Abstract
Hydrocephalus has been reported to occur in spontaneous hypertensive rats (SHRs). The purposes of this study were (1) to use T2 magnetic resonance imaging to examine time of onset, (2) to elucidate potential underlying mechanisms and (3) to determine whether minocycline could prevent hydrocephalus development. Ventriculomegaly was evaluated by T2 imaging in SHRs and Wistar-Kyoto rats from weeks 4 to 7 after birth. Brain histology and transmission electron microscopy were used to assess the periventricular and choroid plexus damage. SHRs were also treated with either vehicle or minocycline. We found that hydrocephalus was observed in SHRs but not in Wistar-Kyoto rats. It occurred at seven weeks of age but was not present at four and five weeks. The hydrocephalus was associated with epiplexus cell (macrophage) activation, choroid plexus cell death and damage to the ventricle wall. Treatment with minocycline from week 5 attenuated hydrocephalus development and pathological changes in choroid plexus and ventricular wall at week 7. The current study found that spontaneous hydrocephalus arises at ∼7 weeks in male SHRs. The early development of hydrocephalus (persistent ventricular dilatation) may result from epiplexus cell activation, choroid plexus cell death and periventricular damage, which can be ameliorated by treatment with minocycline.
Keywords: Choroid plexus, epiplexus cells, hydrocephalus, minocycline, spontaneous hypertensive rats
Introduction
Hydrocephalus often occurs after brain hemorrhage and is associated with poor clinical outcomes.1 Animals studies on adult onset hydrocephalus have generally involved injection of blood or blood components, including red blood cells, thrombin, hemoglobin or iron,2–4 or agents such as kaolin to block CSF flow.5 Only a few chronic and spontaneous adult hydrocephalus models without surgical intervention have been mentioned in literature. In 1986, Ritter and Dinh reported the progressive ventricular dilation in spontaneous hypertensive rats (SHRs) between age of four and eight weeks.6,7 The mechanisms involved in this spontaneous hydrocephalus have not been elucidated and they might give insight into the mechanisms involved in other forms of adult hydrocephalus including those associated with intraventricular and intracerebral hemorrhage and traumatic brain injury. This model may also be an easy and reproducible model for testing potential therapeutics.
The choroid plexus (CP) produces cerebrospinal fluid and has a role in hydrocephalus development.1 There are macrophages that reside on the apical surface of the CP epithelium, called Kolmer epiplexus cells.8 Although epiplexus macrophage activation has been found in a rat model of hydrocephalus, the role of epiplexus macrophages in hydrocephalus is unclear.9,10
Minocycline, a second-generation semi-synthetic tetracycline, has anti-inflammatory properties, including inhibiting macrophage/microglial activation, and inhibiting matrix metalloproteinases.11 It has neurovascular protective effects in animal models of cerebral ischemia and hemorrhage.12–15 Our previous research suggested that iron degraded from hemoglobin may participate in the occurrence of hydrocephalus after intraventricular hemorrhage and minocycline can alleviate iron overload.16–18 Other evidence has shown that minocycline administration can attenuate hydrocephalus, brain edema and gliosis in different hydrocephalic animal models.19–21 Therefore, minocycline may be feasible as a therapeutic targeting hydrocephalus and associated damage.
The present study performed magnetic resonance imaging (MRI) and brain histology on SHR and Wistar Kyoto (WKY) rats to determine the time course of spontaneous hydrocephalus (persistent ventricular dilatation) development and to follow epiplexus macrophage activation and associated periventricular changes. We then evaluated the effects of minocycline on hydrocephalus development in SHRs.
Material and methods
Animals preparation
All the animal studies were approved by the University of Michigan Committee on the Use and Care of Animals and performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A total of 41 male SHRs aged 4- to 5-weeks and 14 age-matched WKY rats (Charles River Laboratories, Massachusetts) were housed in the Animal Facilities of the Unit for Laboratory Animal Medicine under controlled temperature and humidity with a 12/12-h light/dark cycle. The initial body weight of all rats was 90–100 g. Blood pressure was measured using tail cuff method (CODA 6; Kent Scientific Corporation, CT). Systolic blood pressure and mean arterial pressure were recorded at weeks 5, 6 and 7. This study complies with the ARRIVE guidelines for reporting in vivo experiments. Randomization was carried out using odd/even numbers.
Experiment groups
All the animals were distributed into two parts. First, SHRs and WKY rats aged four weeks were housed and received MRI scanning at weeks 4, 5 and 7 to determine the ventricle volumes. SHR (n = 14) and WKY rats (n = 14) were euthanized at weeks 4 and 7 for brain histology and transmission electron microscopy. Second, SHRs (five week old) were treated with vehicle (n = 9), minocycline 10 mg/kg (n = 9) or minocycline 20 mg/kg (n = 9). Animals were euthanized at week 7 and the brains were used for brain histology.
Minocycline administration
Starting from week 5, minocycline (Minocin, The Medicines Company, Parsippany, NJ) was injected intraperitoneally in SHRs every 12 h at either 10 or 20 mg/kg. Dosing was continued for 14 days. Rats in a SHR + vehicle group received intraperitoneal injections of saline. Animals were monitored for adverse reactions such as diarrhea and reduced body weight.
Magnetic resonance imaging and ventricle/brain volume measurement
MRI was performed at weeks 4, 5 and 7. After being anesthetized with 2% isoflurane /air mixture, rats were scanned using a 9.4T MRI (Agilent, Palo Alto, CA). A T2 fast spin-echo sequence was performed with a view field of 35 mm×35 mm, matrix of 256 × 256, 25 coronal slices and 0.5 mm thickness. Ventricle volume was calculated as previously reported.3 Briefly, ventricles were delineated bilaterally on each slice of T2 images from the frontal horns of the lateral ventricles through to the fourth ventricle on Image J software (NIH, Bethesda, MD). Ventricular volumes were calculated by adding the ventricle area on each slice multiplied by slice thickness. Brain tissue volumes were also measured on T2 images from + 3.7 mm to −7.0 mm bregma. All measurements were performed by an observer blinded to treatment.
