Abstract
Recent studies suggest there may be an environmental exposure component to the development and progression of non-alcoholic fatty liver disease (NAFLD) involving the organochlorine (OC) pesticides or their metabolites. However, the roles of OC compounds in the development of NAFLD has not been fully elucidated. Therefore, the current study was designed to determine if exposure to trans-nonachlor, a prevalent OC compound, could promote hepatocyte lipid accumulation and determine potential pro-steatotic mechanisms. McArdle-RH7777 (McA) hepatoma cells were incubated with trans-nonachlor for 24 hours then neutral lipid accumulation was determined by Oil Red O staining. Exposure to trans-nonachlor produced a concentration dependent increase in neutral lipid accumulation. Trans-nonachlor also increased extracellular free fatty acid-induced neutral lipid accumulation which appears to be due at least in part to increased free fatty acid accumulation as evident by increased accumulation of Bodipy labeled dodecanoic acid. Additionally, 14C-acetate incorporation into total cellular lipids was increased by trans-nonachlor implicating increased de novo lipogenesis (DNL) as a potential mediator of trans-nonachlor-induced neutral lipid accumulation. Taken together, the present data indicate exposure to trans-nonachlor has a direct, pro-steatotic effect on hepatocytes to increase lipid accumulation through the combinatorial actions of extracellular free fatty acid accumulation and increased DNL.
Keywords: Trans-nonachlor, hepatic steatosis, triglyceride, de novo lipogenesis, fatty acid uptake, fatty acid oxidation
1. Introduction
Hepatic steatosis or non-alcoholic fatty liver (NAFLD) represents a defect in hepatic lipid metabolism in which lipids accumulate within hepatocytes due to increased fatty acid uptake, increased de novo lipogenesis (DNL), decreased fatty acid oxidation, or overwhelmed VLDL secretion (Choi and Ginsberg, 2011; Diraison et al., 2003). In states of metabolic dysfunction, such as type 2 diabetes, the fatty acids utilized for triglyceride synthesis are derived from increased fatty acid uptake and increased DNL (Donnelly et al., 2005). This increase in triglyceride synthesis is usually accompanied by an increase in VLDL secretion due to increased substrate availability (Lewis, 1997). The formation of hepatic steatosis is a common pathological alteration associated with obesity and type 2 diabetes and studies have shown that increased hepatic steatosis is directly associated with increased risk of CVD development [for reviews see (Francque et al., 2016; Lonardo et al., 2016)].
There is mounting epidemiological evidence that exposure to persistent organic pollutants (POPs) may predispose one to the development of insulin resistance, type 2 diabetes, and metabolic syndrome. In epidemiological studies, elevated plasma or serum concentrations of POPs, including organochlorine (OC) pesticides or their metabolites/contaminants oxychlordane, trans-nonachlor, and p,p′-dichlorodiphenyldichloroethylene (DDE), have been positively correlated with the occurrence of insulin resistance, T2D, and hypertriglyceridemia (Ha et al., 2007; Lee et al., 2007a; Lee et al., 2007b; Lee et al., 2006; Lee et al., 2011; Park et al., 2010). In addition to epidemiological studies, there are a growing number of empirical studies in rodents indicating chronic exposure to OC pesticides either alone or in a mixture with other POPs at environmentally relevant concentrations promote T2D, hepatic steatosis, and dyslipidemia when ingested with a high fat or high fat/high carbohydrate diet (Ibrahim et al., 2011; Ruzzin et al., 2010). Our recent studies (Mulligan et al. (2017)) also demonstrate exposure to a mixture of POPs at environmentally relevant concentrations can promote hepatic steatosis in a genetic model of obesity and type 2 diabetes, the ob/ob mouse (Mulligan et al., 2017). However, the cellular mechanisms by which exposure to oxychlordane, trans-nonachlor, or DDE promote the pathophysiological alterations associated with T2D such as hepatic steatosis and the accompanying dyslipidemias have not been fully determined.
While the previously published studies demonstrate exposure to a mixture of OC pesticides can disrupt normal hepatic lipid metabolism and promote hepatic dysfunction that is prevalent in T2D, it is not known if this effect of OC exposure is due to a direct effect on the hepatocyte itself or a result of an indirect effect such as the OC compound(s) causing an elevation in serum free fatty acids or hyperinsulinemia to drive DNL. Recent in vitro studies indicate exposure to DDE may promote dyslipidemia in the form of hypertriglyceridemia via direct effects on the hepatocyte to promote both triglyceride and apolipoprotein B secretion (Ward et al., 2016). Therefore, the present study seeks to determine if direct exposure to a highly prevalent OC compound trans-nonachlor, which has been implicated in increased T2D pathogenesis as well as increased atherosclerotic risk, can promote intracellular lipid accumulation and the potential effects on hepatic lipid metabolism including fatty acid uptake, fatty acid oxidation, fatty acid secretion, and DNL that could contribute to increased hepatocyte neutral lipid accumulation. To examine the direct effects of OC compound exposure on hepatocyte lipid metabolism, we utilized McArdle-RH7777 (McA) rat hepatoma cells which have been widely used in previous studies examining in vitro hepatocyte function (Caviglia et al., 2011; Chamberlain et al., 2013; DeBose-Boyd et al., 2001; Hansson et al., 2004; Sparks et al., 2016; Yamaguchi et al., 2003).
2. Materials and Methods
2.1 Reagents
McArdle-RH7777 (McA) rat hepatoma cells were obtained from American Type Culture Collection (ATCC) and grown in DMEM with high glucose, 20% FBS, sodium pyruvate, glutamine, penicillin, and streptomycin (normal growth media) for routine culturing. Trans-nonachlor (>99% purity), oleic acid, sodium palmitate, Oil Red O stain, Janus Green stain, and cell viability reagent (Cell Counting Kit-8) were obtained from Sigma Aldrich. Trans-nonachlor stock solutions were made in dimethylsulfoxide (DMSO) at a 4000x concentration to produce DMSO (0.025%) as the final DMSO concentration in vehicle controls. 4,4-difluoro-5-methyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoic acid (BODIPY 500/510 C12; BODIPY-FA) was obtained from Life Technologies for use in fatty acid uptake assays. 14C-acetate was purchased from Perkin Elmer for use in DNL assays. TBARS, glutathione, β-hydroxybutyrate (β-HB), and triglyceride assay kits were purchased from Cayman Chemical.
