau-related biomarkers and the severity of cognitive deficits and tauopathy in animal models. These two aims are to determine: (1) the relevance of the tau pathway to the efficacy of TA and (2) the ability of TA to downregulate tau-related biomarkers in the absence of the tau gene. These aims were evaluated through the usage of two mouse models: (1) mice that are non-carriers for tau (NC) and (2) mice that are carriers for human tau and knockout for murine tau (h-Tau+/−) gene (C). Furthermore, a cellular model of SH-SY5Y human neuroblastoma cells exposed to a series of concentrations of TA was used for further confirmation of treatment efficacy.
Material and methods
Animal exposure
Transgenic (Tg) mice hemizygous for h-Tau, strain B6.Cg-Mapt™1(EGFP)Klt Tg(MAPT)8cPdav/J, were obtained from Jackson Laboratory, Stock No. 005491 (Bar Harbor, ME). These Tg mice are knock-in for h-Tau, and express all six isoforms of the human tau (3R and 4R), and are homozygous knockouts for murine tau. As these mice are carriers of the h-Tau gene, they will be known as carriers (C, +/−). Included in the transgene are the coding sequences, intronic regions, and regulatory elements of the endogenous human promoter region. Mice that are homozygous knockouts for murine tau will be referred to as non-carriers (NC, −/−). The control group (N = 7) received only vehicle (corn oil), while treatment group (N = 7) received TA (dissolved in corn oil) daily for 34 days at a dose of 5 mg/kg via oral gavage. All mice were bred and genotyped in house, on a 12:12 light–dark cycle at the University of Rhode Island (URI). Food and water were made available for all mice ad libitum, and room temperature was maintained at 22 ± 2°C. Male NC, −/− and C+/− mice aged approximately 17–18 months were selected for the study. All animal procedures and protocols were approved by the URI, Institutional Animal Care and Use Committee, and all animals were under the constant supervision of a URI veterinarian for the duration of the study.
Behavioral studies
Mice were tested for learning and memory using the Morris Water Maze task, following the method as described earlier.26 The maze apparatus consisted of a white pool (48″ diameter, 30″ height) filled with water to a depth of 14″. In order to make the water opaque, white, non-toxic paint (Crayola, New York City, NY) was used. The pool was virtually divided into four quadrants (NW, NE, SW, and SE), and distinct visual cues were placed along the sides of the pool. A clear Plexiglas escape platform (10 cm2) was submerged in one of the quadrants, 0.5 cm below the surface of the water. This quadrant was then known as the platform zone (PFZ). Water temperature was maintained at 25 ± 2°C during all procedures.
Mice were allowed to acclimate to the experiment on day 0, in what is known as a habituation trial, by swimming freely for 60 s. Over the following seven days, mice received three daily training sessions with a 20 min inter-trial interval. The starting position was randomly assigned between four possible positions while the platform, and associated PFZ, remained fixed for all trials. Mice were given a maximum of 60 s of swimming to find the platform. For the first three days of the trial, any mouse that failed to locate the platform in time was gently guided toward the platform by the experimenter and allowed to remain on the platform for 30 s. For the remaining four days of the trial, mice were removed from the maze if they failed to locate the platform within 60 s. Following the seventh day of training, probe trials were performed on days 8 and 18 in order to assess memory retention. In probe trials, the platform was removed and mice were allowed to swim for 60 s. A predilection for the platform location would be an indication that the mice had developed memory of the correct quadrant (the quadrant that contained the hidden platform during the previous training sessions). The swim distance and latencies were videotaped and analyzed with a computerized video-tracking system (ObjectScan, Clever Sys. Inc., Reston, VA, USA).
Cell culture
Human neuroblastoma (SH-SY5Y) cells were procured from American Type Culture Collection (Manassas, VA, USA). The cells were grown in Hams F12 media supplemented with heat inactivated fetal bovine serum (Sigma-Aldrich, MO), penicillin (10 IU/ml), streptomycin (10 mg/ml) and 2 mmol/L l‐glutamine. SH5Y cells at a density of 3.0 × 1067 cells/well were seeded in six well plates (CellTreat, Pepperell, MA, USA) and kept in a humidified tissue culture incubator maintained at 5% CO2 and 37°C. After 24 h they were maintained on a six-day differentiation protocol comprising of 10 µM all-trans retinoic acid (Sigma-Aldrich, MO, USA), as described previously.27 The cells were examined for neurite growth periodically with media change after every 48 h. On the seventh day, differentiated neuroblastoma cells were treated with TA according to conditions previously established by our lab.28 Cells were incubated with 5 or 25 µM of TA for 72 h at 37°C.
