Short abstract
Bariatric surgery is on the rise for long-term weight loss and produces various positive metabolic health benefits. The mechanisms that produce surgical weight loss are not yet fully understood. Previous studies showed vertical sleeve gastrectomy prior to gestation resulted in reduced peripheral blood lymphocytes measured during pregnancy due to an undetermined etiology. Further, elevated splenic weight has been associated with vertical sleeve gastrectomy surgery. We hypothesized that perhaps altered splenic filtration was trapping circulating lymphocytes and thus reducing peripheral blood lymphocytes in circulation and contributing to increased spleen weight. We posited whether removal of the spleen concomitant with the stomach surgery may result in an improved immune phenotype. We evaluated female long Evans rats having received Sham surgery or vertical sleeve gastrectomy, with or without splenectomy to determine the contribution of the spleen on metabolic and immune factors after vertical sleeve gastrectomy. Vertical sleeve gastrectomy animals lost significant amounts of body mass and fat mass and ate less in comparison to Sham females during the first five post-operative weeks, but there was no specific effect of the loss of spleen on body mass, fat mass, or food intake. During the post-operative week 6, animals were euthanized and blood recovered for cell sorting of immune cells. There was a reduction in CD3+ total T cells, CD3/CD4+ helper T cells, and CD3/CD8+ cytotoxic T cells, main effect of both bariatric surgery (P < 0.0001) and splenectomy (P < 0.01). Furthermore, there was a significant increase in CD45RA+ B cells as a result of splenectomy (P < 0.001), but a significant reduction in B cells as a result of VSG surgery (P < 0.05). The changes in total T cells but not B cells were strongly correlated with fat mass. Further studies are needed to understand the cause of the immune changes after surgical weight loss.
Impact statement
Bariatric surgery and in particular vertical sleeve gastrectomy are the fastest growing means for robust amelioration of the comorbidities of obesity. The ramifications of the surgeries on immune health are of important consequence because of the connectivity of immunity with every organ system. The current work reports on the impact of the surgery on the spleen, thymus, and peripheral blood in a rodent model that mimics human surgical outcomes. We demonstrate altered immune health in the VSG rat.
Keywords: Vertical sleeve gastrectomy, spleen, thymus, immune
Introduction
Obesity is a disease of chronic low-grade inflammation1,2 and along with its comorbidities is associated with elevations in circulating white blood cells (WBCs) and particularly lymphocytes and neutrophils.3–5 Aiming to identify risk factors for elevated WBC counts, a study of 14,961 healthy women found that obesity contributed significantly to the risk for high circulating WBCs.6 In addition, obesity itself is associated with an enlarged spleen.7 Though a host of potential causes for elevated WBC counts in obesity exist, the specific etiologies have not entirely been identified. One potential factor is leptin, an adipokine secreted in proportion to the level of adipose tissue mass and is greatly elevated in obesity. Leptin has direct effects on hematopoietic proliferation8 and in particular T cell expansion.8 Furthermore, adipocytes themselves produce and release a host of inflammatory mediators, and with the expanded adipose mass of obesity, higher levels of pro-inflammatory mediators are released consequently which then influence circulating levels of WBC.9 In parallel, as the adipose tissue expands in obesity, pro-inflammatory immune cells infiltrate the adipose tissue and in turn produce cytokines that are released into circulation9–12 specifically interleukin 6 (IL6), tumor necrosis factor alpha (TNFα), Il1β and C-reactive protein.13 The homing of these immune cells to the adipose tissue occurs partly through the influence of increased leptin secretion from the expanding fat tissue.14 Taken together, elevated leptin, the altered adipose immune cell populations and generalized pro-inflammatory cytokine production may be contributors to the elevations in WBC counts that both contribute to and propagate the metabolic dysfunction of obesity.
Evidence exists that weight loss through non-surgical (e.g. lifestyle modifications and pharmacologic aids) and surgical methods has direct impact on peripheral blood lymphocyte populations and cytokine production.3,15,16 In a study of women who reduced their bodyweight by 10% through a multidisciplinary program of diet, exercise, and behavioral counseling, cytokine levels were significantly reduced.15 Alternately, in a study following patients two years after laparoscopic banding, both circulating lymphocyte and neutrophil levels declined in proportion to BMI reduction.3 Similarly, one year after gastric bypass, WBC counts and C-reactive protein levels are significantly reduced in contrast to levels prior to surgery.16 Finally, four months after laparoscopic greater curvature plication, a substantial reduction in CD4+ and CD8+ T cells was reported.17 Taken together, reduction of bodyweight through surgical and non-surgical means reduces the circulating cytokine burden and lowers the total number of WBC. However, specific studies to determine whether the levels of cytokines and WBC are lower than or similar to control subjects who are of similar weight and had never been overweight have not been undertaken.
Vertical sleeve gastrectomy (VSG) is the most common form of surgical weight loss procedure performed currently in the United States and its popularity is increasing. Thus, understanding the impact of VSG on immune health is of great importance. Using a rodent model of VSG, we previously reported reductions in peripheral blood leukocytes (PBL) in gestational day 19 pregnant females in comparison to lean and obese sham dams.18 Despite inclusion of both weight- and diet-matched control dams that received sham surgery in this study, we measured reduced PBL in the VSG dams. At the time, we did not know whether this leukopenia was specifically the consequence of VSG or a side effect of pregnancy following VSG. Additionally, during the course of our studies using VSG, we have observed slightly elevated spleen weights following VSG in both male and female cohorts despite overall weight loss and treatment of metabolic syndrome. This finding of elevated spleen weight had been previously reported by others,19 but, in general, remarks concerning the spleen have not been included in any rodent or human literature following studies of VSG. Considering the role of the spleen in both the maturation and filtration of WBC, the relationship between these two immune findings was considered.
The spleen functions as the major site of destruction of abnormal cell components in the blood.20 It is also the major site of antibody production.20 Disease within the spleen can cause either symptomatic splenomegaly resulting in lymphoproliferative or myeloproliferative disorders or hypersplenism resulting in anemia, neutropenia, or thrombocytopenia due to enlargement of the spleen.20,21 Further, pooling of platelets can occur in the enlarged spleen, and some evidence exists that in this case, platelet survival is shortened in splenomegaly.22 In the current work, we hypothesize that the splenic enlargement we identified in animals that received VSG surgery is producing lymphocyte entrapment within the spleen resulting in sequestration of PBLs. We performed splenectomy in parallel with VSG and followed animals post-operatively to determine if removal of the spleen improved the lymphopenia. We characterized the peripheral blood lymphocytes as a function of splenectomy and bariatric surgery and further, sought to understand the molecular and histologic impact to the VSG spleen.