2.2 Intracellular lipid accumulation
McA cells were exposed to trans-nonachlor to determine if exposure altered intracellular lipid accumulation under normal growth conditions as previously performed (Howell III et al., 2016). The concentration related effects of trans-nonachlor exposure on intracellular lipid accumulation was determined by exposing cells (1.0 × 105 cells/ml) to vehicle (DMSO; 0.025%) or trans-nonachlor (0.02, 0.2, 2, 20, or 80 μM) for 24 hours in normal growth media. Following exposure, cells were washed with PBS (pH 7.4) then either fixed with 10% buffered formalin for Oil Red O staining or were harvested in PBS for subsequent intracellular triglyceride quantification. Intracellular triglyceride levels were determined per the manufacturer’s protocol (Cayman Chemical) and expressed as μmole TG/μg cellular protein.
2.3 Free fatty acid-induced neutral lipid accumulation
The effects of trans-nonachlor on free fatty acid-induced neutral lipid accumulation as a model for hepatic steatosis was performed as previously performed with minor modifications (Howell III et al., 2016). Briefly, McA cells were exposed to either vehicle (DMSO 0.025%) or trans-nonachlor (20 μM) in serum free media for 24 hours with or without oleic acid (OA; 200 μM) and palmitic acid (PA; 100 μM) or fatty acid free bovine serum albumin (BSA; 0.5%) to simulate extracellular free fatty acid-induced hepatic steatosis (Araya et al., 2004; Gomez-Lechon et al., 2007). Following exposure, cells were washed twice with PBS and fixed with 10% buffered formalin for Oil Red O staining.
2.4 Oil Red O staining
Following fixation, cell monolayers were subjected to Oil Red O staining as previously performed (Howell and Mangum, 2011; Howell III et al., 2016). Briefly, cells were washed with 60% isopropanol and Oil Red O stain (100 μl per well) was added for 15 minutes at room temperature. Cells were washed 4 times with deionized water then allowed to air dry overnight. Intracellular Oil Red O stain was extracted with 100% isopropanol and the absorbance (520 nm) measured to determine the amount of Oil Red O that was released from the monolayer. To normalize for cell number following Oil Red O staining, cell monolayers were stained with Janus Green for 5 minutes. Janus Green was extracted from the cells with 0.5 N HCl and the absorbance was measured at 595 nm. Data are expressed as the ratio of Oil Red O absorbance (520 nm) to Janus Green absorbance (595 nm) and denoted as ORO/JG.
2.5 Fatty acid uptake assay
The effect of trans-nonachlor exposure on fatty acid uptake was determined in McA cells following 24 hours of exposure to either vehicle (DMSO 0.025%) or trans-nonachlor (20 μM) as previously performed with minor modifications (Howell and Mangum, 2011; Howell III et al., 2016). Following 24 hours of exposure, cell media was removed and replaced with serum free DMEM containing 0.2% fatty acid free BSA and 2.5 μM of 4,4-difluoro-5-methyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoic acid (BODIPY 500/510 C12; BODIPY-FA; Molecular Probes) for 1 or 60 minutes as previously described (Howell and Mangum, 2011; Howell III et al., 2016). Cellular lysates were made in 0.2% SDS and the lysate fluorescence intensity was determined at an excitation of 490 nm and emission of 510 nm. Protein content of the lysate was measured by Bradford assay and data are expressed as fatty acid RFU/μg protein.
2.6 Measurement of cellular fatty acid oxidation
Cellular β-hydroxybutyrate levels were measured as an index of cellular fatty acid oxidation as previously performed (Howell III et al., 2016). Briefly, McA cells were treated with vehicle or trans-nonachlor (20 μM) for 24 hours with fatty acid free BSA (0.5%) or OA (400 μM) stimulation for the last 8 hours in serum free growth media then cellular levels of β-hydroxybutyrate, a metabolic by product of fatty acid oxidation which is widely measured as an index of hepatic fatty acid oxidation, were determined per the manufacturer’s protocol (Cayman Chemical) and data expressed as μmole/mg protein.
2.7 VLDL/triglyceride secretion assay
Trans-nonachlor mediated effects on VLDL secretion were determined as previously performed with minor modifications (Howell III et al., 2016). Briefly, media triglyceride concentrations were measured following exposure to trans-nonachlor (20 μM) or vehicle (DMSO 0.025%) with oleic acid (OA; 400 μM) or fatty acid free BSA (0.5%) stimulation for the last 8 hours of trans-nonachlor exposure. Following BSA or OA stimulation, 500 μl of cell culture media was extracted via the Folch method and the organic phase was dried in a speed-vac (Eppendorf). Dried lipids were resuspended in 100% methanol and subjected to triglyceride measurement via commercially available colorimetric triglyceride assay (Cayman Chemical). Data are expressed as μmole TG/mg of monolayer cellular lysate for normalization.
2.8 Cellular ApoB measurement by SDS-PAGE and Western Blot
To determine the effects of trans-nonachlor on cellular ApoB100 production, McA cells were exposed to trans-nonachlor (0 or 20 μM) for 24 hours in serum free media containing fatty acid free BSA (0.5%) with additional exposure to OA (400 μM) for the last 8 hours then lysed in RIPA buffer with protease and phosphatase inhibitors for whole cell lysates. Cellular proteins (50–75 μg/well) were separated by SDS-PAGE, transferred to PVDF membranes, and subjected to immunoblot analysis with mouse anti-ApoB (Novus Biologicals) primary antibody then goat anti-mouse HRP conjugated secondary antibody. B-actin was used as a loading control. Proteins were visualized with chemiluminescent reagent (Millipore) and digital images captured with the ChemiDoc XRS+ imaging system (Bio-Rad). The integrated density of each band were determined using ImageJ (NIH) image analysis software. ApoB100 integrated densities were normalized to β-actin integrated density.
2.9 De novo lipogenesis assay
Trans-nonachlor mediated alterations in cellular DNL were determined as previously performed with minor modifications (Howell et al., 2009; Howell III et al., 2016). Briefly, McA cells were exposed to either vehicle (DMSO 0.025%) or trans-nonachlor (20 μM) for 24 hours in serum free DMEM containing fatty acid free BSA (0.5%) with or without insulin stimulation (100 nM) for the final 8 hours. Following exposures, cells were then treated with 2 μCi/ml 14C-acetate for 3 hours. After 14C acetate incubation, monolayers were washed twice with ice cold PBS, scraped in 200 μl PBS, and total cellular lipids were extracted with chloroform:methanol (2:1; 1 ml) via the Folch method (Folch et al., 1957). Organic phase (600 μl per sample) radioactivity was measured by liquid scintillation counting and expressed as counts per minute/mg cellular protein. Previous studies in our lab have shown that the majority of 14C-acetate radioactivity in total cellular lipids is incorporated into cellular triglyceride fractions (Howell et al., 2009).
