Abstract
Background:
Microsatellite instability (MIN) has been studied in a variety of carcinomas and gynecologic sarcomas, but never in musculoskeletal sarcomas.
Methods:
We evaluated 16 skeletal and soft tissue sarcomas at nine genetic loci from chromosomal regions 1q, 5q, 7q, 12p, 13q, 17p, 19q, and two at 11p—all potential regions of interest regarding musculoskeletal sarcomas.
Results:
MIN was identified at one or more loci in seven of the cancers studied (44%). Three tumors had more than one locus with MIN and one tumor, a high-grade osteogenic sarcoma, had five of nine loci positive for MIN.
Conclusion:
These results indicate that musculoskeletal sarcomas show instability in areas inside and outside the loci of known oncogenes. Areas of mismatch repair, as heralded by MIN, may contribute to the vast heterogeneity of these neoplasms.
Keywords: Microsatellite instability, Musculoskeletal oncology, Musculoskeletal sarcoma, Neoplasm, PCR, Sarcoma
Sarcomas are a structurally diverse group of rare, malignant connective tissue neoplasms; a total of 2500 skeletal and 6400 soft tissue sarcomas are diagnosed annually in the United States.1 Osteogenic sarcoma (OGS), the most common skeletal sarcoma, illustrates the heterogeneity seen in these cancers. It can occur sporadically, or in association with Paget’s disease, fibrous dysplasia, osteochondromatosis, osteoblastoma, Ollier’s disease, hereditary retinoblastoma, Li-Fraumeni syndrome, or after treatment with radiation or chemotherapy. OGS develops in 12% of bilateral retinoblastoma patients—approximately 2000 times the normal rate.2–4 Seventy percent of OGS patients are known to have a dysfunctional retinoblastoma gene.5,6 Li-Fraumeni syndrome is an inherited disorder caused by a germ-line mutation of the p53 gene. People with this mutation have an unusually high incidence of sarcomas of bone and soft tissue, breast cancer, and other tumors, with 6% of those afflicted developing OGS.7,8
Microsatellites are short oligonucleotide repeats that are dispersed throughout the human genome and exhibit length polymorphism. These microsatellites vary in length from person to person, but are identical in any one person. Some tumor cells show changes in the length of these microsatellites compared with normal cells, indicating that the DNA sequences have gained or lost base pairs during tumor replication. This change in allelic size is termed microsatellite instability (MIN). Because microsatellites are highly polymorphic and are easily analyzed by polymerase chain reaction (PCR), they are valuable genetic markers for the altered phenotypes seen in cancers. Microsatellite instability has been shown to be a signal of defective DNA mismatch repair in several neoplasms. It has been reported in tumors as different as hereditary nonpolyposis colorectal cancer and melanoma. It is not known if mismatch repair, as evidenced by MIN, plays a role in the oncogenesis of skeletal or soft tissue sarcomas.
To determine whether MIN is present in musculoskeletal sarcomas with a particular focus on OGS, we performed microsatellite analysis on neoplastic and normal tissue from several patients with these tumors. In this paper we report our findings and compare the rate of MIN in musculoskeletal sarcomas to that seen in other neoplasms.
METHODS
Fresh or fresh-frozen neoplastic and normal control tissue was obtained intraoperatively from 16 patients (8 males and 8 females) with musculoskeletal sarcomas. Institutional Review Board approval was obtained for use of specimens from human subjects. The histogenesis of the six skeletal sarcomas and 10 soft tissue sarcomas included the following: osteogenic sarcoma (4); malignant fibrous histiocytoma (3); spindle cell sarcoma (2); liposarcoma (2); neurofibrosarcoma (1); chondrosarcoma (1); synovial cell sarcoma (1); fibrosarcoma (1); and hemangiopericytoma (1). The average age of the patients was 53 years (range 8 to 88 years; Table 1). DNA was extracted routinely after tissue pulverization.9,10 Standard PCR amplification was performed on the DNA from both normal and tumor specimens at nine separate microsatellites localized to specific chromosome regions implicated in malignancies: 1q32-q41 (D1S158); 5q11.2–13.3 (D5S107); 7q (D7S594); 11p13-p14 (D11S904); 11p15.2 (D11S861); 12p13.2-p13.3 (vWF-TNR); 13q14.2 (D13S170); 17p13.1 (D17S786); and 19q13.3 (DM). Heterozygosity indices were 89%, 82%, 84%, 83%, 70%, 73%, 90%, 77%, and 73%, respectively. These loci were chosen because they were areas of instability seen in other tumors studied for MIN or because they were in areas of reported cytogenetic abnormalities, such as RB and p53, which are seen in OGS.11–15 Oligonucleotide primers in our study were obtained from Research Genetics (Huntsville, AL).
