Abstract
Background
The role of bone marrow-derived mesenchymal stem cells (BM-MSCs) in liver fibrosis remains poorly understood. This study aimed to use a mouse model of carbon tetrachloride (CCL4)-induced liver fibrosis to investigate the effects of BM-MSCs during liver hypoxia and the involvement of the transforming growth factor beta 1 (TGF-β1) and SMADs pathway.
Material/Methods
Thirty C57BL/6 mice were randomly divided into the control group (n=10), the model group (n=10), and the BM-MSC-treated model group (n=10). In the model group, liver fibrosis was induced by intraperitoneal injection of CCl4. BM-MSCs were transplanted after 12 weeks of CCl4 treatment. The serum biochemical parameters and histological changes in the liver, using histochemical stains, were investigated. The expression of collagen type I (collagen I), alpha-smooth muscle actin (α-SMA), TGF-β1, SMAD3, SMAD7, hypoxia-inducible factor 1 alpha (HIF-1α), and vascular endothelial grow factor (VEGF) were assessed by immunohistochemistry and quantitative real-time polymerase chain (RT-qPCR) reaction.
Results
Treatment with BM-MSCs reduced the expression of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) compared with the model group, and reduced liver fibrosis determined histologically using hematoxylin and eosin (H&E) and Masson’s trichrome staining compared with the model group. The area of liver fibrosis decreased after BM-MSCs treatment (p<0.05). Protein expression of HIF-1α and VEGF were decreased after BM-MSCs treatment (p<0.05). Transplantation of BM-MSCs reduced the mRNA expression of TGF-β1, collagen I, α-SMA, and SMAD3 (p<0.05).
Conclusions
BM-MSC transplantation reduced CCl4-induced murine liver fibrosis, indicating that in a hypoxic microenvironment, BM-MSCs may inhibit the TGFβ-1/SMADs pathway.
MeSH Keywords: Cell Hypoxia; Liver Cirrhosis, Experimental; Mesenchymal Stromal Cells
Background
Cirrhosis is the combination of liver regeneration and fibrosis and can occur due to chronic liver diseases. Liver fibrosis has systemic complications that include liver failure, hepatic encephalopathy, upper gastrointestinal bleeding, and portal hypertension [1]. Global epidemiological studies have shown that deaths from cirrhosis increased from around 760,600 in 1980 to more than 1 million in 2010 [2]. Currently, the most effective treatment for advanced cirrhosis is liver transplantation, but this has several limitations, including a shortage of the liver donors, surgical complications, risk of transplant rejection, and the high costs involved [3–5]. Therefore, new approaches to reduce fibrotic liver disease are needed.
Recently, mesenchymal stem cell (MSC) transplantation has been suggested as an alternative to liver transplantation for the treatment of cirrhosis. MSCs are derived from the bone marrow, umbilical cord, adipose tissue, and fetal tissue, and are transplanted by intravenous, intrahepatic, intraperitoneal [6] and intrasplenic injection [7]. Bone marrow-derived mesenchymal stem cells (BM-MSCs) are the most promising cells in the field of stem cells therapy due to their ease of acquisition, ability to proliferate, low immunogenicity, and potential for multiple cell differentiation [8,9]. Several studies using animal models [10–12] or human clinical trials [8,13] have shown that BM-MSC transplantation might improve liver function and reduce hepatic fibrosis. The mechanisms involved in the role of BM-MSC include their ability to induce proliferation of mature hepatocytes by the secretion of growth factors and differentiation into hepatocytes and other cells that have roles in liver regeneration [14]. However, the specific mechanisms by which BM-MSCs may prevent or reverse liver fibrosis remain unclear.
Recent studies have shown that the activation of hepatic stellate cells is an important mechanism in the development of cirrhosis [15]. Hepatic stellate cells can be activated under hypoxic conditions, and they secrete vimentin and alpha-smooth muscle actin (α-SMA), and participate in the development of liver fibrosis [16]. Liver fibrosis and cirrhosis are associated with hypoxia [17], and with the further development of liver fibrosis, increased hepatic sinusoidal obliteration will lead to increased liver hypoxia [18]. Therefore, reducing hypoxia may delay the progression of liver fibrosis.
Previous studies have shown that transforming growth factor beta 1 (TGF-β1) is the most important fibrogenic factor for the activation of hepatic stellate cells [19]. Also, the SMADs protein family is a key downstream signaling molecule of the TGF-β1 signaling pathway. In our previous study, we found that co-culture of BM-MSCs and hepatic stellate cells could inhibit the activation of hepatic stellate cells by inhibiting the TGFβ-1/SMAD signaling pathway [20].
However, few studies have simultaneously investigated the effects of BM-MSCs on the hypoxic microenvironment and TGFβ-1/SMAD signaling pathway in models of liver fibrosis and cirrhosis. Therefore, this study aimed to use a mouse model of carbon tetrachloride (CCL4)-induced liver fibrosis to investigate the effects of BM-MSCs during liver hypoxia and the involvement of the TGF-β1 and SMADs signaling pathway.
