Abstract
Extensive literature suggests that adverse experiences in early childhood may deleteriously impact later health. These effects are thought to be related to the impact of persistent or chronic stress on various biological processes, mediated by dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, and ultimately irregularities in cortisol levels. Ameliorating persistent stress in young children requires accurately measuring the chronicity of physiologic stress, which is difficult in young children because of unreliable self-report and the burden and inaccuracy associated with using invasive acute-stress biomeasures. A better way to approximate persistent stress in young children is measuring hair cortisol concentration (HCC), as it only requires one noninvasive collection to measure months of HPA-axis activity or experienced stress. However, few studies measure HCC in young children despite wide use in adult stress research. This article reviews and synthesizes research that uses HCC to approximate persistent stress in healthy children, 12–60 months of age. Reviewed studies indicate that HCC is elevated in young children who are experiencing forms of persistent stress such as low socioeconomic status and maternal distress. Hair cortisol is thus a promising measure of early childhood persistent stress, but due to the limited use of HCC in this population, much research is still needed. Specifically, nurse researchers may need to measure several factors associated with early childhood persistent stress and HCC to identify which children are at risk for stress-related disease.
Keywords: hair cortisol, chronic stress, young children
Currently, no consensus exists on the best way to measure persistent (also referred to as chronic) stress in young children (Vanaelst et al., 2012) particularly because young children are unreliable reporters of their symptoms. However, a promising way to measure early childhood persistent stress may be use of the biomeasure hair cortisol concentration (HCC). Researchers have used HCC widely to approximate chronic physiological stress responses in adults (Sauve, Koren, Walsh, Tokmakejian, & Van Uum, 2007; Stalder & Kirschbaum, 2012), but the measure is just emerging in early childhood (12–60 months of age) persistent stress research. To address this gap, in this article, we will first provide background information on early childhood stress and hair cortisol and then synthesize the science that uses hair cortisol to measure chronic physiological stress in children between 12 and 60 months of age. The findings from this article will advance nursing science by providing a better understanding of hair cortisol as a measure of persistent physiologic stress in early childhood.
Extensive literature suggests that adverse experiences and persistent stress in early childhood can have deleterious consequences on health later in the life course (Shonkoff, Boyce, & McEwen, 2009), for instance premature mortality (Anda et al., 2009), limited brain development in areas that predict academic success (Danese & McEwen, 2012), adverse health behaviors (Felitti et al., 1998), and chronic diseases such as cardiopulmonary disease, cancer, liver disease, and skeletal fractures (Felitti et al., 1998). Unfortunately, adverse experiences and persistent stress are typically measured many years after their occurrence, introducing recall bias and limiting intervention effectiveness due to delayed implementation (Halfon & Hochstein, 2002).
Authors have suggested that the pathophysiological effect of adverse childhood experiences on target organs is mediated by persistent stress (Everly & Lating, 2002). Persistent stress is the cumulative result of several stress responses over time. Stress responses are the individual’s cognitive appraisal of and subsequent adjustment to a stressor. Finally, a stressor is the real or perceived environmental stimulus that triggers an individual’s stress-response system to initiate actions to overcome or “survive” the stressor (Everly & Lating, 2002; Marshall, Davis, & Sherbourne, 1999; Monroe, 2008; Smith, Everly, & Johns, 1993). Excessively chronic or repeated stressors (collectively culminating in persistent stress) are more prone to causing psychophysiological pathology than small doses of stressors, collectively known as tolerable (Shonkoff, 2012) or manageable stress (Hostinar & Gunnar, 2013), which can stimulate adaptive responses that optimize health and performance or help an individual avoid danger (e.g., fight or flight response; McEwen, 2007). Examples of potential persistent stressors in early childhood are child abuse (Monnat & Chandler, 2015), low socioeconomic status (SES), and maternal distress (symptoms of stress and/or depression in the child’s mother; Brody et al., 2013; McCoy & Raver, 2014; Ziol-Guest & McKenna, 2014). These persistent issues are considered stressors in part because they interfere with young children’s ability to have their basic needs met. Early inputs of chronic stress experiences can become embedded in the young child’s developing psychoneurobiological framework if they occur during the sensitive period of neurodevelopment (approximately 0–60 months of age; Briggs-Gowan, Carter, & Ford, 2012; Carter, Dubois, Tremblay, & Taljaard, 2013; Shonkoff, Garner, The Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, & Section on Developmental and Behavioral Pediatrics, 2012; Wodtke, 2013). This embedding compromises optimal development of the child’s psychoneurobiological framework, or scaffolding (Ayoub, Vallotton, & Mastergeorge, 2011), such as brain areas responsible for the stress-response (Shonkoff, 2010) and self-regulation systems (McEwen & Gianaros, 2010; Tottenham et al., 2010), stimulating processes that underpin later chronic disease (Halfon, Larson, Lu, Tullis, & Russ, 2014; National Center for Chronic Disease Prevention and Health Promotion, 2009; World Health Organization [WHO], 2005). Several interdisciplinary models help illustrate this early maladaptive adjustment to persistent stress, such as the allostatic load model (McEwen, 2007), the ecobiodevelopmental framework (Shonkoff, 2010), the life course health development framework (Halfon et al., 2014), and the expanded biobehavioral interaction model for nursing (Kang, Rice, Park, Turner-Henson, & Downs, 2010).
Each child processes and responds to persistent stress differently due to variations in gene–environment interactions (Shonkoff et al., 2012). These variations necessitate a tool for reliably measuring children’s stress response, or how children are experiencing persistent stress, so that nurse researchers and clinicians can identify those who are at risk for pathophysiologic adjustment and in need of interventions to relieve persistent stress. Measuring a child’s stress response can be difficult for several reasons. First, paper-and-pencil measures of persistent stress experiences have limited utility in children because young children have limited ability to recall events, identify persistent psychological stress, and clearly articulate emotions (Gow, Thomson, Rieder, Van Uum, & Koren, 2010). In addition, invasive physiologic measures of the stress response, such as serum, urinary, or salivary cortisol, may be burdensome to the child or fraught with testing bias (Stalder, Hucklebridge, Evans, & Clow, 2009; Steudte et al., 2011). Instead, researchers and clinicians should consider using easy-to-collect, noninvasive physiological measures of a child’s retrospective stress response such as HCC.
Cortisol, known as the stress hormone, is one of the end products of the hypothalamic–pituitary–adrenal (HPA) axis. Cortisol release occurs in a daily pattern, facilitating physiologic diurnal regulation (Lightman et al., 2008; Tsigos & Chrousos, 2002; Wust et al., 2000), and in bursts in response to stressors (Gow et al., 2010). For diurnal rhythm regulation, the secretion rate of cortisol is generally highest around 30 min after awakening (Wust et al., 2000) and declines until the following wake-up period the next day (Lightman et al., 2008). After a cortisol surge in response to exposure to an acute stressor, serum cortisol levels typically peak in 15–30 min; these surges have a half-life of approximately 70 min (Baum & Grunberg, 1997). A stress-induced cortisol burst increases the cortisol concentration above the level that would occur naturally according to the diurnal pattern, elevating overall cortisol levels (Blair, Berry, Mills-Koonce, Granger, & F. L. P. Investigators, 2013; Blair et al., 2008, 2011; Essex, Klein, Cho, & Kalin, 2002). However, in adults persistent/chronic stress can sometimes be associated with a blunted diurnal cortisol pattern (reflected in lower levels of cortisol; Steudte et al., 2011), suggesting HPA-axis dysregulation from pathophysiologic adjustment to chronic stressors (Brooks & Robles, 2009).