TABLE 1.
Clinical and biochemical data from patients with skeletal and soft tissue sarcomas
| Patient | Age (y) | Sex | Histogenesis | Grade | Location | Surgery | Microsatellite instability | Pretreatment | Status |
|---|---|---|---|---|---|---|---|---|---|
| Skeletal sarcomas | |||||||||
| 1 | 8 | M | Osteosarcoma | High | Femur | Resection | Chemo | CDFS for 6 mo | |
| 2 | 48 | M | Osteosarcoma | High | Tibia | Resection | Chemo | CDFS for 4 mo | |
| 3 | 69 | M | Osteosarcoma | High | Femur | Amputation | D1S158, D11S861, D7S594, D17S786, D5S107 | Chemo | DWD after 11 mo |
| 4 | 53 | F | Osteosarcoma | Low | Femur | Resection | None | CDFS for 6 mo | |
| 5 | 60 | M | Hemangiopericytoma | Low | Scapula | Resection | DM | None | CDFS for 27 mo |
| 6 | 47 | M | Chondrosarcoma | High | Femur | Amputation | D13S170 | None | DWD after 24 mo |
| Soft tissue sarcomas | |||||||||
| 7 | 65 | F | Malignant fibrous histiocytoma | High | Thigh | Resection | XRT | AWD after 16 mo | |
| 8 | 65 | F | Malignant fibrous histiocytoma* | High | Deltoid | Resection | Chemo | DWD after 2 mo | |
| 9 | 88 | F | Malignant fibrous histiocytoma | High | Leg | Resection | None | AWD after 22 mo | |
| 10 | 36 | F | Spindle cell sarcoma | Intermediate | Pelvis | Resection | DM | None | CDFS for 10 mo |
| 11 | 34 | M | Spindle cell sarcoma | Low | Deltoid | Resection | D5S107 | None | CDFS for 15 mo |
| 12 | 56 | F | Liposarcoma (recurrence) | Low | Thigh | Resection | None | CDFS for 25 mo | |
| 13 | 57 | M | Liposarcoma | High | Arm | Resection | XRT | CDFS for 10 mo | |
| 14 | 54 | M | Fibrosarcoma | Intermediate | Thigh | Resection | D13S170, DM | None | CDFS for 22 mo |
| 15 | 77 | F | Neurofibrosarcoma (recurrence) | High | Thigh | Resection | D11S861, D11S904 | XRT | DWD after 7 mo |
| 16 | 33 | M | Synovial sarcoma | High | Foot | Amputation | Chemo | CDFS for 5 mo | |
AWD, alive with disease; CDFS, continuous disease-free survival; Chemo, chemotherapy; DWD, dead with disease; XRT, radiotherapy.
Metastases on presentation.
PCR was performed on 40 ng of neoplastic and control DNA following established protocols.10 The forward primer was end-labeled with gamma 32P-ATP (Amersham Cooperation, Arlington Heights, IL) for each microsatellite. A single PCR machine, model PTC-150 (MJ Research, Inc., Watertown, MA) was used throughout this analysis.
The 10-μL reaction mixture contained 1.25 mM dNTP, 12 mM MgCl2, 10x Taq buffer, Taq DNA polymerase, nuclease-free water, 2 μm forward and reverse oligonucleotide primers, and 0.4 μm end-labeled forward primer. End-labeling was carried out in a 0.6-mL Eppendorf tube with nuclease-free water, 5x kinase buffer, 2 μm forward primer, gamma 32P-ATP, and T4 polynucleotide kinase (Promega, Madison, WI). Previous experience has shown that conditions for PCR vary depending on which primer is used. For example, primer D11S861 was cycled at 25 step cycles with 1 minute at 94°C, 2 minutes at 55°C, 2.5 minutes at 72°C, and 10 minutes at 72°C. Primer 17S786 underwent 27 step cycles with 1 minute at 94°C, 2 minutes at 57°C, and 2 minutes at 72°C followed by 10 minutes at 72°C. Primer DM was cycled at 35 step cycles with 30 seconds at 94°C, 75 seconds at 60°C, 15 seconds at 72°C, and 6 minutes at 72°C. All other primers were cycled at 26 step cycles with 30 seconds at 94°C, 75 seconds at the respective annealing temperature, 15 seconds at 72°C, and 6 minutes at 72°C with the annealing temperatures as follows: D1S158—60°C; D5S107—55°C; D7S594—58°C; D11S904—55°C; D13S170—55°C; and vWF-TNR—50°C. At the conclusion of PCR, 1.5 μL of sample was added to an Eppendorf tube containing 3 μL of stop solution (250 1 of bromphenol blue xylene cyanole dye and 1 mL of formamide). These samples were then denatured for 7 minutes at 95°C.