Protein extraction and Western blotting
Euthanization of mice was performed by CO2 inhalation 24 h after the final dose of TA was administered. Brains were dissected and stored at −80°C, awaiting analysis. Brain cortices and cells collected following exposure were homogenized or lysed in radio-immunoprecipitation assay lysis buffer (Sigma Aldrich, MO, USA) supplemented with 0.1% protease inhibitor cocktail and 5 µL phosphatase inhibitor per 1 mL lysis buffer (Sigma-Aldrich, MO, USA). The samples were incubated on ice for 30 min in order to allow efficient lysis of the samples. The samples were sonicated and vortexed for 5 min before centrifugation at 10,000 × g for 20 min at 4°C and supernatants were collected and stored at −80°C until further use. Protein concentration was determined by Pierce bicinchoninic assay kit (Thermo Scientific, Waltham, MA, USA).
For the determination of protein expression of various biomarkers, 40 µg of the total protein samples was loaded onto an SDS-Page gel and run for 2 h at 100 mV, and then transferred to polyvinylidene fluoride (PVDF) membranes (GE, Piscataway, NJ, USA). Non-specific binding was blocked by incubation with 5% BSA in Tris buffer saline + 0.1% Tween 20 (TBST) at room temperature for 1 h. Membranes were incubated overnight at 4°C with the following primary antibodies at a dilution of 1:1000 (Mouse Anti-APP, Rabbit Anti-CDK5, Rabbit Anti-COX2, Rabbit Anti-GAPDH, Rabbit Anti-p35/25) (Cell Signaling Technology, Danvers, MA, USA). On the following day, membranes were washed and exposed for 1 h to IRDye®800LT Infrared Dye (LI-COR Biotechnology, NE), goat anti-mouse/goat anti-rabbit diluted at 1:10,000. The images were developed using Odyssey infrared imaging system (LI-COR Biotechnology, NE, USA). As a control for equal protein loading, membranes were stripped and re-probed with rabbit GAPDH antibody (diluted at 1:2500, Cell Signaling Tech, MA) and exposed to anti-rabbit IRDye® 680LT Infrared Dye. After transferring to a PVDF membrane, the gel was stained with Bio-safe Coomassie blue stain (Bio-Rad, CA, USA) to assess the loading of the samples.
Statistical treatment
The results are given as means ± SEM. Data for molecular tests were analyzed by Student’s t-test followed by Bonferroni’s post-hoc test to compare the effects among various treatments, whereas behavioral data were analyzed by two-way analysis of variance (ANOVA) followed by Duncan’s post-hoc test to compare the effects among various treatments. GraphPad Prism 8.0 computer software (La Jolla, CA, USA) was used for analysis. The level of significance was set at P < 0.05.
