Abstract
Heart failure (HF) is responsible for more 30-day readmissions than any other condition. Minorities, particularly African American males (AAM), are at much higher risk for readmission than the general population. In this study, demographic, social, and clinical data were collected from the electronic medical records of 132 AAM patients (control and intervention) admitted with a primary or secondary admission diagnosis of HF. Both groups received guideline-directed therapy for HF. Additionally the intervention group received a pharmacist-led intervention. Data collected from these patients were used to develop and validate a predictive model to evaluate the impact of the pharmacist-led intervention, and identify predictors of readmission in this population. After propensity score matching, the intervention was determined to have a significant impact on readmission, as a significantly smaller proportion of patients in the intervention group were readmitted as compared to the control group (11.5% vs. 42.9%; p = .03). A predictive model for 30-day readmission was developed using K-nearest neighbor (KNN) classification algorithm. The model was able to correctly classify about 71% patients with an AUROC of 0.70. Additionally, the model provided a set of key patient attributes predictive of readmission status. Among these predictive attributes was whether or not a patient received the intervention. A relative risk analysis identified that patients who received the intervention are less likely to be readmitted within 30 days. This study demonstrated the benefit of a pharmacist-led intervention for AAM with HF. Such interventions have the potential to improve quality of life for this patient population.
Keywords: machine learning, predictive modeling, heart failure, readmission, pharmacist intervention
Heart failure (HF) affects about 5.7 million adults in the United States, and as the population continues to age, this number is expected to grow to more than 8 million by 2030 (Benjamin et al., 2017; Heidenreich et al., 2013).The increasing prevalence of HF is alarming as the cost of care for HF patients is high, and this places a significant burden on the health-care system. The total direct medical cost of HF is estimated to be $20.9 billion, and is expected to more than double by 2030 (Heidenreich et al., 2013). A significant portion of these costs is attributable to frequent hospitalizations and readmissions. HF is the leading cause of 30-day readmission among Medicare beneficiaries, and the second most common cause of readmission for all payers combined (Strom et al., 2017). According to the Agency for Healthcare Research and Quality, nearly one in every four HF patients will be readmitted within 30 days contributing more than $2.7 billion to aggregate hospital costs annually (Fingar & Washington, 2006).
Minorities, particularly African Americans or Blacks, are disproportionately affected by HF. The Multi-Ethnic Study of Atherosclerosis (MESA) reported that Blacks had a higher incidence of HF than other populations. The annual incidence of HF per 1000 person-years in this study was 4.6 for Blacks compared with 3.5, 2.4, and 1.0 for Hispanic, White, and Chinese Americans, respectively (Bahrami et al., 2008). Not only is the incidence of HF higher for Blacks, but incidences of HF also tend to occur earlier in this population. Racial differences in the prevalence of HF were investigated in the Coronary Artery Risk Development in Young Adults (CARDIA) study. It was reported that HF occurred 20 times more frequently in Blacks than Whites younger than 50 years of age (Bibbins-Domingo et al., 2009). Additionally, within the African American community, African American males (AAM) tend to be at the highest risk, having both a greater incidence and prevalence of HF at age >60 years than African American females (Benjamin et al., 2017).
Blacks are at higher risk of readmission than Whites. A 2011 study by Joynt et al. reported that Blacks are up to 20% more likely to be readmitted to the hospital within 30 days after being treated for HF compared to White patients depending on the site of care (Joynt, Orav, & Jha, 2011). Another study investigating the incidence of hospitalization due to acute decompensated HF (ADHF) reported that AAM had the highest annual rate of hospitalization due to ADHF events compared with all other populations (Chang et al., 2014). Moreover, Blacks also tend to have poorer outcomes after hospitalization for HF. A Yale University study examining differences between HF outcomes in African Americans and Whites reported that African Americans have a 45% higher risk of either death or functional decline after hospitalization for HF compared to Whites (Vaccarino et al., 2002). A similar study conducted in 2008 based on the Atherosclerosis Risk in Communities (ARIC) Study patient cohort also reported that African Americans had a statistically greater 5-year mortality rate compared with Caucasians (Loehr, Rosamond, Chang, Folsom, & Chambless, 2008).
It is widely accepted that clinical factors such as left ventricular function, atrial fibrillation, renal dysfunction as well as certain biomarkers such as B type natriuretic peptide (BNP) and troponin have prognostic value in disease progression, severity, and mortality. There is currently no consensus on what factors contribute most toward readmission in AAM with HF (Yancy et al., 2013, 2017). It is thought that modifiable risk factors such as hypertension, elevated lipid and blood glucose levels, and smoking may have a more significant impact on HF risk in African Americans than other populations (Benjamin et al., 2017). The high prevalence of these modifiable risk factors in the African American community and their significance toward increased readmission risk affords opportunities for interventions through disease state education, medication management, and counseling.