The samples were immediately placed on ice, and 3.0 μL of the samples were added to a 6% acrylamide gel. The gels were run at 95 watts for 1.5 to 2.5 hours, depending on the probe. After cooling, they were transferred to gel blot paper (Mid-West Scientific, St. Louis, MO). The gel was then covered with cellophane wrap and dried at 60°C for 1 hour on a dryer. The gel was placed in an autoradiography cassette (Fisher Scientific, Pittsburgh, PA) and exposed to radiographic film for 1 to 5 days at −70°C.
RESULTS
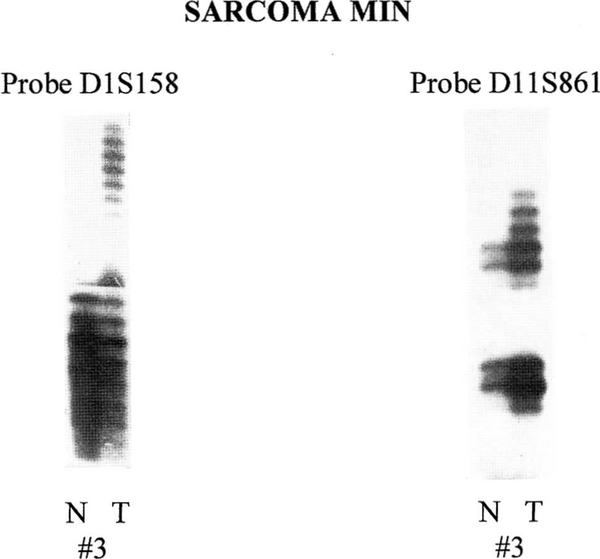
We examined 16 sarcomas and their matched normal tissues as controls, searching for microsatellite instability at nine separate loci. Table 1 displays the clinical and biochemical data of the patients studied, including cancer histogenesis, grade, tumor location, surgical treatment, whether the specimen was pre-exposed to a potentially gene-altering antineoplastic therapy (i.e., chemotherapy or radiation), and current clinical status of the patient. Altered mobility of alleles, indicative of MIN, was seen in 7 of 16 tumors (44%), with three of these tumors showing MIN at multiple loci. Fifty percent of the skeletal sarcomas had MIN, and one high-grade OGS exhibited MIN at five of nine loci. Loss of heterozygosity was not evident in any of these tumors. Figures 1 and 2 show representative microsatellite data generated by PCR amplification of genomic DNA from normal and tumor tissue from skeletal and soft tissue sarcoma patients.
FIG. 1.
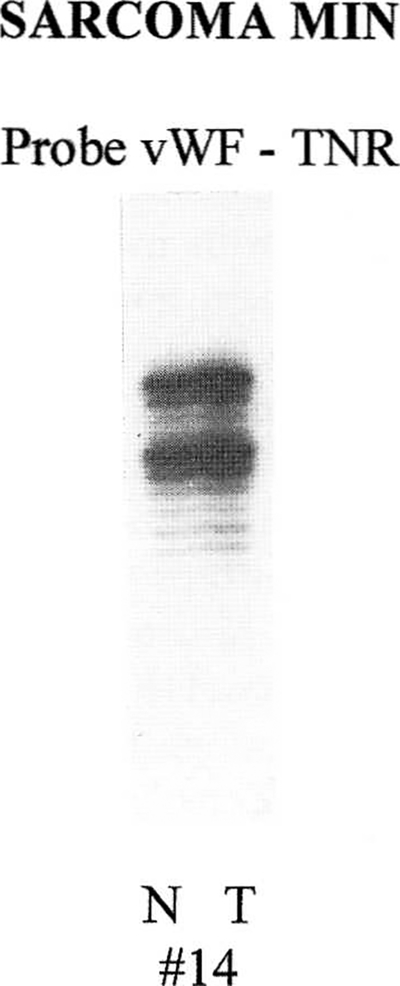
Microsatellite instability study showing no change in allele size at locus 12p (vWF-TNR) when comparing normal (N) and tumor (T) from patient 14.
FIG. 2.
Patient 3 shows microsatellite instability at loci 1q (D1S158) and 11p (D11S861) when comparing normal (N) and tumor (T) tissue.