Materials and methods
Animals
All procedures for animal use complied with the Guidelines for the Care and Use of Laboratory Animals by the National Institutes of Health and were reviewed and approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Female long Evans rats (200–225 g) (Harlan, Indianapolis, IN) were initially multiply housed and maintained in a room on a 12/12-h light/dark cycle at 25°C and 50–60% humidity with ad libitum access to water. After one week of acclimatization to the vivarium, female rats were placed on palatable high-fat diet (HFD) (#D03082706, Research Diets, New Brunswick, NJ, 4.54 kCal/g; 40% fat, 46% carbohydrate, 15% protein) for four weeks prior to surgery. Animals were divided into sham-VSG or VSG groups and further destined to remain with the spleen intact or with splenectomy. In total, we used sham-intact, n = 7; sham-splenectomy, n = 5; VSG-intact, n = 7; VSG-splenectomy, n = 7.
Surgical procedures
Pre-operative care
Four days prior to surgery, body composition was assessed using EchoMRI analyzer (Houston, TX). Animals were fed Osmolite OneCal liquid diet (Abbott Laboratories, IL) but no solid-food for 24 h prior to surgery.
VSG
As previously described,18,22 VSG consisted of a midline laparotomy with exteriorization of the stomach. The stomach was easily articulated by removing ligaments and connective tissue. The lateral 80% of the stomach was excised using an ENDO GIA Ultra Universal stapler (#EGIAUSHORT, Covidien, MA) coupled with an ENDO GIA Auto Suture Universal Articulating Loading Unit, 45 mm–2.5 mm (#030454, Covidien, MA). A sleeve made of gastric tissue in continuity with the esophagus and duodenum thus remained. This gastric sleeve was then reintegrated into the abdominal cavity and internal musculature is sutured and external skin and fascia stapled using wound clips.
Sham-VSG.
Sham surgery consisted of a midline laparotomy with exteriorization of the stomach. The stomach was pressed with forceps for 3 s and then reintroduced into the cavity. The overlying musculature and skin were closed in layers using suture and followed by wound clips.
Splenectomy
In the case of splenectomy, when the stomach was removed from the abdominal cavity during Sham or VSG, the spleen was exteriorized, ligaments and the arterial supply ligated and the spleen resected.
Post-operative care
Following surgery, all rats received care for three days, consisting of once-daily subcutaneous injections of 5 mL saline, 0.10 mL Buprenex® (0.05 mg/kg), and 0.25 mL carprofen (5 mg/kg). Animals were maintained on Osmolite until food was returned three days following surgery.
Body weight, composition, and food intake
Body weight was measured daily during the first post-operative week and weekly until the end of the study. Echo magnetic resonance imaging (echoMRI) whole-body composition analysis (EchoMedical Systems, Houston, TX) was performed on all rats at one week prior to surgery and five weeks post-surgery to determine fat and lean body composition.
Fasting plasma glucose testing
During post-operative week 5, rats were fasted 6 h after the onset of the light cycle. Baseline tail vein blood glucose was measured by Accu-Chek Aviva Plus™ glucometer and glucose strips (Roche, Indianapolis, IN).
Cell isolation protocol
During the sixth post-operative week, animals were euthanized by conscious decapitation and total blood was collected for cell sorting at approximately 5 h after the start of the light cycle. Erythrocytes were lysed using 1× PharmLyse (BD Biosciences) according to the manufacturer’s instructions. Peripheral blood leukocytes (PBL) were washed and resuspended in PBS pH 7.4 containing 2% FCS and 0.09% sodium azide (stain buffer); 5 × 105 cells were stained with immune cell specific antibodies (BD Biosciences, Franklin Lakes, NJ) as follows: CD8a (#561611), CD4 (#554837), CD3 (#554833), CD161a (#555009) or CD45RA (#554881) at a concentration of 1:100 diluted in stain buffer for 30 min on ice. Cells were then washed two times with 2 mL stain buffer and centrifuged at 350 ×g for 5 min at 4°C. Cells were resuspended in 400 μL of stain buffer and immediately analyzed using a Beckman Coulter Gallios analyzer at the UMMC Cancer Institute Flow Cytometry Core Facility. Data were analyzed using Kaluza software.
RNA processing and real-time PCR
Tissue (spleen and thymus) was flash frozen on methylbutane cooled on dry ice, then stored in −80°C until further processing. RNA was extracted using a QIAGEN miniprep RNA kit (QIAGEN, Inc., Valencia, CA), and complementary DNA was transcribed using an iScript complementary DNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Quantitative polymerase chain reaction was performed on a step-one plus real-time PCR machine coupled with StepOne Software (v2.3) (Applied Biosystems) using TaqMan inventoried gene expression assays (Life Technologies, Foster City, CA).
Protein extraction and Western blot procedure
Splenic tissue was flash frozen on methylbutane cooled on dry ice, then stored at −80°C until use. Protein was extracted using the Santa Cruz RIPA lysis buffer system (Santa Cruz Biotechnology, Dallas, TX). Concentrations were determined using a Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL), and spectrometry was performed with a Tecan Infinite 200 PRO. Protein was combined at a 1:1 ratio with Laemmli sample buffer (BioRad Laboratories, Hercules, CA) and denatured at 95° C for 5 min. Protein (40 μg) was loaded onto BioRad 4–20% polyacrylamide Mini Protean TGX gels, and electrophoresis was performed in a BioRad Tetra-Cell 2 gel system. Protein was then transferred to PVDF membranes using a BioRad Trans-Turbo transfer system. Membranes were blocked for 1 h at room temperature with pierce protein-free (TBS) blocking buffer (Thermo Scientific, Rockford, IL). Primary antibodies used were as follows: rabbit NFκB p65 antibody (C-20): (1:1000, #sc-372, Santa Cruz Biotechnology, Inc., Dallas, TX), rabbit anti-hemoglobin subunit alpha antibody (1:1000, #ab92492, Abcam, Cambridge, MA), and mouse CD68 antibody, (1:1000, MCA341GA, Bio-Rad Inc.) Primary antibodies were incubated overnight at 4°C. In between incubations, membranes were washed with TBS with 0.05% Tween. Anti-rabbit HRP conjugate (1:5000, #R1006, Kindle Biosciences, Greenwich, CT) and anti-mouse HRP conjugate (1:5000, #R1005, Kindle Biosciences, Greenwich, CT) were used to incubate membranes for 1 h at room temperature before applying KwikQuant Clean Western Blot Detection Kit HRP substrate. Images were taken with a KwikQuant Imager system and analyzed using KwikQuant Image Manager software.
Paraffin embedding, standard stains, and TUNEL assay
Paraformaldehyde post-fixed spleen was subjected to standard paraffin-embedding, and then sectioned at 5 µm on to glass slides for staining with hematoxylin and eosin (H&E) and Sirus red and additional slides processed for TUNEL staining with Click-iT Plus TUNEL assay for in situ apoptosis detection, Alexa Fluor® 488 dye (#C10617, Molecular Probes, Inc., Eugene, OR), according to the manufacturer’s specifications. Bright field and fluorescent microscopy photographs were obtained with 10× magnification.