In multidisciplinary research, investigators typically measure cortisol in saliva, urine, and more recently, hair. In children, researchers have most commonly measured cortisol via saliva due to its nonpainful sampling and ease of storage. However, salivary cortisol only measures an acute stress response and/or a cross-sectional time point of diurnal cortisol release, which can vary in young children due to stage of development of the HPA axis (Blair, Granger, Willoughby, & Kivlighan, 2006; Gunnar & Donzella, 2002; Kaufman et al., 1997), naptime (Gunnar & Donzella, 2002; Watamura, Donzella, Kertes, & Gunnar, 2004), and developing temperament, a product of gene–environment interactions (Blair, 2010; Chen & Schmidt, 2015; Rothbart, 2011; Rowe, Jacobson, & Van den Oord, 1999). Further, research with salivary cortisol sampling typically excludes nighttime measures of cortisol, and researchers have found reactionary testing effects from salivary cortisol sampling (Steudte et al., 2011). In addition, the capturing of persistent stress responses requires repeated measurements of salivary cortisol (Lee, Kim, & Choi, 2015) to approximate average cortisol levels over time, which may be costly, burdensome to the child, or biased due to sampling error from repeated collections across stages of HPA-axis development, varying naptimes, and testing reactions.
A better way to measure persistent stress responses via cortisol production is through HCC, as one measure of HCC averages the daily fluctuations of cortisol and reduces the burden associated with invasive or repeated measures. Although the exact mechanism for how cortisol is diffused into the hair is unknown, researchers primarily hypothesize that unbound, free cortisol passively diffuses into the hair shaft from its follicular vasculature (Pragst & Balikova, 2006; Russell, Koren, Rieder, & Van Uum, 2012), though a more controversial hypothesis (Grass et al., 2015; Russell, Koren, Rieder, & Van Uum, 2014) is that cortisol from sebaceous and eccrine gland secretions coats the hair follicle (Raul, Cirimele, Ludes, & Kintz, 2004). HCC is obtained by cutting a small sample of hair (the size of the tip of a shoelace) as close as possible to the scalp in the area of the posterior vertex (D’Anna-Hernandez, Ross, Natvig, & Laudenslager, 2011). The HCC in the most proximal 1 cm of hair to the scalp approximates mean cortisol output from the HPA axis (diurnal and in response to stressors) over the prior 1 month of growth (Russell et al., 2015; Sauve et al., 2007; Wennig, 2000) because hair grows approximately 1 cm each month (Wennig, 2000). HCC can be reliably measured in up to 6 cm of proximal hair growth, as it declines distally (Dettenborn, Tietze, Bruckner, & Kirschbaum, 2010), likely due to hair washing and other hair care practices (Hamel et al., 2011; Hoffman, Karban, Benitez, Goodteacher, & Laudenslager, 2014; Kirschbaum, Tietze, Skoluda, & Dettenborn, 2009). Research has also shown that HCC is confounded by health conditions associated with HPA-axis dysregulation (e.g., Cushing’s disease; Starkman, Gebarski, Berent, & Schteingart, 1992), corticosteroid use (Kamps et al., 2014), and chemical hair processing (Hamel et al., 2011; Hoffman et al., 2014; Kirschbaum et al., 2009).
Studies demonstrate that researchers have reliably measured HCC in laboratories worldwide and across various extraction and analysis procedures (i.e., immunoassay vs. liquid chromatography; Russell et al., 2015; Slominski, Rovnaghi, & Anand, 2015). HCC measures correlate well with intraperson averages of three salivary cortisol time-point measures in adults (awakening, 30 min after awakening, and at bedtime; r = .61, p = .01) and is a more reliable test–retest measure (month-to-month HCC: r = .84, p < .001) than the three time-point measures of salivary cortisol week to week (r = .38–.61, p = .13–.01; Short et al., 2016). However, these findings may not be as valid in young children because of factors such as a developing HPA axis, naptime variability, and difficulty obtaining accurate and repeated saliva samples. In the only study that, to our knowledge, correlated salivary cortisol and HCC in young children, Vanaelst et al. (2012) correlated measures from a 6-cm strand of hair with a 2-day average of salivary cortisol in elementary school-aged girls, limiting the validity of their findings. Generally, these findings indicate that, despite the lack of HCC reference norms and different HCC analysis techniques, HCC information within and across individuals should be comparable. However, much remains to be learned regarding HCC measurement in young children, as it is an emerging biomeasure in this population. Thus, in the present review, we will examine the current state of the science on HCC as a biomeasure of persistent stress in young, healthy children.
Methods
We searched all published literature in early August 2016 using 14 databases: PubMed, CINAHL Plus with Full Text, Academic Search Complete, Academic Search Premier, Health and Psychosocial Instruments, MEDLINE, Psychology and Behavioral Sciences Collection, PsycINFO, Social Sciences Abstracts (H. W. Wilson), Social Work Abstracts, SocINDEX with Full Text, Sociological Collection, Cochrane, and Scopus. The database search formula we used was (child OR pediatric OR toddler OR preschool OR youth) AND (stress OR nervous OR nervousness OR psychogenic OR anxiety OR depression OR distress) AND cortisol AND hair. Limits set (when available) included English language, humans, and scholarly (peer-reviewed) journals. We considered peer-reviewed experimental, quasi-experimental, and observational studies for this review.
In an effort to better understand stress-related hair cortisol in normally developing, living human children 12–60 months of age, we excluded child populations who experienced factors that could possibly alter normal HPA-axis reactivity, including those born prematurely (Grunau et al., 2013; Yamada et al., 2007), those experiencing adverse developmental conditions, those with chronic diseases such as Addison’s (De Leo, Cozzolino, Colao, & Pivonello, 2012) and asthma (Kamps et al., 2014), and those using steroids (Smy et al., 2015). If a study’s sample included children aged within our target age range along with children aged above or below that range, we included the study in this review but attempted to extract and focus on data from these studies that corresponds to the target age group when possible.
Results
See Figure 1 for a flowchart depicting the search and selection process for this review. Of the nine studies included in this review, six measured HCC alongside other stress-related measures (e.g., child-abuse risk, family SES, maternal distress, or child temperament or behavior) in children (Groeneveld et al., 2013; Karlen, Frostell, Theodorsson, Faresjo, & Ludvigsson, 2013; Karlen et al., 2015; Palmer et al., 2013; Vaghri et al., 2013; Vliegenthart et al., 2016), while the remaining three examined reference ranges and HCC confounders in young, healthy children (Dettenborn, Tietze, Kirschbaum, & Stalder, 2012; Noppe et al., 2014; Slominski et al., 2015).
Figure 1.
Article search and selection procedure.
Table 1 provides a summary of the studies reviewed. In general, investigators in all of the studies used a type of immunoassay to detect the HCC, and none reported difficulties obtaining sufficient hair or internal review board approval. Because we were primarily interested in stress-related measures associated with child HCC, we have organized our review of these studies by stress-related measurement category (maternal distress and other dyad associations, SES, child temperament or behavior), pooled results of HCC values (by age, race, and gender/sex), and other associations.