Brain histology, hematoxylin and eosin staining and macrophage immunostaining
Rats were anesthetized by pentobarbital (60 mg/kg i.p.) and perfused with 4% paraformaldehyde in 0.1 M PBS, pH 7.4. Brains were removed and placed in paraformaldehyde overnight. They were then transferred into 30% sucrose in 0.1 M PBS at 4℃ until they sank to the bottom. After embedding in a mixture of 30% sucrose and optimal cutting temperature compound (Sakura Finetek, Inc., Torrance, USA), brains were sliced into 18 μm thick frozen sections. Macrophage immunostaining and H & E staining were performed as previously described.22 For Iba1 staining, the primary antibody was goat anti-Iba1 (1:300 dilution; Abcam, Cambridge, MA, USA). The secondary antibody was rabbit anti-goat IgG (H+L) (1:500 dilution; Vector Laboratories, Burlingame, CA, USA). For CD68 staining, the primary antibody was mouse anti-CD68 (1:100 dilution; Abcam, Cambridge, MA, USA). The secondary antibody was horse anti-mouse IgG (1:500 dilution; Vector Laboratories, Burlingame, CA, USA). For Iba1 and CD68-positive cell counting, CPs at ×20 magnification images were selected and counted bilaterally by an observer blinded to the experimental group. Iba1-positive cells are presented as a percentage of all CP cells. Macrophage soma area was measured by capturing image at ×100 magnification and by manual tracing the outline of at least 10 randomly selected cells/section using Image J software.
Quantification of ventricular wall damage
Ventricular wall damage was analyzed using a previously described method.23 In brief, for each rat, sections of three layers of ventricular system were stained by H & E staining and were used for calculation. Layer 1 was ∼0.4 mm posterior to bregma at sagittal midline, demonstrating bilateral frontal ventricular horn. Layer 2 was located ∼1.2 mm posterior to bregma and showed the bilateral lateral ventricle and its connection with the anterior third ventricle. Layer 3 was ∼4.0 mm posterior to bregma which revealed posterior bilateral lateral ventricles and the body of the third ventricle. The perimeter of ventricle wall was outlined bilaterally on each layer in Image J software. The length of damaged ventricular wall was then determined and expressed as a % of total ventricular wall. All analyses were performed by an observer blinded to the experimental group.
Transmission electron microscopy
Transmission electron microscopy (TEM) was used to investigate CP ultrastructure as previously described.24 Rats were perfused with 4% paraformaldehyde and 2.5% glutaraldehyde in 0.1 mol/L Sorensen's buffer (pH 7.4). Brains were cut though midline and bilateral CPs were removed from the lateral ventricle. Tissue was incubated in Karnovski's fixative for at least 1 h at room temperature (RT), then overnight at 4℃. Samples were washed with 20 × volume Sorenson's buffer 3×, before post-fixing in 2% osmium tetroxide in Sorenson's buffer for 1 h at RT. Tissue was washed again 3 × with 20 × volume Sorenson's buffer, then dehydrated through ascending concentrations of ethanol, treated with propylene oxide, and embedded in EMbed 812 epoxy resins. Semi-thin sections were stained with toluidine blue for tissue identification and ultra-thin sectioned 70 nm in thickness and post stained with uranyl acetate and Reynolds lead citrate. A JEOL JEM 1400 + transmission electron microscope was used for imaging.
Terminal deoxynucleotidyl transferase dUTP nick end labeling staining and cell counting
A Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) staining kit (Millipore) was used to detect double-stranded DNA fragmentation as previous described.25 To assess the number of positive cells, the CP in layer 1 of each brain was examined under high-power magnification and counted bilaterally. The number of TUNEL-positive cells was expressed as a % of all cells. Counting was performed in Image J software by an observer blinded to the experimental groups.
Statistical analysis
All data are presented as mean ± SD. Differences between groups were analyzed by Student t tests, one-way ANOVA and two-way ANOVA with Tukey post hoc test. Statistical significance between groups was set at P < 0.05 and P < 0.01. The sample size of minocycline treatment was determined based on the data on SHRs (ventricular volume at week 7: 35.4 ± 7.7 mm3) in the first part of this study. An n = 9 per group would give 90% power for detecting a 25% decrease in ventricular volume with minocycline.
Results
Spontaneous hydrocephalus occurred at week 7 in SHRs
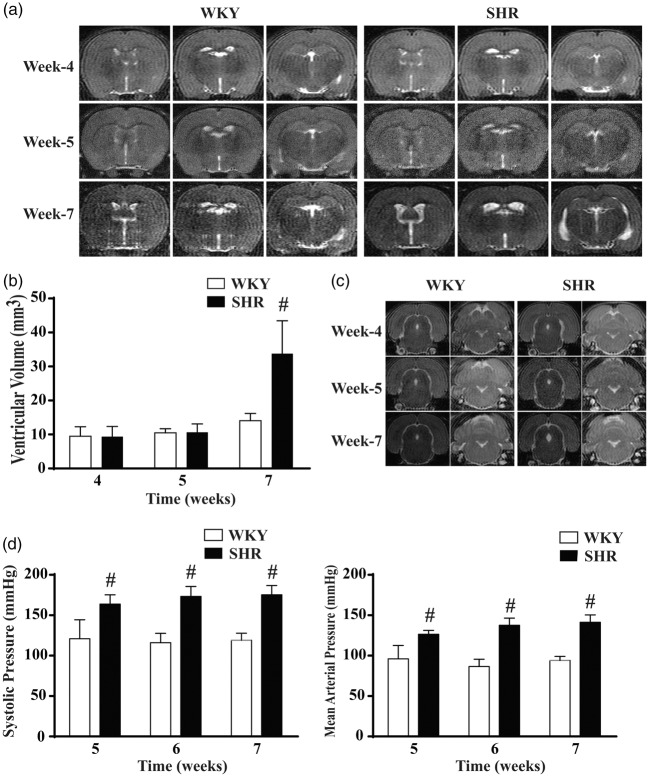
A previous histological study revealed that ventricular dilation occurs somewhere between weeks 4 and 8 in SHRs and resulted in permanent hydrocephalus.7 We performed MRI on WKY and SHR groups at weeks 4, 5 and 7 to determine the time point of hydrocephalus onset. T2 Images revealed that at weeks 4 and 5 there were similar ventricular volumes in both WKY rats and SHRs, whereas obvious ventricular enlargement was observed in SHRs at week 7 (Figure 1(a)). Quantification of ventricular volume confirmed that there was no significant difference between SHRs and WKY rats at weeks 4 and 5. However, SHRs developed spontaneous hydrocephalus at week 7 with a more than 2-fold larger ventricular volume (35.4 ± 7.7 mm3) compared to WKY rats (14.1 ± 2.1 mm3; Figure 1(b), P < 0.01). As well as dilated lateral ventricles, coronal MRIs showed the presence of dilated aqueduct and 4th ventricles in SHRs at week 7 (Figure 1(c)) suggesting non-obstructive hydrocephalus. However, brain (two hemispheres from + 3.7 mm to −7.0 mm bregma) tissue volumes were similar at week 7 in SHRs (1088 ± 20 mm3) and WKY rats (1106 ± 33 mm3, n = 6, P = 0.277).
