Abstract
Prader-Willi syndrome (PWS) is characterized by early childhood obesity, mental deficiency, hypogonadism, hypotonia, hypopigmentation, short stature, small hands and feet, and a characteristic face. It is the most common genetic cause of obesity and obesity is the most significant health problem for PWS patients. Ob protein (leptin), which is produced by adipose tissue, is thought to play a significant role in obesity; thus, unusually low plasma leptin levels, or relative loss of sensitivity to leptin in PWS subjects, could be an important factor in their obesity. We measured plasma leptin levels in 19 obese and 14 non-obese PWS patients [mean body mass index (BMI) 37.2 and 22.0, respectively] and compared these levels to those of 28 obese controls (mean BMI 35.5) and 16 non-obese control individuals (mean BMI 21.6). The mean plasma leptin concentration (ng/ml) for obese PWS subjects was 33.4 and 23.6 for non-obese PWS subjects. Obese control leptin was 36.2 ng/ml and non-obese control was 9.9.
Among the control groups, leptin levels in females were significantly higher than those in males; the obese males and females had significantly higher leptin than their respective non-obese counterparts. These differences did not hold true for the PWS subjects. Leptin levels in obese PWS males and females were similar, and the same was true of the non-obese PWS males and females. The differences between obese and non-obese PWS subjects of both sexes were small and not significant. Comparing control groups with their PWS counterparts revealed no significant differences, with one exception: circulating plasma leptin levels in non-obese PWS males were nearly five times higher than in non-obese control males with similar BMI. This difference may reflect a more female pattern of fat distribution and hypogonadism, which are characteristic of PWS males. Leptin levels in PWS patients were not obviously correlated with the chromosome 15 finding seen in the patients.
Keywords: Prader-Willi syndrome, obese controls, body mass index (BMI), leptin
INTRODUCTION
The Prader-Willi syndrome (PWS), generally sporadic in occurrence, comprises infantile hypotonia, early childhood obesity, mental deficiency, hypogonadism, hypopigmentation, short stature, small hands and feet, and minor facial anomalies [Butler et al., 1986; Butler and Meaney, 1987; Butler, 1990]. While 70% of PWS patients have an interstitial deletion of the proximal long arm of chromosome 15 (15q11q13), 20 to 25% have maternal disomy of chromosome 15 and 5 to 10% have biparental inheritance of normal-appearing chromosomes, submicroscopic deletions, or genetic imprinting mutations [Butler et al., 1986; Butler, 1990; Mascari, et al., 1992; Buiting et al., 1995]. The deletion is paternal in origin, although parental chromosomes are generally normal [Butler et al., 1986; Butler, 1990].
PWS is the most common genetic cause of obesity and obesity is the most significant health problem in affected individuals [Butler et al., 1986; Meaney and Butler, 1989; Butler, 1990], Several factors contribute to obesity in PWS, including hyperphagia, decreased physical activity, decreased metabolic rate, and an inability to vomit [Butler, 1990; Hill et al., 1990]. PWS individuals may have 40 to 50% body fat which is 2–3 times more than in normal individuals and they have a sex reversed fatness pattern (i.e., males have more fat than females) [Butler and Meaney, 1987; Meaney and Butler, 1988, 1989]. Characterization of obesity in PWS subjects by fatness pattern analysis shows abnormally high Z score skinfold variables for all skinfold sites but with the heaviest deposition of subcutaneous fat in the trunk and limbs [Meaney and Butler, 1989].