Table 1.
Characteristics of Reviewed Studies.
| Study Characteristics Study (Year) Location Design Aims | Child Sample # Children in Target Age Race/Ethnicity Ages (extracted ages if >60 mos in sample? Y/N) | HCC Characteristics Detection Method Concentration (pg/mg) Trend | Measures SES Maternal Distress Child Behavior/Temperament Additional Measures | HCC Findings Significant Nonsignificant |
|---|---|---|---|---|
| Dettenborn, Tietze, Kirschbaum, and Stalder (2012) Germany Cross sectional HCC confounders | n = 28 Not reported 1–9 y.o. (mean 3.6 y.o., SD = 2.5; could not extract target ages) | CLIA Mean = 40.91 (SD = 21.6) in all 28 children Higher HCC in younger children | None None None Sex, age, education, medication use, psychiatric issues, hair washing frequency, hair color | Highest HCC in youngest children (r = −.428, p = .023). Decreasing HCC from scalp end to distal end. Sex and HCC: ages 1–9 years, F(1, 51) = 5.304, p = .025; = 0.078, although had too little power to detect sex effects Hair color, hair washing in first 2 proximal hair segments (6 cm) |
| Groeneveld et al. (2013) the Netherlands Cohort HCC changes pre-/poststarting school | n = 42 Not reported Preschool-aged children; mean = 50.1 mos (SD = 0.42) | ELISA (Mean) Prior to school = 27.50 (SD = 13.94); after school = 30.02 (SD = 14.05); Overall = 28.76 N/A | Parent education None CBQ Hair color, hair washing, steroid use in last 6 mos, medications, chronic disease, day-care history | HCC less before school than after school (d = 0.52); HCC higher in children who scored higher on CBQ fearfulness subscale, Pillais F(1, 19) = 4.67, p = .04, = 0.20. Hair color, gender, frequency of hair washing, medication use, corticosteroid use, fearfulness, hours in group care, parent education |
| Karlen et al. (2015) Sweden Prospective cohort Relationship between early psychosocial risks, early HCC levels, and later child health | n = 209 Not reported 1–10 y.o. (HCC obtained at age 12 mos) | Radio IA Boys = 2.45; girls = 2.79 (unsure if mean/median); boy/girl mean = 2.62 + relationship between HCC and psychosocial vulnerability score (β = .40, p < .001), controlled for birth size | Residence type, type of child care, parent education Psychosocial vulnerability survey None Health care, birth size, gender, foreign origin | Positive relationship between psychosocial vulnerability exposures and HCC at age 12 mos. Increasing odds ratios of children with higher HCC affected by different common childhood illnesses Father’s occupation, child gender, child weight |
| Karlen et al. (2013) Sweden Cohort Association of HCC in young children and their pregnant mothers with perinatal and SES factors | n = 100 Not reported 1, 3, 5 y.o. (repeated measures; also 8 y.o included in overall study) | Radio IA Means per age (years) (95% CI): 1 y.o. = 19.87 (13.55–29.13); 3 y.o. = 11.30 (8.28–15.42); 5 y.o. = 6.77 (5.08–9.03) − relationship with age | Family makeup, type of residence (apartment/house), type of child care, foreign origin, education level (binary) Had a “serious life event,” social support, or depression None Child BMI, delivery type | Child HCC decreased over time and positively correlated from 1 to 3 years of age (r = .30, p = .002), 3 to 5 years (r = .39, p < .001). Maternal HCC in the 2nd and 3rd trimesters correlated with child’s levels at 1 and 3 years of age. + relationship between HCC and birth weight (β =.224, p = .020), apartment (β = .200, p = .049), inappropriate size for gestational age (β = .231, p = .017) Maternal HCC in first trimester, type of delivery, longer birth length, gender, maternal education, foreign birth |
| Noppe et al. (2014) the Netherlands Cross sectional Identify HCC reference ranges and confounders (age, gender, hair care) | n = 27 2 non-Caucasian “by appearance”; the rest Caucasian 4–5 y.o. child data extracted (total sample 128 children of ages 4–14 y.o.) | ELISA Children 4–5 y.o.: mean 5.0 (95% CI 0–43.4) + relationship with age | None None None Hair washing, hair color, hair products/treatments, scalp sweat, BMI, Tanner stages, waist and hip circumference, gender | HCC had + relationship with age 4–14 y.o. (log transformed β = .03, r = .18, p = .04); waist circumference associated with HCC (r = .10, p = .04). BMI, waist: height ratio, hair care, distal and proximal hair segments (3 cm), gender, puberty |
| Palmer et al. (2013) Tennessee (CANDLE) Cohort Identify factors (demographic, maternal, child) associated with chronic stress and socioemotional issues in 1 year olds | n = 297 175 Black, 122 White children 1 y.o. | ELISA Median = 13.5 ng/mg (IQR 16.0 ng/mg) for all children. Black median = 16.5 ng/mg (IQR 14.6 ng/mg). White median = 6.6 ng/mg (IQR 11.3 ng/mg) N/A | Health insurance Brief Symptom Inventory, Parenting Stress Index Short Form, Child Abuse Potential Inventory, EPDS, TEMPS BITSEA Bayley SITD, marital status, ethnicity, race, parity, child age, prepregnancy height/weight, sex and body-size percentiles | 32.9% Black mothers and 10.2% White mothers reported SE problems in their 1-year-olds (p < .001); HCC in Black children higher than in White children (p < .001); higher HCC quartiles associated with higher BITSEA total problem scores (p = .01); In White children, HCC positively correlated with parenting stress (r = .24; p = .008), 4-week EPDS scores (r = .22; p = .02) and 1-year EPDS scores (r = .20; p = .03); in Black children, HCC + correlated with dysthymic scores (r = .19; p = .01). Predictors of HCC: less maternal depression at child’s first birthday, increased parenting stress, shorter length at birth, and increased height at 1 year. Shorter birth length and parenting stress at child’s first birthday associated with increased HCC. Child BMI and parental marital status; SES and sex associations not reported |
| Slominski et al. (2015) Tennessee (CANDLE) Cross sectional HCC extraction value | n = 16 Not reported 12–64 months (combined into one age group) | ELISA and LCMS Concentration varied dependent upon method Higher HCC in fine cutting versus milling samples and in ELISA versus LCMS | None None None None | HCC milled versus fine cutting r = .951, p < .01, with higher HCC in fine cutting (p < .05); 4 extractions yielded 0.04% more cortisol than 3; HCC via LCMS and ELISA correlated (rs = .737, p < .0001); higher HCC in ELISA samples versus LCMS Alcohol washing |
| Vaghri et al. (2013) Vancouver, Canada Cross-sectional Examine relationship between child HCC and family SES | n = 254 (total n = 339 with 85 additional children > 60 mos) 150 Caucasian, 98 Chinese, 25 East Indian, 45 other, 21 mixed Age groups: <51 mos, 51–55 mos, 56–60 mos (n = 85 > 60 mos) | ELISA Range 10–70 N/A | SES index, zip codes, 2006 census, 2004 tax-filer data, parent education, and annual income None None Sex, age, hair zinc, ethnicity | Maternal and paternal education significantly correlated with HCC (r = −.18; p = .001); HCC correlated with hair zinc (r = −.15, p = .006). Parent income, ethnicity, sex/gender |
| Vliegenthart et al. (2016) Netherlands Cross sectional Find relationship between SES and hair cortisol and cortisone | n = 270 (%) Dutch = 68.9; NA, Indonesia, Oceana, Japan = 10; Asia, Africa, South America, Turkey = 15.6 4–18 y.o. (Could not extract target ages) | LCMS “Elementary School” mean = 3.05 (range 0.28–38.26) N/A | Postal codes, parent education None None Child sex, height, weight | Postal code associated with HCC (β = −.103, p = .007, 95% CI [−0.179, −0.028]), controlled for sex and age. HCC correlated with maternal education (r = −.164, p < .05). HCC and parental/maternal/paternal education via linear regression, sex differences not reported |
Note. Bayley SITD = Bayley Scales of Infant and Toddler Development, 3rd ed. screener; BITSEA = Brief Infant-Toddler Social Emotional Assessment; BMI = Body Mass Index; CANDLE = Conditions Affecting Neurocognitive Development and Learning in Early Childhood study; CBQ = Child Behavior Questionnaire; CI = confidence interval; CLIA = chemiluminescence immunoassay; ELISA = enzyme-linked immunoassay; EPDS = Edinburgh Postnatal Depression Scale; ETOH = alcohol; NA = North America; N/A = Not Applicable; HCC = hair cortisol concentration; mos = months; IA = immunoassay; IQR = interquartile range; LCMS = liquid chromatography–mass spectrometry; SE = socioemotional; SES = socioeconomic status; TEMPS = Temperament Evaluation of Memphis, Pisa, Paris, and San Diego; Y/N = Yes/No?; y.o. = years old.