Figure 1.
Spontaneous hydrocephalus occurs in SHRs at seven weeks of age. (a) Representative T2-weighted MRI images showing ventricular enlargement in SHRs at week 7 compared with age-matched WKY rats. (b) Quantification of ventricular volume on T2 images at weeks 4, 5 and 7. Values are means ± SD, n = 6; #P < 0.01 compared with WKY group. (c) MRI T2 showing evidence of the aqueduct and 4th ventricular enlargement in SHRs at week 7. (d) SHRs had significant higher systolic blood pressure (SBP) and mean arterial pressure (MAP) than WKY rats at weeks 5, 6 and 7. #P < 0.01 compared with WKY group, n = 6.
At weeks 5, 6 and 7, WKY rats had significant lower systolic blood pressures than SHRs (121 ± 24 vs. 164 ± 11 mmHg at week 5; 116 ± 11 vs. 173 ± 12 mmHg at week 6; 119 ± 9 vs. 175 ± 11 mmHg at week 7. P < 0.01). Similar differences were also found in mean arterial pressure (Figure 1(d)). Thus, hydrocephalus development in the SHR occurs after the establishment of hypertension.
Activation of epiplexus macrophage and alterations of CP structure in SHRs
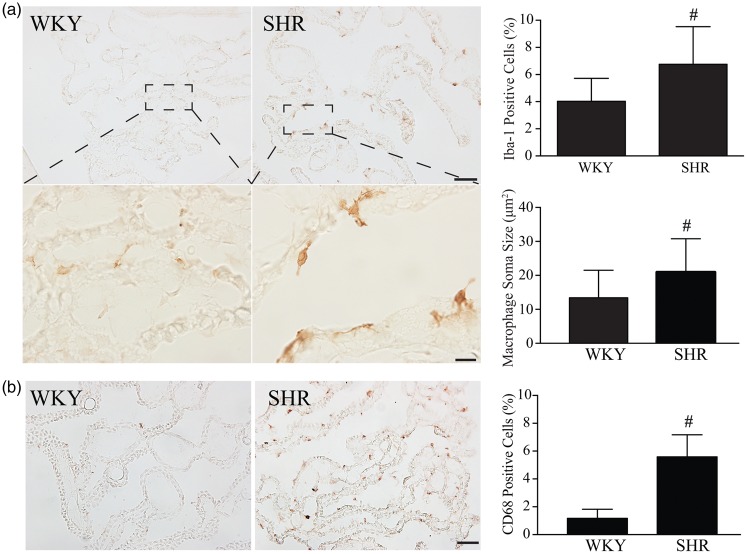
Iba1 was used to stain epiplexus macrophages. At week 4, there was a tendency of increase of Iba1-positive epiplexus macrophage numbers in SHRs (7.3 ± 3.3% vs. 6.3 ± 1.6 % in WKY rats, n = 6, P = 0.382). However, epiplexus macrophage soma areas were larger in SHRs at week 4 (19.5 ± 8.9 µm2 vs. 11.4 ± 6.9 µm2, n = 6, P < 0.01). Activation of epiplexus macrophages was found in SHRs at week 7 with both increased numbers of epiplexus macrophages and larger soma size in SHRs compared to WKY rats (Figure 2(a)). Iba1 results were confirmed by another macrophage marker, CD68. There was a significant increase of CD68-positive epiplexus macrophage in SHRs at week 7 (5.6 ± 1.6% vs. 1.2 ± 0.7 % in WKY rats, n = 6, P < 0.01, Figure 2(b)).
Figure 2.
SHRs have evidence of epiplexus macrophage activation compared to WKY rats. (a) Examples of choroid plexus (CP) Iba-1 immunohistochemistry in SHRs and WKY rats at week 7 (low and high magnification). Note the presence of more and larger Iba-1-positive cells on the apical surface of the CP in the SHRs. Bar graphs show quantification of the number of Iba-1 cells (as a % of all cells) in both WKY rats and SHRs and measurement of the size of the macrophages. Values are means ± SD, #P < 0.01 compared with WKY group, n = 6. Scale bar = 50 μm (low magnification) and 10 μm (high magnification). (b) CD68-positive epiplexus macrophages in SHRs and WKY rats at week 7. Values are means ± SD, #P < 0.01 compared with WKY group, n = 6. Scale bar = 50 μm.
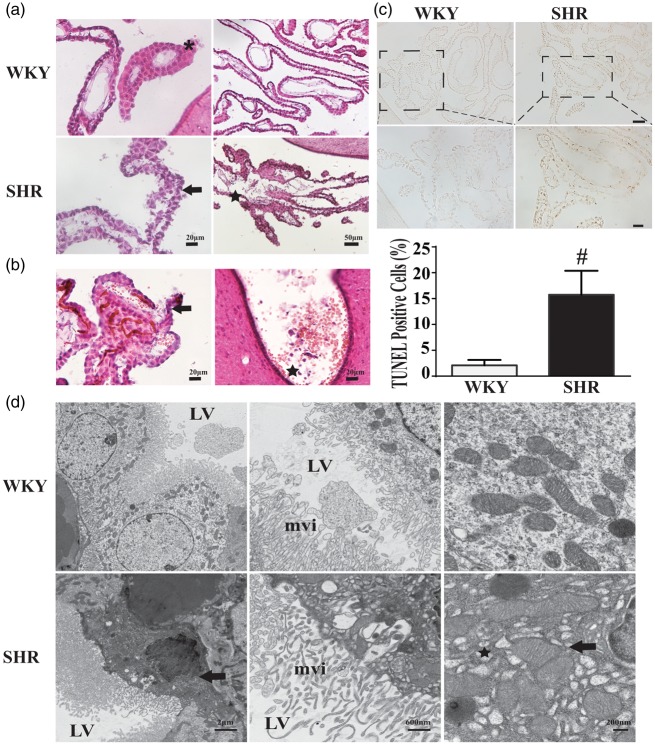
Alteration in CP structure was also examined using H & E-stained sections at week 7 (Figure 3(a)). In WKY rats, CP epithelial cells were regularly spaced with round nuclei. In SHRs, some areas of the epithelium had chaotically arranged cells with abnormal shape and some cells were undergoing karyopyknosis. In WKY rats, the CP capillaries were intact and associated with the inner surface of the epithelial cells. In contrast, in SHRs, the capillaries appeared less associated with the epithelium and there was evidence of erythrocytes outside the capillaries potentially either through extravasation or capillary destruction.