A recently discovered secreted protein, leptin, produced in adipose tissue [Zhang et al., 1994] is thought to initiate its effect on feeding behavior and metabolic rate [Pelleymounter et al., 1995] by binding to its receptor, OB-R [Tartaglia et al., 1996], in the brain. When leptin is injected into obese or normal mice, the animals show a dose-dependent and sustained weight loss. A total deficiency of, or resistance to leptin causes extreme obesity in rodents [Zhang, et al., 1994; Campfield et al., 1995; Halaas et al., 1995]. Leptin is apparently involved with the regulation of body weight and fat deposition through its effects on appetite, and in the ob / ob mice on metabolism, and, thus, may play a key role in obesity, perhaps including the obesity found in PWS subjects [Maffei et al., 1995; Pelley-mounter et al., 1995; Halaas et al., 1995; Campfield et al., 1995]. Here we report the first study of leptin in Prader-Willi syndrome subjects who meet the diagnostic criteria established by Holm et al. [1993], and a comparison with obese and non-obese controls.
MATERIALS AND METHODS
We analyzed leptin levels in the fasting plasma of 33 (19 males and 14 females) PWS patients {18 with 15q11q13 deletion and 7 with maternal disomy 15 (6 with maternal heterodisomy and 1 with maternal isodisomy); 6 had normal chromosomes [i.e., maternal disomy (parental DNA unavailable) or imprinting mutations] and 2 had translocations involving chromosome 15 (1 with reciprocal translocation of 15 and 19 and 1 with Robertsonian translocation of 15 and 15) with an average age of 16.2 years and age range of 2 to 39 years}. Twenty-eight obese subjects (9 males and 19 females) without a known cause of obesity were studied with an average age of 21.4 years (age range 5 to 38 years) and 16 non-obese subjects (8 males and 8 females) were also analyzed, with an average age of 21.4 years (age range 5 to 32 years). Fasting plasma samples were freshly frozen at −70°C until assayed for leptin concentration by a sandwich ELISA method employing affinity-purified polyvalent antibodies, following protocols described by Rosenbaum et al. [1996].
Height and weight were obtained on all individuals and body mass index (BMI) calculated (weight in kilograms divided by the height in meters squared). Obese subjects were classified as those individuals with a BMI ⩾ 27 and non-obese subjects had BMI < 27. The obese and non-obese control subjects were healthy. PWS patients were also divided into obese (13 males and 6 females) and non-obese groups (6 males and 8 females). The PWS patients judged to be non-obese were on diet restriction and exercise programs and not treated with hormone therapy (e.g., growth hormone, testosterone, or estrogen). Averages and standard errors, independent t-tests, and Fisher paired least significant difference tests were calculated or utilized for statistical analysis of the data in this study. The characteristics of individuals in whom leptin levels were measured are shown in Table I, and the leptin data are plotted in Figure 1 along with statistical analyses of intergroup differences. Figures 2 and 3 are scatterplots showing the relationship of leptin to BMI for males and females, respectively.
TABLE I.
Characteristics of Prader-Willi Syndrome and Control Subjects
| Subject groupa | Sex M/F | N | Age (year) X ± SEM | BMI (kg/m2) X ± SEM | Leptin (ng/ml) X ± SEM |
|---|---|---|---|---|---|
| PWS obeseb | M | 13 | 18.9 ± 3.4 | 36.9 ± 2.9 | 32.5 ± 8.7 |
| PWS obese | F | 6 | 20.4 ± 3.4 | 37.5 ± 1.8 | 34.3 ± 6.9 |
| PWS non-obese | M | 6 | 15.3 ± 3.7 | 22.7 ± 1.4 | 25.2 ± 6.6 |
| PWS non-obese | F | 8 | 10.2 ± 2.9 | 21.3 ± 1.6 | 22.1 ± 5.6 |
| Obese controls | M | 9 | 20.1 ± 4.0 | 34.0 ± 1.9 | 26.0 ± 7.4 |
| Obese controls | F | 19 | 22.8 ± 2.1 | 37.0 ± 2.5 | 46.4 ± 6.3 |
| Non-obese controls | M | 8 | 19.0 ± 4.8 | 20.8 ± 1.7 | 5.4 ± 2.7 |
| Non-obese controls | F | 8 | 23.9 ± 3.4 | 22.5 ± 1.4 | 14.4 ± 4.5 |
Obese, BMI > or = 27; non-obese, BMI < 27.