Results
Treatment with TA improves memory retention only in the presence of the tau gene
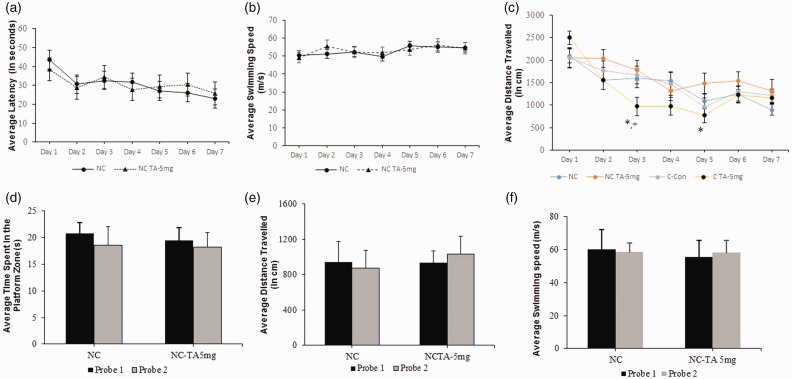
The time taken and distance travelled reaching the platform allows for assessment of memory retention, as those mice that remembered the location of the platform will cover less distance in reaching it. Learning, assessed by the reduction in latency to find the hidden platform during seven days of training clearly indicated no significant difference among control and TA-treated mice that were knockout for murine tau gene (Figure 1(a)); no significant difference was also observed between swim velocity in both groups during daily trails (Figure 1(b)). Furthermore, mice treated with TA and possessing the h-Tau gene swam less distance to reach the platform, with significance of p < 0.05 on day 3 and 5 compared to their counterparts that were untreated by TA; these mice also showed significance (p < 0.05) when compared to TA-treated mice that were knockout for murine tau gene (Figure 1(c)). Differences in cognitive function between NCs treated with 5 mg/kg of TA and their respective controls were assessed utilizing two probe trials spanning 10 days. Probe trials measured the amount of time spent inside of or outside of the correct PFZ after a latency period, which can allow for assessment of reference and working memory. In addition, we also measured distance travelled and swimming velocity during the probe trials. Statistical analysis revealed no significant difference between the treatment group and aged-matched control group, demonstrating the drug as ineffective in this experimental paradigm (Figure 1(d) to (f)). However, these findings should be compared against the results of the same trials reported in a previous study by Chang et al.,29 in which h-Tau carriers treated with TA performed significantly better compared to their untreated, control counterparts.
Figure 1.
Memory retention in 18 mo. knock out (NC) and h-Tau carrier (C) mice after treatment with TA. Mice (N= 7/group) were tested on functions of learning and memory as stated in the “Behavioral studies” section. (a) and (b) average latency in seconds and the swimming velocity in m/s during the seven days of acquisition training, (c) represents average distance swam, in centimeters, during the seven days of acquisition training. Each data point is representative of the average latency, velocity, or distance swam per day. Values are expressed as mean ± standard error of the mean. P < 0.05 considered significant by ANOVA, noted by “*” (C-CTRL versus C-TA) and “+” (NC-TA versus C-TA). Significance was only seen during average distance travelled and (d) to (f) memory retention, average distance, and swimming velocity were assessed by a 60 s probe trial on day 8 (Probe trial 1), following the last day of acquisition testing. Probe trial was repeated after day 18 (Probe trial 2), to create a 10-day latency period. Bar diagram represents the average total time, in seconds, spent by mice in the PFZ, average distance (in cm) and average velocity (in m/s). Values are expressed as mean ± standard error of the mean. No significance was observed.
NC: non-carriers for tau; TA: tolfenamic acid.
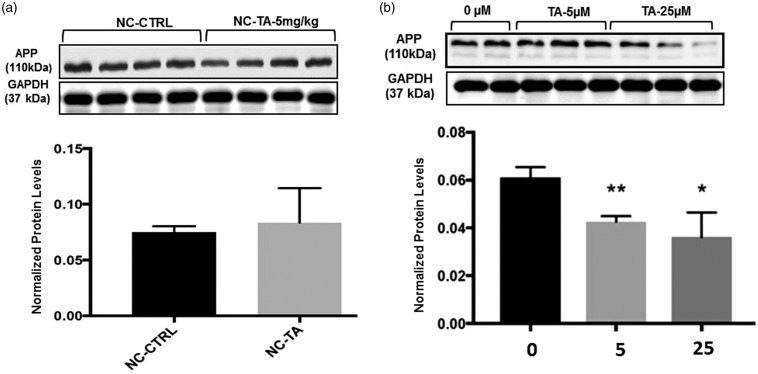
TA alters the protein levels of APP only in the presence of the tau gene
In aged 18-month-old NC mice, TA loses its ability to alter APP levels. NC-C showed no significant difference in levels of APP protein expression, relative to GAPDH, when compared to NC-TA (Figure 2(a)). Antibodies directed against APP were used to measure its levels via Western blot in differentiated human SHSY-5Y cells treated with TA at 5 and 25 µM for 72 h. The genome of these cells was not altered experimentally and contains genes encoding both APP and tau. Consistent with past reports,28 our results confirmed that a significant decrease in the normalized protein levels of APP occurs in naïve cells treated with 5 µM of TA (p = 0.007) and 25 µM of TA (p = 0.05) compared to control, that is untreated with TA (Figure 2(b)).
Figure 2.