Figure 3.
Choroid plexus (CP) ultrastructural changes and CP cell death in SHRs and WKY rats at week 7. (a) H & E staining of WKY sections showed organized CP epithelial cells with homogeneous nuclei at week 7 (*), while CP cells were sometimes disoriented with atrophic nuclei (arrow) in SHRs. Endothelium of capillaries were intact and closely associated with the epithelium in WKY rats but sometimes fragmented in SHRs (star). (b) Microbleeding of CP in the SHR at week 7. There was evidence of erythrocytes leaking out of endothelium and epithelium into ventricle (arrow) and hematoceles (star). (c) TUNEL staining and quantification showed abundant TUNEL-positive cells in CP of SHRs at week 7. #P < 0.01 compared with WKY group, n = 6. Scale bar = 50 μm (low magnification) and 20 μm (high magnification). (d) CP ultrastructure in SHRs and WKY rats at seven weeks of age as assessed by transmission electron microscopy. Low-magnification image showing CP epithelia in WKY rats characterized by round nuclei and clear cell cytoplasm. In contrast, in SHRs, some epithelial cells had atrophied nuclei (arrow) surrounded with abnormal cytoplasm (star). In WKY rats, mitochondria were normal in shape and scale, while in SHRs, mitochondria appear slightly swollen (arrow) and in some cells were surrounded by multiple vesicles/cysts (star). LV: lateral ventricle; mvi: microvilli.
We also detected some evidence of hemorrhage in CP at week 7. Red blood cells were found leaking out from endothelial cells and epithelium cells, then breaking into the lateral ventricle. Other types were identified as hemorrhage or hematocele (Figure 3(b)).
To further assess CP injury, TUNEL staining was performed to examine potential cell death. SHRs had significantly more TUNEL-positive cells in the CP compared to WKY rats (15.7 ± 1.3 vs. 2.1 ± 0.3 % per ventricle, P < 0.01, n = 6. Figure 3(c)), demonstrating severe cell injury in CP at week 7 in SHRs. From the location of those TUNEL positive, they were not endothelial cells and epiplexus macrophages. By location, the TUNEL-positive cells appear to be the cuboidal epithelial cells. This was investigated further using transmission electron microscopy to examine CP epithelial ultrastructure in seven-weeks-old SHRs and WKY rats. There were some differences in ultrastructure between the strains. In particular, some SHR epithelial cells had misshapen nuclei, dark cytoplasm with many vesicles/cysts (Figure 3(d)). At a higher magnification, some SHR epithelial cells showed many small vesicles surrounding mitochondria and those mitochondria appeared slightly swollen compared to WKY counterparts (Figure 3(d)).
Hydrocephalus was accompanied by ventricular wall damage in SHRs
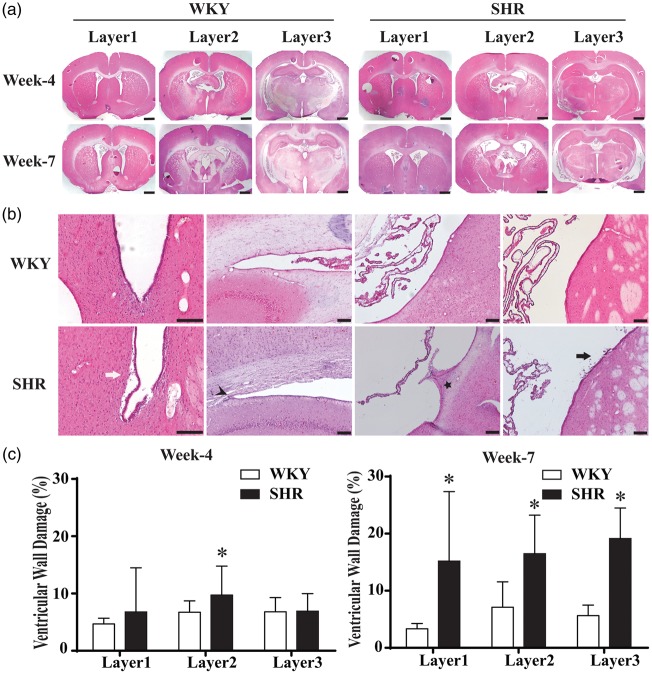
To investigate periventricular changes that might be associated with hydrocephalus development in SHRs, ventricular wall damage was analyzed using H & E-stained sections. Three levels (Figure 4(a)) were examined representing anterior lateral ventricle, connection of lateral ventricle and third ventricle, and posterior part of lateral ventricle. Periventricular damage was found in the SHR including ependymal cells detaching from subventricular zone, periventricular white matter injury, bulging of the ependymal layer into the ventricle, and cell shedding from the ependymal. Examples are shown in Figure 4(b). Quantification of ventricular wall damage demonstrated that SHRs had significantly more periventricular injury than WKY rats in layer 2 at four weeks (9.8 ± 5.1 vs. 6.6 ± 2.0%, P < 0.05; Figure 4(c)). However, at week 7, ventricular wall damage was much more severe in SHRs compared to WKY rats in all three layers (15.2 ± 11.6 vs. 3.4 ± 0.9% on layer 1, 16.5 ± 6.5 vs. 7.0 ± 3.9% on layer 2 and 19.1 ± 5.1 vs. 5.8 ± 1.7% on layer 3, P < 0.01, n = 6; Figure 4(d)). Thus, hydrocephalus in the SHR may cause or be the result of periventricular injury.
Figure 4.
Ventricular wall damage in SHR and WKY rats at weeks 4 and 7. (a) Hematoxylin and eosin- (H & E) stained sections were examined at three different layers of ventricular system for ventricular wall damage. Scale bar = 1 mm. (b) Typical ventricle wall injury including detachment of ependymal layer (white arrow), sparse white matter (arrowhead), bulge (star) and cells shedding (black arrow) was found in SHRs at week 7. Scale bar = 50 μm (high magnification) and 100 μm (low magnification). (c) Quantification of ventricle wall damage (percentage of total perimeter). SHRs had more damage on layer 2 at week 4 and on all the three layers at week 7. Values are means ± SD; *P < 0.05 compared with WKY group; n = 6.