Plasma analytes
The following analytes were measured in plasma according to the manufacturer’s specifications: cytokines (#171K1001M, Bio-Plex Pro Rat Cytokine 24-Plex Immunoassay, BioRad, Laboratories, Hercules, CA), CRP ELISA (#80670 Crystal Chem, Elk Grove Village, IL), RANTES (#MMR00, R&D Systems, Minneapolis, MN).
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 6.07 (GraphPad Software, San Diego, California) USA. Differences between two surgeries were assessed by using unpaired Student's t test and two-tailed distribution. To observe time-wise differences, two-way ANOVA (variables: surgical group and time) with Bonferroni post hoc test was used. All results are given as means ± SEM. Results were considered statistically significant when P < 0.05.
Results
Post-surgical metabolic parameters
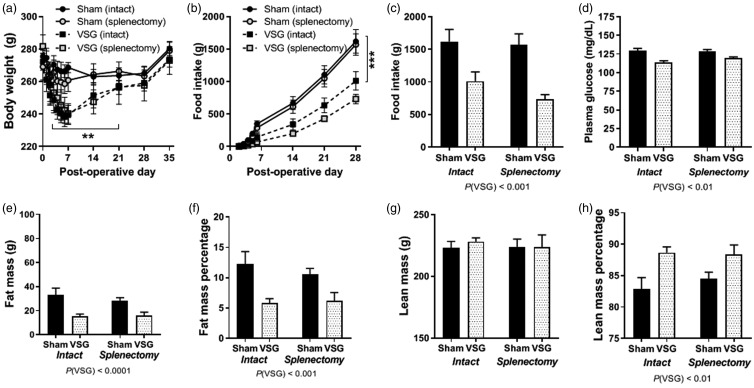
All female rats were placed on HFD for four weeks prior to surgery resulting in increased bodyweight. Four groups of animals were generated: Sham + intact spleen, Sham +Splenectomy, VSG + Intact spleen and VSG + splenectomy. Animals were placed on chow diet after surgery. All animals lost some weight during the initial seven post-operative days (POD), irrespective of the surgery (Sham or VSG, intact or splenectomy) (Figure 1(a)). VSG (intact and splenectomized) rats lost the most weight in the first seven days post-surgery (*P < 0.05) (Figure 1(a)). By POD 28, all animals had weight stabilized and had similar body weights. Thus, in the present study, the Sham group serves as a body weight-matched control for the VSG, but not for body composition.
Figure 1.
Baseline metabolic parameters. (a) Body weight following surgery for female rats receiving Sham or VSG surgery receiving splenectomy or remaining intact. (b) Average daily food intake in grams during the first four weeks after surgery. (c) Cumulative food intake during first four weeks post-surgery. (d) Fasting blood glucose after five weeks of recovery. (e) Fat mass measured by EchoMRI. (f) Fat mass percentage by normalizing to body weight. (g) Lean mass measured by EchoMRI. (h) Lean mass percentage by normalizing to body weight. Two-way ANOVA with repeated measures, two-way ANOVA by surgery/spleen status. Data are presented as mean ±SEM. **P < 0.01, P < 0.001.
Sham rats (intact and splenectomized) consumed a similar amount of calories to each other and this was significantly more kCals cumulatively (Figure 1(b) and (c)) than VSG (intact and splenectomized) in the four weeks post-operatively (***P(VSG)<0.001). Fasting blood glucose measured during week 5 post-operatively showed that the VSG animals had reduced glucose levels in comparison to Sham (**P(VSG)<0.01) (Figure 1(d)).
By post-operative week 5, body composition analysis by EchoMRI showed that VSG (both intact and splenectomy) had significantly reduced fat mass (****P < 0.0001) (Figure 1(e)) and fat mass percentage (Figure 1(f)) (***P < 0.001). There was no impact of surgery on lean mass among the groups (Figure 1(g)), but with respect to lean mass percentage, VSG animals had higher lean mass percentage than Sham rats (Figure 1(h)) (***P < 0.01).
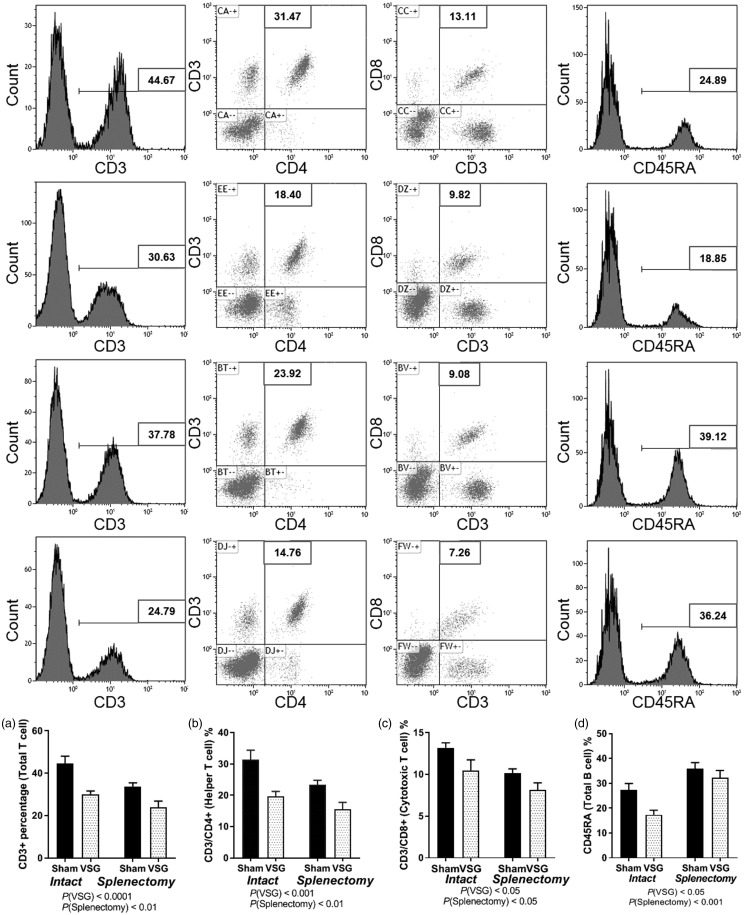
VSG and splenectomy alters peripheral blood lymphocyte populations
Terminal blood was obtained for flow cytometric analysis of total circulating T cells, helper T cells, cytotoxic T cells, and B cells. Total T cells, identified by obtaining percentage of CD3+ cells were significantly reduced as a result of the main effect of surgery, P(VSG < 0.001) and due to the main effect of splenectomy P(Splenectomy < 0.05) (Figure 2(a)). Helper T cells identified by labeling cells for CD3+/CD4+, were also reduced in PBL as a main effect of surgery, P(VSG < 0.001) and main effect of splenectomy, P(Splenectomy < 0.05) (Figure 2(b)). Percentage of cytotoxic T cells, identified by labeling cells for CD3+/CD8+ were also reduced in PBL as a main effect of surgery P(VSG < 0.001) and a main effect of splenectomy, P(Splenectomy < 0.05) (Figure 2(c)). Total B cells, identified by labeling for CD45RA do show a reduction with VSG surgery that is reversed with splenectomy (Figure 2(d)).