APP protein expression after exposure to TA: Levels of APP were measured as stated in the “Protein extraction and Western blotting” section. All values ((a) and (b)) are expressed as mean ± standard error of the mean. (a) Aged 17- to 18-month-old mice (N = 7/group), homozygous knockouts for tau (NC), were administered with vehicle (corn oil) or 5 mg/kg TA (dissolved in corn oil) for 34 consecutive days. Quantification of APP in the frontal cortex was normalized against GAPDH; each bar in the histogram is representative of mean densitometric values of four individual mice per group (n = 4) from each experimental group, no significance was observed and (b) differentiated SHSY-5Y human neuroblastoma cells after 72 h exposure to 0, 5, or 25 µM of TA. Quantification of APP was normalized against GAPDH, each data point in the histogram is representative of three independent analysis (n′ = 3). P ≤ 0.05 considered significant by Student’s t-test, noted by “*”, p ≤0.01 noted as “**”. For 5 µM, p = 0.007 (**). For 25 µM, p = 0.05 (*).
NC: non-carriers for tau; TA: tolfenamic acid.
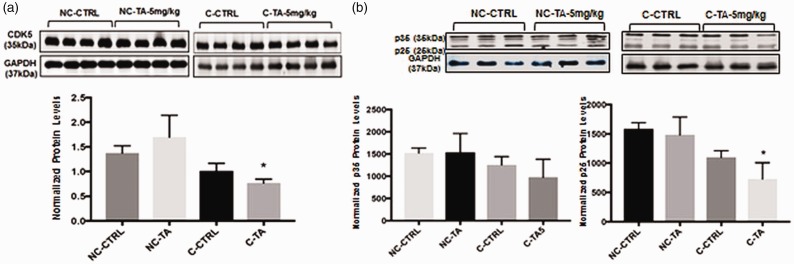
TA impacts CDK5 and p25/35 only in the presence of the tau gene
Quantification of protein expression relative to GAPDH by Western blotting across the four groups of mice revealed that only those murine models that possessed h-Tau and were treated with TA displayed a significant decrease in levels of CDK5 (p = 0.03) (Figure 3(a)) and p25 (p = 0.05) (Figure 4). On the other hand, those NC-TA mice did not demonstrate the same downregulation of CDK5 and p25, even though they had received identical treatment with TA. No significant difference between controls and respective treatment groups (C and NC) was seen for p35 (Figure 3(b)).
Figure 3.
CDK5 and p35/p25 protein expression in frontal cortex after exposure to TA: Protein levels were measured as stated in “Protein extraction and Western blotting” section. Mice (N = 7/group) were administered with vehicle (corn oil) or 5 mg/kg TA dissolved in corn oil for 34 consecutive days. Quantification of protein in the frontal cortex of mice aged 17–18 months normalized against GAPDH. P < 0.05 is considered significant via Student’s t-test, noted by “*”. All values are expressed as mean ± standard error of the mean. (a) Quantification of CDK5 normalized against GAPDH; each bar in the histogram is representative of mean densitometric value of four individual mice per group (n = 4). (NC-CTRL, NC-TA) p > 0.05. (C-CTRL, C-TA) p = 0.03. (b) Quantification of p35 normalized against GAPDH, n = 3, p > 0.05 for all groups. Quantification of p25 normalized against GAPDH, each bar in the histogram is representative of three independents analysis (n′ = 3). (NC-CTRL, NC-TA) p > 0.05. (C-CTRL, C-TA) p = 0.05.
NC: non-carriers for tau; TA: tolfenamic acid.
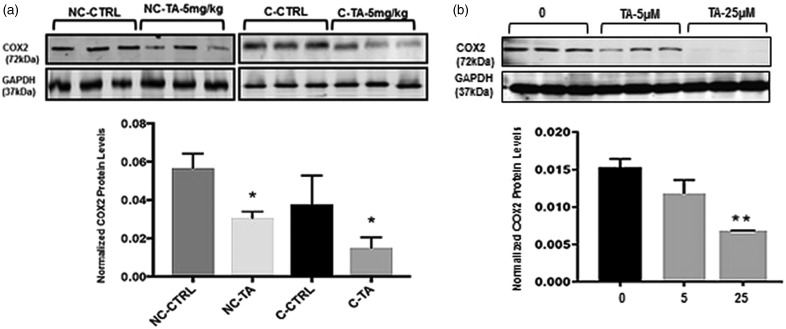
Figure 4.