Material and Methods
Isolation and culture of mouse bone marrow-derived mesenchymal stem cells (BM-MSCs)
Thirty male C57BL/6 mice aged between 8 to 10 weeks were obtained from the School of Medicine of Lanzhou University. All animal experiments were approved by the Animal Experiment Committee of Lanzhou University (Approval No. LDYYLL2019-187). Isolation and culture of bone marrow-derived mesenchymal stem cells (BM-MSCs) were performed as previously described [21].
Briefly, bone marrow cells were collected under complete aseptic conditions by flushing the femur and tibia of mice using culture medium, which was then centrifuged at 1000 rpm for 10 minutes. After centrifugation, the supernatant was carefully aspirated, and the cells were washed three times with phosphate buffered saline (PBS). The cells were resuspended in Dulbecco’s modified Eagle’s medium (DMEM) containing 1% penicillin and streptomycin and 10% fetal bovine serum (FBS) (Biological Industries, Kibbutz Beit-Haemek, Israel) and seeded in a 25 cm2 cell culture flask. The cells were incubated at 37°C, in 5% CO2 and saturated humidity. The culture medium was replaced once every three days. When the cells approached 80–90% confluence, the cells were digested using 0.25% trypsin/EDTA (Biological Industries, Kibbutz Beit-Haemek, Israel). The cells were resuspended and transferred to a new culture flask with the appropriate cell density. Cells from the third to fifth passage were used for further experiments.
Animal groups and BM-MSC transplantation
The mice were housed under standard conditions at a temperature of 25°C, a relative humidity of 70%, and a 12 hourly light and dark cycles. The mice were given food and water ad libitum throughout the experiments, and were adapted to the laboratory conditions for one week before the study began.
Cirrhosis was induced by intraperitoneal injection with 40% carbon tetrachloride (CCl4) in olive oil at a dose of 1 ml/kg twice a week for 12 weeks, according to the method previously described [22]. Thirty C57BL/6 mice were randomly divided into the control group (n=10), the model group (CCl4+PBS) (n=10), and the BM-MSC-treated model group (CCl4+BM-MSCs) (n=10). Mice were infused with 1×106 BM-MSCs in suspension via the tail vein to establish the BM-MSCs transplantation group. After the experiments, all mice were euthanized, peripheral blood was taken for serum liver function tests, and fresh liver tissue was frozen in liquid nitrogen to extract the RNA. Liver tissue was fixed with 4% formaldehyde and routinely embedded in paraffin wax for histology and immunohistochemical detection of protein expression.
Liver function tests
Blood samples were taken from all experimental mice after treatment with BM-MSCs. The blood samples were sent to the Infectious Diseases Laboratory of the First Hospital of Lanzhou University for serum measurement of alanine transaminase (ALT) and aspartate transaminase (AST) using an automated biochemical analyzer (Beckman Coulter, Brea, CA, USA), according to the manufacturer’s instructions.
Liver histopathology
The liver samples were fixed with 4% formaldehyde and then embedded in paraffin wax blocks. Liver sections were cut at a thickness of 5 μm onto glass slides and were stained with hematoxylin and eosin (H&E) and Masson’s trichrome stain and examined histologically, as previously described [23]. Photomicrographs were taken, and analysis was conducted by a liver pathologist who was unaware of the study grouping, and the degree of fibrosis was assessed semi-quantitatively by ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Quantitative real-time polymerase chain (RT-qPCR) reaction
RT-qPCR was performed to detect collagen I, alpha-smooth muscle actin (α-SMA), transforming growth factor beta 1 (TGF-β1), SMAD3, and SMAD7 mRNA expression. β-actin was used as a control. The total RNA of the liver tissue was extracted by TRIzol reagent, according to the manufacturer’s protocols. The genomic DNA (gDNA) was removed according to the instructions in the Prime Script RT reagent Kit with the gDNA Eraser kit to synthesize cDNA (Takara, Minato-ku, Tokyo, Japan). The reaction conditions were 85°C for 5 s, 37°C for 15 min, and the reaction volume was 20 μl. For the RT-qPCR, amplification was performed to measure the mRNA levels of TGF-β1, collagen I, α-SMA, SMAD3, and SMAD7. RT-qPCR was performed using the SYBR Premix EX Taq II kit in the LightCycler Real-Time PCR instrument (Takara, Minato-ku, Tokyo, Japan) with a reaction system of 10 μl, reaction conditions of 95°C for 30 s, 45 cycles of incubation at 95°C for 5s, 60°C for 30 s, and 60°C to 95°C for 5 s. Data processing was performed using 2−ΔΔCT. Primer sequences are shown in Table 1.
Table 1.