The current clinical status of patients in the study is as follows: four patients dead of disease; two patients alive with disease; and ten continuous disease-free survivors. Neither of the patients alive with disease had any loci positive for MIN, nor did six of the ten continuous disease-free survivors. Three of the four deceased patients showed MIN. Preoperatively, two of seven patients with MIN had treatment, one with chemotherapy and the other with radiation therapy.
DISCUSSION
Microsatellite instability, not previously explored in musculoskeletal sarcomas, was identified in 44% of our patients. MIN was seen in 50% of the skeletal sarcomas. This compares to previous studies, which identified the rate at which repetitive DNA segments undergo length changes in normal human somatic cells as 0.29%,16 and the incidence of MIN in sporadic carcinomas, which ranges from 11% to 34%.17–22 Hereditary nonpolyposis colon cancer (HNPCC) is a common syndrome with a predisposition for endometrial, gastric, and breast cancer.23–25 Studies have shown MIN to exist in 29% to 54% of colon tumors26,27 and 75% of endometrial carcinomas in patients with HNPCC.28 In addition, four DNA mismatch repair genes (hMSH2, hMLH1, hPMS1, and hPMS2) are known to be mutated in the germ line cells of patients with HNPCC.29
Two previous studies on sarcomas have been conducted. One examined a small number of soft tissue sarcomas and found 11.1% with MIN,14 and the other reported MIN in 25% of gynecologic sarcomas.12 Contrary to studies that show HNPCC patients to have MIN at multiple loci while having a more favorable prognosis, sarcoma patients with MIN were shown to have a worse prognosis, with the majority having MIN at a single locus.12,14 Our current data support this finding. Of the 12 patients in our study who are alive, 66% had no evidence of MIN, whereas 75% of the 4 deceased patients showed MIN.
Also contrary to the previous study on gynecologic sarcomas, three of our seven patients displayed MIN at multiple loci. The presence of MIN outside the loci of known tumor suppressor oncogenes RB (13q14.2) and p53 (17p13–13.3), and the presence of MIN at multiple loci demonstrate that many mechanisms may contribute to the oncogenesis of the numerous types of skeletal and soft tissue sarcomas and to the heterogeneity of these neoplasms.
The inherent ability of PCR to assess microsatellites facilitates the evaluation of the many types of sarcomas, including OGS. This technology may help in the genetic classification of the clinically diverse subtypes of skeletal and soft tissue sarcomas, and, therefore, help to identify significant prognostic factors that could alter treatment plans. Further studies of MIN in sarcomas and its role in oncogenesis, prognosis, and clinical management are warranted as musculoskeletal sarcomas are added to the growing list of malignancies that demonstrate somatic alterations in the repeat length of microsatellites.
Acknowledgments:
We would like to thank Janie Falkenberg and Greg S. Martin, MD, for help with preparation of the manuscript. Support for this project was received by a grant from the Vanderbilt University Research Council.
REFERENCES
- 1.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. CA Cancer J Clin 1996;46:5–27. [DOI] [PubMed] [Google Scholar]
- 2.Dryja TP, Rapaport JM, Epstein J, Goorin AM, Weichselbaum R, Koufos A, Cavenee WK. Chromosome 13 homozygosity in osteosarcoma without retinoblastoma. Am J Hum Genet 1986; 138:59–66. [PMC free article] [PubMed] [Google Scholar]
- 3.Gallie BL. Retinoblastoma gene mutations in human cancer. N Engl J Med. 1994;330:786–7. [DOI] [PubMed] [Google Scholar]
- 4.Hansen MF, Koufos A, Gallie BL, et al. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sei USA 1985;82:6216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goddard AD, Balakier H, Canton M, et al. Infrequent genomic rearrangement and normal expression of the putative RB1 gene in retinoblastoma tumors. Mol Cell Biol 1988;8:2082–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen MF. Molecular genetic considerations in osteosarcoma. Review. Clin Orthop 1991;270:237–46. [PubMed] [Google Scholar]
- 7.Li FP, Fraumeni JF, Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA, Miller RW. A cancer family syndrome in twenty-four kindreds. Cancer Res 1988;48:5358–62. [PubMed] [Google Scholar]
