Abstract
Examining how multiple concomitant factors interact to augment HIV transmission risk is needed to inform more effective primary and secondary HIV prevention programs for men who have sex with men (MSM) in the United States. The development of a “taxonomy” of long-term sexual and drug-related risk behavior profiles may have important implications for resource allocation and targeted HIV prevention programming. A secondary data analysis was conducted to explore longitudinal HIV transmission risk profiles among 423 MSM living with HIV enrolled in the Study to Understand the Natural History of HIV and AIDS in the Era of Effective Therapy (SUN Study). Between March 2004 and February 2012, participants completed semiannual, audio computer-assisted self-interviews that included demographics, employment status, medical information, alcohol use, stimulant use, sexual risk, and depression. Latent class analysis was used to identify patterns of risky behavior over time with respect to sexual risk, heavy drinking, and stimulant (i.e., methamphetamine and cocaine) use taken collectively. Three classes were identified: (a) High Sustained Heavy Drinker Class (33%), (b) High Mostly Stable Sexual Risk Class (17%), and (c) Overall Low Risk Class. (50%). Post hoc comparisons between classes revealed that men in Classes 1 (p = .03) and 2 (p = .02) were significantly younger than those in Class 3. In comparison to those in Classes 1 and 3, those in Class 2 were less likely to report being a racial/ethnic minority (p = .04) and had the highest self-reported sexually transmitted infections (p < .001). Findings indicate the need to better integrate sexual and substance use risk reduction strategies, including brief interventions and engagement in addiction treatment, for MSM living with HIV in the United States.
Keywords: HIV, transmission risk, MSM, SUN study, longitudinal data
Gay, bisexual, and other men who have sex with men (MSM) are disproportionately affected by HIV in the United States. Although MSM comprise roughly 2% of the national population, 67% of the total estimated new diagnoses in the United States were among MSM in 2016 (Centers for Disease Control and Prevention [CDC], 2018). Prior research suggests that a sizable proportion of MSM living with HIV engage in sexual risk behaviors (Crepaz et al., 2009; Durham et al., 2013; Morin et al., 2007), such as condomless anal intercourse (CAI), and may do so at higher rates than their uninfected peers (Carey et al., 2009). Some research has pointed to the presence of a “syndemic” of HIV infection, illicit drug use, and hazardous alcohol use, such that the concentration and specific combination of these overlapping epidemics can potentiate HIV transmission among MSM subgroups (Santos et al., 2014).
Frequent drug and alcohol use have been documented among some populations of MSM living with HIV (Patterson, Semple, Zians, & Strathdee, 2005). One multicity study seeking to engage MSM living with HIV in medical care revealed that 72% of the study sample used illicit drugs or engaged in heavy alcohol use in the previous 12 months (Sohler et al., 2007). Studies indicate that MSM living with HIV may be more likely than those living without HIV to report the use of “club drugs” (e.g., cocaine, ecstasy, poppers, γ-Hydroxybutyric acid or GHB, crystal methamphetamine, and ketamine) as well as polydrug use—including mixing alcohol and other drugs (Patterson et al., 2005). Numerous studies have documented associations between drug and alcohol use and HIV transmission risk (Mayer et al., 2010; Sander et al., 2013; Skeer et al., 2012); explicit mechanisms underlying the observed associations between alcohol use and HIV transmission risk, however, remain poorly understood (Woolf & Maisto, 2009). Alcohol and substance misuse are associated with poor adherence to antiretroviral therapy (ART), which has important implications for both HIV transmission and HIV-related clinical outcomes (Chander et al., 2006; King et al., 2009; Mellins et al., 2009).
HIV-related risk behaviors (e.g., condomless sex and substance use prior to or during sex) may also result in the acquisition of sexually transmitted infections (STIs). Prior research (Mayer et al., 2010; Skeer et al., 2012) has identified a high prevalence of STIs (e.g., syphilis, gonorrhea, and chlamydia) and substance use among MSM living with HIV in the United States (Mayer et al., 2010; Morin et al., 2007; Skeer et al., 2012). Positive associations have also been reported between substance use and HIV transmission risk behaviors among MSM living with HIV receiving medical care (Mayer et al., 2010; Morin et al., 2007; Skeer et al., 2012).
Collectively, these previous studies speak to the need to better understand long-term patterns of specific behavioral risk factors among MSM living with HIV that might contribute to onward HIV transmission as well as acquisition of other STIs. Developing a more complete understanding of risk profiles among MSM living with HIV, including how multiple concomitant factors interact to augment HIV transmission risk, is needed to inform more effective and targeted HIV prevention programs for specific subgroups within this population (Heath, Lanoye, & Maisto, 2012). Amelioration of the adverse consequences associated with such risk profiles may have important implications for HIV disease progression and achieving optimal clinical outcomes such as viral load suppression.