Several published studies have demonstrated the benefit of pharmacist engagement in HF management (Cheng, 2017; Parajuli, Franzon, McKinnon, Shakib, & Clark, 2017). A study conducted by Jack et al. showed that having a clinical pharmacist conduct a follow-up call with patients 2 to 4 days after discharge lowered the rate of hospital utilization compared to those receiving usual care (0.314 vs. 0.451 visits per person per month; Jack et al., 2009). In a recent study, the impact of a pharmacist as part of a multidisciplinary team was evaluated in a pharmacist-led intervention to optimize HF medication during hospitalization (Suzuki et al., 2018). In this study, the pharmacist’s contribution to the multidisciplinary team included guideline-directed optimization of the patient’s medication regimen and providing comments on the appropriateness of prescribed medications. The intervention was associated with an increase in beta-blocker prescriptions and a decrease in medications that should be avoided in HF. Other studies have demonstrated that pharmacists can contribute to improved medication adherence as well as reductions in readmissions, mortality, and length of hospital stay (Ponniah, Anderson, Shakib, Doecke, & Angley, 2007; Szkiladz et al., 2013).
Predictive models are tools that can relate a patient’s characteristics to the probability of developing some future clinical event (Hendriksen, Geersing, Moons, & de Groot, 2013; Kuziemsky, 2016). There is currently a great deal of interest in applying such models in health care as an approach to support clinical decision making and improve patient outcomes. Statistical modeling and machine learning are two approaches to predictive modeling, and the choice between these two approaches is largely dependent on the particular application. As opposed to traditional statistical modeling, machine learning requires fewer prior assumptions about the nature of the underlying relationships between variables. This affords machine learning models greater flexibility to capture interactions between patient characteristics that may be contributing toward observed clinical outcomes. This study aims to assess the impact of a pharmacist-led intervention in reducing 30-day readmission rates in AAM with HF. Additionally, machine learning approaches will be used to identify patient characteristics that are predictive of all-cause readmission within 30 days in this patient population.
Methods
Study Design, Population, and Data Collection
Data were collected from AAM patients admitted to WellStar Atlanta Medical Center (WAMC), a major metropolitan academic hospital in Atlanta, Georgia, between May 2012 and December 2015 with a primary or secondary admission diagnosis of HF as defined by the following International Classification of Diseases (ICD) 9th/10th revision codes: 428 and/or I50. Complete details on the study design, intervention, and patient baseline demographics were outlined in our previous publication (Moye, Chu, Pounds, & Thurston, 2018). Briefly, patients were divided into two groups: a historical control group (n = 58) and an intervention group (n = 74). Both groups received standard of care for HF and prescribed medications according to the most recent practice guidelines. Standard of care at WAMC meant that all HF patients received a scale, an invitation to attend a cardiovascular educational class, a 72-hr postdischarge call by a nurse, and an attempt to schedule a follow-up appointment with a physician. The standard of care protocol does not include a pharmacist.
In addition to the standard of care, the intervention group received medication reconciliation, medication cost/formulary review, discharge medication counseling, self-monitoring resources (a packet of printed material included fluid/sodium intake log, daily symptom log, daily weight log, a list of patients’ current medications, and health-care providers contact information), and 14/30-day postdischarge telephone follow-up by pharmacy staff (see Supplemental Digital Content, CHF Telephone Questionnaire). The primary outcome of the study was all-cause readmission within 30 days which was defined as any return hospitalization to an acute care hospital following a prior discharge from the same or another acute care facility within a period of 30 days. Readmission status for the historical control group was determined from the medical records whereas the readmission status for the intervention group was determined either using the medical records or as reported by the patient during the 30-day telephone follow-up. Patients who received the intervention provided written consent, and both the patient and the health-care institution retained a copy of the signature page for their records. This protocol was approved by both WAMC and Mercer University (Protocol: H1105099).
Demographic, social, and clinical data were retrieved from the patients’ electronic health record, EPIC (EPIC Systems Corporation, Verona, WI), and analyzed for inclusion in the predictive model. The collected data contained a total of 39 attributes. In preparing the dataset for modeling, several characteristics were manually removed from the dataset due to correlation to other attributes, or lack of adequate distribution of attribute values among the study population. Candidate attributes with missing data were imputed with the class-specific median value. The final dataset contained a total of 29 characteristics common to both the control and intervention groups. The dependent variable was readmission within 30 days encoded as a binary variable.
Statistical Analysis
Categorical attributes were summarized by event frequency. Statistical comparison of these attributes between groups was done by relative risk analysis and chi-square tests. Continuous attributes were summarized by median and standard deviation and compared by Mann–Whitney U (Wilcoxon rank-sum) test. Statistical significance was defined for a p value < .05 for all analyses.
Statistically relevant differences in baseline attribute distributions between groups were addressed through propensity score matching (PSM). PSM is a statistical technique that attempts to correct for these imbalances in attribute distributions that arise due to the limitations of observational studies and may result in a biased estimate of the intervention’s impact on readmission rates. A logistical model was fit assigning patient attributes as covariates to predict the probability of group membership (i.e., control group vs. intervention group). The propensity score (predicted probability of group membership) was then used to match subjects in the intervention group to subjects in the control group with similar attribute distributions. Matching was performed with replacement. Improvement in attribute balance was assessed statistically and visually using diagnostic plots. Impact analysis was performed based on the propensity adjusted dataset.
Predictive Model Development
The dataset was modeled using the machine learning tools available in Scikit-learn version 0.19.1 (Pedregosa et al., 2011). Attribute selection was performed before classification to filter nonpredictive attributes from the dataset, and to inform the list of attributes identified as predictive of readmission status. Attribute selection was carried out using either a greedy sequential selection or embedded algorithm. Choice of an algorithm for attribute selection depended on the classifier used for predictive modeling. The following classifiers were explored for predictive modeling: K-nearest neighbor (KNN), randomized K-nearest neighbor (rKNN), support vector machine (SVM), random forest, Lasso regression, and gradient boosting machine (GBM). All models were trained under 10-fold cross-validation using two-third of the dataset as training data. The remaining one-third of the data were included in an external test set to assess the performance of the trained model.