2.10 Determination of cytotoxicity
The effects of trans-nonachlor on cellular viability was measured via commercially available reagent (Cell Counting Kit-8; Sigma-Aldrich) as previously performed (Howell III et al., 2016). Briefly, cells were treated for 24 hours with vehicle (DMSO 0.025%) or trans-nonachlor (2, 20, and 80 μM) in phenol red free regular growth media then exposed to the cell viability reagent for 2 hours which utilizes the water-soluble tetrazolium salt WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,monosodium salt]. This salt forms a water soluble formazan dye in the presence of live cells and which was measured at 450 nm and data were expressed as the percent viability of vehicle treated cells.
2.11 Assessment of oxidative stress
Both cellular lipid peroxidation and glutathione levels were measured to assess if exposure to trans-nonachlor alters cellular oxidative stress and antioxidant capacities. Following exposure to vehicle (DMSO 0.025%) or trans-nonachlor (20 μM) for 24 hours in normal growth media, cellular levels of malondialdehyde (MDA), a widely used indicator of oxidative stress, was measured via thiobarbituric acid reactive substances (TBARS) assay and cellular levels of glutathione were measured via the manufacturer’s protocol (Cayman Chemical). Data were expressed as nmole MDA/mg protein for the TBARS assay and μg glutathione/mg protein.
2.12 Statistical analysis
Data are expressed as the means ± the standard error of the mean (SEM). For experiments with three or more groups, data were analyzed with a one-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test (SigmaPlot 12.5) with pairwise comparisons. In experiments with only two groups, data were analyzed by Student’s t-test. For experiments with two different exposures, data were analyzed with a two-way ANOVA followed by a Tukey’s post hoc test to determine if there was significant differences between groups and potential interactions between exposures. A P-value less than or equal to 0.05 was considered the threshold of statistical significance.
3. Results
3.1 Trans-nonachlor exposure increases intracellular neutral lipid accumulation
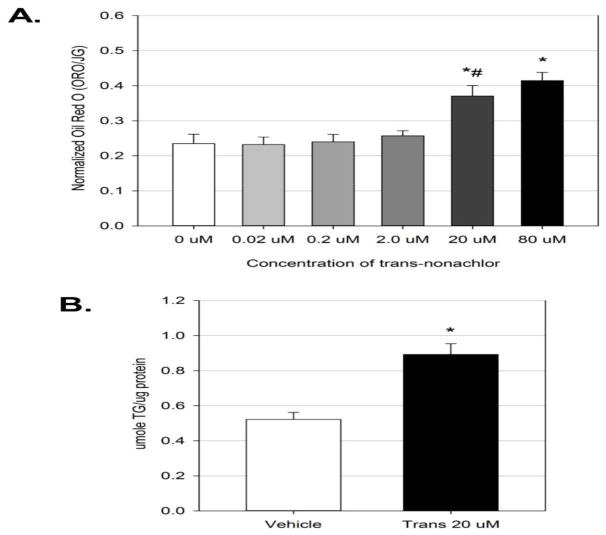
To determine if exposure to trans-nonachlor alters intracellular neutral lipid accumulation, McA cells were exposed to trans-nonachlor (0.02–80 μM) for 24 hours in normal growth media and subjected to Oil Red O staining as previously performed (Howell III et al., 2016). Exposure to trans-nonachlor (20 and 80 μM) resulted in significant increases of neutral lipid accumulation compared to vehicle (DMSO 0.025%; Figure 1A). Trans-nonachlor (20 μM; 0.37 ± 0.03 ORO/JG) significantly increased neutral lipid staining by 1.61-fold whereas the highest concentration of transnonachlor (80 μM; 0.41 ± 0.02 ORO/JG) significantly increased neutral lipid staining by 1.78-fold compared to vehicle (0 μM; 0.23 ± 0.03 ORO/JG). There were no significant differences between the 20 μM and 80 μM concentrations of trans-nonachlor suggesting a plateau in the steatotic effect. It should be noted that these concentrations did not produce any significant cytotoxicity (Supplementary figure 1). Thus, we utilized a concentration of 20 μM to confirm the neutral lipid stain was associated with intracellular triglyceride accumulation and for subsequent studies examining trans-nonachlor-mediated alterations of major cellular processes in hepatic lipid metabolism that would promote hepatic steatosis.
Figure 1.
Exposure to trans-nonachlor increases intracellular neutral lipid accumulation. McA cells were exposed to trans-nonachlor (0–80 μM) for 24 hours then subjected to Oil Red O staining for (A.) neutral lipid accumulation or to trans-nonachlor (0 or 20 μM) for (B.) cellular triglyceride measurement. Data represent the mean ± SEM of n=8/group for Oil Red O and n=5/group for triglyceride measurement. Data analysis was performed by one-way ANOVA with Tukey’s post hoc test in figure 1A and by Student’s t-test in figure 1B. *P<0.05 vs. vehicle; #P<0.05 vs. 2 μM
To confirm that the observed Oil Red O staining was associated with intracellular triglyceride accumulation, which is a hallmark of hepatic steatosis, cellular triglyceride levels were determined following trans-nonachlor exposure for 24 hours in normal growth media (Figure 1B). Trans-nonachlor (20 μM; 0.89 ± 0.06 μmole/μg protein) significantly increased intracellular triglyceride levels by 1.71-fold compared to vehicle (0 μM; 0.52 ± 0.04 μmole/μg protein). Therefore, these data indicate the intracellular neutral lipid staining is due at least in part to an increase in intracellular triglyceride accumulation.