The current study sought to characterize subgroups among MSM living with HIV based on their longitudinal patterns of risky behaviors for sexual risk, heavy drinking, and stimulant use. This study specifically focuses on heavy drinking and stimulant use as they most often lead to sexual risk behaviors among MSM (Heath et al., 2012). Examining the clustering of behavioral risks within this population can provide a useful taxonomy to guide prevention and intervention efforts. Identifying the long-term sexual and drug-related risk behavior profiles may have important implications for resource allocation and targeted programming for this at-risk population.
Methods
The SUN Study: Overview
Data from the Study to Understand the Natural History of HIV and AIDS in the Era of Effective Therapy (SUN Study) were analyzed, which is a closed, prospective cohort study conducted by the CDC of 699 patients living with HIV receiving care at HIV specialty clinics in Denver, Colorado; Minneapolis, Minnesota; Providence, Rhode Island; and St. Louis, Missouri. Participants were enrolled between March 2004 and June 2006 and were followed every 6 months until February 2012. Of the full sample, data were included from 423 participants who identified MSM as their primary risk factor (60.5% of the full sample) for the current analysis. Detailed data collection methods have been described elsewhere (Vellozzi et al., 2009) and a complete list of inclusion criteria for the original study can be found online at https://clinicaltrials.gov/ct2/show/NCT00146419. The study protocol was approved and reviewed annually by the institutional review boards of the CDC and all participating institutions listed on the National Institutes of Health ClinicalTrials.gov website. All participants provided written informed consent.
Measures
Measures assessed for this analysis included abstracted clinical information (e.g., on ART) from medical records. Participants completed an audio computer-assisted self-interview (ACASI) at enrollment (baseline) and every 6 months for the duration of their participation in the study. The ACASI questionnaire could be taken in English or Spanish. Participant data collected by ACASI included in the study’s analyses were sociodemographics (e.g., age, race, ethnicity, and employment status), alcohol use, stimulant use, sexual risk (e.g., CAI), and STIs.
For the baseline, alcohol use was defined as self-reported number of drinks per day and number of days that alcohol was consumed by participants in the past 30 days. Stimulant use was defined as self-reported history of ever using methamphetamine only, cocaine only, or both. Sexual risk was defined as self-reported multiple sex partners (>4) and CAI in the past 6 months. STI was defined as self-reported history of having any STI, gonorrhea, chlamydia, or syphilis. Depression was measured using the Patient Health Questionnaire-9 (PHQ-9) following Tedaldi et al., 2012. The PHQ-9 has sound psychometric properties for reliability and validity (Kroenke, Spitzer, & Williams, 2001). Prior research conducted with persons living with HIV using this measure has produced a Cronbach’s α of .93, which denotes high internal consistency with this population (van den Berg et al., 2016).
For the follow-ups, heavy drinking was defined as self-reported history of having at least one heavy drinking day (≥5 drinks/day) over the past 30 days. Stimulant use was defined as self-reported use of methamphetamine or cocaine in the past 30 days. Sexual risk was defined as self-reported history of any CAI in the past 60 days. STI was based on self-reported gonorrhea, syphilis, or chlamydia at any study visit. Depression was measured using the PHQ-9 scores at baseline. For this analysis, data were examined from the baseline through the 96-month follow-up period.
Statistical Analysis
Baseline data for the MSM sample (N = 423) were summarized using means (standard deviations) for continuous variables and percentages (N) for categorical variables. As interest was in the frequency of sexual risk, heavy drinking, and stimulant use over time, the proportions of visits with these reported behaviors were summarized for the whole sample. All three variables were binary indicators of risky behavior.
Latent class analysis (LCA) was used to identify patterns of risky behavior over time with respect to sexual risk, heavy drinking, and stimulant use taken collectively. Latent class models (LCMs) are a data reduction technique to subdivide a population of participants into classes reflecting similar response profiles (Bartholomew, Knott, & Moustaki, 2011). LCMs estimate the response probabilities (probability of risky behavior conditional on class) as well as the distribution of classes. LCMs provide an objective means for determining subgroups of the population over a study period. LCMs result in a vector of probabilities for each participant, corresponding to the probability of belonging to each of the latent classes. Models use an expectation-maximization (EM) algorithm for estimation and thus are likelihood based, and make use of all available data without directly imputing missing values (Little & Rubin, 2002).