Figure 2.
Peripheral blood flow cytometry. (a) CD3+ percentage (total T Cells). (b) CD3/CD4+ (helper T Cells). (c) CD3/CD8+ (cytotoxic T cells). (d) CD45RA (total B Cells). Two-way ANOVA by surgery/spleen status. Data are presented as mean ±SEM.
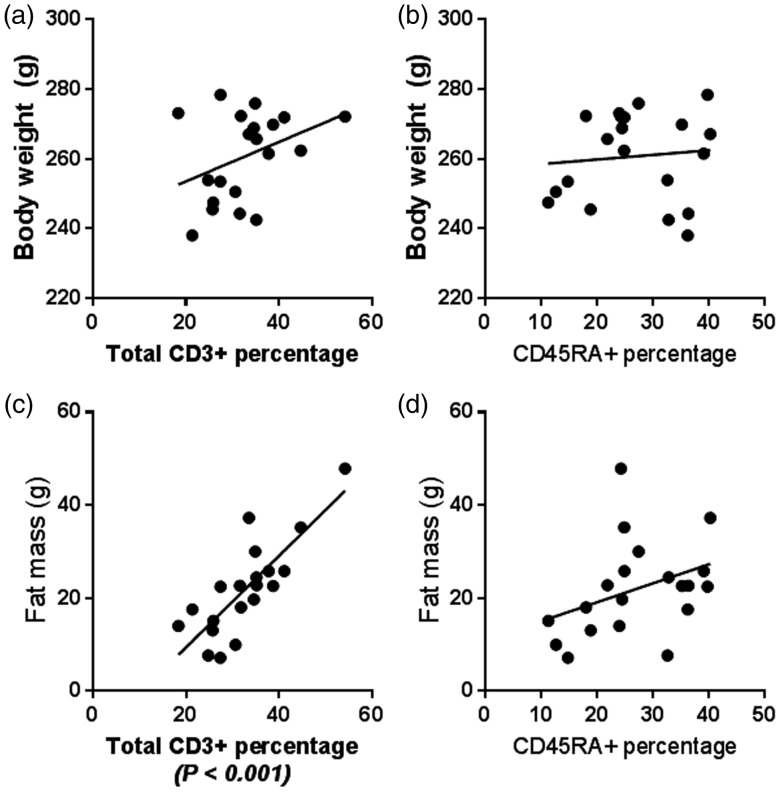
Peripheral T cells are associated with higher fat mass but not body weight
Using cell population percentages in peripheral blood presented in Figure 2, we determined the relationship of body weight and fat mass with T and B cell levels. There was no relationship between T cell percentage and body weight (Figure 3(a)) or B cell percentage and body weight (Figure 3(c)) or body fat (Figure 3(d)). On the other hand, total T cell percentages were highly associated with the level of fat mass (****P < 0.0001, R = 0.644, F32.62) (Figure 3(c)) as well as CD3+/CD4+ and CD3+/CD8+ (not shown).
Figure 3.
Peripheral cell lymphocytes relationship with body weight and fat mass (a) Total T cell percentage as a function of body weight. (b) B cell percentage as a function of body weight. (c) Total T cell percentage as a function of body weight. (d) B cell percentage as a function of body weight. Linear regression analysis was performed using FACS analysis and echoMRI body weight and fat mass.
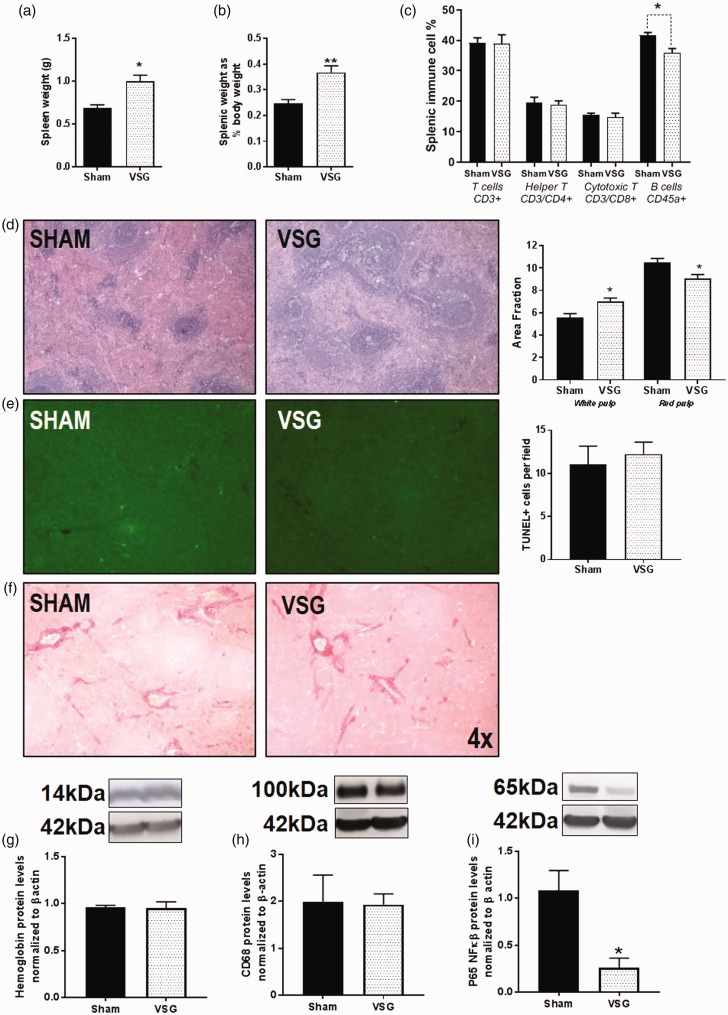
VSG leads to an enlarged spleen
The spleen weight is considerably increased in VSG animals in comparison to Sham in animals that did not receive a splenectomy (*P < 0.05) (Figure 4(a)). When normalized as a percent of body weight, the VSG spleen was also enlarged in comparison to Sham (**P < 0.01) (Figure 4(b)). We used a portion of the splenic tissue to perform cell dissociation for flow to determine the phenotype of the splenic cells. No differences were observed in CD3+ T cells, CD3+/CD4+ helper T cells, or CD3+/CD8+ cytotoxic T cells (Figure 4(c)). CD45RA+ B cells in the VSG spleen were reduced in comparison to Sham (**P < 0.01) (Figure 4(c)). H&E staining of the spleen of Sham and VSG (Figure 4(d)) did show increased area of white pulp and reduced area of red pulp (Figure 4(d)) in the VSG animals. To determine if the spleen was infiltrated by apoptotic cells, we performed TUNEL staining on adjacent sections and analyzed for fluorescent apoptotic bodies. There was no difference in the numbers of immune-positive staining (Figure 4(e)). In support of this result, there was also no change in gene expression of the apoptotic signaling marker, caspase-3 (Table 1). We further questioned whether the spleen might be fibrotic following surgery, resulting in an increased weight. Sirius Red staining for fibrosis showed no gross differences in fibrosis between VSG and sham animals (Figure 4(f)). We then queried whether red blood cells were pooling in the spleen by using surrogate marker hemoglobin subunit α and observed no differences between the two groups (Figure 4(g)).