Eighteen Prader-Willi syndrome (PWS) patients with 15q11q13 deletion; 13 with normal chromosomes or maternal disomy 15; two with chromosome 15 translocations.
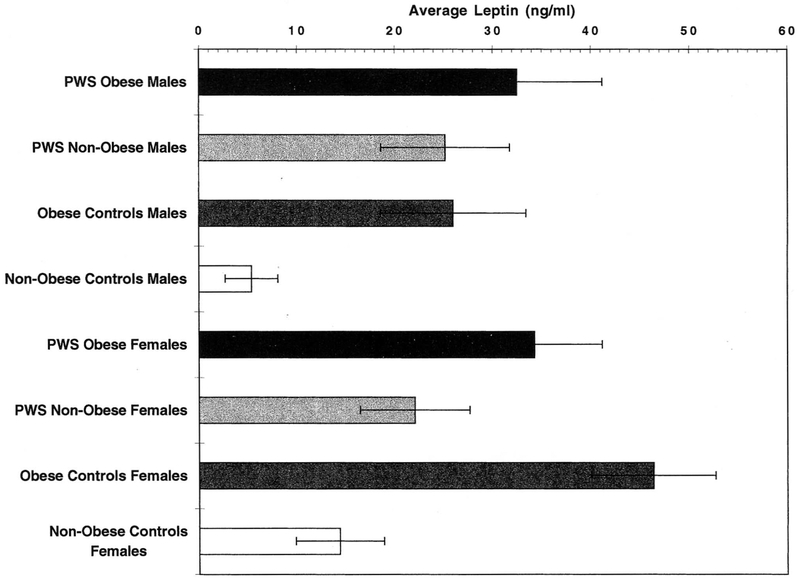
Fig. 1.
Histogram showing plasma leptin levels in Prader-Willi syndrome and control subjects. Significant differences in plasma leptin levels were found (P < 0.05) using the Fishers paired least significant difference test for the following: 1) Non-obese control females > non-obese control males; 2) Obese control females > non-obese control females; 3) Obese control males > non-obese control males; and 4) PWS non-obese males > non-obese control males.
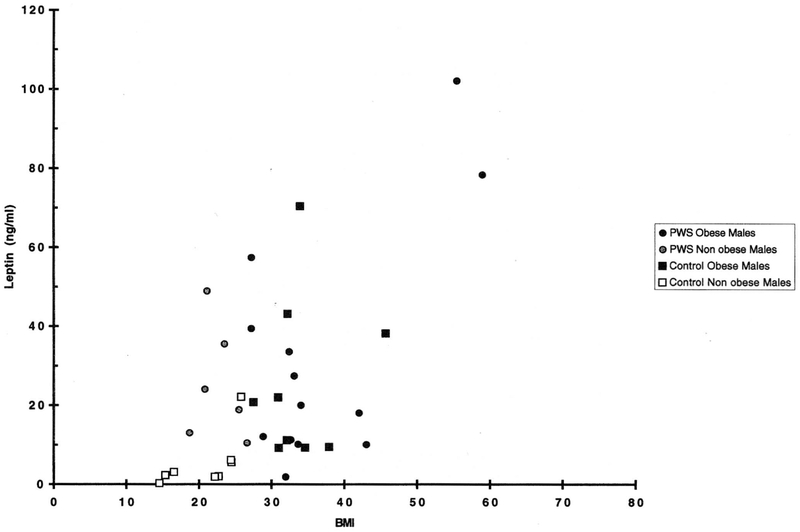
Fig. 2.
Scatterplot of body mass index (BMI) and leptin levels for Prader-Willi syndrome and control males.