For this study, a series of LCMs were fit, with number of classes ranging from two to five. Models were compared using the Bayesian Information Criteria (BIC), with lower values indicative of better model fit. Posterior probabilities were summarized across the cohort of study participants. Using a multinomial distribution, most likely class was drawn for each participant. Analysis of variance (ANOVA) was used to compare key demographic and medical variables between classes. Patterns of risky behavior over time were calculated and plotted for ease of interpretation. All analyses were carried out in SAS Version 9.3.
Results
Study Visits
At the time of these analyses, 423 MSM completed Visit 1 (baseline) followed by 330 (78.0%) at Visit 2, 315 (74.5%) at Visit 3, 301 (71.2%) at Visit 4, 281 (66.4%) at Visit 5, 274 (64.8%) at Visit 6, 256 (60.5%) at Visit 7, 245 (57.9%) at Visit 8, 247 (58.4%) at Visit 9, 225 (53.2%) at Visit 10, 228 (53.9%) at Visit 11, and 219 (51.8%) at Visit 12.
Baseline Characteristics
A full description of the sample is provided in Table 1. Participants were 41.0 years of age on average (SD = 9.0), predominantly White (n = 333, 78.7%), and employed (n = 290, 68.6%). More than half of participants reported having none/minimal depression at baseline (PHQ-9 score range 0–4). The vast majority (n = 403, 95.3%) of participants were on ART at the time of their baseline visit and (n = 29, 6.9%) self-reported an STI diagnosis at baseline. With respect to alcohol use, the average number of drinks per day was two (SD = 1.9) and the average number of days during which alcohol was consumed in the past 30 days was 6 (SD = 7.2) at baseline.
Table 1.
Baseline Demographics and Behavioral Risk Among MSM in the SUN (N = 423).
| Mean (SD) or N (%) | |
|---|---|
| Age | 41 (9.0) |
| Race | |
| White | 333 (78.7) |
| Black | 58 (13.7) |
| Other | 32 (7.6) |
| Ethnicity | |
| Hispanic | 36 (8.5) |
| Non-Hispanic | 380 (91.5) |
| Employed at baseline visit | |
| Yes | 290 (68.6) |
| On HIV (ART) medications at baseline visit | |
| Yes | 403 (95.3) |
| Sexually transmitted infection (STI) at baseline visit | |
| Any STIa | 29 (6.9) |
| Gonorrhea (GC) | 22 (5.2) |
| Chlamydia (Chly) | 0 (0.0) |
| Syphilis (RPR) | 8 (1.9) |
| Alcohol use at baseline visit | |
| Drinks per day | 2 (1.9) |
| Number of days during which alcohol was consumed in past 30 days | 6 (7.2) |
| Stimulant use at baseline visit | |
| Ever used methamphetamine (Meth) | 48 (11.4) |
| Ever used cocaine (Coke) | 43 (10.17) |
| Ever used any of the above stimulants (Meth or Coke) | 84 (19.9) |
| Sexual risk behaviors at baseline visit | |
| Had multiple sex partners (>4) within past 6 months | 189 (44.7) |
| Had condomless anal intercourse within past 6 months | 306 (72.3) |
| Depression screening by PRIME-MD1 * (PHQ-9 scores) | |
| None/minimal (total score = 0–4) | 218 (52.7) |
| Mild (total score = 5–9) | 103 (24.9) |
| Moderate (total score = 10–14) | 53 (12.8) |
| Moderately severe (total score = 15–19) | 23 (5.6) |
| Severe (total score = 20–27) | 17 (4.1) |
| Median depression screening score (PHQ-9) | 4.0 |
Note. ART = antiretroviral therapy; MSM = men who have sex with men; PHQ-9 = Patient Health Questionnaire-9; SUN = Study to Understand the Natural History of HIV and AIDS in the Era of Effective Therapy.
STI includes gonorrhea (GC), chlamydia (Chly), and syphilis (RPR). Total scores are based on all nine PRIME-MD depression screening questions. Nine participants had missing values. Following Tedaldi et al. (2012), the ENERGY question was recoded to construct the TIRED question for the baseline visit.
Approximately one fifth of participants (n = 84, 19.9%) reported ever having used stimulants, such as methamphetamine or cocaine, at baseline. Finally, almost half of participants (n = 189, 44.7%) reported having four or more sexual partners in the 6 months prior to their baseline visit, and nearly three fourths (n = 306, 72.3%) indicated that they had engaged in condomless sex (of any kind) within 6 months of their baseline visit.