COX2 protein expression in frontal cortex after exposure to TA: Protein levels were measured as stated in the “Protein extraction and Western blotting” section. Mice (N = 7/group) were administered with vehicle (corn oil) or 5 mg/kg TA dissolved in corn oil for 34 consecutive days. (a) Quantification of COX2 in the frontal cortex from aged mice 17–18 months was normalized against GAPDH. Values are expressed as mean ± standard error of the mean, each bar in the histogram is representative of mean densitometric value of three individual mice per group (n = 3). P < 0.05 is considered significant via Student’s t-test, noted by “*” (NC-CTRL, NC-TA) p = 0.003. (C-CTRL, C-TA) p = 0.006. (b) SHSY-5Y neuroblastoma cells after 72 h exposure to 0, 5, or 25 µM of TA. Quantification of APP normalized against GAPDH, each data point is representative of three independents analysis (n′= 3). P ≤ 0.05 considered significant by Student’s t-test, noted by “*”, p ≤0.01 noted as “**”.
NC: non-carriers for tau; TA: tolfenamic acid.
TA alters COX2 levels regardless of the presence or absence of tau
To confirm that TA still retains its COX-related anti-inflammatory activity, COX2 protein levels were measured across all four groups relative to GAPDH. Results indicated a significant decrease in the protein levels of COX2 in both the C (p = 0.03) and NC (p = 0.003) treated groups (Figure 4(a)).
Likewise, differentiated SH-SY5Y cells with prior treatment of TA for 72 h at a concentration of 5 and 25 µM were analyzed for changes in the COX2 protein levels. Our results indicated significant (p < 0.01) reduction in the normalized levels of COX-2 by TA treatment at a concentration of 25 µM compared to control (Figure 4(b)).
Discussion
Recently, Chang et al.29 reported that TA-treated h-Tau knock-in mice displayed a significant improvement in cognitive function and long-term memory compared to their non-treated counterparts. In addition, TA was shown to reduce site-specific hyperphosphorylation of tau at threonine-181 and serine-396, known sites of hyperphosphorylation in AD.30 All of the studies have explored the effect of TA on both wild-type and transgenic mice, both of which possessed the MAPT and the APP gene. The findings of the above studies as a collective, however, did not make it clear whether TA had simultaneous effects on the amyloid and tau pathways, or whether it selectively targeted one of these pathways in a way which impacted the other. The present study was designed to test whether the cognitive improvements and reduction in AD-associated biomarkers after TA treatment are contingent on the presence of tau. Analysis of the behavioral data revealed no impact on cognitive performance in the absence of tau (Figure 1). Thus, the functional impact of TA appears to require the presence of tau.
Previously published work by our lab has shown that administration of TA to wild-type mice or human APP knock-in mice results in a significant reduction in APP levels.24,28 The presence of tau may be necessary for this action to be productive, particularly when it relates to TA-induced downregulation of the amyloid pathway. As seen in Figure 2, only in an experimental model in which the tau pathway is present (Figure 2(a)), TA is able to work on the amyloid pathway and downregulate levels of APP protein. When the tau pathway has been removed, TA is no longer able to alter the APP protein. This was further verified by our in vitro model, wherein TA treatment reduced APP protein levels in human neuroblastoma containing human tau and APP genes, respectively (Figure 2(b)). Unlike wild-type mice that do not develop NFTs due to sequence differences between mouse and human tau (only 88% sequence homology) and the fact that adult mice only express 4R isoforms, not a mixture of 3R and 4R isoforms that are present in human, it becomes relevant to scrutinize the action of TA in an AD paradigm.
The calpain driven transformation of p35–p25 is responsible for the activation of CDK5, thus any aberrations to CDK5 are subsequent to aberrations in p25.12 Studies have found accumulations of p25 in the neurons of postmortem brains of AD patients, directly correlated with an increase in CDK5 activity.12 Thus, there is need for a therapeutic agent with an ability to modulate p25, which would eventually slow the phosphorylative activity of CDK5 and force it to contribute less to overall phosphorylation. The ability of TA to attenuate hyperphosphorylation via reduction of CDK5 and p25 levels is lost in concordance with the loss of the tau gene (Figure 3).