Minocycline ameliorates hydrocephalus in SHRs
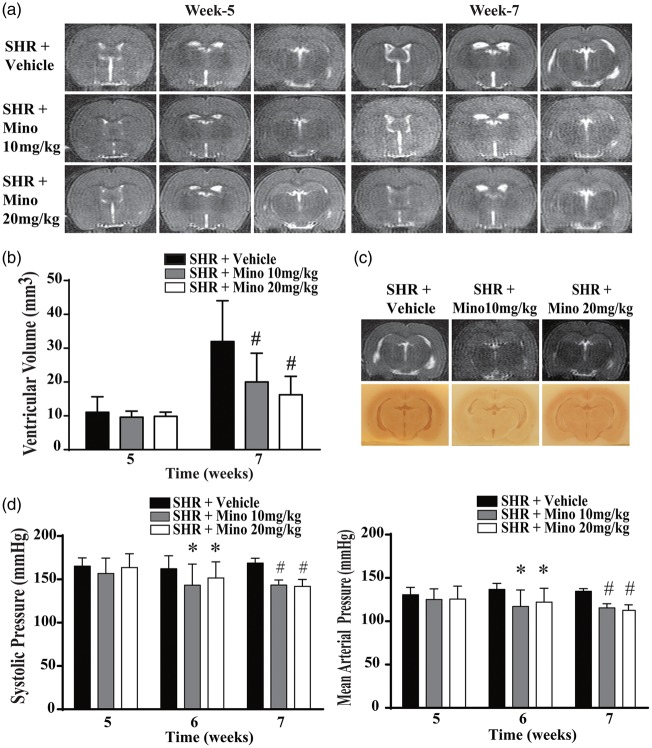
Previous studies have suggested that minocycline can reduce kaolin- and germinal matrix hemorrhage-induced hydrocephalus.20,26 In addition, the results presented above indicate that the hydrocephalus in SHRs is associated with epiplexus macrophage activation and minocycline can block macrophage/microglial activation.27 Therefore, the effectiveness of minocycline on reducing hydrocephalus in SHRs was examined. Two different dosages (10 and 20 mg/kg) were used in SHRs, starting at five weeks of age, with treatment for two weeks. No side effects and no body weight loss were noticed at either dose. At week 7, both doses of minocycline strikingly reduced ventricular dilation on T2 images compared to the SHR + Vehicle groups (Figure 5(a)). The ventricular volume in the SHR +Vehicle, SHR + Mino 10 mg/kg and SHR + Mino 20 mg/kg groups (n = 9 per group) were 32.0 ± 12.0, 20.0 ± 8.5 and 16.2 ± 5.5 mm3, respectively (Figure 5(b), P < 0.01 vs. vehicle). No significant difference was found between the SHR + Mino 10 mg/kg and SHR + Mino 20 mg/kg groups.
Figure 5.
Minocycline reduced hydrocephalus and hypertension in SHRs at week 7. T2 images (a) and quantification of ventricular volume (b) at weeks 5 and 7 in animals treated with vehicle, 10 or 20 mg/kg minocycline (mino) from week 5. Both minocycline doses reduced the ventricular enlargement found at week 7. (c) The effects of minocycline on hydrocephalus were confirmed by examining coronal sections of frozen brains. Representative MRIs and frozen sections from the three groups. (d) Minocycline treatment also significantly reduced systolic and mean arterial pressures at weeks 6 and 7. Values are means ± SD, *P < 0.05 compared with SHR + vehicle group, #P < 0.01 compared with SHR + vehicle group; n = 9.
Interestingly, minocycline treatment reduced blood pressure. Thus, at week 6 (one week of treatment), systolic blood pressure was significantly reduced in SHR +Mino 10 mg/kg group and SHR + Mino 20 mg/kg group compared to SHR + Vehicle group (143 ± 24 and 152 ± 19 mmHg vs. 166 ± 15 mmHg, P < 0.05) as it was at week 7 after two weeks of treatment (143 ± 6 and 142 ± 8 mmHg vs. 172 ± 7 mmHg, P < 0.01; Figure 5(d)). Similar reductions were also found in mean arterial pressure (Figure 5(d)).
Minocycline reduced epiplexus macrophage activation and protected against CP cell death in SHRs
Immunohistochemistry showed that minocycline reduced epiplexus macrophages (both Iba1 and CD68 positive) in the CP in SHRs. We also found that epiplexus macrophage soma sizes were smaller in minocycline-treated groups (Figure 6, P < 0.01 vs. the vehicle-treated group).
Figure 6.
Minocycline reduced epiplexus macrophage activation in SHRs. (a) Examples of choroid plexus (CP) Iba-1 immunohistochemistry (low and high magnification) in SHRs treated with 10 or 20 mg/kg minocycline (mino). Rats were treated for two weeks and examined at week 7. Note the reduction in the Iba-1 immunoreactivity in cells on the apical CP surface with minocycline treatment. Bar graphs showing quantification of the number of Iba-1 cells (as a % of all cells) with and without minocycline treatment and measurement of the size of the macrophages. Values are means ± SD, #P < 0.01 compared with SHR + Vehicle group, n = 9. Scale bar = 50 μm (low magnification) and 10 μm (high magnification). (b) CD68 immunohistochemistry in SHRs treated with 10 or 20 mg/kg minocycline (mino). Values are means ± SD, #P < 0.01 compared with SHR + Vehicle group, n = 9. Scale bar = 50 μm.
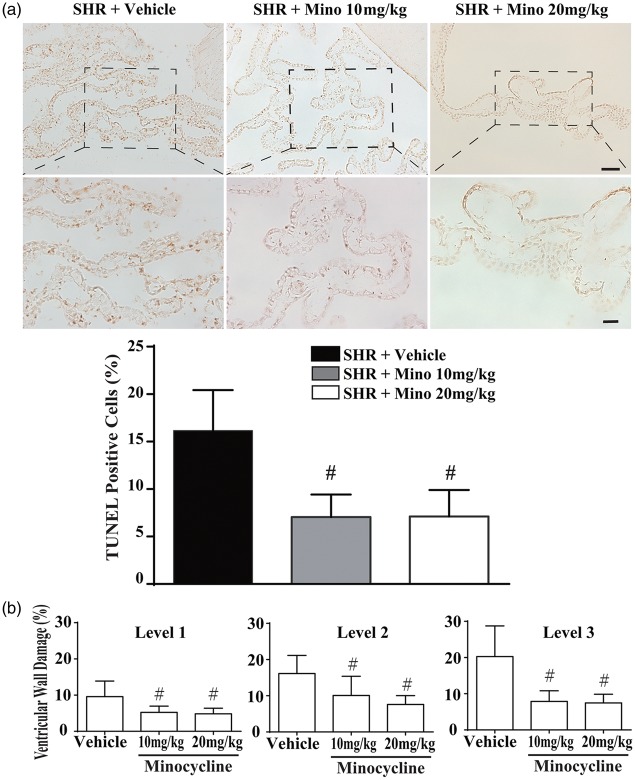
To examine CP damage further, double-stranded DNA fragmentation was analyzed by TUNEL staining at week 7. Cell death in the SHR CP was ameliorated by minocycline treatment (10 or 20 mg/kg) compared to vehicle (Figure 7(a)). Quantification of the number of TUNEL-positive cells showed reduced numbers with minocycline treatment (7.1 ± 2.3% in SHR + Mino 10 mg/kg group and 7.1 ± 2.7% in SHR + Mino 20 mg/kg group compared to 16.8 ± 4.5% in the SHR + Vehicle group; P < 0.01; Figure 7(a)). No significant difference was observed between the two-minocycline doses.