HIV-Related Risk Behavior During the Study Period
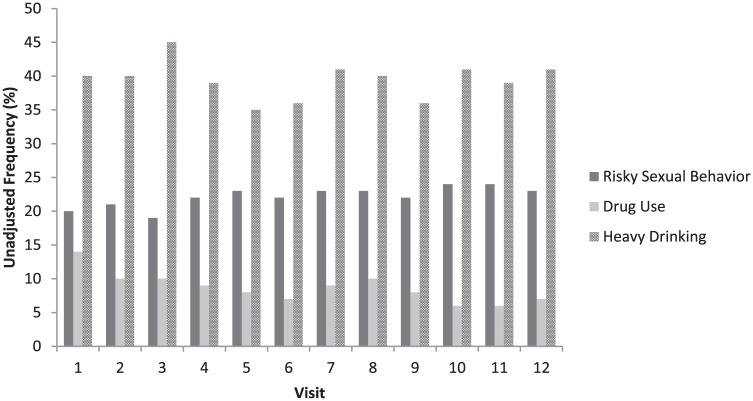
Over three quarters of participants (n = 326, 77.0%) self-reported STI diagnosis at some point over the study period, with the most common STI being syphilis. Stimulant use was reported on at least one visit over the study for more than half of participants, and a high number of participants (n = 286, 67.6%) reported at least one heavy drinking day at some point during the study. Unadjusted frequencies of risky behavior (sexual risk, heavy drinking, and stimulant use) across all 12 study visits are provided in Figure 1. Of note is the high frequency of reported sexual risk and heavy drinking across visits.
Figure 1.
Unadjusted frequency of risky behavior by visit.
Note. At Visit 1, N = 423, Visit 2, N = 330, Visit 3, N = 315, Visit 4, N = 301, Visit 5, N = 281, Visit 6, N = 274, Visit 7, N = 256, Visit 8, N = 245, Visit 9, N = 247, Visit 10, N = 225, Visit 11, N = 228, Visit 12, N = 219; Total N = 3,344.
Latent Class Analysis of HIV Risk Categories
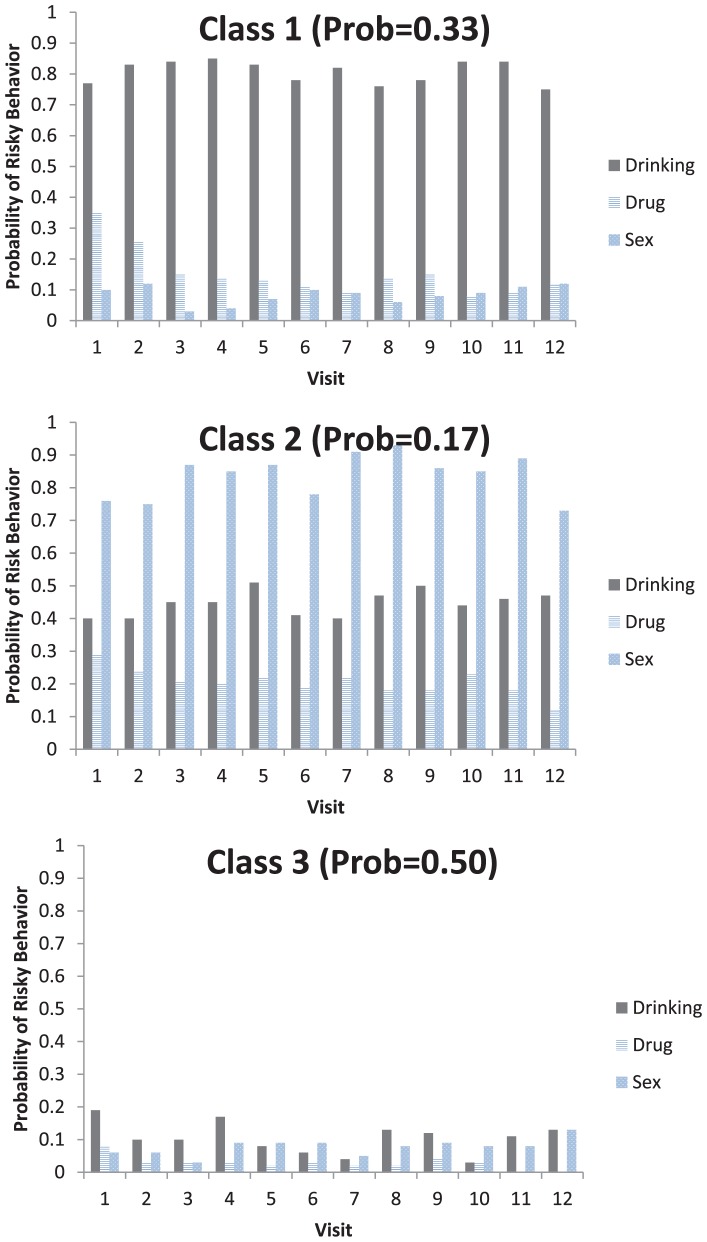
A series of LCMs were fit, the results of which suggested a three-class model was best supported by the data (lowest BIC value). The distribution of classes was such that 139 participants or 33% belonged to Class 1 (High Sustained Heavy Drinker Class), 72 participants or 17% belonged to Class 2 (High Mostly Stable Sexual Risk Class), and 212 participants or 50% belonged to Class 3 (Overall Low Risk Class). The average response profiles for each of the behaviors are provided across time and stratified by class in Figure 2.