Figure 4.
Changes in the spleen with VSG (a) Splenic weight in g. (b) Spleen weight normalized to body weight. (c) Flow cytometry of splenic lymphocytes. (d) H&E staining of the spleen. (e) TUNEL staining in spleen. (f) Sirius Red staining of spleen. (g) Protein levels for hemoglobin subunit alpha. (h) Protein levels for CD68 (i) Protein levels for NFKB p65 subunit. Student T test, two-way ANOVA by surgery and spleen status. Data are presented as mean ±SEM. *P < 0.05, **P < 0.01.
Table 1.
Spleen gene qPCR.
| Sham | VSG | ||||
|---|---|---|---|---|---|
| Gene | Gene symbol | Probe # | Mean±SEM | Mean±SEM | P value |
| Il6 | Interleukin 6 | Rn01410330_m1 | 100±31.8 | 124.50±24.6 | 0.493 |
| Il1β | Interleukin 1β | Rn00580432_m1 | 100±11.1 | 83.18±21.9 | 0.663 |
| TNFα | Tumor necrosis factor α | Rn01525859_g1 | 100±20.5 | 141.60±20.9 | 0.225 |
| CD68 | Cluster of differentiation 68 | Rn01495634_g1 | 100±28.0 | 73.40±14.5 | 0.095 |
| HIF1α | Hypoxia inducible factor 1α | Rn01472831_m1 | 100±17.6 | 89.15±16.8 | 0.796 |
| CASP3 | Caspase-3 | Rn00563902_m1 | 100±25.0 | 98.51±15.1 | 0.958 |
| ITGAX | Integrin subunit αX | Rn01511082_m1 | 100±27.1 | 101.80±25.4 | 0.965 |
| IL10 | Interleukin 10 | Rn01483988_g1 | 100±23.0 | 205.80±41.4 | 0.077 |
| ITGB3 | Integrin subunit β3 | Rn00596601_m1 | 100±24.3 | 135.00±34.1 | 0.476 |
| NFKB | Nuclear factor κ light chain enhancer of activated B cells | Rn01399572_m1 | 100±20.8 | 135.00±26.0 | 0.365 |
| MPO | Myeloperoxidase | Rn01460205_m1 | 100±29.5 | 1554.00±740.8 | 0.155 |
Note: All day are normalized to ribosomal gene RPL32 and expressed as relative units. Two-way ANOVA by surgery and spleen status. Data are presented as mean ±SEM.
VSG: vertical sleeve gastrectomy.
Because we observed increased spleen weight and increased levels of white pulp in the spleen despite a decrease in B cells and no T cell changes in the spleen, we further queried whether the spleen weight could be due to increases in splenic macrophages. We performed gene expression and Western blot analyses for macrophage marker CD68 (cluster of differentiation 68), and there were no differences in mRNA levels (Table 1) or protein for CD68 (Figure 4(h)). We further questioned whether the master transcription regulator of cytokine signaling, nuclear factor kappa-light-chain-enhancer of activated B cells (NFκβ), was elevated by mRNA and protein. No differences could be detected in mRNA levels (Table 1). By Western blot, the functionally active subunit, NFκβ p65 subunit was reduced in VSG (Figure 4(i)).
Gene expression analysis by rtPCR of the spleen did not show differences in genes for major pro-inflammatory cytokine genes, IL6, IL1β, and TNFα (Table 1). We questioned whether potential ischemia would elevate gene expression for hypoxia-induced factor alpha (HIFα) but did not see any differences (Table 1). We further asked if dendritic cells were homing to the spleen by probing for dendritic marker, integrin alpha X (ITGAX) but did not observe any differences (Table 1). We tested whether there was increased neutrophil infiltration by probing with granulocyte marker, myeloperoxidase (MPO) (Table 1) and observed a modest trend towards increased expression that was not significant. Finally, we measured expression of ITGB3 (integrin β3/CD61), gene marker for platelets but there were no differences (Table 1). Because interleukin 10 (IL1-10) is a powerful anti-inflammatory cytokine produced by splenic immune cells, we sought to determine whether IL10 levels were altered in the spleen. Trends towards increased IL10 were also observed in VSG spleen (Table 1).
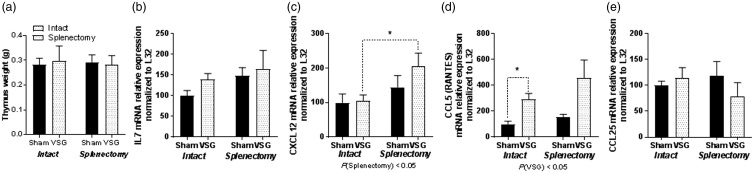
Thymic genes for T cell maturation are changed by VSG and splenectomy
Thymus was dissected at the time of sacrifice. There were no differences in thymic weight based on either VSG or splenectomy (Figure 5(a)). Because IL7 and CCL25 (TEC, thymus-expressed chemokine) are secreted by the thymus to increase hematopoiesis and the maturation of T lymphocytes, we measured gene expression for IL7 and CCL25 in the thymus. No differences in mRNA levels were measured for interleukin 7 (Figure 5(b)) or CCL25 (Figure 5(e)) that are important in T cell maturation. However, CCL12 (Figure 5(c)) also known as monocyte chemotactic protein 5 (MCP-5), which is important for T cell lineage commitment in the thymus, was significantly elevated as a result of splenectomy. CCL5 also known as RANTES (Figure 5(d)) was elevated in the thymus as a result of VSG irrespective of whether the spleen was removed or not.
Figure 5.
Thymic gene expression. (a) Il7 mRNA expression. (b) CXCL12 mRNA expression. (c) CCL5 mRNA expression. (d) CCL25 mRNA expression. Two-way ANOVA by surgery and spleen status. Two-way ANOVA by surgery and spleen status and Student T test for individual effects. Data are presented as mean ±SEM. *P < 0.05.