3.2 Exposure to trans-nonachlor increases free fatty acid-induced lipid accumulation
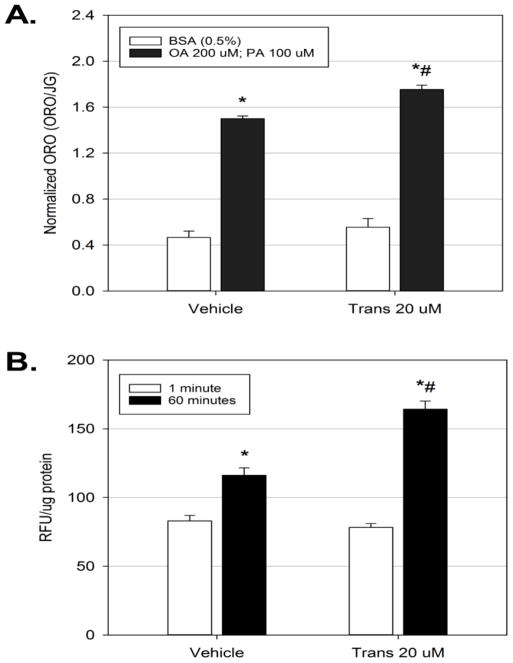
In obesity and type 2 diabetic states, a common dyslipidemia that occurs is an elevation in serum free fatty acids, which will promote hepatic steatosis. To determine if exposure to trans-nonachlor would promote lipid accumulation in conditions that would mimic hepatic steatosis in these disease states, McA cells were exposed to either vehicle or trans-nonachlor (20 μM) for 24 hours in serum free media with or without treatment with a combination of OA (200 μM) and PA (100 μM) to mimic free fatty acid-induced lipid accumulation (Figure 2A). Treatment with the OA/PA combination significantly increased neutral lipid staining by 3.17-fold compared to BSA treatment of vehicle cells (1.49 ± 0.02 vs. 0.47 ± 0.05 ORO/JG, respectively). Interestingly, trans-nonachlor exposure in combination with OA/PA (1.75 ± 0.04 ORO/JG) increased this steatotic effect of OA/PA by 3.72-fold compared to BSA-treated vehicle cells (0.47 ± 0.05 ORO/JG). Additionally, the increased in lipid accumulation following trans-nonachlor exposure with OA/PA was significantly greater than that observed in vehicle-exposed, OA/PA-treated cells. These data indicate that trans-nonachlor potentiates free fatty acid-induced hepatocyte lipid accumulation.
Figure 2.
Trans-nonachlor exposure significantly increases free fatty acid accumulation. McA cells were treated with trans-nonachlor (0 or 20 μM) for 24 hours in serum free media with or without OA/PA for the final 8 hours then subjected to Oil Red O staining (A.) or with Bodipy labeled dodecanoic acid for 1 minute or 60 minutes (B.). Data represent the mean ± SEM of n=7–8/group for figure 2A and of n=8/group for figure 2B. Data analysis was performed by two-way ANOVA with Tukey’s post hoc test. *P<0.05 vs. vehicle + BSA and #P<0.05 vs. vehicle + OA/PA in figure 2A. *P<0.05 vs. vehicle @ 1 minute and #P<0.05 vs. vehicle @ 60 minutes in figure 2B.
Since increased free fatty acid uptake is a major mechanism through which hepatic steatosis is induced, the effect of trans-nonachlor exposure on uptake and accumulation of Bodipy labeled dodecanoic acid was determined as previously performed (Howell and Mangum, 2011; Howell III et al., 2016). Exposure to trans-nonachlor for 24 hours did not significantly alter free fatty acid uptake at 1 minute following addition of the Bodipy labeled free fatty acid (Figure 2B). However, trans-nonachlor exposure did significantly increase the accumulation of labelled free fatty acid at 60 minutes (164.3 ± 5.9 RFU/μg protein) by 1.41-fold following addition of the Bodipy labelled free fatty acid compared to vehicle exposed cells after 60 minutes (116.2 ± 5.4 RFU/μg protein). Based on our previous kinetic studies with the Bodipy labeled free fatty acid, the current data indicate trans-nonachlor exposure does not alter a saturable, transporter-mediated fatty acid uptake, but exacerbates free fatty acid accumulation (Howell III et al., 2016).
3.3 Fatty acid oxidation is upregulated following trans-nonachlor exposure
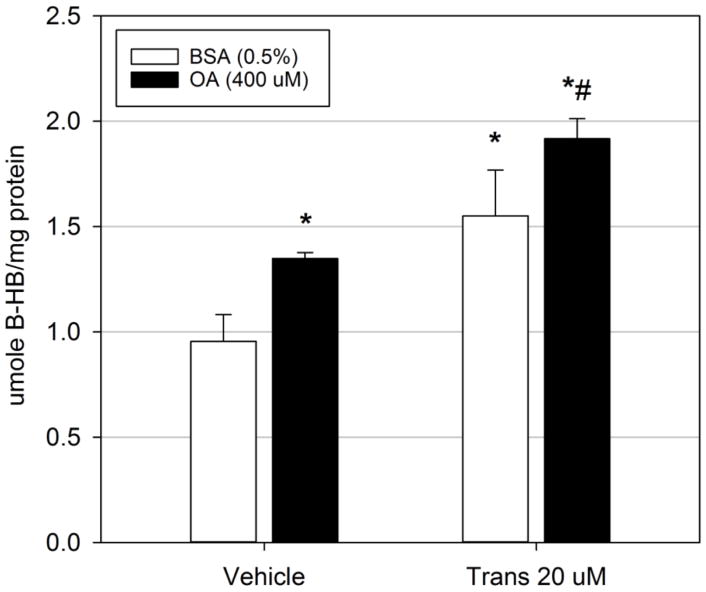
To examine if alterations in fatty acid oxidation may mediate trans-nonachlor-induced neutral lipid accumulation, McA cells were exposed to trans-nonachlor (0 or 20 μM) for 24 hours in serum free media with or without the addition of OA (400 μM) for the last 8 hours of incubation then cellular levels of β-HB, a widely utilized cellular and systemic indicator of fatty acid oxidation, was measured as an index of fatty acid degradation (Figure 3). As anticipated, addition of OA significantly increased cellular levels of β-HB compared to BSA-treated vehicle cells (1.41-fold; 1.35 ± 0.03 μmole/μg protein vs. 0.96 ± 0.13 μmole/μg protein, respectively). Trans-nonachlor exposure (1.55 ± 0.22 μmole/μg protein) significantly increased cellular β-HB levels in BSA-treated vehicle cells by 1.62-fold and in OA-treated cells (1.92 ± 0.1 μmole/μg protein) by 1.42-fold compared to their corresponding DMSO vehicle treated controls. These data suggest that trans-nonachlor does not significantly decrease either basal or stimulated fatty acid oxidation as a potential steatotic mechanism.
Figure 3.