Maternal Distress and Child HCC and Other Dyadic Associations
Evidence suggests that child HCC is correlated with established measures of maternal distress. Palmer et al. (2013) found that in White dyads, child HCC was positively correlated with parenting stress (via the Parenting Stress Index; r = .24, p = .008), 4-week maternal depression scores (via the Edinburgh Postnatal Depression Scale [EPDS]; r = .22, p = .02), and 1-year maternal depression scores (via the EPDS; r = .20; p = .03), while in Black dyads, child HCC was positively correlated with maternal dysthymic scores (r = .19; p = .01), measured by the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego. Karlen, Frostell, Theodorsson, Faresjo, and Ludvigsson (2013) did not find associations between maternal distress and child HCC, but their distress assessment was based on asking three nonvalidated binary questions in a sample that reported no depression or psychiatric medication use.
Researchers also identified associations between child levels of HCC and maternal variables other than distress. Karlen et al. (2013) found that maternal HCC in the second and third trimesters positively correlated with their child’s HCC levels at 1 and 3 years of age (second trimester and 1-year-old child r = .36, p = .002; second trimester and 3-year-old child r = .25, p = .033; third trimester and 1-year-old child r = .23, p = .024; third trimester and 3-year-old child r = .27, p = .007). They also discovered that higher birth weight (β = .224, p = .020) and having an inappropriate size for gestational age (β = .231, p = .017) were positively associated with HCC.
SES and Child HCC
Child HCC was associated with several SES measures. In the Vaghri et al. (2013) study, parental income was not associated with HCC, but parental education was (r = −.18; p = .001), even after controlling for demographic variables and neighborhood clustering. However, Vaghri et al. (2013) sometimes had low participant response rates (<50%) per neighborhood, as they attempted to obtain a neighborhood quota sample in Vancouver. Vliegenthart et al. (2016) found that child HCC was associated with neighborhood SES (via postal code; β = −.103, p = .007, 95% CI [−0.179, −0.028]) and parental education (specifically maternal education had a correlation value of r = −.164 [p < .05]). However, Vliegenthart et al. (2016) reported that the finding regarding parental education was no longer significant with linear regression. Karlen et al. (2013) also reported no statistically significant association between maternal education and HCC. However, in a later study, Karlen et al. (2015) found that cumulative psychosocial vulnerability (parental education, parental employment, residence type, and binary questions on maternal distress and perceived support) was positively associated with child HCC at 12 months, after controlling for birth size (β = .40, p < .001).
Child Stress Measures and Child HCC
Groeneveld et al. (2013) found that child HCC was significantly less before the start of school (M = 27.50 pg/mg, SD = 13.94) than 2 months after school started (M = 30.02 pg/mg, SD = 14.05, d = 0.52) and that HCC was significantly higher in children who scored “high” on the fearfulness subscale of the Child Behavior Questionnaire Short Form than those who scored “low,” Pillais F(1, 19) = 4.67, p = .04, = 0.20. In another paper-and-pencil observation of child temperament, Palmer et al. (2013) found higher HCC quartiles associated with higher Brief Infant-Toddler Social and Emotional Assessment total problem scores (p = .01), with no difference between Black and White children.
Pooled Child HCC Values With Age, Race, and Gender/Sex
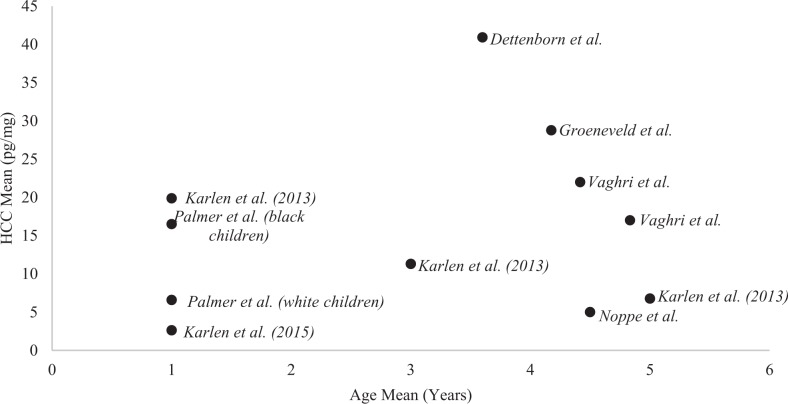
Collectively, there was high variability in HCC results among age, race, and gender/sex within the reviewed studies. In regard to age, we plotted a collection of estimated mean and median HCC values from each study that reported both age and HCC values in Figure 2 using Microsoft Excel® (seven studies). When plotting these values, we noted that Palmer et al. (2013) reported a median HCC value of 13.5 ng/mg (13,500 pg/mg), which is an extreme outlier compared to the plotted results from the other six studies. Instead of reporting HCC as ng/mg, we suspect that Palmer et al. (2013) meant to report their HCC values in terms of pg/mg. In regard to race, Palmer et al. (2013) reported higher HCC in Black compared to White children (median 16.5 ng/mg vs. 6.6 ng/mg, respectively, p < .001), and Vaghri et al. (2013) found no significant correlation between child HCC and the “ethnicity variables” (p. 336) of Caucasian, Chinese, East Indian, other ethnicities, and mixed. Researchers found varying evidence for HCC associations with gender. Dettenborn, Tietze, Kirschbaum, and Stalder (2012) found higher HCC in male compared to female children, ages 1–9 years, F(1, 51) = 5.304, p = .025; = 0.078, although they reported too little power to detect effects. In five other studies, researchers found no differences by sex (Groeneveld et al., 2013; Karlen et al., 2013, 2015; Noppe et al., 2014; Vaghri et al., 2013); and in three studies, researchers failed to report on sex associations with HCC (Palmer et al., 2013; Slominski et al., 2015; Vliegenthart et al., 2016).