Figure 7.
Cell death in the choroid plexus and ventricular wall damage were alleviated by minocycline. (a) Examples of TUNEL staining in the choroid plexus in SHRs treated with vehicle or 10 or 20 mg/kg minocycline. Note the reduced prevalence of TUNEL-positive cells with minocycline treatment. Quantification of the number of TUNEL-positive cells in the choroid plexus (at level 1). Values are #P < 0.01 compared with SHR + vehicle group; n = 9. Scale bar = 50 μm (low magnification) and 20 μm (high magnification). (b) Quantification of ventricular damage in the three groups. Both minocycline groups had reduced ventricular wall damage compared to the vehicle group. Values are means ± SD, #P < 0.01 compared with SHR + vehicle group, n = 9.
Minocycline reduced periventricular damage in SHRs
We further evaluated whether minocycline would reduce ventricular wall damage in SHRs. H & E staining showed that injuries, such as detachment and cell loss, were attenuated by minocycline. Quantification confirmed a reduction in ventricular wall damage in both the 10 mg/kg and 20 mg/kg minocycline groups compared to vehicle (Figure 7(b)); for example, at layer 3 (7.9 ± 2.8 and 7.5 ± 2.3% vs. 20.3 ± 8.2% with vehicle, P < 0.01). However, there was no significant difference in efficacy between the two-minocycline doses (Figure 7(b)).
Discussion
The major findings of this study are: (1) spontaneous non-obstructive hydrocephalus occurred in SHRs at seven weeks of age but not at four or five weeks, (2) the onset of hydrocephalus was associated with activation of epiplexus macrophages, CP injury and periventricular damage; (3) administration of either 10 or 20 mg/kg minocycline for two weeks significantly reduced the ventriculomegaly, epiplexus macrophage activation, CP cell death and ventricular wall damage at week 7 in SHRs.
In the current study, all SHRs had ventriculomegaly by week 7, but not at four and five weeks. In 1986, Ritter et al. measured ventricular areas in SHR, WKY and Sprague-Dawley rats on three different coronal sections at weeks 4, 8, 12, 16, 21 and 56, and reported progressive ventricular dilation in SHRs that dramatically expanded at week 8 and persisted long term.6 In a subsequent study, an angiotensin-converting enzyme inhibitor, captopril (started at week 3), reduced blood pressure in SHRs but failed to attenuate the ventriculomegaly that started from week 8.7 However, consecutive measurements of ventricular volume were not performed in those studies. The ventriculomegaly in SHRs at three months of age was confirmed by MRI.28 Another study also noticed hydrocephalus as well as white matter injury by using MRI at age of 35 weeks in SHRs.29 These studies indicate the potential of the SHR as a model of spontaneous hydrocephalus. In this study, we used MRI T2 image to consecutively evaluate the early phase of hydrocephalus in SHRs in comparison to the parent WKY strain. Consistent with previous studies, no obvious ventricular dilation was observed at weeks 4 and 5. However, a more than 2-fold enlargement in ventricular size and serious damage to the ventricular wall and CP occurred in all SHRs at week 7, demonstrating week 7 is an important time point for hydrocephalus onset in SHRs.
Brain tissue loss can result in ventricular enlargement (i.e. hydrocephalus ex vacuo). It is important to differentiate hydrocephalus ex vacuo from communicating/noncommunicating hydrocephalus. In the young SHRs (week 7), we found significant changes in CP and the ventricular wall, as well as enlarged ventricles. We then measured brain tissue volumes of two hemispheres and found there were no differences between WKY rats and SHRs at week 7. It should be noted that marked brain tissue loss occurs in older SHRs (e.g. week 35).29 This suggests the possibility that the hydrocephalus (or underlying mechanisms) may be the cause rather than the result of brain tissue loss.
The precise underlying mechanisms of the hydrocephalus in SHR and why it should start to develop around week 7 have not been elucidated. There are a number of events occurring in the SHRs during development including high blood pressure, CP cell death, epiplexus cell activation, ventricular wall damage and ventricular enlargement. Which events trigger other events is still not certain. One possibility was that it might be related to hypertension. However, as noted above, Ritter et al.7 found that reducing blood pressure in the SHR did not reduce the ventriculomegaly. Our data also provide support for the concept that hypertension is not the direct cause of the hydrocephalus as the onset of ventricular dilatation occurred at seven weeks even though hypertension in SHRs developed much earlier. An alternate hypothesis is that the hydrocephalus may be related to activation of epiplexus macrophages, periventricular damage and/or CP injury. The role of CP hemorrhage and cell death in hydrocephalus development needs to be examined further. It is noticeable that in the current study the reduction in hydrocephalus with minocycline treatment was associated with reductions in each of those parameters. It is interesting, however, that there is evidence that perivascular macrophage activation occurs in animal models of hypertension and that targeting those macrophages reduces both neurovascular and cognitive dysfunction.30 In combination with our results, this suggests a critical role of macrophages at the interface between blood and brain/CSF in brain injury.