Figure 2.
Average trajectory of risky behavior by class.
Note. Class 1 = High Sustained Drinker Class; Class 2 = High Mostly Stable Risk Sex Class; and Class 3 = Overall Low Risk Class.
Study results indicate that the High Sustained Heavy Drinker Class was characterized by high probability of at least 1 day over the past 30 days of drinking five or more drinks consistently over time, and a decreasing probability of stimulant use (any methamphetamine or cocaine use over the past 30 days) and sexual risk (any CAI over the past 60 days). In the High Sustained Heavy Drinker Class, the probability of using methamphetamine or cocaine at least once in the past 30 days was less than 40% early in the study (within the first three visits) and decreased over time. By comparison, the High Mostly Stable Sexual Risk Class was defined by high and mostly stable sexual risk (i.e., CAI, moderate drinking behavior, and low-to-moderate stimulant use). There was a low correlation (using the Spearman rank order test, rho <0.17) between sexual risk and stimulant use in the High Mostly Stable Sexual Risk Class as compared to the High Sustained Heavy Drinker Class (rho <0.40). The Overall Low Risk Class was defined by consistently low-risk behavior (sexual risk, heavy drinking, or stimulant use) over the study period.
Post hoc comparisons between classes suggest a significant difference between classes with respect to mean age of participants as indicated in Table 2. Participants in the High Mostly Stable Sexual Risk Class were significantly younger than those in the Overall Low Risk Class (p = .02) and those in the High Sustained Drinker Class were significantly younger than those in the Overall Low Risk Class (p = .03). There was no significant age difference between those in the High Sustained Heavy Drinker Class and the High Mostly Stable Sexual Risk Class. Participants in the High Mostly Stable Sexual Risk Class were less likely to report being a racial/ethnic minority compared to the High Sustained Heavy Drinker Class and the Overall Low Risk Class (p = .04) and had the highest number of STIs compared to the other two classes (p < .001).
Table 2.
Between-Class Differences in Key Demographic and Medical Variables.
| Class 1 (N = 140) | Class 2 (N = 72) | Class 3 (N = 211) | |
|---|---|---|---|
| Age (mean, SD)* | 40.34 (8.24) | 39.73 (7.10) | 42.83 (8.96) |
| Race/ethnicity (%)* | |||
| White | 69.5% | 83.8% | 77.6% |
| Black | 14.8% | 8.1% | 15.4% |
| Hispanic | 12.5% | 6.8% | 7.0% |
| Other | 3.1% | 1.4% | 0% |
| STI (over study period)* | 18.6% | 44.0% | 14.4% |
| HIV meds, % yes | 80.5% | 72.0% | 82.2% |
| Depressed, % at least several days | 58.3% | 49.3% | 46.5% |
Note. Class 1 = High Sustained Heavy Drinker Class; Class 2 = High Mostly Stable Sexual Risk Class; and Class 3 = Overall Low Risk Class. STI = sexually transmitted infection.
p < .05 for between-class differences.
Discussion
This study showed that nearly one fifth of the MSM living with HIV in the sample reported engaging in multiple potential HIV risk behaviors concurrently throughout the study period, such as sexual risk, heavy drinking, and stimulant use. The correlation between specific risky behaviors varied among subsets (i.e., latent classes) of men. These long-term behavioral risk profiles have important implications for both HIV transmission and for HIV-related clinical outcomes. Findings from this study point to the need for brief sexual and substance use risk reduction interventions and addiction treatments that address individual factors and the impact of the combination of these factors on MSM living with HIV to reduce their engagement in risky behaviors that contribute to the higher prevalence of HIV among MSM in the United States.
Sexual Risk
This sample of MSM living with HIV enrolled in the SUN study had significant sexual risk during the study period as evidenced by three quarters self-reporting an STI diagnosis at some point during their participation. This finding is consistent with previously published reports of high rates of STIs among samples of MSM living with HIV receiving primary care for HIV (Mayer et al., 2010; Poynten, Templeton, & Grulich, 2011). As recommended by the CDC, screening, diagnosis, and treatment of STIs for sexually active individuals living with HIV are important both for the health of persons living with HIV and for HIV prevention, and findings from this sample of MSM living with HIV provide further support for this recommendation (CDC, 2015).