Gene primer sequences.
| Gene | Primer sequences |
|---|---|
| β-actin | Forward: AAGATCAAGATCATTGCTCCTCCTG |
| Reverse: AGCTCAGTAACAGTCCGCCT | |
| Collagen I | Forward: GAGAGGTGAACAAGGTCCCG |
| Reverse: AAACCTCTCTCGCCTCTTGC | |
| α-SMA | Forward: CCACCATGTACCCAGGCATT |
| Reverse: GTGTGCTAGAGGCAGAGCAG | |
| TGFβ-1 | Forward: ACTGGAGTTGTACGGCAGTG |
| Reverse: GGCTGATCCCGTTGATTTCC | |
| SMAD3 | Forward: CCTTCTGGTGCTCCATCTCC |
| Reverse: ACCTCTCCCAATGTGTCGC | |
| SMAD7 | Forward: CCTCGGAAGTCAAGAGGCTG |
| Reverse: CAGCCTGCAGTTGGTTTGAG |
Immunohistochemistry
Paraffin-embedded sections of mouse liver tissues were used to detect the expression of proteins, according to the manufacturer’s protocol. Tissue sections were incubated at 4°C overnight with the following rabbit anti-mouse primary antibodies, anti-TGFβ-1 (1: 400) (Bioworld Technology Inc., St. Louis Park, MN, USA), anti-SMAD3 (1: 400) (Bioworld Technology Inc., St. Louis Park, MN, USA), anti-SMAD7 (1: 400) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), anti-HIF-1α (1: 200) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), and anti-VEGF (1: 200) (Santa Cruz Biotechnology Inc., Dallas, TX, USA). The tissue sections were then incubated with biotinylated IgG at room temperature for 1 h and incubated with a goat anti-rabbit secondary antibody for 15 minutes. Tissue sections were washed three times with PBS, incubated with streptavidin for 15 minutes, rinsed with PBS, and incubated in the 3,3-diaminobenzidine (DAB) chromogen, and the reaction was stopped with tap water. The tissue sections were counterstained with hematoxylin and then coverslipped. Photomicrographs were taken by senior pathologists. The proteins were semi-quantified with Image J analysis software (National Institutes of Health, USA).
Statistical analysis
Data were analyzed using SPSS version 24.0 software (IBM Corp, Armonk, NY, USA). All measurements were performed in triplicate. The results were expressed as the mean ± standard deviation (SD). The data that did not satisfy the normal distribution and homogeneity of variance underwent nonparametric log-rank testing. A P-value <0.05 was considered to be statistically significant.
Results
Phenotypic characterization of bone marrow-derived mesenchymal stem cells (BM-MSCs) bone marrow-derived mesenchymal stem cells (BM-MSCs) identified microscopically by their spindle-shaped morphological features (Figure 1)
Figure 1.
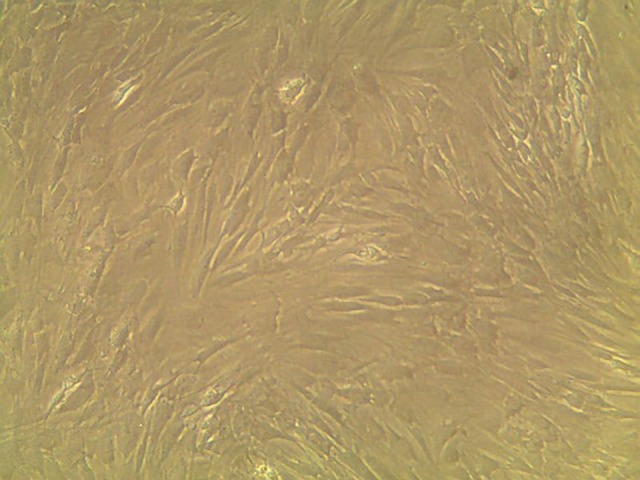
The morphology of bone marrow-derived mesenchymal stem cells (BM-MSCs) at the fifth passage showed spindle-shaped morphology (magnification ×100).