Model accuracy on the test set is one of the most-frequently cited point measures of classifier performance; however, classifier accuracy may provide a biased assessment of classifier performance for datasets with imbalances in the number of patients in each outcome category. A more informative measure of the performance of a binary classifier under these conditions is area under the receiver operating characteristic curve (AUROC). This metric is robust to the imbalances in patient outcomes. Model performance was compared based on AUROC on the external validation set.
Results
Baseline Characteristics
Table 1 presents the distribution of baseline attributes among AAM patients in the intervention and control groups. The median age, length of stay, and BMI of patients in the control group and intervention group were all similar, 60 years versus 58.5 years, 4 days versus 4.5 days, and 32.6 versus 30, respectively. Several attributes were determined to have a statistically significant difference in baseline distribution between groups. Subjects in the intervention group were more likely to have: anemia, arrhythmia, dyslipidemia/coronary artery disease (CAD), and renal disease. Additionally, subjects were also more likely to be on aldosterone antagonists, direct oral anticoagulants (DOAC), calcium channel blockers, and a lipid-lowering agent than subjects assigned to the control group. Subjects in the control group were more likely to have a primary admission diagnosis of HF than subjects in the intervention group.
Table 1.
Comparison of the Baseline Attribute Distributions Among Subjects in the Control and Intervention Group.
A.
| Categorical attributes | No. of control patients (%) | No. of intervention patients (%) | p value | ||
|---|---|---|---|---|---|
| Primary admission diagnosis of HF | 52 (89.7) | 35 (47.3) | p < .001 | ||
| Ejection fraction <40 | 33 (56.9) | 32 (43.2) | .150 | ||
| Anemia | 6 (10.3) | 26 (35.1) | .002 | ||
| Arrhythmia | 6 (10.3) | 29 (39.2) | p < .001 | ||
| Diabetes | 27 (46.6) | 30 (40.5) | .610 | ||
| Dyslipidemia/CAD | 14 (24.1) | 41 (55.4) | .001 | ||
| Hypertension | 53 (91.4) | 68 (91.9) | .830 | ||
| Obesity | 19 (32.8) | 17 (23.0) | .290 | ||
| Pacemaker/ICD | 7 (12.1) | 19 (25.7) | .084 | ||
| Heart attack | 5 (8.6) | 12 (16.2) | .300 | ||
| Renal disease | 16 (27.6) | 38 (51.4) | .010 | ||
| Beta blocker | 53 (91.4) | 70 (94.6) | .700 | ||
| ACE inhibitor | 35 (60.3) | 38 (51.4) | .390 | ||
| ARB | 4 (6.9) | 6 (8.11) | .940 | ||
| Diuretics | 39 (67.2) | 59 (79.7) | .150 | ||
| Digoxin | 5 (8.6) | 5 (6.76) | .940 | ||
| Aldosterone antagonist | 7 (12.1) | 23 (31.1) | .017 | ||
| Nitrates | 16 (27.6) | 30 (40.5) | .170 | ||
| Warfarin | 9 (15.5) | 21 (28.4) | .120 | ||
| Other anticoagulants | 3 (5.2) | 13 (17.6) | .058 | ||
| Aspirin | 36 (62.1) | 49 (66.2) | .760 | ||
| Lipid lowering agents | 24 (41.4) | 55 (74.3) | p < .0015 | ||
| Calcium channel blockers | 8 (13.8) | 24 (32.4) | .023 | ||
| Other cardiovascular medications | 29 (50.0) | 37 (50.0) | 1.000 | ||
| Tobacco use | 19 (32.8) | 30 (40.5) | .470 | ||
| Alcohol consumption | 37 (34.5) | 23 (31.1) | .810 | ||
| Readmission within 30 days | 22 (37.9) | 16 (21.6) | .039 | ||
| B. | |||||
| Continuous attributes | Control | Intervention | p value | ||
| Median | SD | Median | SD | ||
| Age | 60.0 | 14.93 | 58.5 | 14.1 | .773 |
| Length of stay | 4.00 | 2.87 | 4.50 | 7.31 | .097 |
| Body mass index | 32.6 | 9.44 | 30.0 | 10.8 | .134 |
Note. Baseline distribution of categorical (A) and continuous (B) attributes among subjects in the control and intervention group. Categorical attributes compared through chi-squared test. Continuous attributes compared through Mann–Whitney U test. Significance declared for p < .05. Other cardiovascular meds includes hydralazine, amiodarone/other antiarrhythmics, alpha-1 antagonists, and clonidine. CAD = coronary artery disease; ACE = angiotensin-converting enzyme; ICD = implantable cardioverter-defibrillator; ARB = angiotensin receptor blocker; HF = heart failure; SD = standard deviation.
Unadjusted Analysis
During the study period, a total of 38 subjects were readmitted within 30 days of discharge representing an overall readmission rate of 28.8% for this patient cohort. A comparison of readmission rates between the control and intervention group identifies a significant reduction in all-cause 30-day readmission favoring the intervention group. Of the 38 total readmissions, 22 were in the control group (n = 58) and 16 were in the intervention group (n = 74) representing a readmission rate of 37.9% and 21.6% control versus intervention, respectively [RR = 0.57 (0.33–0.98); p = .04].