- 8.Toguchida J, Yamaguchi T, Dayton SH, et al. Prevalence and spectrum of germline mutations of the p53 gene among patients with sarcoma. N Engl J Med 1992;326:1301–8. [DOI] [PubMed] [Google Scholar]
- 9.Butler MG, Sciadini M, Hedges LK, Schwartz HS. Chromosome telomere integrity of human solid neoplasms. Cancer Genet Cytogenet 1996;86:50–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheiner M, Hedges L, Schwartz HS, Butler MG. Lack of microsatellite instability in giant cell tumor of bone. Cancer Genet Cy-togenet 1996;88:35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoogerwerf WA, Hawkins AL, Perlman EJ, Griffin CA. Chromosome analysis of nine osteosarcomas. Genes Chromosomes Cancer 1994;9:88–92. [DOI] [PubMed] [Google Scholar]
- 12.Risinger JI, Umar A, Boyer JC, Evans AC, Berchuck A, Kunkel TA, Barrett JC. Microsatellite instability in gynecological sarcomas and in hMSH2 mutant uterine sarcoma cell lines defective in mismatch repair activity. Cancer Res 1995;55:5664–9. [PubMed] [Google Scholar]
- 13.Talwalkar VR, Scheiner M, Hedges LK, Butler MG, Schwartz HS. Microsatellite instability in malignant melanoma. Cancer Genet Cytogenet 1998. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wooster R, Cleton-Jansen AM, Collins N, et al. Instability of short tandem repeats (microsatellites) in human cancers. Nat Genet 1994;6:152–6. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi T, Toguchida J, Yamamuro T, et al. Allelotype analysis in osteosarcomas: frequent allele loss on 3q, 13q, 17p, and 18q. Cancer Res 1992;52:2419–23. [PubMed] [Google Scholar]
- 16.Hackman P, Gabbani G, Osterholm AM, Hellgren D, Lambert B. Spontaneous length variation in microsatellite DNA from human T-cell clones. Genes Chromosomes Cancer 1995;14:215–9. [DOI] [PubMed] [Google Scholar]
- 17.Cawkwell L, Li D, Lewis FA, Martin I, Dixon MF, Quirke P. Microsatellite instability in colorectal cancer: improved assessment using fluorescent polymerase chain reaction. Gastroenterology 1995;109:465–71. [DOI] [PubMed] [Google Scholar]
- 18.Katabuchi H, van Rees B, Lambers AR, et al. Mutations in DNA mismatch repair genes are not responsible for microsatellite instability in most sporadic endometrial carcinomas. Cancer Res 1995; 55:5556–60. [PubMed] [Google Scholar]
- 19.Robledo M, Martinez B, Arranz E, Trujillo MJ, Gonzalez AA, Rivas C, Benitez J. Genetic instability of microsatellites in hematological neoplasms. Leukemia 1995;9:960–4. [PubMed] [Google Scholar]
- 20.Shibata D, Peinado MA, Ionov Y, Malkhosyan S, Perucho M. Genomic instability in repeated sequences is an early somatic event in colorectal tumorigenesis that persists after transformation. Nat Genet 1994;6:273–81. [DOI] [PubMed] [Google Scholar]
- 21.Shridhar V, Siegfried J, Hunt J, del Mar Alonso M, Smith DI. Genetic instability of microsatellite sequences in many non-small cell lung carcinomas. Cancer Res 1994;54:2084–7. [PubMed] [Google Scholar]
- 22.Yee CJ, Roodi N, Verrier CS, Pari FF. Microsatellite instability and loss of heterozygosity in breast cancer. Cancer Res 1994;54: 1641–4. [PubMed] [Google Scholar]
- 23.Bergthorsson JT, Egilsson V, Gudmundsson J, Arason A, Ingvarsson S. Identification of a breast tumor with microsatellite instability in a potential carrier of the hereditary non-polyposis colon cancer trait. Clin Genet 1995;47:305–10. [DOI] [PubMed] [Google Scholar]
- 24.Modrich P. Mismatch repair, genetic stability, and cancer. Science 1994;266:1959–60. [DOI] [PubMed] [Google Scholar]
- 25.Peltomäki P, Lothe RA, Aaltonen LA, et al. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res 1993; 53:5853–5. [PubMed] [Google Scholar]
- 26.Jacoby RF, Marshall DJ, Kailas S, Schlack S, Harms B, Love R. Genetic instability associated with adenoma to carcinoma progression in hereditary nonpolyposis colon cancer. Gastroenterology 1995;109:73–82. [DOI] [PubMed] [Google Scholar]
- 27.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816–9. [DOI] [PubMed] [Google Scholar]
- 28.Risinger JI, Berchuck A, Kohler MF, Watson P, Lynch HT, Boyd J. Genetic instability of microsatellites in endometrial carcinoma. Cancer Res 1993;53:5100–3. [PubMed] [Google Scholar]
- 29.Nicolaides NC, Papadopoolos N, Liu B, et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 1994;371:75–80. [DOI] [PubMed] [Google Scholar]