It is interesting to note that participants in Class 2, who were mostly White and younger, had significantly higher rates of STIs relative to the other two classes, both of which had much lower overall sexual risk. Class 2 participants were also characterized by moderate alcohol use but relatively low stimulant use. Brief interventions that are tailored to MSM living with HIV who fit into this class should focus on reducing their alcohol use and high-risk sexual behaviors, and explore how engagement in these behaviors both individually and in combination with one another may increase the likelihood of transmitting HIV to others and/or acquiring other STIs.
Alcohol and Stimulant Use
Alcohol and stimulant use among participants varied across study visits. While most participants reported some stimulant and/or heavy drinking, it is difficult to determine the degree to which such use contributed to HIV transmission risk. For example, a third of participants were classified as having a high probability of heavy drinking consistently over time, but a relatively low probability of stimulant use and sexual risk such as those in Class 1, who were more likely to identify as Black and Latino. MSM living with HIV who fit into this class might benefit from brief interventions that focus on reducing and treating heavy alcohol use and reinforce not using stimulants and engaging in risky sexual behaviors. This finding is notable, given that racial and ethnic subgroups within the MSM population are at disproportionately higher risk for HIV than their MSM peers (CDC, 2018).
Use of stimulants, including cocaine and methamphetamine, has been reported to be associated with risk for HIV transmission among MSM living with HIV (Blumenthal et al., 2014; Wade, O’Cleirigh, Mayer, & Safren, 2013). Substance use among partners of MSM living with HIV has also been reported to be associated with poor HIV disease management. Carrico, Woolf-King, Neilands, Dilworth, and Johnson (2014) examined the role of stimulants in HIV disease management among MSM couples and reported that having a stimulant-using partner was associated with lower odds of ART adherence and having an undetectable viral load. Woolf-King, Neilands, Dilworth, Carrico, and Johnson (2014) identified similar results among MSM couples in which one partner was classified as a hazardous drinker. Although use of stimulants was reported by participants in this study throughout the study period, it was lower than expected based upon prior research with this population. Given the high degree of reported sexual activity among MSM living with HIV in the SUN study, the current study’s findings suggest the need to better integrate sexual and substance use risk reduction strategies, including brief interventions and engagement in treatment, if indicated.
Understanding Multiple Risks
Findings from this study also suggests that MSM living with HIV may exhibit complex and varied risk profiles characterized by sexual risk and substance use behaviors over time that are fairly consistent. While most MSM in the sample had relatively low HIV transmission risk, half of the sample was classified as having a mixture of sexual and substance use–related behaviors, which implies that other cofactors may be influencing the relationship between these behaviors. The use of the syndemic theory may help to contextualize these cofactors as their co-occurrence and synergistic effect may contribute to a stronger and more intense adverse health outcome for these men than if these factors were experienced alone (Singer & Clair, 2003). For example, Santos et al. (2014) analyzed a global sample of nearly 4,000 MSM with one or more syndemic conditions defined as depression, substance use, violence, sexual stigma, and homelessness, and reported greater odds of HIV infection compared with MSM without such conditions. They also identified a dose–response relationship such that MSM with a greater number of syndemic conditions had a greater likelihood of both CAI and HIV infection. Further work is needed to determine whether syndemic conditions predict long-term substance use and sexual risk behavior profiles among MSM living with HIV.
There are some limitations to the current study that are important to highlight. First, not all participants completed all follow-up interviews. Losses to follow-up and missing data may introduce bias, particularly with respect to HIV-related risk behaviors over time. Missing data were accounted for by using LCM, which mitigates this effect. Second, risk factors included in the LCA were based on self-reported measures, such as CAI and stimulant and alcohol use, which may introduce social desirability bias or mistakes. At the same time, the study employed ACASI to minimize the potential for any biases or errors and to encourage participants to answer questions honestly and carefully. Third, the analytic sample included predominately White patients enrolled across four HIV primary care clinics. Nevertheless, approximately 30% of the sample were Black, Hispanic, or Other. While clinics included in this study are located in geographically diverse settings, the study’s results cannot necessarily be generalized to all MSM living with HIV in care in the United States or elsewhere.
Despite these limitations, this study is among the first to characterize long-term risk profiles of MSM living with HIV in the United States. Identifying particular subgroupings of men according to their risk behaviors (e.g., high sustained heavy drinkers, high and mostly stable sexual risk behaviors, and overall low-risk behaviors) is especially important for targeted primary and secondary HIV prevention programs for MSM. The development, implementation, and evaluation of brief interventions that address these risky behaviors at different levels and to decrease their potential synergistic effects on health outcomes is critical for reducing high morbidity and mortality rates among MSM in the United States.