To further evaluate the impact of the intervention on readmission status subjects in the control and intervention groups were combined into a single dataset, and the two populations were distinguished by a dummy attribute for intervention (0 = control, 1 = intervention). Attribute selection was performed to identify a list of attributes predictive of readmission status and the performance of each classifier was observed on this dataset (Table 2). The KNN classifier outperformed all other classifiers on the combined dataset and was able to classify readmission status in 77.3% patients correctly. The KNN model was also able to achieve modest discrimination between patients predicted to be readmitted versus not readmitted as evidenced by an AUROC of 0.77. The intervention was found to be predictive of readmission status. A relative risk analysis revealed that patients who received the intervention are less likely to be readmitted within 30 days (see Supplemental Digital Content, Figure S1.). Other attributes identified as predictive of readmission status by the KNN model were age, anemia, arrhythmia, dyslipidemia, pacemaker, beta-blocker use, nitrate use, aspirin use, and other cardiovascular medication use.
Table 2.
Classifier Performance on the Combined Control and Intervention Dataset.
| Combined group | ||||||
|---|---|---|---|---|---|---|
| Classifier | KNN | rKNN | SVM | Forest | GBM | Lasso |
| Accuracy | 0.773 | 0.477 | 0.545 | 0.682 | 0.614 | 0.614 |
| AUROC | 0.768 | 0.469 | 0.496 | 0.616 | 0.589 | 0.576 |
| False positive rate | 0.214 | 0.500 | 0.321 | 0.143 | 0.321 | 0.286 |
| False negative rate | 0.250 | 0.563 | 0.688 | 0.625 | 0.500 | 0.563 |
Note. KNN = K-nearest neighbor; rKNN = randomized K-nearest neighbor; AUROC = area under the receiver operating characteristic curve; SVM = support vector machine; GBM = gradient boosting machine.
Adjusted Analysis
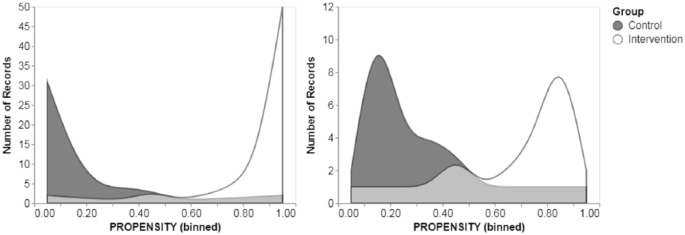
Due to the imbalanced distribution of baseline patient characteristics among subjects in the intervention and control group, PSM was performed to limit bias in the estimation of the effect size of the intervention. Attributes determined to have a statistically significant difference in baseline distribution between groups, as well as all other clinically relevant characteristics were included as covariates in the PSM adjustment. Figure 1 presents the difference in propensity score distributions between the control and intervention subjects before and after PSM adjustment. Subjects with extremely high (approximately >0.9) or low propensity scores (approximately <0.1) were removed from the dataset. The impact of the PSM adjustment on attribute balance was evaluated through statistical analysis, and no significant difference was observed in attribute distributions between subjects in the matched control and intervention dataset indicating improved attribute balance (see Supplemental Digital Content, Table S1).
Figure 1.
Distribution of propensity scores before (left panel) and after (right panel) trimming the dataset. Light gray region represents overlapping area of propensity scores.
Figure 2 presents the comparison of readmission rates between subjects in the adjusted versus the unadjusted group. A more extreme difference in readmission rates between groups after adjustment by PSM, 42.9% and 11.5% control versus intervention, respectively [RR = 0.28 (0.09–0.88); p = .03]. Impact analysis was also performed through matching to investigate the heterogeneity in intervention effect between the control and intervention group. The estimated average treatment effect (ATE), or average effect of the treatment, on the total population was −0.165 (95% CI [–0.593, 0.266]). The ATE on the treated (ATT) was higher than the ATE on the control (ATC), –0.210 (95% CI [–0.660, 0.240]), and −0.123 (95% CI [–0.624, 0.377]), respectively.
Figure 2.
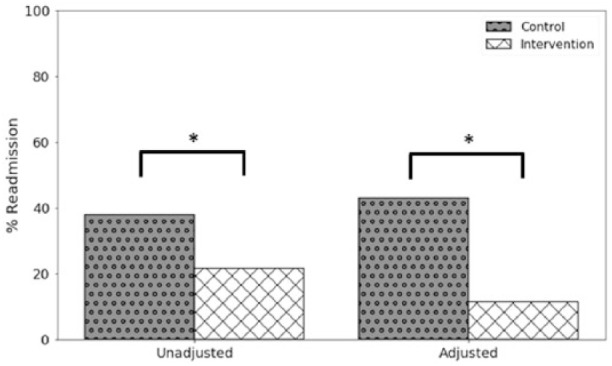
Percent readmission in the control and the intervention group for the unadjusted and adjusted analysis. *Significance at the alpha = .05 level.