BM-MSCs treatment reduced liver fibrosis in the mouse model treated with carbon tetrachloride (CCl4)
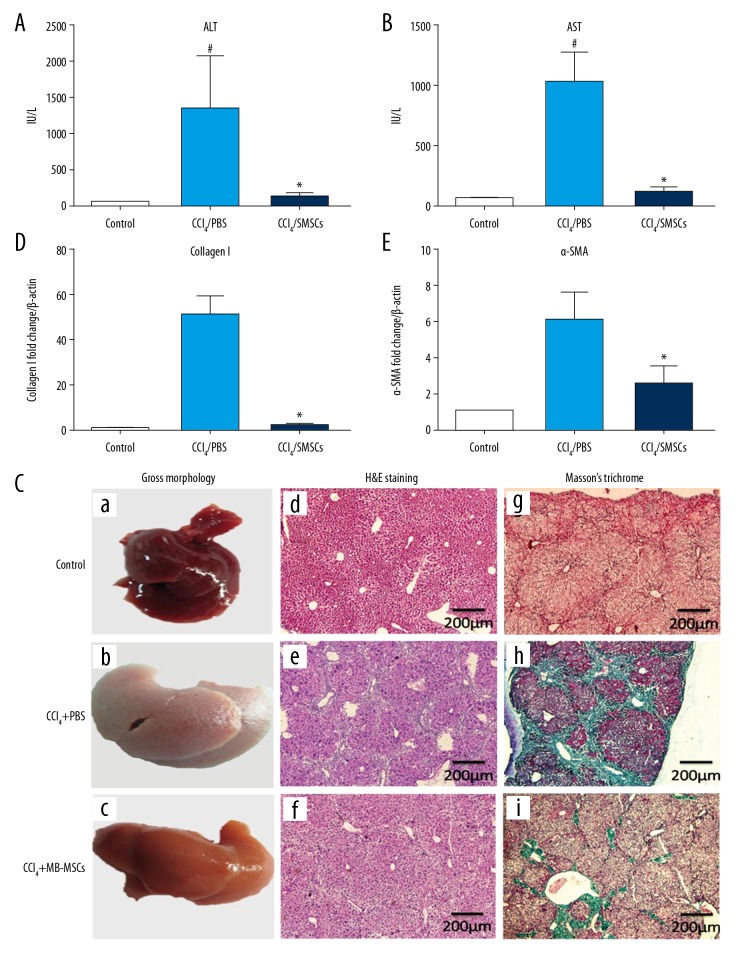
The results showed that the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were significantly increased in the CCl4-treated mouse group compared with the control group. ALT and AST levels were reduced after BM-MSCs transplantation (p<0.05) (Figure 2A, 2B).
Figure 2.
Antifibrotic effects of bone marrow-derived mesenchymal stem cells (BM-MSCs) in the mouse model of liver fibrosis. Thirty C57BL/6 mice were randomly divided into the control group (n=10), the model group (n=10), and the BM-MSC-treated model group (n=10). (A) Liver injury was assessed by measurement of serum levels of alanine aminotransferase (ALT). (B) Liver injury was assessed by serum levels of aspartate aminotransferase (AST). (C) The gross morphology of the mouse liver (a–c) (magnification ×100). Photomicrographs of the mouse liver stained with hematoxylin and eosin (H&E) (d–f) (magnification ×100). Photomicrographs of the mouse liver stained with Masson’s trichrome (g–i) (magnification ×100). (D) Expression of collagen I in each group. (E) Expression of alpha-smooth muscle actin (α-SMA) in each group. # Compared with the control group (p<0.05); * compared with the model group (p<0.05).
When compared with the control group, the gross morphology of the mouse liver was pale, dull, and coarser and the liver was small and nodular. In the BM-MSCs transplantation group, the liver was pink, shiny, and with small uniform nodules. Histology showed that the liver cells in the control group were normal. In the model group, the liver histology showed edema, hepatocyte swelling, steatosis, bridging necrosis, hepatocyte disarray, and a large number of collagen fibers were deposited in the portal tracts and involved the hepatic lobules in forming pseudolobules. In the BM-MSCs transplantation group, there was a small amount of liver edema, steatosis, necrosis, and a small number of collagen fibers were deposited in the portal area (Figure 2C). Masson’s trichrome staining showed no fibrous deposition in the portal area of the control group, but a large number of collagen fibers were deposited in the portal area and invaded the hepatic lobules to form pseudo lobes in the model group. When compared with the model group, there were fewer fibrotic in the liver of the BM-MSCs treated group (Figure 2C).
Masson’s trichrome staining showed that the expression of collagen in the model group was significantly increased compared with the control group (p<0.000). The expression of collagen in BM-MSCs transplantation group was significantly lower than that in the model group (p=0.029) (Table 2). Masson’s trichrome staining showed the expression of collagen I in the model group was increased compared with the control group, while the expression of collagen I in BM-MSCs transplantation group was significantly lower than that in the model group (p<0.05) (Figure 2D). Compared with the control group, the expression of α-SMA was increased in the model group, which indicated the activation of hepatic stellate cells. The expression of α-SMA in the BM-MSCs transplantation group was significantly down-regulated compared with the model group (p<0.05) (Figure 2E).
Table 2.
Semi-quantitative evaluation of collagen fibers.
| Groups | Collagen fibers |
|---|---|
| Control group | 0.00 (0.00, 0.00) |
| Model group | 5.59 (11.83, 41.28)# |
| BM-MSCs group | 4.66 (2.57, 5.73)* |
Compared with the control group, P <0.05;
compared with the model group, P <0.05.
BM-MSCs – bone marrow-derived mesenchymal stem cells.