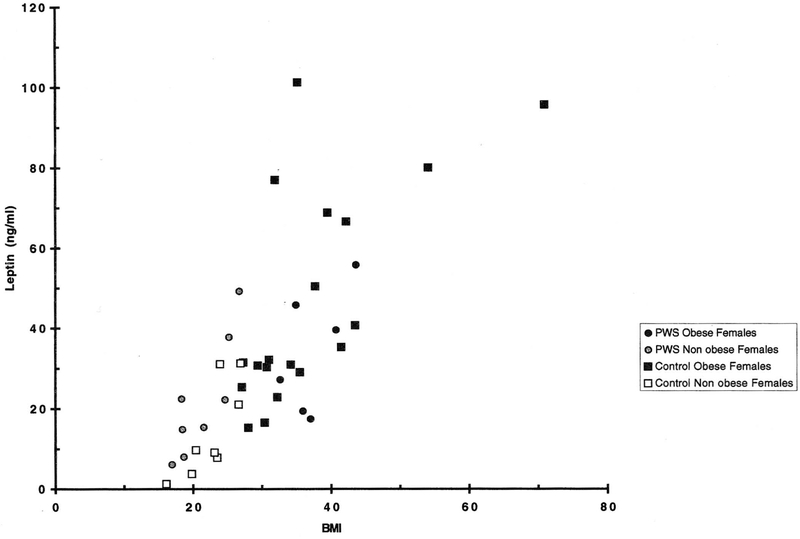
Fig. 3.
Scatterplot of body mass index (BMI) and leptin levels for Prader-Willi syndrome and control females.
RESULTS AND DISCUSSION
In the non-obese control groups, the mean leptin level was significantly higher (P < 0.05) in females than in males. The obese control males and females had significantly higher leptin levels than their non-obese control counterparts (P < 0.05). These results confirmed earlier reports of the relationship of body fat to plasma leptin levels [Maffei et al., 1995] and the tendency for leptin levels in females to be higher than in males at the same BMI.
The striking differences observed in mean leptin levels between obese and non-obese, and males and females in our control groups were not duplicated in the PWS subjects. In the PWS subgroups, the mean leptin level in females was the same (obese) or insignificantly lower (non-obese) than that of the corresponding male group. Similarly, and also in contrast to the controls, mean leptin levels were comparable for obese and non-obese PWS males. PWS obese females had somewhat higher (but not significant) leptin levels than those found in their non-obese counterparts.
A comparison of corresponding subgroups between the PWS subjects and controls showed insignificantly different leptin levels for non-obese females, obese females, and for obese males. The one outstanding difference between PWS and controls occurred in the non-obese male subgroups, where the mean leptin level for the controls was 5.4 ± 2.7 ng/ml and for PWS was 25.2 ± 6.6 ng/ml (P < 0.05). It appears that normal weight PWS males have leptin levels which are nearly five times those of corresponding control males, and similar to those of obese males of both groups and normal females of both PWS and control groups, despite similar mean BMI values for non-obese PWS males and non-obese control males (22.7 vs. 20.8 kg/m2, respectively). Although there were discrepancies in mean age between the subjects in the control and PWS groups and the sample numbers in some cases were small, these cannot account for the significantly higher leptin levels in non-obese PWS males compared with the equivalent control group. The short stature of the PWS patients might account for body fat differences relative to BMI, as compared with control populations, and BMI is not the most reliable indicator of body fat mass, but this did not appear to be the case for the other three PWS subgroups (non-obese females, and obese males, and females). There were no significant correlations between PWS leptin levels and specific chromosome 15 changes (15q11q13 deletion, or normal appearance).
In comparison with control groups, the mean plasma level of non-obese PWS males was not significantly lower than that of their obese counterparts (25.2 vs. 32.5 ng/ml), even though the mean BMI of non-obese PWS males was 22.7 vs. 36.9 kg/m2 for the obese PWS males. In general, the leptin levels found in the obese PWS groups were not significantly different from those of the obese controls, and the non-obese females of PWS and control groups had leptin levels that were not significantly different from one another (22.1 vs. 14.4 ng/ml, respectively). As in adults, obese children have high concentrations of serum leptin, which are closely correlated with BMI and increased adipose tissue [Hassink et al., 1996].