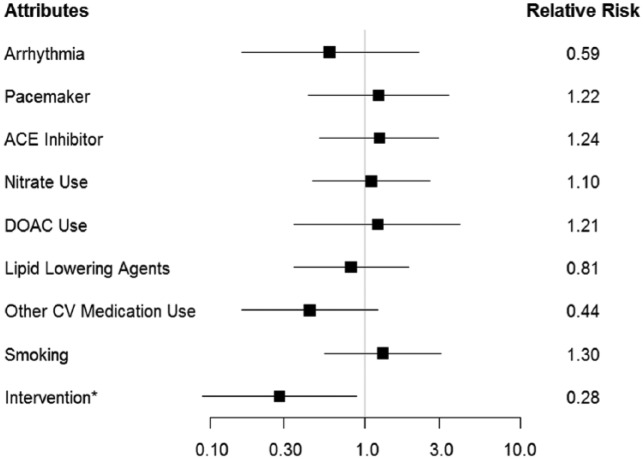
A predictive model was developed using the KNN classifier on the propensity score matched combined adjusted dataset. The model performed similarly to the model built on the unadjusted dataset. The KNN model was able to correctly classify readmission status in 70.8% of patients with an AUROC of 0.70. The model also had a false positive and false negative rate of 0.333 and 0.273 respectively. The intervention was again found to be predictive of readmission status. A relative risk analysis showed that patients who received the intervention are less likely to be readmitted within 30 days. Other attributes found to be predictive of readmission status were arrhythmias, pacemaker/implantable cardioverter-defibrillator (ICD), smoking, and use of the following medications: angiotensin-converting enzyme (ACE) inhibitor, nitrate, DOAC, and lipid-lowering agents. Relative risk analysis of these attributes is presented in Figure 3.
Figure 3.
PSM-adjusted readmission risk by attribute. *Statistical significance at the α = .05 level.
Discussion
The impact of pharmacist-led interventions in patients with HF has become a topic of considerable interest to hospitals and health systems seeking to reduce readmission rates in this group. In a 2006 study, Lopez-Cabezas et al. assessed the impact on a multifactorial educational intervention led by pharmacists in patient admitted for HF (Lopez Cabezas et al., 2006). Patients assigned to the intervention group received disease state education, drug therapy and diet education, and active telephone follow-up. The authors reported a significant reduction in hospital readmissions at 2 months and 6 months, and trend toward lower readmission rates at 12 months for patients enrolled in the intervention group. A study conducted in 2015 by Jackevicius et al. examined the impact of a pharmacist intervention as part of a multidisciplinary team (Jackevicius et al., 2015). The pharmacist’s contribution consisted of an assessment of the patient’s progress and medication regimen, prevention of drug–drug interactions, renal dose adjustment, and patient education on medication-related adverse effects. This study reported significantly lower all-cause 90-day readmission rates for HF favoring the intervention group. Similar benefits of the pharmacist intervention were observed in the present study (42.9% and 11.5% control versus intervention, respectively) as compared to previously published studies, despite differences in the pharmacist role in the intervention as well as targeted readmission time point. These results suggest that a pharmacist-led intervention including medication regimen optimization, counseling, and follow-up as well as patient education are important to reducing readmission risk in patients with HF.
There have been several attempts over the past two decades to develop predictive models of 30-day readmission in patients with HF using both statistical and machine learning methods. Several researchers have aimed to use large patient databases in conjunction with machine learning techniques to improve predictive accuracy over traditional statistical models. In 2016, Mortazavi et al. developed logistic regression, Poisson regression, GBM, SVM, and random forest models to predict all-cause readmission within 30 days in patients with HF (Mortazavi et al., 2016). The study included 236 attributes including socioeconomic, demographic, laboratory results, physical exams, and patient survey information collected from a diverse population of subjects. GBM was determined to be the best performing model with an AUROC of 0.615. In a similar study also conducted in 2016, Yang et al. developed a LASSO, GBM, and an artificial neural network (ANN) model to predict all-cause readmission within 30 days in a population of HF patients (Yang, Delcher, Shenkman, & Ranka, 2016). Candidate attributes for their predictive models included patient demographics, admission and discharge information, clinical, disease severity, hospital, and previous admission information. All three models performed similarly with an AUROC of 0.657, 0.663, and 0.662 for the Lasso, GBM, and ANN models, respectively. In comparison, in this study, the KNN model in the unadjusted analysis was able to achieve an AUROC of 0.77. The modest improvement in predictive performance of the KNN model compared to models published in previous studies is likely a reflection of selection bias due to the inherent disadvantages of the observational study design, and the limited population diversity of the dataset. After adjustment by PSM, which was shown to improve attribute balance between the control and intervention group, the results fell in line with previous published studies. The discussion is focused on the adjusted analysis because it is likely to provide a more accurate estimate of the effect of the intervention as it reduces bias due to confounding variables.
Prediction of all-cause readmission is a potentially dubious target as reasons for 30-day readmission vary by subject and are often not directly associated with HF. In a study examining the diagnoses and timing of 30-day readmission after hospitalization for HF, it was observed that only about 35% patients re-hospitalized within 30 days after discharge for HF were readmitted for the same condition (Dharmarajan et al., 2013). The remaining two-thirds of patients were readmitted due to varying comorbidities. This heterogeneity in readmission profile may partially explain the marginally improved performance of the KNN classifier over other classifiers tested in this study. The KNN classifier works by classifying each observation in the test set by majority vote of the K most similar observations to the training examples. It is possible that patients likely to be readmitted within 30 days are stratified by readmission diagnosis with patients who were readmitted due to HF having similar attributes to each other, but different attributes compared to patients readmitted for renal disease. The KNN model was likely able to identify some of these patterns, classifying the readmission status of a patient based on the similarity to other patients in the dataset.