Immunohistochemistry findings and the effects of BM-MSCs
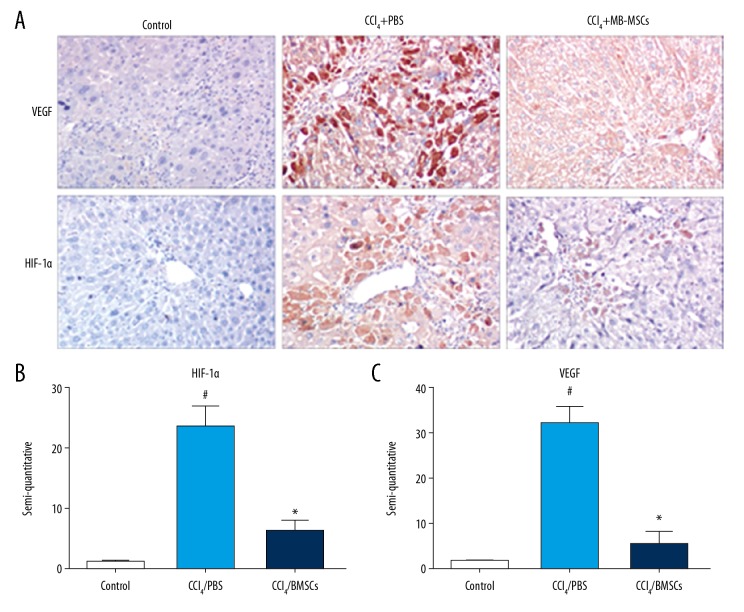
Cirrhosis is often accompanied by an increase in the expression of hypoxia-inducible factor 1 alpha (HIF-1α) and vascular endothelial grow factor (VEGF) [24]. In this study, HIF-1α was mildly expressed in the liver tissues of mice in the control group. In the model group, the positive staining of HIF-1α was mainly found in the cytoplasm and part of the nucleus (Figure 3A). Positive staining of VEGF was mainly present in the cytoplasm (Figure 3A). The expression of HIF-1α and VEGF in the model group were increased compared with the control group (p<0.05). After BM-MSCs transplantation, the expression of HIF-1α and VEGF were decreased significantly compared with the model group, and the difference was statistically significant (p<0.05) (Figure 3B, 3C).
Figure 3.
Expression of vascular endothelial grow factor (VEGF) and hypoxia-inducible factor 1 alpha (HIF-1α) in each group. Thirty C57BL/6 mice were randomly divided into the control group (n=10), the model group (n=10), and the BM-MSC-treated model group (n=10). (A) Photomicrograph shows the expression of VEGF and HIF-1α (magnification ×200). (B) Semi-quantitative expression of HIF-1α in each group. (C) Semi-quantitative expression of VEGF in each group. # Compared with the control group (p<0.05); * compared with the model group (p<0.05).
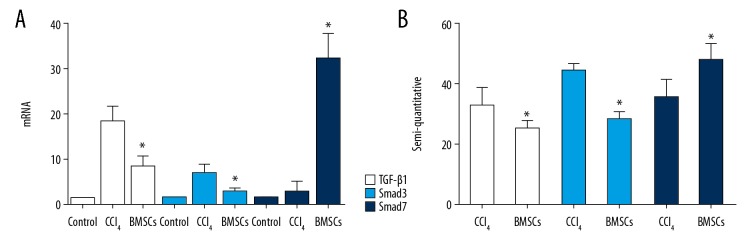
The mRNA expression of transforming growth factor beta 1 (TGF-β1), SMAD3 and SMAD7 following treatment with BM-MSCs
Compared with the control group, the mRNA expression of transforming growth factor beta 1 (TGF-β1) and SMAD3 in the model group was increased, and the expression of TGF-β1 and SMAD3 in the BMSCs transplantation group was significantly down-regulated compared with the model group (p<0.05). The mRNA expression of SMAD7 in the BMSCs transplantation group was significantly upregulated compared with the model group (p<0.05) (Figure 4A). The protein expression of these genes was consistent with the result of the mRNA expression, mainly through the semi-quantitative analysis of the immunohistochemistry (Figure 4B).
Figure 4.
Effects of bone marrow-derived mesenchymal stem cells (BM-MSCs) on the expression of transforming growth factor beta 1 (TGF-β1), SMAD3, and SMAD7. Thirty C57BL/6 mice were randomly divided into the control group (n=10), the model group (n=10), and the BM-MSC-treated model group (n=10). (A) The mRNA expression of TGF-β1, SMAD3, and SMAD7 in each group. (B) The protein expression of TGF-β1, SMAD3, and SMAD7 in each group. * Compared with the model group (p<0.05).
Discussion
The aim of this study was to use a mouse model of carbon tetrachloride (CCL4)-induced liver fibrosis to investigate the effects of bone marrow-derived mesenchymal stem cells (BM-MSCs) during liver hypoxia and the involvement of the transforming growth factor beta 1 (TGF-β1) and SMADs pathway. The findings showed that treatment with BM-MSCs improved the hypoxic liver microenvironment in CCl4-induced fibrosis, BM-MSCs improved the liver function and reduced fibrosis modulated the TGF-β1/SMADs signaling pathway by reducing TGF-β1 and SMAD3 expression and increased SMAD7 expression. This study used an established mouse model of liver fibrosis with hypoxia to investigate the association between TGF-β1/SMADs signaling, liver fibrosis, and BM-MSC treatment.