Conclusion
Further research is necessary to test the risk profiles identified in the current study with larger and more racially and ethnically diverse samples of MSM living with HIV in the United States. Results from this study indicate that there are different levels of risk profiles among these men that may work synergistically to impact their health outcomes, but future research is needed to determine how this process occurs. Findings also denote the need to better integrate sexual and substance use risk reduction strategies, including brief interventions and engagement in treatment for MSM living with HIV in the United States. Health-care providers (e.g., psychologists, nurses, social workers) located in HIV clinical settings would be in the ideal position to deliver these interventions and provide treatment for MSM living with HIV who fit within the three different classifications identified in this study.
Acknowledgments
The authors especially thank the CDC and all the MSM living with HIV who participated in the SUN study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from the National Institutes of Health including U24AA022000 (PI: Operario) and P30AI042853 (PI: Cu-Uvin).
References
- Bartholomew D. J., Knott M., Moustaki I. (2011). Latent variable models and factor analysis: A unified approach (3rd ed.). West Sussex, United Kingdom: John Wiley & Sons Ltd. [Google Scholar]
- Blumenthal J., Haubrich R., Jain S., Sun X., Dube M., Daar E., … Morris S. (2014). Factors associated with high transmission risk and detectable plasma HIV RNA in HIV-infected MSM on ART. International Journal of STDs and AIDS, 25(10), 734–741. doi: 10.1177/0956462413518500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey J. W., Mejia R., Bingham T., Ciesielski C., Gelaude D., Herbst J. H., … Stall R. (2009). Drug use, high-risk sex behaviors, and increased risk for recent HIV infection among men who have sex with men in Chicago and Los Angeles. AIDS & Behavior, 13(6), 1084–1096. doi: 10.1007/s10461-008-9403-3 [DOI] [PubMed] [Google Scholar]
- Carrico A. W., Woolf-King S. E., Neilands T. B., Dilworth S. E., Johnson M. O. (2014). Stimulant use and HIV disease management among men in same-sex relationships. Drug and Alcohol Dependence, 139, 174–177. doi: 10.1016/j.drugalcdep.2014.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2018). HIV and gay and bisexual men. Retrieved from https://www.cdc.gov/hiv/pdf/group/msm/cdc-hiv-msm.pdf
- CDC. (2015). Sexually transmitted diseases treatment guidelines, 2015. Morbidity and Mortality Weekly Report Recommendations and Reports, 64(RR3), 1–137. [PMC free article] [PubMed] [Google Scholar]
- Chander G., Lau B., Moore R. D. (2006). Hazardous alcohol use: A risk factor for non-adherence and lack of suppression in HIV infection. Journal of Acquired Immune Deficiency Syndromes, 43(4), 411–417. doi: 10.1097/01.qai.0000243121.44659.a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepaz N., Marks G., Liau A., Mullins M. M., Aupont L. W., Marshall K. J., … Wolitski R. J. (2009). Prevalence of unprotected anal intercourse among HIV-diagnosed MSM in the United States: A meta-analysis. AIDS, 23(13), 1617–1629. [DOI] [PubMed] [Google Scholar]
- Durham M. D., Buchacz K., Richardson J., Yang D., Wood K., Yanqco B., … Brooks J. T. (2013). Sexual risk behavior and viremia among men who have sex with men in the HIV Outpatient Study, United States, 2007–2010. Journal of Acquired Immune Deficiency Syndrome, 63(3), 372–378. doi: 10.1097/QAI.0b013e31828c20d8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath J., Lanoye A., Maisto S. A. (2012). The role of alcohol and substance use in risky sexual behavior among older men who have sex with men: A review and critique of the current literature. AIDS & Behavior, 16(3), 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W. D., Larkins S., Hucks-Ortiz C., Wang P. C., Gorbach P. M., Veniegas R., Shoptaw S. (2009). Factors associated with HIV viral load in a respondent driven sample in Los Angeles. AIDS & Behavior, 13(1), 145–153. doi: 10.1007/s10461-007-9337-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R. L., Williams J. B. (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R. J. A., Rubin D. B. (2002). Statistical analysis with missing data (2nd ed.). Hoboken, NJ: Wiley & Sons, Inc. [Google Scholar]