Figure 2.
The variability of hair cortisol concentration (HCC) values from studies that provided mean age with HCC value (except that Palmer et al. reported median age and is included here in the chart as a mean only for visual representation). Each study in the figure used immunoassay to detect HCC. When age ranges were reported, we averaged the absolute maximum and minimum (except for the age range of less than 51 months in Vaghri et al. 2013, as the authors did not report a minimum age). Palmer et al. (2013) reported a median HCC value of 13.5 ng/mg (13,500 pg/mg), indicating an extreme outlier or possibly an error reporting the HCC units. Assuming that Palmer et al. (2013) intended to report their HCC values in pg/mg instead of ng/mg, we included the values from that study in pg/mg.
Additional Associations With Child HCC
Hair care practices and hair length
In the two studies that aimed to discover HCC confounders (Groeneveld et al., 2013; Noppe et al., 2014), researchers discovered that hair care and hair washing had a nonsignificant effect on HCC. Groeneveld et al. (2013) measured approximately 5 cm of hair, and Noppe et al. (2014) measured approximately 3 cm. However, Dettenborn et al. (2012) found that after 6 cm (i.e., the distal third part of the 9 cm hair segment they sampled), hair had lower cortisol concentration and HCC was associated with hair washing frequency (p < .335 for the proximal 2/3 segment and p < .008 for the distal 1/3 segment).
Health-related demographics
In the Karlen et al. (2015) study, findings revealed increasing odds ratios of different common childhood illnesses in children with higher HCC. Noppe et al. (2014) found an association of HCC with waist circumference (r = .10, p = .04) but not body mass index (BMI) or waist/height ratio.
Discussion
In this review, we aimed to better understand HCC as a biomeasure of persistent stress in young children. We generally found that HCC in young children is elevated with sources of persistent stress (low SES and maternal distress). We are not able to provide HCC reference ranges to guide nurse researchers on which children may actually be experiencing persistent stress specifically because there are no known cohort studies that link early childhood HCC values with adverse health outcomes later in life. Nevertheless, the reviewed data suggest that HCC may be a promising biomeasure for nurse researchers to use to approximate persistent physiologic stress in young children, especially because HCC averages fluctuations in cortisol output from the HPA axis of the developing child, includes measures of overnight cortisol secretions, avoids the reactionary testing effects that can confound salivary cortisol sampling (Steudte et al., 2011), and reduces the burden associated with repeated salivary measures. However, given the heterogeneous nature of the studies reviewed and the limited concomitant use of other measures to approximate persistent stress outcomes (e.g., temperament or behavior) and sources (e.g., SES and maternal distress within the dyad), our ability to draw specific inferences from this review is constrained.
As stated above, our main finding is that HCC is elevated in children who are exposed to hypothesized sources of persistent stress (i.e., low family SES and/or maternal distress) or who have perceived poorer temperaments or behavior (i.e., are more fearful or have socioemotional problems). This finding aligns with salivary cortisol research in young children but not necessarily with adult HCC research. In adults, HCC may sometimes be lower with persistent stress, such as in individuals with post-traumatic stress disorder (PTSD) (Steudte-Schmiedgen et al., 2015) or generalized anxiety disorder (GAD; Steudte et al., 2011). We suspect that in early childhood, the HPA axis is still appropriately secreting more cortisol during stress responses as opposed to the blunted stress responses that may be associated with PTSD or GAD in adults. We did not find the latter type of HPA-axis dysregulation in children between 12 and 60 months of age in our review.
The lack of diversity in the study samples limits our ability to draw conclusions about HCC across children from the wide range of racial and ethnic backgrounds present in the United States. For example, only one sample was derived from children in the United States, while the other studies were conducted in countries with universal health care, which is associated with better health outcomes (Bradley & Taylor, 2013) and may help reduce SES-related disparities seen more frequently in the United States. Further, the diversity of the populations in the United States differs from those in Europe, and the stress associated with racism in the United States may influence HCC levels. In the only study measuring stress in Black and White children from the United States, Palmer et al. (2013) found that HCC levels differed between the two racial groups and that these levels were correlated with different measures of maternal distress. These findings imply that the stress response in these children differs by race and/or that Black and White children are exposed to different stressors. The studies in this review also lacked Latino/Hispanic participants, which limits their applicability to a large and growing component of the U.S. population.
Despite the heterogeneity of the studies in this review, it is interesting that only one study found a possible sex difference in HCC, but that study did not have sufficient power to detect effects in their young-child subsample (Dettenborn et al., 2012). It is possible that sex differences in HCC vary by developmental period. For example, in young children, sex differences in HCC may not yet be apparent due to low prepubertal levels of sex hormones or, possibly, to parental perception or incomplete socialization regarding how males and females are “supposed” to respond to stress. Socially suppressing stress may have different biological outcomes as compared to talking about stress. Socialization of stress responses may also have important implications for understanding the stress responses among residents of recent-immigrant enclaves in the United States, who sometimes have better health than those of acculturated later generations (Alegria et al., 2008; Schwartz, Unger, Zamboanga, & Szapocznik, 2010).
Another limitation to drawing pooled inferences from this review is that not all studies concomitantly measured possible sources or outputs of persistent stress in early childhood. For example, the racial differences Palmer et al. (2013) found in the associations of maternal distress measures with HCC cannot be compared to comparable findings from any other study. In addition, studies that did measure SES used different variables to do so, and not all SES measures were associated with HCC levels. For some of the studies, however, the results involving SES may have been biased. For example, Vaghri et al. (2013) were limited in their measure of neighborhood SES in that neighborhood boundaries are sometimes self-defined and are not necessarily correlated with city records and they had low response rates from some neighborhoods, which may have biased their results.
One way to overcome the limitations of individual measures of persistent stress in early childhood is to quantify several variables. This strategy aligns with the methods of Karlen et al. (2015) who used a novel method to quantify psychosocial vulnerability and found that the score on this overall psychosocial vulnerability scale was associated with HCC in early and later-childhood health. It may be of little surprise, then, that these authors found a statistically significant relationship between HCC and psychosocial vulnerability (β = .40, p < .001) when using this scale yet did not find significant relationships between HCC and individual measures of psychosocial vulnerability in earlier analyses (Karlen et al., 2013). These findings suggest that individual risk factors associated with persistent stress in early childhood may not activate the child’s HPA axis, but rather it is the accumulation of such risk factors that may significantly activate and possibly later disrupt regulation of the child’s HPA axis.
Noppe et al. (2014) also had an interesting finding on the (albeit small) correlation between child HCC and waist circumference but not BMI. This finding may highlight early life precursors of disease associated with waist circumference, such as the metabolic syndrome, which is typically associated with both chronic stress and HCC levels in adulthood (Kuehl et al., 2015; Stalder et al., 2013). The pathways among early central adiposity, the HPA axis, and later adverse health call for further investigation.