TA presents itself as promising in other modes, as neurodegenerative diseases are highly inflammatory in nature. In AD alone, increases in levels of neuronal COX2, microglial COX1, mPGES1, and parenchymal PGE2 have been observed.31 All of the aforementioned are markers of inflammation, and as such, an effective therapeutic for AD should possess some anti-inflammatory properties, which TA does. The data shown confirm that TA can lower COX levels regardless of whether tau is present or absent (Figure 4). This shows that the anti-inflammatory action of TAs is disassociated from its impact on the tau pathway.
In summary, TA exhibits itself as a drug with a broad spectrum of activity, as it is capable of lowering several AD-associated proteins and inflammation-associated pathways, and is able to cross the blood–brain barrier.32 The present data revealed that TA does not cause any improvement in cognitive function or change the expression of the amyloid pathway in the absence of tau. The ability of TA to decrease AD-related biomarkers is only present in models which contain either the endogenous or human form of the tau gene. Altogether, these results suggest that tau is a requirement for the action of TA in an AD paradigm.
Authors’ contributions
All authors contributed in the design, analysis of the data, and review of the manuscript. AL and SWB conducted western blot and cell culture. AM, JKC, and ALO conducted behavioral experiments. SWB and ALO analyzed the behavioral data. AL, SWB, and NZ wrote the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Institutes of Health (NIH) and by the NIH grants ES13022, AG027246, 1R56ES01568670-01A1, and 1RO1ES015867-01A2 awarded to NHZ. The research was made possible by the use of the RI-INBRE Research Core Facility, supported by the NIGMS/NIH Grant #P20GM103430.
References
- 1.Moodley KK, Chan D. The hippocampus in neurodegenerative disease. Front Neurol Neurosci 2014; 34:95–108 [DOI] [PubMed] [Google Scholar]
- 2.Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 2014; 88:640–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jho YS, Zhulina EB, Kim MW, Pincus PA. Monte Carlo simulations of tau proteins: effect of phosphorylation. Biophys J 2010; 99:2387–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer D, Mukrasch MD, Biernat J, Bibow S, Blackledge M, Griesinger C, Mandelkow E, Zweckstetter M. Conformational changes specific for pseudophosphorylation at serine 262 selectively impair binding of tau to microtubules. Biochemistry 2009; 48:10047–55 [DOI] [PubMed] [Google Scholar]
- 5.Ishihara T, Zhang B, Higuchi M, Yoshiyama Y, Trojanowski JQ, Lee VM. Age-dependent induction of congophilic neurofibrillary tau inclusions in tau transgenic mice. Am J Pathol 2001; 158:555–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, Gao S, Unverzagt FW, Langa KM, Larson EB, White LR. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement 2011; 7:80–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farlow M. A clinical overview of cholinesterase inhibitors in Alzheimer’s disease. Int Psychogeriatr 2002; 14: 93–126 [DOI] [PubMed] [Google Scholar]
- 8.Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 2014; 71:505–8 [DOI] [PubMed] [Google Scholar]
- 9.Duka V, Lee JH, Credle J, Wills J, Oaks A, Smolinsky C, Shah K, Mash DC, Masliah E, Sidhu A. Identification of the sites of tau hyperphosphorylation and activation of tau kinases in synucleinopathies and Alzheimer’s diseases. PLoS One 2013; 8:e75025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larson ET, Ojo KK, Murphy RC, Johnson SM, Zhang Z, Kim JE, Leibly DJ, Fox AM, Reid MC, Dale EJ, Perera BG, Kim J, Hewitt SN, Hol WG, Verlinde CL, Fan E, Van Voorhis WC, Maly DJ, Merritt EA. Multiple determinants for selective inhibition of apicomplexan calcium- dependent protein kinase CDPK1. J Med Chem 2012; 55:2803–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner U, Utton M, Gallo JM, Miller CC. Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. J Cell Sci 1996; 109:1537–43 [DOI] [PubMed] [Google Scholar]
- 12.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 1999; 402:615–22 [DOI] [PubMed] [Google Scholar]