The heterogeneity in patient readmission profile was also reflected in the analysis on the impact of the pharmacist intervention. Pharmacist intervention was associated with a significant decrease in readmission in the unadjusted analysis. After correcting for selection bias through PSM, the effect of the intervention was strengthened providing further support that this intervention was effective in reducing 30-day readmissions in this population; however, impact analysis revealed that the effect size of the intervention was not consistent across the entire patient population. The ATE is an estimate of the impact of the intervention on 30-day readmission over the entire population (i.e., both the control and intervention groups, had the control group received the intervention). It is the weighted average of the ATC and ATT, which represents the estimated effect of the intervention on the control and intervention group, respectively. All three estimates are expected to be equivalent when: (a) the distribution of known and unknown predictors is balanced between the control and intervention group, and (b) there is no effect measure modification by these attributes (Wang, Nianogo, & Arah, 2017). While all three estimates showed the intervention to have a consistent protective impact on readmission, the estimated effect of the intervention on the intervention group was almost twice as large as its effect on the control group had this group received the intervention. This suggests that there may be additional unmeasured attributes that contribute to an increased risk of 30-day readmission. It is possible that these unmeasured attributes could influence the effectiveness of the intervention as well. Predictive modeling found the pharmacist intervention to be predictive of 30-day readmission status in both the adjusted and unadjusted analysis; however, the 95% confidence interval of the effect size estimates suggests that the effect of the intervention varied widely among the population. This observation is likely the result of the intervention impacting some individuals more than others, depending on the patient-specific characteristics and the reason for readmission. In the design of a future intervention, the effectiveness of the intervention may be improved by stratifying patients by risk factors.
Aside from the intervention, several other attributes were found to be predictive of readmission status. Arrhythmia, lipid lowering agents, and other cardiovascular medications (e.g., hydralazine) were associated with decreased risk of readmission, and pacemaker/ICD, ACE inhibitor use, nitrate use, DOAC use, and smoking were associated with increased risk of readmission in relative risk analysis. It is suspected that the association of arrhythmia with decreased readmission might be because patients with an arrhythmia may have increased contact with the health-care system allowing practitioners to intervene early, thereby avoiding preventable readmissions. The increased risk of readmission observed in smokers, patients with pacemakers, and patients using DOACs was expected as smoking is a well-known risk factor for HF exacerbation, pacemakers, and other implantable devices are associated with sicker patients and increase the risk of thromboembolism, and DOAC use is associated with an increased bleeding risk. The association of nitrate use with increased readmission was not surprising as it is well known that the cardiovascular benefits of nitrates such as isosorbide are short-term in monotherapy due to the rapid development of tolerance (Gupta et al., 2013). Currently, HF guidelines recommend a combination of nitrates with hydralazine due to its ability to attenuate nitrate tolerance (Yancy et al., 2013, 2017). This explains why both nitrates and other cardiovascular medications were found to be predictive of readmission status as the interaction of these two attributes is significant. The association of ACE inhibitor use with increased risk of readmission was the most interesting. ACE inhibitors are currently recommended first line in the treatment of Stage C HF with reduced ejection fraction due to the observed morbidity and mortality benefits in several randomized control trials (CONSENSUS Trial Study Group, 1987; Garg & Yusuf, 1995; SOLVD Investigators et al., 1991; Yancy et al., 2013, 2017). There is much debate over the effectiveness of ACE inhibitors in the African American population; however, data from a 2015 retrospective cohort study conducted by Ogedegbe et al. reported ACE inhibitor therapy to be less effective in Blacks than Whites, and was even associated with poorer cardiovascular outcomes for these patients (Ogedegbe et al., 2015). Similarly, findings from this current study support this conclusion and add further support to the growing body of evidence against the use of ACE inhibitors in African Americans.
A limitation of this work is the small sample size and relatively homogenous population. This drawback limits generalization of these results to a broader population. A second limitation of this work is that readmission could only be confirmed for patients admitted to hospitals in the Atlanta metro area; thus actual 30-day readmission rates may be higher than observed. Another limitation of this study is the use of historical controls. Historical control subjects are often used to support a comparative conclusion of an intervention’s effectiveness, particularly in situations where it is unreasonable or unethical to randomize patients into a true control arm. Inclusion of historical patients, however, poses a significant risk for bias in the estimation of the intervention’s effectiveness due to potential variations in prognostic factor distributions, standards of care, diagnostic criteria, and assessment procedures between enrollment periods.
In conclusion, analysis by machine learning showed the pharmacist-led intervention to be predictive of reduced 30-day readmission in AAM with HF. Several patient-specific factors were determined to be predictive of 30-day readmission; however, model performance and results from impact analysis suggest that there are still additional predictors outside the scope of routinely collected patient data such as biomarkers of disease severity or genetic factors that play a significant role in 30-day readmission rates. The design of a future intervention should include these potentially informative attributes, and also take into account the effect of heterogeneity on readmission risk, by stratifying and tailoring interventions to the likely cause of readmission. Future studies are warranted to validate these findings further and to explore whether such an intervention can potentially reduce readmission rates in a broader population of HF patients.