Future Directions
Because of nursing’s wide and diverse scope of practice, nurse researchers are in a unique position to identify children who are experiencing persistent stress. The biomeasure of HCC may be an appropriate and relatively easy tool to use for such identification, yet this review confirms that more research is needed in order to understand the full potential of HCC in this context. Because many individual measures had weak associations with child HCC or demonstrated racial differences in the reviewed studies, nurse researchers may need to use multiple types of measures to determine the factors associated with persistent stress in early childhood and be able to identify which children may be at risk for stress-related adverse health outcomes, as in the Karlen et al. (2015) study discussed above. For example, to understand HCC associations with SES, nurse researchers should measure both parent education and parent income. Moreover, they should measure additional factors already known to be significantly associated with HCC, including dyad characteristics (e.g., birth size and pregnancy cortisol levels), age, hair washing, medication use, health, sex/gender, and waist circumference, to improve the quality of their study. Finally, nurse researchers should consider measuring cortisol in no more than 6 cm of hair length, as the reviewed studies revealed that longer lengths may provide less reliable HCC measures. Improving the quality of HCC research in young children may eventually help identify HCC reference ranges across multiple attributes, develop sensitivity and specificity data, and allow the use of HCC to transition from research to clinical practice.
Conclusions
In this article, we reviewed all known studies that measured HCC in young, normally developing children from age 12 to 60 months. In general, we found that early childhood HCC is elevated with sources of persistent stress. We hope this review will help nurses understand factors associated with HCC and persistent stress in early childhood, an area of research crucial for lifelong health.
Footnotes
Author Contribution: R. Bates contributed to conception and design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. P. Salsberry contributed to design, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. J. Ford contributed to conception and design, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Randi Bates is supported by a graduate research associateship with the Crane Center for Early Childhood Research and Policy and by a scholarship from the Jonas Doctoral Nurse Scholar Program.
References
- Alegria M., Canino G., Shrout P. E., Woo M., Duan N., Vila D.…Meng X. L. (2008). Prevalence of mental illness in immigrant and non-immigrant U.S. Latino groups. American Journal of Psychiatry, 165, 359–369. doi:10.1176/appi.ajp.2007.07040704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda R. F., Dong M., Brown D. W., Felitti V. J., Giles W. H., Perry G. S.…Dube S. R. (2009). The relationship of adverse childhood experiences to a history of premature death of family members. BMC Public Health, 9, 106 doi:10.1186/1471-2458-9-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub C., Vallotton C. D., Mastergeorge A. M. (2011). Developmental pathways to integrated social skills: The roles of parenting and early intervention. Child Development, 82, 583–600. [DOI] [PubMed] [Google Scholar]
- Baum A., Grunberg N. (1997). Measurement of stress hormones In Cohen S., Kessler R. C., Gordon L. U. (Eds.), Measuring stress: A guide for health and social scientists (pp. 175–192). New York, NY: Oxford University Press. [Google Scholar]
- Blair C. (2010). Stress and the development of self-regulation in context. Child Development Perspectives, 4, 181–188. doi:10.1111/j.1750-8606.2010.00145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., Berry D., Mills-Koonce R., Granger D., & F. L. P. Investigators. (2013). Cumulative effects of early poverty on cortisol in young children: Moderation by autonomic nervous system activity. Psychoneuroendocrinology, 38, 2666–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., Granger D. A., Kivlighan K. T., Mills-Koonce R., Willoughby M., Greenberg M. T.…Fortunato C. K. (2008). Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology, 44, 1095–1109. [DOI] [PubMed] [Google Scholar]
- Blair C., Granger D., Willoughby M., Kivlighan K. (2006). Maternal sensitivity is related to hypothalamic-pituitary-adrenal axis stress reactivity and regulation in response to emotion challenge in 6-month-old infants. Annals of the New York Academy of Sciences, 1094, 263–267. [DOI] [PubMed] [Google Scholar]
- Blair C., Granger D. A., Willoughby M., Mills-Koonce R., Cox M., Greenberg M. T.…Fortunato C. K. (2011). Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development, 82, 1970–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley E. H., Taylor L. A. (2013). The American health care paradox: Why spending more is getting us less. New York, NY: Public Affairs. [Google Scholar]
- Briggs-Gowan M. J., Carter A. S., Ford J. D. (2012). Parsing the effects violence exposure in early childhood: Modeling developmental pathways. Journal of Pediatric Psychology, 37, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G. H., Yu T., Chen Y. F., Kogan S. M., Evans G. W., Beach S. R.…Philibert R. A. (2013). Cumulative socioeconomic status risk, allostatic load, and adjustment: A prospective latent profile analysis with contextual and genetic protective factors. Developmental Psychology, 49, 913–927. doi:10.1037/a0028847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks K. P., Robles T. F. (2009). Recent depressive and anxious symptoms predict cortisol responses to stress in men. Psychoneuroendocrinology, 34, 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. A., Dubois L., Tremblay M. S., Taljaard M. (2013). The influence of place on weight gain during early childhood: A population-based, longitudinal study. Journal of Urban Health, 90, 224–239. doi:10.1007/s11524-012-9712-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Schmidt L. A. (2015). Temperament and personality In Lerner R. M., Lamb M. E. (Eds.), Handbook of child psychology and developmental science, socioemotional processes (7th ed, Vol. 33, pp. 152–200). Hoboken, NJ: Wiley. [Google Scholar]
- Danese A., McEwen B. S. (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology and Behavior, 106, 29–39. doi:10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- D’Anna-Hernandez K. L., Ross R. G., Natvig C. L., Laudenslager M. L. (2011). Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: Comparison to salivary cortisol. Physiology and Behavior, 104, 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo M., Cozzolino A., Colao A., Pivonello R. (2012). Subclinical Cushing’s syndrome. Best Practice & Research: Clinical Endocrinology, 26, 497–505. [DOI] [PubMed] [Google Scholar]
- Dettenborn L., Tietze A., Bruckner F., Kirschbaum C. (2010). Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology, 35, 1404–1409. [DOI] [PubMed] [Google Scholar]
- Dettenborn L., Tietze A., Kirschbaum C., Stalder T. (2012). The assessment of cortisol in human hair: Associations with sociodemographic variables and potential confounders. Stress, 15, 578–588. [DOI] [PubMed] [Google Scholar]
- Essex M. J., Klein M. H., Cho E., Kalin N. H. (2002). Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry, 52, 776–784. [DOI] [PubMed] [Google Scholar]