We demonstrated that epiplexus macrophage activation occurs in SHRs developing hydrocephalus. In another rat model of hydrocephalus, that induced by a maternal injection of 6-aminonicotinamide, the morphology of epiplexus macrophages was also changed dramatically.9 The physiological/pathophysiological roles of epiplexus cells, also known as Kolmer cells, are largely unknown.31 They may act as a surveillance mechanism, responding to the entry of compounds or organisms across the CP epithelium into CSF. Thus, the activation of these cells in the SHR might reflect damage to the CP. However, macrophages/microglia also have a role in inducing brain injury after cerebral hemorrhage and inflammation has a role in hydrocephalus development.1 It is well known that M1 macrophage polarization can produce reactive oxygen species,32 which might cause double-strain DNA damage (i.e. TUNEL positive). In the current study, we found that significant M1 polarization occurred in epiplexus macrophages and many epiplexus macrophages were CD68 positive. Thus, these cells might participate in CP injury. Although it is uncertain whether activation of epiplexus macrophages contributes to hydrocephalus, we found that minocycline, a known inhibitor of macrophage activation, reduced epiplexus cell activation and attenuated hydrocephalus development in SHRs. Future studies should determine if epiplexus cells have a key role in cerebrospinal fluid production and hydrocephalus.
In the current study, we noticed CP cell death and hemorrhage in SHRs at seven weeks. TEM showed evidence of damaged epithelial cells with many vesicles. We also noticed a marked increase in the numbers of cyst spaces in CP cells which is in line with a previous report.33,34 Importantly, TUNEL staining demonstrated increased CP cell death in SHRs compared to WKY rats. CP damage might contribute to hydrocephalus by disrupting blood–CSF barrier and promoting bleeding. However, bleeding in CP may also result in CP cell death. Hydrocephalus is commonly found in intraventricular hemorrhage, and clinically is a risk factor for poor outcome in both ICH and SAH patients.35 Blood components have a key role in the process of hydrocephalus and ependymal cells damage. We previously demonstrated that intraventricular injection of hemoglobin and iron resulted in significant ventricular enlargement in neonatal rats.3 We also observed hydrocephalus after intraventricular injection of lysed red blood cells, iron and thrombin in adult rats.2,16 On the other hand, thrombin also contributes to hydrocephalus development following intraventricular hemorrhage. In the current study, in addition to CP damage, there was some evidence of bleeding from the CP in SHRs.
The current study also found significant damage to the ventricle wall (ependyma) in SHRs. Whether this is the cause or the result of the hydrocephalus is still uncertain. It has been hypothesized that ependymal loss may contribute to the development of hydrocephalus via a variety of mechanisms including loss of cilia and altered CSF flow, and dysregulation of communication across the ependymal affecting underlying structures such as the subventricular zone and periventricular white matter.36
Minocycline is a blood–brain barrier permeable tetracycline. It has been shown to have anti-inflammatory and anti-apoptotic activities, it can inhibit proteolysis, and regulate immune cell activation and proliferation in different models of CNS disease.37 In SHRs, minocycline prevented decreases in brain endothelial tight junction proteins, such as claudin-5, occludin, and ZO-1, while increasing angiogenic factors and oligodendrocyte proliferation in neuroinflammatory white matter, and showed efficacy in blocking blood–brain barrier disruption associated with chronic hypoxia.38 Moreover, minocycline can attenuate HIF-1α-induced neuroinflammation in white matter by reducing MMP-9 expression and secondary blood–brain barrier leakage in ischemic SHR stroke prone rats.39 In relation to hydrocephalus, minocycline reduced ventricular extension and periventricular reactive gliosis (upregulation of GFAP and Iba-1) in different hydrocephalic animal models.19–21 The current study shows that minocycline can reduce ventriculomegaly as well as CP cell death and periventricular damage in SHRs at the early stage of hydrocephalus formation. The mechanism underlying this protection may relate to the ability of minocycline to inhibit inflammation and/or chelate iron. Our previous studies have shown that iron has a role in intraventricular hemorrhage-induced hydrocephalus. Deferoxamine, an iron chelation agent, successfully reduced enlarged ventricular size after intraventricular injection of iron and hemoglobin.3,16 In addition, minocycline can attenuate iron overload after intracerebral hemorrhage and iron-induced brain injury.17 However, there is also considerable evidence on the anti-inflammatory actions of minocycline.37 For example, minocycline inhibited the MMP-9 pathway preserving endothelial junctions and blood–brain barrier integrity in condition of hydrocephalus.39,40 Future studies should examine whether MMPs have a role in CP damage and hydrocephalus development.
There are several limitations of this study. (1) The role of sex in hydrocephalus development in SHRs was not examined. It is important to understand if hydrocephalus occurs in female SHRs. 92) The effects of minocycline on hydrocephalus development were only tested in male rats. Although minocycline reduces cerebral hemorrhage-induced brain injury in both males and females,18,41 it only reduces ischemic brain injury in males.42 (3) Minocycline was given before hydrocephalus developed. It is important to know whether minocycline can attenuate hydrocephalus development after ventricular dilatation has started (e.g. week 7 in SHR). (4) No functional outcomes were measured. It is time to develop sensitive behavioral tests for early stage of hydrocephalus development. (5) A causal relationship between epiplexus macrophage activation and hydrocephalus has not established.
In conclusion, this study provides evidence that spontaneous non-obstructive hydrocephalus (persistent ventricular dilatation) occurs by week 7 in male SHRs. Epiplexus macrophage activation, CP cell death, hemorrhage and periventricular damage were observed in concert with the ventricular dilation. Minocycline treatment reduced hydrocephalus, epiplexus macrophage activation, as well as choroid plexus cell death and periventricular damage in SHRs suggesting it may be a therapeutic approach in some forms of hydrocephalus.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants NS-073595, NS-091545, NS-090925, NS-096917, and NS-106746 from the National Institutes of Health (NIH).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
Conception and design of the study: GX, YH and RFK. Acquisition and analysis of data: CG, XH, YH, JL and GX. Drafting the text or preparing the figures: CG, XH, YH, RFK, and GX. Drs. Gu and Hao contributed equally in this study.