BM-MSCs have recently been shown to have a potential therapeutic role in liver disease from in vitro [25] and in vivo [26] studies and clinical studies, including in chronic hepatitis C [27], chronic hepatitis B [28], and alcoholic liver disease [29], which have suggested that transplantation of MSCs may be a promising treatment option. In our study, we established a mouse model of liver cirrhosis using an intraperitoneal injection of CCl4, which generates free radicals that trigger a cascade liver damaging effects [30]. This study showed that BM-MSC treatment reduced the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (Figure 2A, 2B), to reduce liver injury. Histology provided supportive evidence for the biochemical findings (Figure 2C). The results of quantitative real-time polymerase chain (RT-qPCR) suggested that BM-MSCs reduced the expression of alpha-smooth muscle actin (α-SMA) and collagen I, which further confirmed that BM-MSCs could reverse cirrhosis to some extent. Our results showed that the transplantation of BM-MSCs significantly reduces cirrhosis compared with PBS group and are consistent with the above reports.
Currently, several studies have confirmed that BM-MSCs can reverse liver fibrosis, but the precise mechanisms of its treatment remain controversial. Several reports have indicated that MSCs can reduce cirrhosis through degrading collagen deposition via secreting the matrix metalloproteinases (MMPs) such as MMP-9 [31], MMP-13 [32], MMP-1 [33]. Other studies have suggested that the anti-fibrosis effects of BM-MSCs mainly by increasing the anti-inflammatory factors such as IL-10 [34] and suppressing the expression of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-alpha) and IL-6 [35]. In recent years, hypoxia has played an important role in liver fibrosis by upregulating the expression of hypoxia-inducible factor 1 alpha (HIF-1α), vascular endothelial grow factor (VEGF), α-SMA, TGF-β1 in LX-2 human hepatic stellate cells [36].
HIF-1α acts as an essential transcription factor of the cellular response to hypoxia [37] and is significantly upregulated during hypoxic conditions. HIF-1α can also upregulate the expression of VEGF and other factors to promote liver fibrosis. Currently, the effect of BM-MSCs on the liver hypoxia microenvironment in mice with cirrhosis is still unclear. Wang et al. [38] showed that mesenchymal stem cells derived from adipose tissue could improve liver microcirculation to a certain extent and reduced the degree of hepatic fibrosis, as demonstrated by computed tomography (CT) perfusion scanning. To clarify the role of BM-MSCs on the hypoxic microenvironment in the liver in cirrhosis, we established a mouse model of cirrhosis and measured the expression of HIF-1α and its downstream factor VEGF. BM-MSCs restored the increased expression of HIF-1α and VEGF induced by CCl4. This finding indicated that BM-MSCs improved the hypoxic microenvironment of cirrhosis to a certain extent and alleviated the degree of cirrhosis.
The TGF-β1/SMADs signaling pathway is the main pathway mediating the activation of hepatic stellate cells, which promotes the secretion of extracellular matrix by hepatic stellate cells and inhibits the activity of matrix metalloproteinases (MMPs) [39]. Blocking this signaling pathway might be an effective target for the treatment of cirrhosis. In this study, we investigated the effects of BM-MSCs on the expression of TGF-β1, SMAD3, and SMAD7. Our results showed that the transplantation of BM-MSCs reduced the expression of TGF-β1 and SMAD3, but increased the expression of SMAD7. The findings showed that BM-MSCs could reverse cirrhosis by inhibiting the TGF-β1/SMADs signaling pathway, which was consistent with our previous findings [20], and those previously reported by Jang et al. [40].
Conclusions
In this study, a mouse model of carbon tetrachloride (CCL4)-induced liver fibrosis was used to study the effects of bone marrow-derived mesenchymal stem cells (BM-MSCs) during liver hypoxia and the involvement of the transforming growth factor beta 1 (TGF-β1) and SMADs pathway. The findings showed that BM-MSC transplantation reduced CCl4-induced murine liver fibrosis, indicating that in a hypoxic microenvironment, BM-MSCs may inhibit the TGFβ-1/SMADs pathway. A limitation of this study was that CCl4 was used to establish liver fibrosis and cirrhosis in a mouse model, which differs from human cirrhosis that develops during a long period of time. However, further long-term studies are recommended to investigate the role of BM-MSCs and the mechanisms of their effects on liver fibrosis.
Acknowledgments
The authors wish to thank Mr. Xie, Mr. Chen, and clinical staff.
Footnotes
Source of support: National Natural Science Foundation of China (31570509); National Natural Science Foundation of China (81800528); Major Science and Technology Projects in Gansu Province (1602FKDA001); Open Project of Key Laboratory of Biotherapy and Regenerative Medicine of Gansu Province (zdsyskfkt-201701)
Conflict of interest
None.