- Everly G. S., Jr, Lating J. M. (2002). A clinical guide to the treatment of the human stress response (2nd ed). New York, NY: Kluwer Academic/Plenum. [Google Scholar]
- Felitti V. J., Anda R. F., Nordenberg D., Williamson D. F., Spitz A. M., Edwards V.…Marks J. S. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) study. American Journal of Preventive Medicine, 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Gow R., Thomson S., Rieder M., Van Uum S., Koren G. (2010). An assessment of cortisol analysis in hair and its clinical applications. Forensic Science International, 196, 32–37. [DOI] [PubMed] [Google Scholar]
- Grass J., Kirschbaum C., Miller R., Gao W., Steudte-Schmiedgen S., Stalder T. (2015). Sweat-inducing physiological challenges do not result in acute changes in hair cortisol concentrations. Psychoneuroendocrinology, 53, 108–116. doi:10.1016/j.psyneuen.2014.12.023 [DOI] [PubMed] [Google Scholar]
- Groeneveld M. G., Vermeer H. J., Linting M., Noppe G., van Rossum E. F., van I. M. H. (2013). Children’s hair cortisol as a biomarker of stress at school entry. Stress, 16, 711–715. [DOI] [PubMed] [Google Scholar]
- Grunau R. E., Cepeda I. L., Chau C. M. Y., Brummelte S., Weinberg J., Lavoie P. M.…Turvey S. E. (2013). Neonatal pain-related stress and NFKBIA genotype are associated with altered cortisol levels in preterm boys at school age. PloS One, 8, 1–10. doi:10.1371/journal.pone.0073926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M. R., Donzella B. (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27, 199–220. [DOI] [PubMed] [Google Scholar]
- Halfon N., Hochstein M. (2002). Life course health development: An integrated framework for developing health, policy, and research. Milbank Quarterly, 80, 433–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon N., Larson K., Lu M., Tullis E., Russ S. (2014). Lifecourse health development: Past, present and future. Maternal and Child Health Journal, 18, 344–365. doi:10.1007/s10995-013-1346-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel A. F., Meyer J. S., Henchey E., Dettmer A. M., Suomi S. J., Novak M. A. (2011). Effects of shampoo and water washing on hair cortisol concentrations. Clinica Chimica Acta, 412, 382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M. C., Karban L. V., Benitez P., Goodteacher A., Laudenslager M. L. (2014). Chemical processing and shampooing impact cortisol measured in human hair. Clinical and Investigative Medicine, 37, 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar C. E., Gunnar M. R. (2013). Future directions in the study of social relationships as regulators of the HPA axis across development. Journal of Clinical Child and Adolescent Psychology, 42, 564–575. doi:10.1080/15374416.2013.804387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps A. W. A., Molenmaker M., Kemperman R., Veen B. S., Bocca G., Veeger N. J. G. M. (2014). Children with asthma have significantly lower long-term cortisol levels in their scalp hair than healthy children. APA Acta Paediatrica, 103, 957–961. [DOI] [PubMed] [Google Scholar]
- Kang D. H., Rice M., Park N. J., Turner-Henson A., Downs C. (2010). Stress and inflammation: A biobehavioral approach for nursing research. Western Journal of Nursing Research, 32, 730–760. [DOI] [PubMed] [Google Scholar]
- Karlen J., Frostell A., Theodorsson E., Faresjo T., Ludvigsson J. (2013). Maternal influence on child HPA axis: A prospective study of cortisol levels in hair. Pediatrics, 132, e1333–e1340. [DOI] [PubMed] [Google Scholar]
- Karlen J., Ludvigsson J., Hedmark M., Faresjo A., Theodorsson E., Faresjo T. (2015). Early psychosocial exposures, hair cortisol levels, and disease risk. Pediatrics, 135, e1450–e1457. doi:10.1542/peds.2014-2561 [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Perel J., Dahl R. E., Moreci P., Nelson B.…Ryan N. D. (1997). The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biological Psychiatry, 42, 669–679. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Tietze A., Skoluda N., Dettenborn L. (2009). Hair as a retrospective calendar of cortisol production—Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology, 34, 32–37. doi:10.1016/j.psyneuen.2008.08.024 [DOI] [PubMed] [Google Scholar]
- Kuehl L. K., Hinkelmann K., Muhtz C., Dettenborn L., Wingenfeld K., Spitzer C.…Otte C. (2015). Hair cortisol and cortisol awakening response are associated with criteria of the metabolic syndrome in opposite directions. Psychoneuroendocrinology, 51, 365–370. doi:10.1016/j.psyneuen.2014.09.012 [DOI] [PubMed] [Google Scholar]
- Lee D. Y., Kim E., Choi M. H. (2015). Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Reports, 48, 209–216. doi:10.5483/BMBRep.2015.48.4.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman S. L., Wiles C. C., Atkinson H. C., Henley D. E., Russell G. M., Leendertz J. A.…Conway-Campbell B. L. (2008). The significance of glucocorticoid pulsatility. European Journal of Pharmacology, 583, 255–262. doi:10.1016/j.ejphar.2007.11.073 [DOI] [PubMed] [Google Scholar]
- Marshall G. N., Davis L. M., Sherbourne C. D. (1999). A review of the scientific literature as it pertains to Gulf War illnesses: Vol. 4. Stress. Santa Monica, CA: RAND; Retrieved from https://www.rand.org/natsec_area/products/gulf.html [Google Scholar]
- McCoy D. C., Raver C. C. (2014). Household instability and self-regulation among poor children. Journal of Children and Poverty, 20, 131–152. doi:10.1080/10796126.2014.976185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S. (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87, 873–904. doi:10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Gianaros P. J. (2010). Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186, 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnat S. M., Chandler R. F. (2015). Long term physical health consequences of adverse childhood experiences. Sociological Quarterly, 56, 723–752. doi:10.1111/tsq.12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S. M. (2008). Modern approaches to conceptualizing and measuring human life stress. Annual Review of Clinical Psychology, 4, 33–52. doi:10.1146/annurev.clinpsy.4.022007.141207 [DOI] [PubMed] [Google Scholar]
- National Center for Chronic Disease Prevention and Health Promotion. (2009). The power of prevention: Chronic disease…the public health challenge of the 21st century, 1–16. Atlanta, Georgia: Centers for Disease Control and Prevention; Retrieved from https://www.cdc.gov/chronicdisease/pdf/2009-power-of-prevention.pdf [Google Scholar]
- Noppe G., Van Rossum E. F., Koper J. W., Manenschijn L., Bruining G. J., de Rijke Y. B., van den Akker E. L. (2014). Validation and reference ranges of hair cortisol measurement in healthy children. Hormone Research in Paediatrics, 82, 97–102. doi:10.1159/000362519 [DOI] [PubMed] [Google Scholar]
- Palmer F. B., Anand K. J. S., Graff J. C., Murphy L. E., Qu Y., Völgyi E.…Tylavsky F. A. (2013). Early adversity, socioemotional development, and stress in urban 1-year-old children. Journal of Pediatrics, 163, 1733–1739. doi:10.1016/j.jpeds.2013.08.030 [DOI] [PubMed] [Google Scholar]
- Pragst F., Balikova M. A. (2006). State of the art in hair analysis for detection of drug and alcohol abuse. Clinica Chimica Acta, 370, 17–49. doi:10.1016/j.cca.2006.02.019 [DOI] [PubMed] [Google Scholar]
- Raul J. S., Cirimele V., Ludes B., Kintz P. (2004). Detection of physiological concentrations of cortisol and cortisone in human hair. Clinical Biochemistry, 37, 1105–1111. [DOI] [PubMed] [Google Scholar]