- Mayer K. H., O’Cleirigh C., Skeer M., Covahey C., Leidolf E., Vanderwarker R., Safren S. A. (2010). Which HIV-infected men who have sex with men in care are engaging in risky sex and acquiring sexually transmitted infections: Findings from a Boston community health centre. Sexually Transmitted Infections, 86(1), 66–70. doi: 10.1136/sti.2009.036608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins C. A., Havens J. F., McDonnell C., Lichtenstein C., Uldall K., Chesney M., …. Bell J. (2009). Adherence to antiretroviral medications and medical care in HIV-infected adults diagnosed with mental and substance abuse disorders. AIDS Care, 21(2), 168–177. doi: 10.1080/09540120802001705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin S. F., Myers J. J., Shade S. B., Koester K., Maiorana A., Rose C. D. (2007). Predicting HIV transmission risk among HIV-infected patients seen in clinical settings. AIDS & Behavior, 11(5 Suppl), S6–S16. doi: 10.1007/s10461-007-9253-4 [DOI] [PubMed] [Google Scholar]
- Patterson T. L., Semple S. J., Zians J. K., Strathdee S. A. (2005). Methamphetamine-using HIV-positive men who have sex with men: Correlates of polydrug use. Journal of Urban Health, 82(1 Suppl. 1), i120–i126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynten I. M., Templeton D. J., Grulich A. E. (2011). Sexually transmissible infections in aging HIV populations. Sexual Health, 8(4), 508–511. doi: 10.1071/SH11027 [DOI] [PubMed] [Google Scholar]
- Sander P. M., Cole S. R., Stall R. D., Jacobson L. P., Eron J. J., Napravnik S., … Ostrow D. G. (2013). Joint effects of alcohol consumption and high-risk sexual behavior on HIV seroconversion among men who have sex with men. AIDS, 27(5), 815–823. doi: 10.1097/QAD.0b013e32835cff4b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos G. M., Do T., Beck J., Makofane K., Arreola S., Pyun T., … Ayala G. (2014). Syndemic conditions associated with increased HIV risk in a global sample of men who have sex with men. Sexually Transmitted Infections, 90(3), 250–253. doi: 10.1136/sextrans-2013-051318 [DOI] [PubMed] [Google Scholar]
- Singer M., Clair S. (2003). Syndemics and public health: Reconceptualizing disease in bio-social context. Medical Anthropology Quarterly, 17(4), 423–441. doi: 10.1525/maq.2003.17.4.423 [DOI] [PubMed] [Google Scholar]
- Skeer M. R., Mimiaga M. J., Mayer K. H., O’Cleirigh C., Covahey C., Safren S. A. (2012). Patterns of substance use among a large urban cohort of HIV-infected men who have sex with men in primary care. AIDS & Behavior, 16(3), 676–689. doi: 10.1007/s10461-011-9880-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohler N. L., Wong M. D., Cunningham W. E., Cabral H., Drainoni M. L., Cunningham C. O. (2007). Type and pattern of illicit drug use and access to health care services for HIV-infected people. AIDS Patient Care and STDS, 21(Suppl. 1), S68–S76. doi: 10.1089/apc.2007.9985 [DOI] [PubMed] [Google Scholar]
- Tedaldi E. M., van den Berg-Wolf M., Richardson J., Patel P., Durham M., Hammer J., … Buchacz K. (2012). Sadness in the SUN: Using computerized screening to analyze correlates of depression and adherence in HIV-infected adults in the United States. AIDS Patient Care and STDS, 26(12), 718–729. doi: 10.1089/apc.2012.0132 [DOI] [PubMed] [Google Scholar]
- van den Berg J. J., Neilands T. B., Johnson M. O., Chen B., Saberi P. (2016). Using path analysis to evaluate the healthcare empowerment model among persons living with HIV for antiretroviral therapy adherence. AIDS Patient Care and STDs, 30(11), 497–505. doi: 10.1089/apc.2016.0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellozzi C., Brooks J. T., Bush T. J., Conley L. J., Henry K., Carpenter C. C., … Holmberg S. D. (2009). The study to understand the natural history of HIV and AIDS in the era of effective therapy (SUN study). American Journal of Epidemiology, 169(5), 642–652. doi: 10.1093/aje/kwn361 [DOI] [PubMed] [Google Scholar]
- Wade T. S., O’Cleirigh C., Mayer K. H., Safren S. A. (2013). HIV-infected men who have sex with men who engage in very high levels of transmission risk behaviors: Establishing a context for novel prevention interventions. Psychology, Health, and Medicine, 18(5), 576–587. doi: 10.1080/13548506.2012.756537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf S. E., Maisto S. A. (2009). Alcohol use and risk of HIV infection among men who have sex with men. AIDS & Behavior, 13(4), 757–782. doi: 10.1007/s10461-007-9354-0 [DOI] [PubMed] [Google Scholar]
- Woolf-King S. E., Neilands T. B., Dilworth S. E., Carrico A. W., Johnson M. O. (2014). Alcohol use and HIV disease management: The impact of individual and partner-level alcohol use among HIV-positive men who have sex with men. AIDS Care, 26(6), 702–708. doi: 10.1080/09540121.2013.855302 [DOI] [PMC free article] [PubMed] [Google Scholar]