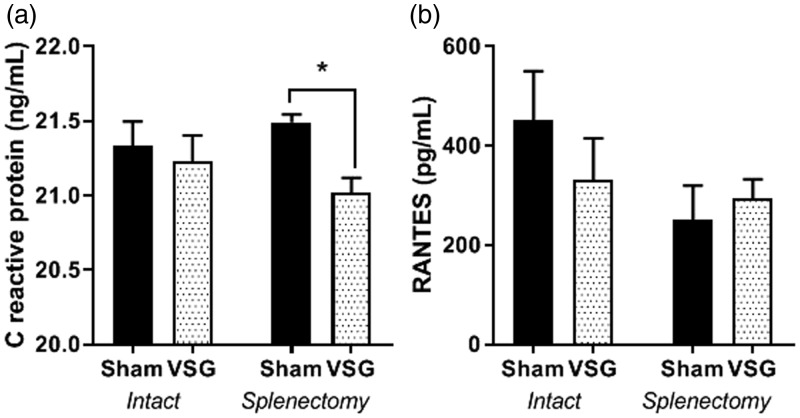
CRP and RANTES in plasma
C-reactive protein (CRP), a generalized marker of inflammation, was reduced in circulation in VSG females that had received a splenectomy (*P < 0.05) but not in VSG with intact spleens (Figure 6(a)). RANTES, (regulated on activation, normal T cell expressed and secreted) is an important chemokine for T cells recruitment. Though RANTES in circulation trends to be reduced in both Sham and VSG (Figure 6(b)) animals having received splenectomy, this change was not significant. Multiplex immunoassay for 24 cytokines was performed on terminal plasma. Increased levels of GRO/KC (growth regulated oncogene) cytokine CCL1 involved in activating neutrophils P(VSG < 0.05), P(Splenectomy < 0.05) There were no other differences in levels of cytokines based on either VSG surgery or splenectomy (Supplemental Table 1).
Figure 6.
Plasma circulating analytes. (a) CRP. (b) RANTES. Two-way ANOVA by surgery and spleen status and Student T test for individual effects. Data are presented as mean ±SEM. *P < 0.05.
Discussion
Surgical weight loss is touted to improve the chronic inflammation that is congruent with long-duration obesity and consumption of diet high in saturated fats and sugar. Reductions in BMI are also accompanied by reduced percentages of peripheral blood lymphocyte populations. Following rodent VSG, we previously reported a reduction in PBL specifically during VSG pregnancy that was lower than weight-matched controls.18 In conjunction, we also identified increased spleen weight in VSG in comparison to sham groups. In the current work, we hypothesized that the VSG spleen contributed to the loss of PBL by altered filtration of the circulating cells thereby enlarging its size. We probed whether removal of the spleen would alter the trajectory of weight loss and immune indices.
Obesity parameters in VSG and splenectomy
Splenectomy in the context of obesity can cause improvements to various indices that complicate excess body weight gain. In a model of monosodium glutamate-induced obesity, splenectomy reversed pre-diabetic insulin hypersecretion, improved insulin sensitivity and reduced hypertrophy of both adipocytes and islets.23 In the current work, splenectomy in the Sham rats did not produce any improvements to metabolic indices including change in body weight, body composition or blood glucose. As previously published,18,22 VSG reduced body weight, cumulative food intake, and blood glucose. Furthermore, fat mass and fat mass percentage were reduced, whereas lean mass percentage was increased with no effect on total unadjusted lean mass. However, there were no further improvements coupling the VSG with splenectomy.
Splenectomy and circulating peripheral lymphocyte populations
Individuals that have either congenital hyposplenism or acquired hyposplenism through incidental splenectomy have chronically reduced T cells levels and dampened immune response.24 Therefore, it is not surprising that splenectomy in our study’s sham-operated animals reduced all the T lymphocyte sub-populations in comparison to the spleen-intact Sham animals. However, contrary to our hypothesis, with respect to total T cells, helper T cells, and cytotoxic T cell percentages, splenectomy in the context of VSG did not ameliorate the low levels of T cells but rather exacerbates it. On the other hand, splenectomy increased overall numbers of CD45RA+ B cells in circulation in both the Sham and VSG animals. In animals that had an intact spleen but also received VSG, we report a significant reduction in the CD45RA+ B cells in comparison to the other groups. So splenectomy was instrumental to increase the circulating B cell levels that appear to be reduced while obtaining VSG. Though this was not directly tested in the current work, we would predict that the levels of IgDhi naïve recirculating B cells in the periphery would be reduced after splenectomy since the spleen is the major site of B cell maturation after migration from the bone marrow.25,26
What is intriguing about the linear regression analysis is that total circulating T cell levels specifically correlated with total fat mass of the rats, measured by echoMRI just prior to euthanasia, but on the other hand, T cell levels did not correlate with body weight. Further, B cell levels did not vary significantly with body weight and fat mass. Because leptin levels are intrinsically connected to an individual’s level of adiposity, it is possible that leptin can specifically affect the maturation of the T cells at the level of the thymus27 or by direct actions of leptin on T cells.14
Though weight loss by various bariatric surgeries appears to reduce PBL, the effect on resident immune cell populations in adipose depots for instance appears to not be reduced but increased after surgical weight loss. For example, in mice that had received VSG, elevated frequencies of CD11c+ macrophages and increased frequencies of T cells were identified specifically in the adipose depots of VSG mice.28 It is indeed possible that the PBL reductions we have identified are due to homing of these peripheral immune cells to other tissues, in this case, the adipose tissue.
The spleen post-VSG
Beyond testing whether the spleen was responsible for the lymphopenia that we identified previously,18 we further sought to determine whether we could identify the etiology of the increased splenic weight; this still remains elusive though we have better characterized the spleen following VSG. However, despite our efforts, there is no evidence of pro-inflammatory activities within the enlarged spleen. We hypothesized that macrophage infiltration could be driving the increased splenic weight; however, there was no identifiable increase of macrophage marker CD68 by mRNA or protein. We next hypothesized that red blood cells (RBC) could be pooling in the post-surgical spleen. But RBC marker hemoglobin 1A was not different by mRNA either. We next asked whether neutrophils could be increasing the weight of the spleen and probed to find a modest trend towards increased gene expression of myeloperoxidase (MPO), as well as increased plasma neutrophil proliferation cytokine GRO/KC. Neutrophils could be localizing to the spleen for a variety of reasons. The innervation and blood flow to the spleen could be affected by gastric resection, causing ischemia. However, neither cell death markers caspase-3 nor TUNEL were elevated. Further, the lymphatic drainage of the stomach to the spleen could be disrupted resulting in splenic damage and homing of the granulocytes. This may be the cause of the increased spleen weight post-VSG in the rodent.