Fatty acid oxidation is increased following trans-nonachlor exposure. McA cells were exposed to trans-nonachlor (0 or 20 μM) for 24 hours in serum free media with or without OA for the final 8 hours then cellular β-HB levels were determined. Data represent the mean ± SEM of 5–6/group and were analyzed by two-way ANOVA with Tukey’s post hoc test. *P<0.05 vs. vehicle + BSA; #P<0.05 vs. vehicle + OA
3.4 Exposure to trans-nonachlor does not alter triglyceride secretion
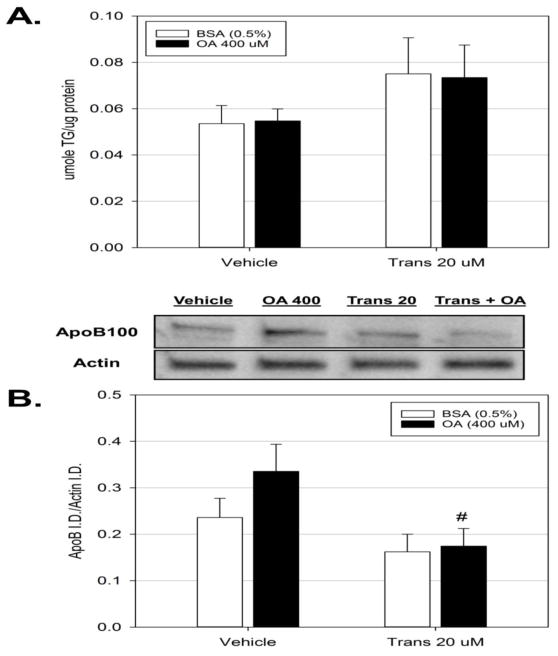
The effects of trans-nonachlor exposure on VLDL/triglyceride secretion was explored to determine if decreased secretion might mediate trans-nonachlor-induced lipid accumulation. McA cells were treated as described above for determination of fatty acid oxidation and media levels of triglyceride were determined as an index of VLDL secretion (Figure 4A). Exposure to trans-nonachlor had no significant effects on media triglyceride levels.
Figure 4.
Exposure to trans-nonachlor decreases cellular ApoB levels. McA cells were exposed to trans-nonachlor (0 or 20 μM) for 24 hours in serum free media with or without OA for the final 8 hours then (A.) media triglyceride levels and (B.) cellular ApoB levels were determined. Data represent the mean ± SEM of n=6/group and were analyzed by two-way ANOVA with Tukey’s post hoc test. #P<0.05 vs. vehicle + OA
As an additional indicator of VLDL stability and potential for secretion, the cellular levels of ApoB100 were determined to examine if trans-nonachlor exposure alters ApoB100 production (Figure 4B). While exposure to trans-nonachlor did not significantly alter basal levels of ApoB100, exposure did significantly decrease the slight increase in cellular ApoB100 levels produced by treatment of vehicle-exposed cells with OA (400 μM) by 2-fold (0.17 ± 0.04 vs. 0.34 ± 0.06 ApoB/β-actin, respectively). Thus, the possibility arises that trans-nonachlor exposure may act to destabilize cellular production of ApoB100.
3.5 Trans-nonachlor significantly increases cellular DNL
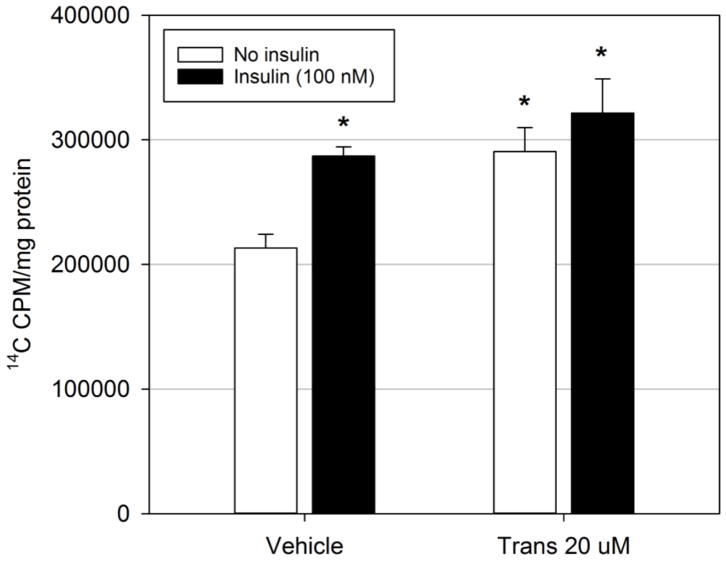
While elevated extracellular free fatty acids and resulting fatty acid accumulation play a major role in the etiology of hepatic steatosis, DNL is the second largest source of intracellular free fatty acid which can be esterified into triglycerides. Thus, the effect of trans-nonachlor exposure on DNL was examined. McA cells were exposed to trans-nonachlor (0 or 20 μM) in serum free media with or without insulin stimulation (100 nM) for the final 8 hours of exposure followed by a lipogenic assay as previously performed (Figure 5). Exposure to trans-nonachlor (290,568 ± 19,316.8 cpm/mg protein) significantly increased basal, but not insulin-stimulated, 14C acetate incorporation into total cellular lipids by 1.36-fold compared to vehicle-treated cells (213,356 ± 10,964.4 cpm/mg protein). These data indicate trans-nonachlor-mediated DNL may contribute to trans-nonachlor mediated neutral lipid accumulation.
Figure 5.
Trans-nonachlor significantly increased de novo lipogenesis. McA cells were exposed to trans-nonachlor (0 or 20 μM) for 24 hours in serum free media with or without insulin stimulation for the final 8 hours then lipogenesis was determined by 14C-acetate incorporation into total cellular lipids. Data represent the mean ± SEM of n=4/group and were analyzed by two-way ANOVA with Tukey’s post hoc test. *P<0.05 vs. vehicle without insulin
3.6 Exposure to trans-nonachlor does not alter lipid peroxidation or cellular glutathione levels
Alterations in cellular oxidative stress levels are well known to contribute to hepatic lipid accumulation and resulting hepatic steatosis. Thus, effects of trans-nonachlor (0 or 20 μM) on MDA levels and cellular glutathione levels were determined following 24 hours of exposure (Supplementary figure 2). There were no significant alterations in either cellular levels of MDA or glutathione indicating that trans-nonachlor exposure does not promote oxidative stress as a mechanism through which it causes neutral lipid accumulation.
4. Discussion
Within the last decade, there is a growing body of evidence that implicates environmental chemical exposures in the pathogenesis of obesity, insulin resistance, and subsequent type 2 diabetes as well as atherosclerosis (Heindel et al., 2017). Among the most highly implicated classes of chemicals in these epidemiological studies are the OC pesticides or their metabolites and the PCBs. In support of these epidemiological studies, recent in vivo and in vitro studies have demonstrated exposure to OC pesticides or PCBs alone or in a mixture can promote hepatic steatosis and alterations in systemic lipid profiles (Ibrahim et al., 2011; Mulligan et al., 2017; Ruzzin et al., 2010; Wahlang et al., 2013). However, the contribution of individual compounds in these chemical groups has not been fully elucidated. Therefore, the goal of the present study was to examine the effects of direct cellular exposure to the OC compound, trans-nonachlor, on hepatocyte lipid metabolism as a potential chemical inducer of NAFLD.