References
- 1.Raafat N, Abdel Aal SM, Abdo FK, El Ghonaimy NM. Mesenchymal stem cells: In vivo therapeutic application ameliorates carbon tetrachloride induced liver fibrosis in rats. Int J Biochem Cell Biol. 2015;68:109–18. doi: 10.1016/j.biocel.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farkas S, Hackl C, Schlitt HJ. Overview of the indications and contraindications for liver transplantation. Cold Spring Harb Perspect Med. 2014;4(5) doi: 10.1101/cshperspect.a015602. pii: a015602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wertheim JA, Petrowsky H, Saab S, et al. Major challenges limiting liver transplantation in the United States. Am J Transplant. 2011;11:1773–84. doi: 10.1111/j.1600-6143.2011.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito T, Tomita K, Haga H, et al. Bone marrow cell-based regenerative therapy for liver cirrhosis. World J Methodol. 2013;3:65–69. doi: 10.5662/wjm.v3.i4.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao W, Li JJ, Cao DY, et al. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol. 2012;18:1048–58. doi: 10.3748/wjg.v18.i10.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Idriss NK, Sayyed HG, Osama A, Sabry D. Treatment efficiency of different routes of bone marrow-derived mesenchymal stem cell injection in rat liver fibrosis model. Cell Physiol Biochem. 2018;48:2161–71. doi: 10.1159/000492558. [DOI] [PubMed] [Google Scholar]
- 8.Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: Short-term and long-term outcomes. Hepatology. 2011;54:820–28. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 9.Bi H, Ming L, Cheng R, et al. Liver extracellular matrix promotes BM-MSCs hepatic differentiation and reversal of liver fibrosis through activation of integrin pathway. J Tissue Eng Regen Med. 2017;11:2685–98. doi: 10.1002/term.2161. [DOI] [PubMed] [Google Scholar]
- 10.Iwamoto T, Terai S, Hisanaga T, et al. Bone-marrow-derived cells cultured in serum-free medium reduce liver fibrosis and improve liver function in carbon-tetrachloride-treated cirrhotic mice. Cell Tissue Res. 2013;351:487–95. doi: 10.1007/s00441-012-1528-z. [DOI] [PubMed] [Google Scholar]
- 11.Nasir GA, Mohsin S, Khan M, et al. Mesenchymal stem cells and Interleukin-6 attenuate liver fibrosis in mice. J Transl Med. 2013;11:78. doi: 10.1186/1479-5876-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang YO, Kim MY, Cho MY, et al. Effect of bone marrow-derived mesenchymal stem cells on hepatic fibrosis in a thioacetamide-induced cirrhotic rat model. BMC Gastroenterol. 2014;14:198. doi: 10.1186/s12876-014-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang YO, Kim YJ, Baik SK, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: A pilot study. Liver Int. 2014;34:33–41. doi: 10.1111/liv.12218. [DOI] [PubMed] [Google Scholar]
- 14.Kochat V, Baligar P, Maiwall R, Mukhopadhyay A. Bone marrow stem-cell therapy for genetic and chronic liver diseases. Hepatol Int. 2014;8:166–78. doi: 10.1007/s12072-013-9499-z. [DOI] [PubMed] [Google Scholar]
- 15.Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473–92. doi: 10.1002/cphy.c120035. [DOI] [PubMed] [Google Scholar]
- 16.Deng J, Huang Q, Wang Y, et al. Hypoxia-inducible factor-1alpha regulates autophagy to activate hepatic stellate cells. Biochem Biophys Res Commun. 2014;454:328–34. doi: 10.1016/j.bbrc.2014.10.076. [DOI] [PubMed] [Google Scholar]
- 17.Cannito S, Paternostro C, Busletta C, et al. Hypoxia, hypoxia-inducible factors and fibrogenesis in chronic liver diseases. Histol Histopathol. 2014;29:33–44. doi: 10.14670/HH-29.33. [DOI] [PubMed] [Google Scholar]
- 18.Copple BL, Bai S, Burgoon LD, Moon JO. Hypoxia-inducible factor-1α regulates the expression of genes in hypoxic hepatic stellate cells important for collagen deposition and angiogenesis. Liver Int. 2011;31:230–44. doi: 10.1111/j.1478-3231.2010.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dooley S, ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347:245–56. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang LT, Fang XQ, Chen QF, et al. Bone marrow-derived mesenchymal stem cells inhibit the proliferation of hepatic stellate cells by inhibiting the transforming growth factor β pathway. Mol Med Rep. 2015;12:7227–32. doi: 10.3892/mmr.2015.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ming LG, Ge BF, Wang MG, Chen KM. Comparison between 8-prenylnarigenin and narigenin concerning their activities on promotion of rat bone marrow stromal cells’ osteogenic differentiation in vitro. Cell Prolif. 2012;45:508–15. doi: 10.1111/j.1365-2184.2012.