- 13.Tomizawa K, Ohta J, Matsushita M, Moriwaki A, Li ST, Takei K, Matsui H. Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J Neurosci 2002; 22:2590–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res 2010; 7:656–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valin A, Cook JD, Ross S, Saklad CL, Gill G. Sp1 and Sp3 regulate transcription of the cyclin-dependent kinase 5 regulatory subunit 2 (p39) promoter in neuronal cells. Biochim Biophys Acta 2009; 1789:204–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bu B, Li J, Davies P, Vincent I. Deregulation of cdk5, hyperphosphorylation, and cytoskeletal pathology in the Niemann-Pick type C murine model. J Neurosci 2002; 22:6515–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santpere G, Nieto M, Puig B, Ferrer I. Abnormal Sp1 transcription factor expression in alzheimer disease and tauopathies. Neurosci Lett 2006; 397:30–4 [DOI] [PubMed] [Google Scholar]
- 18.Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge YW, Lahiri DK, Zawia NH. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J Neurosci 2005; 25:823–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adwan LI, Basha R, Abdelrahim M, Subaiea GM, Zawia NH. Tolfenamic acid interrupts the de novo synthesis of the beta-amyloid precursor protein and lowers amyloid beta via a transcriptional pathway. Curr Alzheimer Res 2011; 8:385–92 [DOI] [PubMed] [Google Scholar]
- 20.Ohshima T, Nagle JW, Pant HC, Joshi JB, Kozak CA, Brady RO, Kulkarni AB. Molecular cloning and chromosomal mapping of the mouse cyclin-dependent kinase 5 gene. Genomics 1995;2:585–8. [DOI] [PubMed] [Google Scholar]
- 21.Ross S, Tienhaara A, Lee MS, Tsai LH, Gill G. GC box-binding transcription factors control the neuronal specific transcription of the cyclin-dependent kinase 5 regulator p35. J Biol Chem 2002; 277:4455–64 [DOI] [PubMed] [Google Scholar]
- 22.Ohshima T, Kozak CA, Nagle JW, Pant HC, Brady RO, Kulkarni AB. Molecular cloning and chromosomal mapping of the mouse gene encoding cyclin-dependent kinase 5 regulatory subunit p35. Genomics 1996; 35:372–5 [DOI] [PubMed] [Google Scholar]
- 23.Sidhu PK, Landoni MF, Lees P. Pharmacokinetic and pharmacodynamic interactions of tolfenamic acid and marbofloxacin in goats. Res Vet Sci 2006; 80:79–90 [DOI] [PubMed] [Google Scholar]
- 24.Subaiea GM, Adwan LI, Ahmed AH, Stevens KE, Zawia NH. Short-term treatment with tolfenamic acid improves cognitive functions in Alzheimer’s disease mice. Neurobiol Aging 2013; 34:2421–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adwan LI, Subaiea GM, Basha R, Zawia NH. Tolfenamic acid reduces tau and CDK5 levels: implications for dementia and tauopathies. J Neurochem 2015; 133:266–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature 1982; 297:681–3 [DOI] [PubMed] [Google Scholar]
- 27.Bihaqi SW, Eid A, Zawia NH. Lead exposure and tau hyperphosphorylation: an in vitro study. Neurotoxicology 2017; 62:218–23 [DOI] [PubMed] [Google Scholar]
- 28.Adwan LI, Subaiea GM, Zawia NH. Tolfenamic acid downregulates BACE1 and protects against lead-induced upregulation of Alzheimer’s disease related biomarkers. Neuropharmacology 2014; 79:596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang JK, Leso A, Subaiea GM, Lahouel A, Masoud A, Mushtaq F, Deeb R, Eid A, Dash M, Bihaqi SW, Zawia NH. Tolfenamic acid: a modifier of the tau protein and its role in cognition and tauopathy. Curr Alzheimer Res 2018; 15:655–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong CX, Iqbal K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr Med Chem 2008; 15:2321–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yagami T, Koma H, Yamamoto Y. Pathophysiological roles of cyclooxygenases and prostaglandins in the central nervous system. Mol Neurobiol 2016; 53:4754–71 [DOI] [PubMed] [Google Scholar]
- 32.Subaiea GM, Alansi BH, Serra DA, Alwan M, Zawia NH. The ability of tolfenamic acid to penetrate the brain: a model for testing the brain disposition of candidate Alzheimer’s drugs using multiple platforms. Curr Alzheimer Res 2011; 8:860–7 [DOI] [PubMed] [Google Scholar]