In the current study, we utilized rat McA cells as a model of hepatocyte function due to the fact they have been widely used to examine various aspects of cellular lipid metabolism including lipogenesis and lipid secretion. Specifically, McA cells have been used in studies examining mechanisms governing VLDL secretion and ApoB regulation because they secrete triglyceride laden VLDL and ApoB in a manner which is similar to that observed in primary hepatocytes (Caviglia et al., 2011; Chamberlain et al., 2013; Sparks et al., 2016; Yamaguchi et al., 2003). In addition, McA cells have been shown to have a lipogenic drive which more closely represents that of the intact liver or freshly isolated hepatocytes in that McA cells express the insulin-sensitive lipogenic mediator sterol response element binding protein-1c (Srebp-1c), which is present in intact liver and freshly isolated primary hepatocytes, as compared to Srebp-1a, which is present in the majority of immortalized hepatocyte/hepatoma cell lines (DeBose-Boyd et al., 2001; Shimomura et al., 1997). However, McA cells have been shown to have increased fatty acid uptake compared to primary hepatocytes (Hansson et al., 2004). Thus, the presently observed trans-nonachlor-mediated alterations in fatty acid uptake/accumulation and lipogenesis need to be confirmed in a primary hepatocyte culture system in future studies.
In the Fourth National Report on Human Exposure to Environmental Chemicals, the mean lipid adjusted serum concentrations of trans-nonachlor were between 41 to 31 nM (18.3 to 14.7 ng/g) for the years 2000 to 2004 (CDC, 2009). However, there are wide variations in serum and tissue concentrations of this compound and other POPs due to geographic location and prevalence of compound use. For instance, the serum concentrations of DDT, a similar bioaccumulative OC compound, was 743 ng/ml or 2.1 μM in Northeast India (Mishra et al., 2011). In addition, the lipophilic nature of these compounds allows for selective bioaccumulation in tissues such as the adipose and liver. Thus, hepatic levels may be higher than those measured in blood with bioaccumulation over time. Our current data utilized a concentration range in the low micromolar range with a 24-hour duration of exposure to establish steatotic potential and utilized the lowest tested concentration of trans-nonachlor (20 μM) that demonstrated significant steatosis to explore potential physiological mechanisms. This concentration is similar to those used in recent in vitro studies to explore cellular mechanisms governing the effects of trans-nonachlor exposure on EGFR activity in HepG2 cells where 10 μM was utilized and the effects of exposure on generation of reactive oxygen species in THP1 macrophages where 10 μM was primarily utilized (Hardesty et al., 2018; Mangum et al., 2015).
Our current data demonstrate exposure to trans-nonachlor produces a concentration-dependent increase in hepatocyte neutral lipid accumulation which is due, at least in part, to increased intracellular triglyceride accumulation. Recent in vivo studies have demonstrated exposure to contaminated fish oil containing a mixture of persistent organic pollutants (POPs), containing both OC pesticides including trans-nonachlor and PCBs, exacerbated high fat diet-induced steatosis in rodent models (Ibrahim et al., 2011; Ruzzin et al., 2010). Additional studies in our lab have demonstrated that a defined mixture of prevalent POPs found in contaminated fish oil, including the five most prevalent OC pesticides and the five most prevalent PCBs, exacerbated hepatic steatosis in a genetically-induced model of obesity and type 2 diabetes, the ob/ob mouse (Mulligan et al., 2017). In vivo studies utilizing only trans-nonachlor determined that chronic oral exposure increases liver weight due in part to hepatocyte hypertrophy and increased triglyceride content (Bondy et al., 2004; Bondy et al., 2000). Therefore, these data indicate exposure to trans-nonachlor, either alone or as part of a mixture, has the potential to promote hepatic lipid accumulation and this accumulation can occur through direct cellular effects on the hepatocyte.
As previously mentioned, studies by Bondy et al. (2004) demonstrated chronic exposure to trans-nonachlor produced hepatocyte hypertrophy which was associated with increased oxidative stress as indicated by increased TBARS reactivity and decreased superoxide dismutase activities in male rats (Bondy et al., 2004). Our current data demonstrate hepatocyte lipid accumulation without a significant increase in TBARS reactivity or decrease in cellular glutathione indicating a lack of oxidative stress in our in vitro model. Thus, the possibility arises that the increased oxidative stress observed in vivo may be due to a pro-inflammatory state of the intact organ. Mangum et al (2015) recently demonstrated that exposure to trans-nonachlor can promote oxidative stress in a human monocyte/macrophage model and thus may increase the inflammatory tone in a whole organ system (Mangum et al., 2015)
Donnelly et al. (2005) demonstrated extracellular free fatty acids are the major source of fatty acids for esterification into intracellular triglyceride in patients with NAFLD (Donnelly et al., 2005). Therefore, we explored the effects of trans-nonachlor exposure on hepatocyte neutral lipid accumulation under conditions of elevated free fatty acids (OA/PA in a 2:1 ratio) which would recapitulate the fatty acid ratio used to model NAFLD in vitro (Gomez-Lechon et al., 2007). Interestingly, trans-nonachlor exposure significantly increased free fatty acid-induced neutral lipid accumulation compared to vehicle-exposed cells. These data indicate exposure to trans-nonachlor may exacerbate hepatic steatosis under conditions of elevated circulating free fatty acid levels which is a common dyslipidemia associated with obesity and type 2 diabetes.
Based on our current data, it appears exposure to trans-nonachlor does not alter rapid transporter-mediated free fatty acid uptake. Prior kinetic studies in our lab have demonstrated that the Bodipy labeled fatty acid compound displays a biphasic uptake profile consisting of a rapid cellular uptake phase up to 1 minute following exposure then a gradual linear accumulation phase from 1 minute to 60 minutes (Howell III et al., 2016). The rapid uptake phase is reflective of unsaturated, transporter mediated uptake whereas the linear accumulation phase is most likely a balanced product of fatty acid cycling through the cellular processes of fatty acid uptake, oxidation, and secretion (Abumrad et al., 1998; Berk and Stump, 1999). Trans-nonachlor exposure significantly increased Bodipy labeled dodecanoic acid accumulation following 60 minutes of free fatty acid treatment. Therefore, we anticipate this increase in labeled fatty acid accumulation to be associated with either decreased fatty acid oxidation or decreased fatty acid secretion in the form of triglyceride laden VLDL [for review see (Angulo, 2002; Marchesini et al., 2001; Marchesini et al., 2003)].