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farouk S, Sabet S, Abu Zahra FA, El-Ghor AA. Bone marrow derived-mesenchymal stem cells downregulate IL17A dependent IL6/STAT3 signaling pathway in CCl4-induced rat liver fibrosis. PLoS One. 2018;13:e0206130. doi: 10.1371/journal.pone.0206130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truong NH, Nguyen NH, Le TV, et al. Comparison of the treatment efficiency of bone marrow-derived mesenchymal stem cell transplantation via tail and portal veins in CCl4-induced mouse liver fibrosis. Stem Cells Int. 2016;2016 doi: 10.1155/2016/5720413. 5720413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Ma X, Wang J, et al. Curcumin protects against CCl4-induced liver fibrosis in rats by inhibiting HIF-1α through an ERK-dependent pathway. Molecules. 2014;19:18767–80. doi: 10.3390/molecules191118767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rengasamy M, Singh G, Fakharuzi NA, et al. Transplantation of human bone marrow mesenchymal stromal cells reduces liver fibrosis more effectively than Wharton’s jelly mesenchymal stromal cells. Stem Cell Res Ther. 2017;8:143. doi: 10.1186/s13287-017-0595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aithal AP, Bairy LK, Seetharam RN, Kumar N. Haemostatic potential of human bone marrow-derived mesenchymal stromal cells in Wistar rats with carbon tetrachloride induced liver cirrhosis. Stem Cell Investig. 2018;5:21. doi: 10.21037/sci.2018.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amer ME, El-Sayed SZ, El-Kheir WA, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011;23:936–41. doi: 10.1097/MEG.0b013e3283488b00. [DOI] [PubMed] [Google Scholar]
- 28.Kim JK, Park YN, Kim JS, et al. Autologous bone marrow infusion activates the progenitor cell compartment in patients with advanced liver cirrhosis. Cell Transplant. 2010;19:1237–46. doi: 10.3727/096368910X506863. [DOI] [PubMed] [Google Scholar]
- 29.Saito T, Okumoto K, Haga H, et al. Potential therapeutic application of intravenous autologous bone marrow infusion in patients with alcoholic liver cirrhosis. Stem Cells Dev. 2011;20:1503–10. doi: 10.1089/scd.2011.0074. [DOI] [PubMed] [Google Scholar]
- 30.Abdel Aziz MT, Atta HM, Mahfouz S, et al. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin Biochem. 2007;40:893–99. doi: 10.1016/j.clinbiochem.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Tanimoto H, Terai S, Taro T, et al. Improvement of liver fibrosis by infusion of cultured cells derived from human bone marrow. Cell Tissue Res. 2013;354:717–28. doi: 10.1007/s00441-013-1727-2. [DOI] [PubMed] [Google Scholar]
- 32.Rabani V, Shahsavani M, Gharavi M, et al. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol Int. 2010;34:601–5. doi: 10.1042/CBI20090386. [DOI] [PubMed] [Google Scholar]
- 33.Du C, Jiang M, Wei X, et al. Transplantation of human matrix metalloproteinase-1 gene-modified bone marrow-derived mesenchymal stem cell attenuates CCL4-induced liver fibrosis in rats. Int J Mol Med. 2018;41:3175–84. doi: 10.3892/ijmm.2018.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier RP, Mahou R, Morel P, et al. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J Hepatol. 2015;62:634–41. doi: 10.1016/j.jhep.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 35.Pulavendran S, Vignesh J, Rose C. Differential anti-inflammatory and anti-fibrotic activity of transplanted mesenchymal vs. hematopoietic stem cells in carbon tetrachloride-induced liver injury in mice. Int Immunopharmacol. 2010;10:513–19. doi: 10.1016/j.intimp.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Shi YF, Fong CC, Zhang Q, et al. Hypoxia induces the activation of human hepatic stellate cells LX-2 through TGF-beta signaling pathway. FEBS Lett. 2007;581:203–10. doi: 10.1016/j.febslet.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Nath B, Szabo G. Hypoxia and hypoxia inducible factors: Diverse roles in liver diseases. Hepatology. 2012;55:622–33. doi: 10.1002/hep.25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Lian F, Li J, et al. Adipose derived mesenchymal stem cells transplantation via portal vein improves microcirculation and ameliorates liver fibrosis induced by CCl4 in rats. J Transl Med. 2012;10:133. doi: 10.1186/1479-5876-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabregat I, Moreno-Càceres J, Sánchez A, et al. TGF-β signalling and liver disease. FEBS J. 2016;283:2219–32. doi: 10.1111/febs.13665. [DOI] [PubMed] [Google Scholar]
- 40.Jang YO, Kim SH, Cho MY, et al. Synergistic effects of simvastatin and bone marrow-derived mesenchymal stem cells on hepatic fibrosis. Biochem Biophys Res Commun. 2018;497:264–71. doi: 10.1016/j.bbrc.2018.02.067. [DOI] [PubMed] [Google Scholar]