References
- 1.Strahle J, Garton HJ, Maher CO, et al. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res 2012; 3: 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao F, Liu F, Chen Z, et al. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab 2014; 34: 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strahle JM, Garton T, Bazzi AA, et al. Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurgery 2014; 75: 696–705. discussion 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Q, Feng Z, Tan Q, et al. Post-hemorrhagic hydrocephalus: recent advances and new therapeutic insights. J Neurol Sci 2017; 375: 220–230. [DOI] [PubMed] [Google Scholar]
- 5.Mao X, Enno TL, Del Bigio MR. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci 2006; 23: 2929–2936. [DOI] [PubMed] [Google Scholar]
- 6.Ritter S, Dinh TT. Progressive postnatal dilation of brain ventricles in spontaneously hypertensive rats. Brain Res 1986; 370: 327–332. [DOI] [PubMed] [Google Scholar]
- 7.Ritter S, Dinh TT, Stone S, et al. Cerebroventricular dilation in spontaneously hypertensive rats (SHRs) is not attenuated by reduction of blood pressure. Brain Res 1988; 450: 354–359. [DOI] [PubMed] [Google Scholar]
- 8.Schwarze EW. The origin of (Kolmer's) epiplexus cells. A combined histomorphological and histochemical study. Histochemistry 1975; 44: 103–104. [DOI] [PubMed] [Google Scholar]
- 9.Ling EA, Tseng CY, Wong WC. An electron microscopical study of epiplexus and supraependymal cells in the prenatal rat brain following a maternal injection of 6-aminonicotinamide. J Anatomy 1985; 140(Pt 1): 119–29. [PMC free article] [PubMed] [Google Scholar]
- 10.Ling EA, Kaur C, Lu J. Origin, nature, and some functional considerations of intraventricular macrophages, with special reference to the epiplexus cells. Microscopy Res Tech 1998; 41: 43–56. [DOI] [PubMed] [Google Scholar]
- 11.Murata Y, Rosell A, Scannevin RH, et al. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke 2008; 39: 3372–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machado LS, Sazonova IY, Kozak A, et al. Minocycline and tissue-type plasminogen activator for stroke: assessment of interaction potential. Stroke 2009; 40: 3028–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yenari MA, Xu L, Tang XN, et al. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke 2006; 37: 1087–1093. [DOI] [PubMed] [Google Scholar]
- 14.Wasserman JK, Schlichter LC. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Exp Neurol 2007; 207: 227–237. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Yang S, Xi G, et al. Minocycline reduces intracerebral hemorrhage-induced brain injury. Neurol Res 2009; 31: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao C, Du H, Hua Y, et al. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab 2014; 34: 1070–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao F, Hua Y, He Y, et al. Minocycline-induced attenuation of iron overload and brain injury after experimental intracerebral hemorrhage. Stroke 2011; 42: 3587–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao S, Hua Y, Keep RF, et al. Minocycline effects on intracerebral hemorrhage-induced iron overload in aged rats: brain iron quantification with magnetic resonance imaging. Stroke 2018; 49: 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang J, Chen Q, Guo J, et al. Minocycline attenuates neonatal germinal-matrix-hemorrhage-induced neuroinflammation and brain edema by activating cannabinoid receptor 2. Mol Neurobiol 2016; 53: 1935–1948. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Tan G, Zhang S, et al. Minocycline reduces reactive gliosis in the rat model of hydrocephalus. BMC Neurosci 2012; 13: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAllister JP, 2nd, Miller JM. Minocycline inhibits glial proliferation in the H-Tx rat model of congenital hydrocephalus. Cerebrospinal Fluid Res 2010; 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang G, Yang Y, Wu G, et al. Early erythrolysis in the hematoma after experimental intracerebral hemorrhage. Transl Stroke Res 2017; 8: 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okubo S, Strahle J, Keep RF, et al. Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke 2013; 44: 547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu S, Xi G, Jin H, et al. Thrombin-induced autophagy: a potential role in intracerebral hemorrhage. Brain Res 2011; 1424: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu R, Cao S, Hua Y, et al. CD163 Expression in Neurons After Experimental Intracerebral Hemorrhage. Stroke 2017; 48: 1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo J, Chen Q, Tang J, et al. Minocycline-induced attenuation of iron overload and brain injury after experimental germinal matrix hemorrhage. Brain Res 2015; 1594: 115–124. [DOI] [PubMed] [Google Scholar]
- 27.Tikka T, Fiebich BL, Goldsteins G, et al. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 2001; 21: 2580–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bendel P, Eilam R. Quantitation of ventricular size in normal and spontaneously hypertensive rats by magnetic resonance imaging. Brain Res 1992; 574: 224–228. [DOI] [PubMed] [Google Scholar]
- 29.Kaiser D, Weise G, Moller K, et al. Spontaneous white matter damage, cognitive decline and neuroinflammation in middle-aged hypertensive rats: an animal model of early-stage cerebral small vessel disease. Acta Neuropathol Commun 2014; 2: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faraco G, Sugiyama Y, Lane D, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest 2016; 126: 4674–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghersi-Egea JF, Strazielle N, Catala M, et al. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol 2018; 135: 337–361. [DOI] [PubMed] [Google Scholar]
- 32.Zhao H, Garton T, Keep RF, et al. Microglia/macrophage polarization after experimental intracerebral hemorrhage. Transl Stroke Res 2015; 6: 407–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanat A, Turkmenoglu O, Aydin MD, et al. Toward changing of the pathophysiologic basis of acute hydrocephalus after subarachnoid hemorrhage: a preliminary experimental study. World Neurosurg 2013; 80: 390–395. [DOI] [PubMed] [Google Scholar]
- 34.Baas D, Meiniel A, Benadiba C, et al. A deficiency in RFX3 causes hydrocephalus associated with abnormal differentiation of ependymal cells. Eur J Neurosci 2006; 24: 1020–1030. [DOI] [PubMed] [Google Scholar]
- 35.Xiang J, Routhe LJ, Wilkinson DA, et al. The choroid plexus as a site of damage in hemorrhagic and ischemic stroke and its role in responding to injury. Fluids Barriers CNS 2017; 14: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jimenez AJ, Dominguez-Pinos MD, Guerra MM, et al. Structure and function of the ependymal barrier and diseases associated with ependyma disruption. Tissue Barriers 2014; 2: e28426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrido-Mesa N, Zarzuelo A, Galvez J. What is behind the non-antibiotic properties of minocycline? Pharmacol Res 2013; 67: 18–30. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Kimura-Ohba S, Thompson JF, et al. Vascular tight junction disruption and angiogenesis in spontaneously hypertensive rat with neuroinflammatory white matter injury. Neurobiol Dis 2018; 114: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jalal FY, Yang Y, Thompson JF, et al. Hypoxia-induced neuroinflammatory white-matter injury reduced by minocycline in SHR/SP. J Cereb Blood Flow Metab 2015; 35: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaturvedi M, Kaczmarek L. Mmp-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol 2014; 49: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai S, Hua Y, Keep RF, et al. Minocycline attenuates brain injury and iron overload after intracerebral hemorrhage in aged female rats. Neurobiol Dis 2018. S0969-9961(18)30173-6. Epub ahead of print 5 June 2018. DOI: 10.1016/j.nbd.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab 2009; 29: 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]