Interestingly, exposure to trans-nonachlor significantly increased, not decreased, cellular fatty acid degradation as indicated by increased cellular β-HB levels under basal and OA-stimulated conditions. Thus, it appears trans-nonachlor does not significantly decrease cellular fatty acid degradation/oxidation in McA cells as a steatotic mechanism. Additionally, trans-nonachlor had no effect extracellular triglyceride levels as an index of triglyceride laden VLDL secretion. This effect on extracellular triglyceride is in contrast to those observed for another highly prevalent OC pesticide, DDE, in which extracellular triglyceride levels were elevated under basal and OA-stimulated conditions (Ward et al., 2016). It should be noted that intracellular ApoB levels following OA stimulation were significantly decreased by exposure to trans-nonachlor in the present study. This decrease could be reflective of decreased ApoB lipidation and resulting stability which will be explored in future studies (Cardozo et al., 2002; Rutledge et al., 2010).
While extracellular fatty acids are the primary source of intracellular free fatty acids in NAFLD, hepatic DNL is the second major source of free fatty acids for esterification in NAFLD and this process is upregulated in type 2 diabetes by hyperinsulinemia (Donnelly et al., 2005; Marchesini et al., 1999; Utzschneider and Kahn, 2006). Therefore, we determined the effects of trans-nonachlor exposure on basal and insulin-induced DNL as previously performed (Howell et al., 2009; Howell III et al., 2016). Exposure to trans-nonachlor significantly increased 14C-acetate incorporation into total cellular lipids under basal conditions but did not significantly enhance insulin-stimulated incorporation. Our previous studies indicate that the majority of radiolabeled acetate is incorporated into the triglyceride fraction of total cellular lipids (Howell et al., 2009). Thus, these data indicate upregulation of hepatic lipogenesis following exposure to trans-nonachlor may contribute to the increased neutral lipid accumulation by providing free fatty acids for esterification. Future studies will be designed to determine the molecular machinery involved in trans-nonachlor mediated lipogenesis and to dissect the overall contribution of DNL to the currently observed pro-steatotic effect of trans-nonachlor exposure.
In summary, the current study has demonstrated that direct exposure to the highly prevalent OC compound trans-nonachlor can promote intracellular neutral lipid accumulation due to increased intracellular triglyceride levels in a rat hepatoma cell model. This steatotic effect is associated with increased free fatty acid accumulation, increased DNL, and increased fatty acid oxidation which may be a compensatory mechanism stemming from elevated intracellular free fatty acid levels. These data shed light on the cellular mechanisms that may govern the pro-steatotic potential of trans-nonachlor as well as further our current understanding of the metabolic alterations which may stem from environmental exposures to OC compounds that have been implicated in the pathogenesis of obesity and type 2 diabetes. Future studies should determine if this direct, pro-steatotic effect of trans-nonachlor occurs in primary hepatocytes as an “ex vivo” model in addition to in vivo studies examining the hepatic effects of trans-nonachlor exposure in normal and NAFLD models to determine if trans-nonachlor exacerbates disease status.
Supplementary Material
Highlights.
Trans-nonachlor exposure promotes a concentration dependent increase in neutral lipid accumulation in McArdle-RH7777 cells
Extracellular free fatty acid-induced lipid accumulation is increased by trans-nonachlor
Exposure to trans-nonachlor significantly increases Bodipy-labeled dodecanoic acid accumulation
Trans-nonachlor significantly increases cellular de novo lipogenesis
Acknowledgments
The present work was funded by grant #1R15ES026791-01 from the National Institute of Environmental Health Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Abumrad N, Harmon C, Ibrahimi A. Membrane transport of long-chain fatty acids: evidence for a facilitated process. J Lipid Res. 1998;39:2309–2318. [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. The New England Journal of Medicine. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, Poniachik J. Increase in long-chain polyunsaturated fatty acid n - 6/n - 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2004;106:635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- Berk PD, Stump DD. Mechanisms of cellular uptake of long chain free fatty acids. Mol Cell Biochem. 1999;192:17–31. [PubMed] [Google Scholar]
- Bondy G, Curran I, Doucet J, Armstrong C, Coady L, Hierlihy L, Fernie S, Robertson P, Barker M. Toxicity of trans-nonachlor to Sprague-Dawley rats in a 90-day feeding study. Food Chem Toxicol. 2004;42:1015–1027. doi: 10.1016/j.fct.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Bondy GS, Newsome WH, Armstrong CL, Suzuki CA, Doucet J, Fernie S, Hierlihy SL, Feeley MM, Barker MG. trans-Nonachlor and cis-nonachlor toxicity in Sprague-Dawley rats: comparison with technical chlordane. Toxicol Sci. 2000;58:386–398. doi: 10.1093/toxsci/58.2.386. [DOI] [PubMed] [Google Scholar]
- Cardozo C, Wu X, Pan M, Wang H, Fisher EA. The inhibition of microsomal triglyceride transfer protein activity in rat hepatoma cells promotes proteasomal and nonproteasomal degradation of apoprotein b100. Biochemistry. 2002;41:10105–10114. doi: 10.1021/bi025749w. [DOI] [PubMed] [Google Scholar]
- Caviglia JM, Gayet C, Ota T, Hernandez-Ono A, Conlon DM, Jiang H, Fisher EA, Ginsberg HN. Different fatty acids inhibit apoB100 secretion by different pathways: unique roles for ER stress, ceramide, and autophagy. J Lipid Res. 2011;52:1636–1651. doi: 10.1194/jlr.M016931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC; Department of Health and Human Services, C.f.D.C.a.P, editor. Fourth national report on human exposure to environmental chemicals. Atlanta, GA: 2009. [Google Scholar]