Receptors for leptin are found in the hypothalamus and in the choroid plexus, the site of cerebrospinal fluid (CSF) production and the location of the blood/CSF barrier [Tartaglia et al., 1996]. Once leptin enters the CSF it is thought to initiate its effect on feeding behavior, and possible energy expenditure, at the level of the hypothalamus. CSF leptin levels are positively correlated with BMI, as is the case with plasma leptin, and are also strongly correlated with plasma leptin levels in a non-linear manner [Schwartz et al., 1996]. If plasma leptin enters the CSF in relation to the body’s fat mass, there seems to be some control over the process which causes the CSF: plasma leptin ratio to be substantially lower in those with the highest, as compared with the lowest, plasma leptin levels. This has been interpreted to suggest that the delivery of leptin to the brain occurs by way of a saturable transport mechanism, and that in obese individuals this system may be reduced in efficiency due to limited transporters, resulting in relative leptin resistance [Schwartz et al., 1996; Caro et al., 1996].
Neuropeptide Y (NPY) is a potential mediator of leptin’s central effects. Administration of leptin has been shown to inhibit the synthesis and release of NYP in the hypothalamus, thereby providing a potential mechanism for the reduction in food intake and subsequent weight loss which occurs in rodent model systems [Stevens et al., 1995], Hypothalamic NPY increases with starvation (and leptin levels are decreased), inhibiting gonadotropins and sex steroids [White and Kershaw, 1989; Ahima et al., 1996], Totally leptin-deficient ob / ob mice display neuroendocrine abnormalities similar to those of starvation. Administration of leptin to male mice during starvation substantially reduces the gonadal, adrenal, and thyroid changes, and in female mice prevents the starvation-induced delay in ovulation [Ahima et al., 1996]. Human recombinant leptin has also been reported to correct the sterility defect in female ob / ob mice [Chehab et al., 1996]. One could speculate that if leptin was transported inefficiently to the hypothalamus, a starvation-like effect might ensue, with an over-production (or lack of inhibition) of NPY and subsequent chronic hyperphagia, accompanied by changes in gonadotropins and sex steroids. Thus, regulation of the neuroendocrine system during starvation could be an important physiological role for leptin [Ahina et al., 1996]. In addition to obesity, two consistent manifestations in Prader-Willi syndrome patients are sterility and hypogonadism, which tempts speculation about possible defects in the leptin transport system of Prader-Will syndrome patients.
In summary, leptin levels in PWS subjects follow, in general, the pattern observed in normal individuals; that is, they are related to fat mass, or in this case to body mass index. However, the large differences in plasma leptin levels between males and females, or between obese and non-obese, which are so characteristic of the normal population, are very much reduced in PWS subjects, creating a more homogeneous group than their normal counterparts. It is clear that PWS plasma leptin concentrations fall well within the range of the normal population, and therefore there is no direct relationship between leptin levels and the clinical syndrome. It remains to be seen whether other differences, involving transport or the response to leptin, might be identified. The one obvious difference in leptin levels between control and PWS non-obese males can be theorized to result from relatively more fat mass, distributed in a female pattern in PWS males, and hypogonadism, resulting in low circulating levels of sex steroids, which might tend to mask the greater sex-related differences in leptin levels observed in control groups as compared with the PWS counterparts.
ACKNOWLEDGMENTS
We thank Verna Wyatt for expert preparation of the manuscript. We acknowledge National Institute of Child Health & Human Development (NICHD) grant (P01HD30329) (MGB).
Contract grant sponsor: National Institute of Child Health & Human Development (NICHD); Contract grant number: P01HD30329.