Since bariatric surgery is often sought additionally for the amelioration of liver-associated diseases29 such as non-alcoholic fatty liver disease and hepatic steatosis,29 CT and MRI scans may be performed as standard of care post-bariatric surgery to further follow-up on the size of the liver. However, the size and characterization of the spleen are not reported either before or after surgery in the clinical literature post-bariatric surgery. There are no reports as to whether its size and its relative proportion to post surgery bodyweight are more closely associated to pre-surgery weight or pro-surgery weight. This may be an important clinical measure that should be carefully explored in future studies.
Though injury to the spleen during bariatric surgery is rare, it has been reported, and in particular ischemia of the spleen has been reported.30 In a few cases, splenectomy has been reported to improve the clinical outcome in these circumstances.31,32 Splenic abscesses following bariatric surgery are also very rare several cases have been reported.33 Thrombotic events involving the portal-splenic-mesenteric venous system are also possible.34,35
Thymus post-VSG
Given the T cell reductions in circulation, we measured single and double positive thymocytes by flow and were not able to measure differences (data not shown). We also measured a variety of genes that would suggest stress to maturation within the thymus. RANTES also known as CCL5 (regulated on activation, normal T cell expressed and secreted) was not altered in circulation but was increased specifically due to VSG in the thymus; again, we predict this is increased in order to drive T cell development and maturation.
Circulating inflammatory factors
In several studies, we measured circulating cytokines post-VSG in the rat model and have observed very limited changes in pro-inflammatory cytokines.18 Whereas CRP was not reduced with VSG-alone, splenectomy coupled with VSG led to reduced circulating CRP, suggesting the spleen may be contributing to some inflammation post-surgery. We also measured RANTES in circulation but did not observe differences in levels. Significant numbers of reports suggest reductions to circulating pro-inflammatory cytokines such as MCP1, IL6, and CRP36–39 following surgical weight loss. But some reports are not so convincing that bariatric surgery reduced inflammation at all. In a study comparing inflammatory parameters in patients either obtaining RYGB or placed on a very-low calorie diet, individuals on very-low calorie diet had far lower levels of circulating cytokines in particular IL2, IL6, and TNFα then the bariatric group.40 This is further complicated by differences in cytokine gene expression differences in peripheral tissues. For instance, in subcutaneous adipose tissue from post-bariatric patients, TNFα and caspase-3 gene expression was reported to be elevated even one year post bariatric surgery;41 the authors’ explanation was that there was an ongoing state of cachexia within the adipose tissue of bariatric individuals;41 though plausible, given the previous study in rodents we discussed earlier which showed elevated immune cells in adipose tissue after VSG,28 an alternate hypothesis would suggest that the increased presence of increased immune cells within the VSG adipose tissue produced produce greater levels of pro-inflammatory cytokines. The etiology of these increased immune cell presence and cytokine production should be carefully studied.
Conclusions and further studies
Whereas initially we hypothesized that the enlarged splenic weight and reduction in PBL post-surgery were interconnected, we currently believe that these may be independent side effects of bariatric surgery. Where reductions in PBL are common with amelioration of obesity and its comorbidities, the reductions in PBL in our work are not linked so much to body weight reduction but specifically to total fat loss; we surmised this might be directly influenced by the dramatic reductions in leptin post-surgery. Further questions that arise concerning this suppressed level of T and B cells are whether individuals who have obtained surgical weight loss procedures respond adequately to challenges to their immune system. Based on the current work, we suspect that those who have received VSG may have shifts in their ability to respond to immune-related stressors.
Supplemental Material
Supplemental Material for Splenectomy fails to attenuate immuno-hematologic changes after rodent vertical sleeve gastrectomy by Alexandra R Himel, Erin B Taylor, Charles L Phillips, Bradley A Welch, Redin A Spann, Sibali Bandyopadhyay and Bernadette E Grayson in Experimental Biology and Medicine
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; ARH, EBT, RAS, CLP, BAW, SB and BEG performed experiments included in this manuscript. ARH, EBT, BEG are responsible for writing the manuscript. ARH, EBT, RAS, CLP, BAW, SB and BEG proofread, edited and approved the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
B.E.G is supported by the General Medical Sciences of the National Institutes of Health under Award Number P20GM121334 and Department of Defense Award Number W81XWH-16–1-0387. Further, research reported in this publication was also supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM104357 and the National Heart, Lung and Blood Institute under Award Number P01HL51971. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Some plasma assays were completed by the Mouse Metabolic Phenotyping Center at the University of Cincinnati, Ohio (www.uc.edu/labs/mmpc.html) supported by GRANT U2C DK059630.
References
- 1.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011; 11:98–107 [DOI] [PubMed] [Google Scholar]
- 2.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006; 116:1793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon JB, O' Brien PE. Obesity and the white blood cell count: changes with sustained weight loss. Obes Surg 2006; 16:251–7 [DOI] [PubMed] [Google Scholar]
- 4.Veronelli A, Laneri M, Ranieri R, Koprivec D, Vardaro D, Paganelli M, Folli F, Pontiroli AE. White blood cells in obesity and diabetes. Effects Weight Loss Normal Glucose Metab 2004; 27:2501–2 [DOI] [PubMed] [Google Scholar]
- 5.Farhangi MA, Keshavarz SA, Eshraghian M, Ostadrahimi A, Saboor-Yaraghi AA. White blood cell count in women: relation to inflammatory biomarkers, haematological profiles, visceral adiposity, and other cardiovascular risk factors. J Health Popul Nutr 2013; 31:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisch IR, Freedman SH. Smoking, oral contraceptives, and obesity: effects on white blood cell count. JAMA 1975; 234:500–6 [PubMed] [Google Scholar]
- 7.Chow KU, Luxembourg B, Seifried E, Bonig H. Spleen size is significantly influenced by body height and sex: establishment of normal values for spleen size at US with a cohort of 1200 healthy individuals. Radiology 2016; 279:306–13 [DOI] [PubMed] [Google Scholar]
- 8.Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol 1996; 6:1170–80 [DOI] [PubMed] [Google Scholar]