Supplemental Material
Supplemental material, Supplemental_Digital_Content for Impact of a Pharmacist-Led Intervention on 30-Day Readmission and Assessment of Factors Predictive of Readmission in African American Men With Heart Failure by DeAngelo McKinley, Pamela Moye-Dickerson, Shondria Davis and Ayman Akil in American Journal of Men’s Health
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
References
- Bahrami H., Kronmal R., Bluemke D. A., Olson J., Shea S., Liu K., … Lima J. A. (2008). Differences in the incidence of congestive heart failure by ethnicity: The multi-ethnic study of atherosclerosis. Archives of Internal Medicine, 168(19), 2138–2145. doi: 10.1001/archinte.168.19.2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin E. J., Blaha M. J., Chiuve S. E., Cushman M., Das S. R., Deo R., … Muntner P. (2017). Heart disease and stroke statistics-2017 update: A report from the American heart association. Circulation, 135(10), e146–e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbins-Domingo K., Pletcher M. J., Lin F., Vittinghoff E., Gardin J. M., Arynchyn A., … Hulley S. B. (2009). Racial differences in incident heart failure among young adults. New England Journal of Medicine, 360(12), 1179–1190. doi: 10.1056/NEJMoa0807265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. P., Chambless L. E., Shahar E., Bertoni A. G., Russell S. D., Ni H. Y., … Rosamond W. D. (2014). Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the atherosclerosis risk in communities study). American Journal of Cardiology, 113(3), 504–510. doi: 10.1016/j.amjcard.2013.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. W. (2017). Current perspectives on the role of the pharmacist in heart failure management. Integrated Pharmacy Research and Practice, 7, 1–11. doi: 10.2147/IPRP.S137882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmarajan K., Hsieh A. F., Lin Z., Bueno H., Ross J. S., Horwitz L. I., … Krumholz H. M. (2013). Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA, 309(4), 355–363. doi: 10.1001/jama.2012.216476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar K., Washington R. (2006). Trends in hospital readmissions for four high-volume conditions, 2009–2013 (HCUP statistical brief No. 196). Rockville, MD: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- Garg R., Yusuf S. (1995). Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure: Collaborative group on ACE inhibitor trials. JAMA, 273(18), 1450–1456. [PubMed] [Google Scholar]
- CONSENSUS Trial Study Group. (1987). Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). New England Journal of Medicine, 316(23), 1429–1435. doi: 10.1056/NEJM198706043162301 [DOI] [PubMed] [Google Scholar]
- Gupta D., Georgiopoulou V. V., Kalogeropoulos A. P., Marti C. N., Yancy C. W., Gheorghiade M., … Butler J. (2013). Nitrate therapy for heart failure: Benefits and strategies to overcome tolerance. JACC: Heart Failure, 1(3), 183–191. doi: 10.1016/j.jchf.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Heidenreich P. A., Albert N. M., Allen L. A., Bluemke D. A., Butler J., Fonarow G. C., … Stroke C. (2013). Forecasting the impact of heart failure in the United States: A policy statement from the American heart association. Circulation: Heart Failure, 6(3), 606–619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen J. M., Geersing G. J., Moons K. G., de Groot J. A. (2013). Diagnostic and prognostic prediction models. Journal of Thrombosis and Haemostasis, 11, 129–141. doi: 10.1111/jth.12262 [DOI] [PubMed] [Google Scholar]
- Jack B. W., Chetty V. K., Anthony D., Greenwald J. L., Sanchez G. M., Johnson A. E., … Culpepper L. (2009). A reengineered hospital discharge program to decrease rehospitalization: A randomized trial. Annals of Internal Medicine, 150(3), 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackevicius C. A., de Leon N. K., Lu L., Chang D. S., Warner A. L., Mody F. V. (2015). Impact of a multidisciplinary heart failure post-hospitalization program on heart failure readmission rates. Annals of Pharmacotherapy, 49(11), 1189–1196. doi: 10.1177/1060028015599637 [DOI] [PubMed] [Google Scholar]
- Joynt K. E., Orav E. J., Jha A. K. (2011). Thirty-day readmission rates for medicare beneficiaries by race and site of care. JAMA, 305(7), 675–681. doi: 10.1001/jama.2011.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziemsky C. (2016). Decision-making in healthcare as a complex adaptive system. Healthcare Management Forum, 29(1), 4–7. doi: 10.1177/0840470415614842 [DOI] [PubMed] [Google Scholar]
- Loehr L. R., Rosamond W. D., Chang P. P., Folsom A. R., Chambless L. E. (2008). Heart failure incidence and survival (from the atherosclerosis risk in communities study). The American Journal of Cardiology, 101(7), 1016–1022. doi: 10.1016/j.amjcard.2007.11.061 [DOI] [PubMed] [Google Scholar]
- Lopez Cabezas C., Falces Salvador C., Cubi Quadrada D., Arnau Bartes A., Ylla Bore M., Muro Perea N., Homs Peipoch E. (2006). Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow-up in patients with heart failure. Farmacia Hospitalaria, 30(6), 328–342. [DOI] [PubMed] [Google Scholar]