- Chamberlain JM, O’Dell C, Sparks CE, Sparks JD. Insulin suppression of apolipoprotein B in McArdle RH7777 cells involves increased sortilin 1 interaction and lysosomal targeting. Biochem Biophys Res Commun. 2013;430:66–71. doi: 10.1016/j.bbrc.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab. 2011;22:353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBose-Boyd RA, Ou J, Goldstein JL, Brown MS. Expression of sterol regulatory element-binding protein 1c (SREBP-1c) mRNA in rat hepatoma cells requires endogenous LXR ligands. Proc Natl Acad Sci U S A. 2001;98:1477–1482. doi: 10.1073/pnas.98.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes & metabolism. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J Hepatol. 2016;65:425–443. doi: 10.1016/j.jhep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Gomez-Lechon MJ, Donato MT, Martinez-Romero A, Jimenez N, Castell JV, O’Connor JE. A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact. 2007;165:106–116. doi: 10.1016/j.cbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Ha MH, Lee DH, Jacobs DR. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: results from the National Health and Nutrition Examination Survey, 1999–2002. Environmental health perspectives. 2007;115:1204–1209. doi: 10.1289/ehp.10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson PK, Asztely AK, Clapham JC, Schreyer SA. Glucose and fatty acid metabolism in McA-RH7777 hepatoma cells vs. rat primary hepatocytes: responsiveness to nutrient availability. Biochim Biophys Acta. 2004;1684:54–62. doi: 10.1016/j.bbalip.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Hardesty JE, Al-Eryani L, Wahlang B, Falkner KC, Shi H, Jin J, Vivace B, Ceresa BP, Prough RA, Cave MC. Epidermal Growth Factor Receptor Signaling Disruption by Endocrine and Metabolic Disrupting Chemicals. Toxicol Sci. 2018 doi: 10.1093/toxsci/kfy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal F. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell G, 3rd, Deng X, Yellaturu C, Park EA, Wilcox HG, Raghow R, Elam MB. N-3 polyunsaturated fatty acids suppress insulin-induced SREBP-1c transcription via reduced trans-activating capacity of LXRalpha. Biochim Biophys Acta. 2009;1791:1190–1196. doi: 10.1016/j.bbalip.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell G, 3rd, Mangum L. Exposure to bioaccumulative organochlorine compounds alters adipogenesis, fatty acid uptake, and adipokine production in NIH3T3-L1 cells. Toxicol In Vitro. 2011;25:394–402. doi: 10.1016/j.tiv.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GE, III, Mulligan C, Young D, Kondakala S. Exposure to chlorpyrifos increases neutral lipid accumulation with accompanying increased de novo lipogenesis and decreased triglyceride secretion in McArdle-RH7777 hepatoma cells. Toxicol In Vitro. 2016;32:181–189. doi: 10.1016/j.tiv.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Fjaere E, Lock EJ, Naville D, Amlund H, Meugnier E, Le Magueresse Battistoni B, Froyland L, Madsen L, Jessen N, Lund S, Vidal H, Ruzzin J. Chronic consumption of farmed salmon containing persistent organic pollutants causes insulin resistance and obesity in mice. PloS one. 2011;6:e25170. doi: 10.1371/journal.pone.0025170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR., Jr Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes care. 2007a;30:622–628. doi: 10.2337/dc06-2190. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR., Jr Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetologia. 2007b;50:1841–1851. doi: 10.1007/s00125-007-0755-4. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR., Jr A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PloS one. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8:146–153. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- Lonardo A, Sookoian S, Pirola CJ, Targher G. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism. 2016;65:1136–1150. doi: 10.1016/j.metabol.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Mangum LC, Borazjani A, Stokes JV, Matthews AT, Lee JH, Chambers JE, Ross MK. Organochlorine insecticides induce NADPH oxidase-dependent reactive oxygen species in human monocytic cells via phospholipase A2/arachidonic acid. Chem Res Toxicol. 2015;28:570–584. doi: 10.1021/tx500323h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- Mishra K, Sharma RC, Kumar S. Organochlorine pollutants in human blood and their relation with age, gender and habitat from North-east India. Chemosphere. 2011;85:454–464. doi: 10.1016/j.chemosphere.2011.07.074. [DOI] [PubMed] [Google Scholar]
- Mulligan C, Kondakala S, Yang EJ, Stokes JV, Stewart JA, Kaplan BL, Howell GE., 3rd Exposure to an environmentally relevant mixture of organochlorine compounds and polychlorinated biphenyls Promotes hepatic steatosis in male Ob/Ob mice. Environ Toxicol. 2017;32:1399–1411. doi: 10.1002/tox.22334. [DOI] [PubMed] [Google Scholar]
- Park SK, Son HK, Lee SK, Kang JH, Chang YS, Jacobs DR, Lee DH. Relationship between serum concentrations of organochlorine pesticides and metabolic syndrome among non-diabetic adults. Journal of preventive medicine and public health = Yebang Uihakhoe chi. 2010;43:1–8. doi: 10.3961/jpmph.2010.43.1.1. [DOI] [PubMed] [Google Scholar]
- Rutledge AC, Su Q, Adeli K. Apolipoprotein B100 biogenesis: a complex array of intracellular mechanisms regulating folding, stability, and lipoprotein assembly. Biochem Cell Biol. 2010;88:251–267. doi: 10.1139/o09-168. [DOI] [PubMed] [Google Scholar]
- Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT, Malde MK, Du ZY, Chavey C, Fajas L, Lundebye AK, Brand CL, Vidal H, Kristiansen K, Froyland L. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environmental health perspectives. 2010;118:465–471. doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99:838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks RP, Guida WC, Sowden MP, Jenkins JL, Starr ML, Fratti RA, Sparks CE, Sparks JD. Sortilin facilitates VLDL-B100 secretion by insulin sensitive McArdle RH7777 cells. Biochem Biophys Res Commun. 2016;478:546–552. doi: 10.1016/j.bbrc.2016.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. The Journal of clinical endocrinology and metabolism. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- Wahlang B, Falkner KC, Gregory B, Ansert D, Young D, Conklin DJ, Bhatnagar A, McClain CJ, Cave M. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J Nutr Biochem. 2013;24:1587–1595. doi: 10.1016/j.jnutbio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AB, Dail MB, Chambers JE. In Vitro effect of DDE exposure on the regulation of lipid metabolism and secretion in McA-RH7777 hepatocytes: A potential role in dyslipidemia which may increase the risk of type 2 diabetes mellitus. Toxicol In Vitro. 2016;37:9–14. doi: 10.1016/j.tiv.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, Gamble MV, Conlon D, Liang JS, Ginsberg HN. The conversion of apoB100 low density lipoprotein/high density lipoprotein particles to apoB100 very low density lipoproteins in response to oleic acid occurs in the endoplasmic reticulum and not in the Golgi in McA RH7777 cells. J Biol Chem. 2003;278:42643–42651. doi: 10.1074/jbc.M306920200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.