- 9.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004; 92:347–55 [DOI] [PubMed] [Google Scholar]
- 10.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117:175–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest 2006; 116:33–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 2014; 105:141–50 [DOI] [PubMed] [Google Scholar]
- 13.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes 2003; 27:S53–5 [DOI] [PubMed] [Google Scholar]
- 14.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998; 394:897–901 [DOI] [PubMed] [Google Scholar]
- 15.Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D'Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 2002; 105:804–9 [DOI] [PubMed] [Google Scholar]
- 16.Chen S-B, Lee Y-C, Ser K-H, Chen J-C, Chen SC, Hsieh H-F, Lee W-J. Serum C-reactive protein and white blood cell count in morbidly obese surgical patients. Obes Surg 2009; 19:461–6 [DOI] [PubMed] [Google Scholar]
- 17.Fathy SM, Morshed G. Peripheral blood lymphocyte subsets (CD4+, CD8+ T cells), leptin level and weight loss after laparoscopic greater curvature plication in morbidly obese patients. Arch Med Sci 2014; 10:886–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spann RA, Lawson WJ, Bidwell GL, 3rd, Zamarripa CA, Maranon RO, Bandyopadhyay S, Taylor ER, Reckelhoff JF, Garrett MR, Grayson BE. Rodent vertical sleeve gastrectomy alters maternal immune health and fetoplacental development. Clin Sci 2018; 132:295–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aktekin A, Kazan K, Gunes P, Yekrek M, Muftuoglu T, Saglam A. Hematological, biochemical, and immunological laboratory and histomorphological effects of sleeve gastrectomy on female rats. Ind J Surg 2015; 77:557–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weledji EP. Benefits and risks of splenectomy. Int J Surg 2014; 12:113–9 [DOI] [PubMed] [Google Scholar]
- 21.Takamatsu Y, Jimi S, Sato T, Hara S, Suzumiya J, Tamura K. Thrombocytopenia in association with splenomegaly during granulocyte-colony-stimulating factor treatment in mice is not caused by hypersplenism and is resolved spontaneously. Transfusion 2007; 47:41–9 [DOI] [PubMed] [Google Scholar]
- 22.Lawson WJ, Shirey K, Spann RA, Zamarripa CA, Hosler JP, Grayson BE. Vertical sleeve gastrectomy improves indices of metabolic disease in rodent model of surgical menopause. Menopause 2017; 24:426–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leite Nde C, Montes EG, Fisher SV, Cancian CR, de Oliveira JC, Martins-Pinge MC, Kanunfre CC, Souza KL, Grassiolli S. Splenectomy attenuates obesity and decreases insulin hypersecretion in hypothalamic obese rats. Metab Clin Exp 2015; 64:1122–33 [DOI] [PubMed] [Google Scholar]
- 24.Wolf HM, Eibl MM, Georgi E, Samstag A, Spatz M, Uranus S, Passl R. Long-term decrease of CD4+CD45RA+ T cells and impaired primary immune response after post-traumatic splenectomy. Br J Haematol 1999; 107:55–68 [DOI] [PubMed] [Google Scholar]
- 25.Vitetta ES, Melcher U, McWilliams M, Lamm ME, Phillips-Quagliata JM, Uhr JW. Cell surface immunoglobulin. XI. The appearance of an IgD-like molecule on murine lymphoid cells during ontogeny. J Exp Med 1975; 141:206–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kearney JF, Cooper MD, Klein J, Abney ER, Parkhouse RM, Lawton AR. Ontogeny of Ia and IgD on IgM-bearing B lymphocytes in mice. J Exp Med 1977; 146:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest 1999; 104:1051–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frikke-Schmidt H, Zamarron BF, O'Rourke RW, Sandoval DA, Lumeng CN, Seeley RJ. Weight loss independent changes in adipose tissue macrophage and T cell populations after sleeve gastrectomy in mice. Mol Metab 2017; 6:317–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laursen TL, Hagemann CA, Wei C, Kazankov K, Thomsen KL, Knop FK, Gronbaek H. Bariatric surgery in patients with non-alcoholic fatty liver disease - from pathophysiology to clinical effects. World J Hepatol 2019; 11:138–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamou KM, Menenakos E, Gomatos IP, Panousopoulos S-G, Smparounis S, Leandros E, Zografos G. Clinical implications of sleeve gastrectomy as a source of spleen infarction or ischemia. Obes Surg 2011; 21:1490–3 [DOI] [PubMed] [Google Scholar]
- 31.Peters TG, Steinmetz SR, Cowan GS., Jr,. Splenic injury and repair during bariatric surgical procedures. South Med J 1990; 83:166–9 [DOI] [PubMed] [Google Scholar]
- 32.Abell TL, Minocha A. Gastrointestinal complications of bariatric surgery: diagnosis and therapy. Am J Med Sci 2006; 331:214–8 [DOI] [PubMed] [Google Scholar]
- 33.Nassour F, Schoucair NM, Tranchart H, Maitre S, Dagher I. Delayed intra splenic abscess: a specific complication following laparoscopic sleeve gastrectomy. Obes Surg 2018; 28:589–93 [DOI] [PubMed] [Google Scholar]
- 34.Rottenstreich A, Elazary R, Kalish Y. Abdominal thrombotic complications following bariatric surgery. Surg Obes Relat Dis 2017; 13:78–84 [DOI] [PubMed] [Google Scholar]
- 35.El Lakis MA, Pozzi A, Chamieh J, Safadi B. Portomesenteric vein thrombosis after laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass: a 36-case series. Surg Endosc 2017; 31:1005–11 [DOI] [PubMed] [Google Scholar]
- 36.Gumbau V, Bruna M, Canelles E, Guaita M, Mulas C, Bases C, Celma I, Puche J, Marcaida G, Oviedo M, Vazquez A. A prospective study on inflammatory parameters in obese patients after sleeve gastrectomy. Obes Surg 2014; 24:903–8 [DOI] [PubMed] [Google Scholar]
- 37.Hakeam HA, O'Regan PJ, Salem AM, Bamehriz FY, Jomaa LF. Inhibition of C-reactive protein in morbidly obese patients after laparoscopic sleeve gastrectomy. Obes Surg 2009; 19:456–60 [DOI] [PubMed] [Google Scholar]
- 38.Coimbra S, Ferreira C, Belo L, Rocha-Pereira P, Catarino A, Monteiro L, Catarino C, Santos-Silva A. Impact of weight loss on inflammation and red blood cell biomarkers after laparoscopic gastric banding surgery. J Investig Med 2018; 66:304–8 [DOI] [PubMed] [Google Scholar]
- 39.Fenske WK, Dubb S, Bueter M, Seyfried F, Patel K, Tam FW, Frankel AH, Le Roux CW. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis 2013; 9:559–68 [DOI] [PubMed] [Google Scholar]
- 40.Lips MA, van Klinken JB, Pijl H, Janssen I, Willems van Dijk K, Koning F, van Harmelen V. Weight loss induced by very low calorie diet is associated with a more beneficial systemic inflammatory profile than by Roux-en-Y gastric bypass. Metab Clin Exp 2016; 65:1614–20 [DOI] [PubMed] [Google Scholar]
- 41.Jurets A, Itariu BK, Keindl M, Prager G, Langer F, Grablowitz V, Zeyda M, Stulnig TM. Upregulated TNF expression 1 year after bariatric surgery reflects a cachexia-like state in subcutaneous adipose tissue. Obes Surg 2017;27(6):1514–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Splenectomy fails to attenuate immuno-hematologic changes after rodent vertical sleeve gastrectomy by Alexandra R Himel, Erin B Taylor, Charles L Phillips, Bradley A Welch, Redin A Spann, Sibali Bandyopadhyay and Bernadette E Grayson in Experimental Biology and Medicine