- Mortazavi B. J., Downing N. S., Bucholz E. M., Dharmarajan K., Manhapra A., Li S. X., … Krumholz H. M. (2016). Analysis of machine learning techniques for heart failure readmissions. Circulation: Cardiovascular Quality and Outcomes, 9(6), 629–640. doi: 10.1161/CIRCOUTCOMES.116.003039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye P. M., Chu P. S., Pounds T., Thurston M. M. (2018). Impact of a pharmacy team-led intervention program on the readmission rate of elderly patients with heart failure. The American Journal of Health-System Pharmacy, 75(4), 183–190. doi: 10.2146/ajhp170256 [DOI] [PubMed] [Google Scholar]
- Ogedegbe G., Shah N. R., Phillips C., Goldfeld K., Roy J., Guo Y., … Bangalore S. (2015). Comparative effectiveness of angiotensin-converting enzyme inhibitor-based treatment on cardiovascular outcomes in hypertensive Blacks versus Whites. Journal of the American College of Cardiology, 66(11), 1224–1233. doi: 10.1016/j.jacc.2015.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli D. R., Franzon J., McKinnon R. A., Shakib S., Clark R. A. (2017). Role of the pharmacist for improving self-care and outcomes in heart failure. Current Heart Failure Reports, 14(2), 78–86. doi: 10.1007/s11897-017-0323-2 [DOI] [PubMed] [Google Scholar]
- Pedregosa F., Varoquaux G., Gramfort A., Michel V., Thirion B., Grisel O., … Duchesnay E. (2011). Scikit-learn: Machine learning in python. Journal of Machine Learning Research, 12, 2825–2830. [Google Scholar]
- Ponniah A., Anderson B., Shakib S., Doecke C. J., Angley M. (2007). Pharmacists’ role in the post-discharge management of patients with heart failure: A literature review. The Journal of Clinical Pharmacy and Therapeutics, 32(4), 343–352. doi: 10.1111/j.1365-2710.2007.00827.x [DOI] [PubMed] [Google Scholar]
- SOLVD Investigators,Yusuf S., Pitt B., Davis C. E., Hood W. B., Cohn J. N. (1991). Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The New England Journal of Medicine, 325(5), 293–302. doi: 10.1056/NEJM199108013250501 [DOI] [PubMed] [Google Scholar]
- Strom J. B., Kramer D. B., Wang Y., Shen C., Wasfy J. H., Landon B. E., … Yeh R. W. (2017). Short-term rehospitalization across the spectrum of age and insurance types in the United States. PLoS ONE, 12(7), e0180767. doi: 10.1371/journal.pone.0180767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Matsue Y., Izumi S., Kimura A., Hashimoto T., Otomo K., … Funakoshi R. (2018). Pharmacist-led intervention in the multidisciplinary team approach optimizes heart failure medication. Heart Vessels, 33(6), 615–622. doi: 10.1007/s00380-017-1099-8 [DOI] [PubMed] [Google Scholar]
- Szkiladz A., Carey K., Ackerbauer K., Heelon M., Friderici J., Kopcza K. (2013). Impact of pharmacy student and resident-led discharge counseling on heart failure patients. Journal of Pharmacy Practice, 26(6), 574–579. doi: 10.1177/0897190013491768 [DOI] [PubMed] [Google Scholar]
- Vaccarino V., Gahbauer E., Kasl S. V., Charpentier P. A., Acampora D., Krumholz H. M. (2002). Differences between African Americans and Whites in the outcome of heart failure: Evidence for a greater functional decline in African Americans. American Heart Journal, 143(6), 1058–1067. doi: 10.1067/mhj.2002.122123 [DOI] [PubMed] [Google Scholar]
- Wang A., Nianogo R. A., Arah O. A. (2017). G-computation of average treatment effects on the treated and the untreated. BMC Medical Research Methodology, 17(1), 3. doi: 10.1186/s12874-016-0282-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy C. W., Jessup M., Bozkurt B., Butler J., Casey D. E., Jr., Colvin M. M., … Westlake C. (2017). 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Journal of the American College of Cardiology, 70(6), 776–803. doi: 10.1016/j.jacc.2017.04.025 [DOI] [PubMed] [Google Scholar]
- Yancy C. W., Jessup M., Bozkurt B., Butler J., Casey D. E., Jr., Drazner M. H., … Wilkoff B. L. (2013). 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation, 128(16), 1810–1852. doi: 10.1161/CIR.0b013e31829e8807 [DOI] [PubMed] [Google Scholar]
- Yang C. L., Delcher C., Shenkman E., Ranka S. (2016). Predicting 30-day all-cause readmissions from hospital inpatient discharge data. In 18th International Conference on E-Health Networking, Applications and Services (Healthcom), IEEE; Retrieved from http://apps.webofknowledge.com/InboundService.do?customersID=ResearchSoft&mode=FullRecord&IsProductCode=Yes&product=WOS&Init=Yes&Func=Frame&DestFail=http%3A%2F%2Fwww.webofknowledge.com&action=retrieve&SrcApp=EndNote&SrcAuth=ResearchSoft&SID=5CfXEfKE5MNSq6nilBB&UT=WOS%3A000391459700035 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Digital_Content for Impact of a Pharmacist-Led Intervention on 30-Day Readmission and Assessment of Factors Predictive of Readmission in African American Men With Heart Failure by DeAngelo McKinley, Pamela Moye-Dickerson, Shondria Davis and Ayman Akil in American Journal of Men’s Health