REFERENCES
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos–Flier E, Flier JS (1996): Role of leptin in the neuroendocrine response to fasting. Nature 382:250–252. [DOI] [PubMed] [Google Scholar]
- Buiting KS, Saitoh S, Gross S, Dittrich B, Schwartz S, Nicholls RD, Horsthemke B (1995): Inherited microdeletions in the Angelman and Prader-Willi syndromes define an imprinting center on human chromosome 15. Nat Genet 9:395–400. [DOI] [PubMed] [Google Scholar]
- Butler MG (1990): Prader-Willi syndrome: Current understanding of cause and diagnosis. Am J Med Genet 35:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Meaney FJ (1987): An anthropometric survey of 38 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet 26:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Meaney FJ, Palmer CG (1986): Clinical and cytogenetic survey of 39 individuals with Prader-Willi syndrome. Am J Med Gen 23:793–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P (1995): Recombinant mouse ob protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science 269:546–549. [DOI] [PubMed] [Google Scholar]
- Caro JF, Kolacznski JW, Nyce MR, Ohannesian JP, Opentanoya I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV (1996): Decreased cerebrospinal-fluid/serum leptin ratio in obesity: A possible mechanism for leptin resistance. Lancet 348:159–161. [DOI] [PubMed] [Google Scholar]
- Chehab FF, Lim ME, Lu R (1996): Correction of the sterility defect in homozygous obese female mice by treatment with human recombinant leptin. Nat Genet 12:318–320. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM (1995): Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546. [DOI] [PubMed] [Google Scholar]
- Hassink SG, Sheslow DV, deLancey E, Opentanova I, Considine RV, Caro JF (1996): Serum leptin in children with obesity: Relationship to gender and development. Pediatrics 98:201–206. [PubMed] [Google Scholar]
- Hill JO, Kaler M, Spetalnick B, Reed G, Butler MG (1990): Resting metabolic rate in Prader-Willi syndrome. Dysmorph Clin Genet 4:27–32. [PMC free article] [PubMed] [Google Scholar]
- Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, Greenberg F (1993): Diagnostic criteria for Prader-Willi syndrome. Pediatrics 91:398–402. [PMC free article] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PO, Friedman JM (1995): Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161. [DOI] [PubMed] [Google Scholar]
- Mascari JF, Gottlieb W, Rogan PK, Butler MG, Weller DA, Armour JAL, Jeffreys RL, Ladda RL, Nicholls RD (1992): The frequency of uniparental disomy in Prader-Willi syndrome. N Engl J Med 326:1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney FJ, Butler MG (1988): The developing role of anthropologists in medical genetics: Anthropometric assessment of the Prader-Labhart-Willi syndrome as an illustration. Med Anthropol 10:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney FJ, Butler MG (1989): Characterization of obesity in the Prader-Labhart-Willi syndrome: Fatness patterning. Med Anthropol Quart 3: 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F (1995): Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel R (1996): Effects of gender, body composition and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab 81:3424–3427. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Peskind E, Raskind M, Boyko E, Porte D Jr (1996): Cerebrospinal fluid leptin levels: Relationship to plasma levels and to adiposity in humans. Nat Med 2:589–593. [DOI] [PubMed] [Google Scholar]
- Stevens TW, Basinski M, Bristow PK, Bue-Vallesky JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kviauciunas A, MacKeller W, Rosteck PR, Schoner B, Smith D, Tinsley FC, Zang X-Y, Heiman M (1995): The role of neuropeptide Y in the anti-obesity action of the obese gene product. Nature 377:530–532. [DOI] [PubMed] [Google Scholar]
- Tartaglia L, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards G, Campfield LA, Clark F, Deeds J, Muir C, Sanker S, Moriarty A, Moore K, Smutko J, Mays G, Woolf E, Monroe C, Tepper R (1996): Identification and expression cloning of a leptin receptor, OB-R. Cell 83:1263–1271. [DOI] [PubMed] [Google Scholar]
- White JD, Kershaw M (1989): Increased hypothalamus neuropeptide Y expression following food deprivation. Mol Cell Neurosci 1:41–48. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994): Positional cloning of the mouse obese gene and it’s human homologue. Nature 372:425–432. [DOI] [PubMed] [Google Scholar]