- Rothbart M. K. (2011). Becoming who we are: Temperament and personality in development. New York, NY: Guilford Press. [Google Scholar]
- Rowe D. C., Jacobson K. C., Van den Oord E. J. (1999). Genetic and environmental influences on vocabulary IQ: Parental education level as moderator. Child Development, 70, 1151–1162. [DOI] [PubMed] [Google Scholar]
- Russell E., Kirschbaum C., Laudenslager M. L., Stalder T., de Rijke Y., van Rossum E. F.…Koren G. (2015). Toward standardization of hair cortisol measurement: Results of the first international interlaboratory round robin. Therapeutic Drug Monitoring, 37, 71–75. [DOI] [PubMed] [Google Scholar]
- Russell E., Koren G., Rieder M., Van Uum S. (2012). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37, 589–601. doi:10.1016/j.psyneuen.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Russell E., Koren G., Rieder M., Van Uum S. H. M. (2014). The detection of cortisol in human sweat: Implications for measurement of cortisol in hair. Therapeutic Drug Monitoring, 36, 30–34. doi:10.1097/FTD.0b013e31829daa0a [DOI] [PubMed] [Google Scholar]
- Sauve B., Koren G., Walsh G., Tokmakejian S., Van Uum S. H. (2007). Measurement of cortisol in human hair as a biomarker of systemic exposure. Clinical & Investigative Medicine, 30, E183–E191. [DOI] [PubMed] [Google Scholar]
- Schwartz S. J., Unger J. B., Zamboanga B. L., Szapocznik J. (2010). Rethinking the concept of acculturation: Implications for theory and research. American Psychologist, 65, 237–251. doi:10.1037/a0019330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J. P. (2010). Building a new biodevelopmental framework to guide the future of early childhood policy. Child Development, 81, 357–367. doi:10.1111/j.1467-8624.2009.01399.x [DOI] [PubMed] [Google Scholar]
- Shonkoff J. P. (2012). Leveraging the biology of adversity to address the roots of disparities in health and development. Proceedings of the National Academy of Sciences of the United States of America, 109, 17302–17307. doi:10.1073/pnas.1121259109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J. P., Boyce W. T., McEwen B. S. (2009). Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association, 301, 2252–2259. doi:10.1001/jama.2009.754 [DOI] [PubMed] [Google Scholar]
- Shonkoff J. P., Garner A. S., The Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, and the Section on Developmental and Behavioral Pediatrics. (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129, e232–e246. [DOI] [PubMed] [Google Scholar]
- Short S. J., Stalder T., Marceau K., Entringer S., Moog N. K., Shirtcliff E. A.…Buss C. (2016). Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology, 71, 12–18. doi:10.1016/j.psyneuen.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski R., Rovnaghi C. R., Anand K. J. (2015). Methodological considerations for hair cortisol measurements in children. Therapeutic Drug Monitoring, 37, 812–820. doi:10.1097/FTD.0000000000000209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. J., Everly G. S., Johns T. R. (1993). The role of stress arousal in the dynamics of the stressor-to-illness process among accountants. CARE Contemporary Accounting Research, 9, 432–449. [Google Scholar]
- Smy L., Shaw K., Smith A., Russell E., Van Uum S., Rieder M.…Koren G. (2015). Hair cortisol as a novel biomarker of HPA suppression by inhaled corticosteroids in children. Pediatric Research, 78, 44–47. [DOI] [PubMed] [Google Scholar]
- Stalder T., Hucklebridge F., Evans P., Clow A. (2009). Use of a single case study design to examine state variation in the cortisol awakening response: Relationship with time of awakening. Psychoneuroendocrinology, 34, 607–614. doi:10.1016/j.psyneuen.2008.10.023 [DOI] [PubMed] [Google Scholar]
- Stalder T. & Kirschbaum C. (2012). Analysis of cortisol in hair--state of the art and future directions. Brain, Behavior, and Immunity, 26(7), 1019–1029. [DOI] [PubMed] [Google Scholar]
- Stalder T., Kirschbaum C., Alexander N., Bornstein S. R., Gao W., Miller R.…Fischer J. E. (2013). Cortisol in hair and the metabolic syndrome. Journal of Clinical Endocrinology and Metabolism, 98, 2573–2580. doi:10.1210/jc.2013-1056 [DOI] [PubMed] [Google Scholar]
- Starkman M. N., Gebarski S. S., Berent S., Schteingart D. E. (1992). Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biological Psychiatry, 32, 756–765. [DOI] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S., Stalder T., Schonfeld S., Wittchen H. U., Trautmann S., Alexander N.…Kirschbaum C. (2015). Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology, 59, 123–133. doi:10.1016/j.psyneuen.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Steudte S., Stalder T., Dettenborn L., Klumbies E., Foley P., Beesdo-Baum K., Kirschbaum C. (2011). Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Research, 186, 310–314. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Hare T. A., Quinn B. T., McCarry T. W., Nurse M., Gilhooly T.…Casey B. J. (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science, 13, 46–61. doi:10.1111/j.1467-7687.2009.00852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C., Chrousos G. P. (2002). Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53, 865–871. [DOI] [PubMed] [Google Scholar]
- Vaghri Z., Guhn M., Weinberg J., Grunau R. E., Yu W., Hertzman C. (2013). Hair cortisol reflects socio-economic factors and hair zinc in preschoolers. Psychoneuroendocrinology, 38, 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaelst B., Huybrechts I., Bammann K., Michels N., de Vriendt T., Vyncke K.…de Henauw S. (2012). Intercorrelations between serum, salivary, and hair cortisol and child-reported estimates of stress in elementary school girls. Psychophysiology, 49, 1072–1081. doi:10.1111/j.1469-8986.2012.01396.x [DOI] [PubMed] [Google Scholar]
- Vliegenthart J., Noppe G., van Rossum E. F. C., Koper J. W., Raat H., van den Akker E. L. T. (2016). Socioeconomic status in children is associated with hair cortisol levels as a biological measure of chronic stress. Psychoneuroendocrinology, 65, 9–14. doi:10.1016/j.psyneuen.2015.11.022 [DOI] [PubMed] [Google Scholar]
- Watamura S. E., Donzella B., Kertes D. A., Gunnar M. R. (2004). Developmental changes in baseline cortisol activity in early childhood: Relations with napping and effortful control. Developmental Psychobiology, 45, 125–133. doi:10.1002/dev.20026 [DOI] [PubMed] [Google Scholar]
- Wennig R. (2000). Potential problems with the interpretation of hair analysis results. Forensic Science International, 107, 1–3. [DOI] [PubMed] [Google Scholar]
- Wodtke G. T. (2013). Duration and timing of exposure to neighborhood poverty and the risk of adolescent parenthood. Demography, 50, 1765–1788. doi:10.1007/s13524-013-0219-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2005). Preventing chronic diseases: A vital investment. Geneva, Switzerland: WHO Press. [Google Scholar]
- Wust S., Wolf J., Hellhammer D. H., Federenko I., Schommer N., Kirschbaum C. (2000). The cortisol awakening response—normal values and confounds. Noise Health, 2, 79–88. [PubMed] [Google Scholar]
- Yamada J., Stevens B., de Silva N., Gibbins S., Beyene J., Taddio A.…Koren G. (2007). Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology, 92, 42–49. [DOI] [PubMed] [Google Scholar]
- Ziol-Guest K. M., McKenna C. C. (2014). Early childhood housing instability and school readiness. Child Development, 85, 103–113. doi:10.1111/cdev.12105 [DOI] [PubMed] [Google Scholar]