Abstract
Background:
Awake fiberoptic intubation (AFOI) is the gold standard technique for managing patients with anticipated difficult airway. Conscious sedation is desirable, not only to make the procedure more tolerable and comfortable for the patient but also to ensure optimal intubating conditions. Ideal sedation regime for AFOI should provide comfort, cooperation, hemodynamic stability, and amnesia along with maintenance of spontaneous respiration. Several sedative agents have been assessed over the past two decades for this purpose but α2 agonists appear to be the favorable choice owing to its sedative, analgesic, amnestic, and sympatholytic properties along with good hemodynamic profile.
Aims:
The present study has been aimed to recognize the characteristics of dexmedetomidine, clonidine, and midazolam and to compare their efficacy in providing optimal intubating conditions as well as hemodynamic stability during AFOI.
Settings and Design:
A prospective double-blind randomized study done in tertiary care hospital.
Materials and Methods:
Sixty patients of American Society of Anesthesiologists physical status Classes I and II aged 18–60 years with anticipated difficult airway were randomly allocated into three groups. All the patients received injection midazolam bolus followed by sedation infusion of midazolam, dexmedetomidine, and clonidine according to the allocated group. Primary outcome includes the time to achieve Ramsay Sedation Score (RSS) ≥2, time taken in intubation, intubation score, comfort score for fiberoptic insertion and intubation, and patient tolerance after intubation. The secondary outcome was hemodynamic, and respiratory variables include changes in heart rate (HR), mean arterial pressure (MAP), oxygen saturation (SpO2), and respiratory rate during the procedure.
Statistical Analysis:
All data were recorded, summarized, tabulated, and statistically analyzed using SPSS 16.0 version (Chicago, Inc., USA). The data were presented in mean ± standard deviation. P < 0.05 was considered as statistically significant.
Results:
All the three groups were comparable in terms of demographic profile. Time to achieve RSS ≥2 and mean intubation time was significantly less in Groups D and C as compared to Group M (P < 0.001). Among groups, Group D took least time to achieve RSS ≥2 (5.53 ± 0.74) and mean intubation time (4.53 ± 0.91). Similarly, overall intubation score, comfort, and patient tolerance score were significantly more in Group M as compared to Groups D and C (P < 0.001). Among the groups, Group D achieved least intubation score (3.80 ± 0.67) and comfort score (2.53 ± 0.74). Although Groups D and C have a lower mean HR and MAP during the procedure and intubation compared to Group M, the incidence of SpO2 is most frequent with clonidine.
Conclusions:
Patients who received α2 agonist were calmer and cooperative with less pain and discomfort than the patients who received midazolam. Dexmedetomidine allows better endurance, stable hemodynamics, and patent airway as compared to clonidine.
Keywords: Awake fiberoptic intubation, clonidine, conscious sedation, dexmedetomidine, midazolam
INTRODUCTION
Awake fiberoptic intubation (AFOI) is the gold standard technique for managing patients with anticipated difficult airway.[1] Failure to maintain patent airway is an important cause of anesthesia-related morbidity and mortality, with subsequent failure of oxygenation and ventilation. AFOI helps in securing airway without losing control over patient's spontaneous respiration, but it can be associated with intense nociceptive stimulation, especially during passage of the endotracheal tube (ETT). Conscious sedation is desirable, not only to make the procedure more tolerable and comfortable for the patient but also to ensure optimal intubating conditions. On the contrary, deeper plain of sedation can result in loss of already compromised airway with serious consequences.[2]
Optimal conditions for AFOI include a patient who is comfortable, cooperative, free from oral or pharyngeal secretions or blood, and are able to maintain their airway and spontaneous ventilation.[2,3] Several agents have been utilized to provide sedation for AFOI. These include benzodiazepines, ketamine, propofol, sevoflurane, remifentanil, clonidine, and dexmedetomidine.[3,4] However, there are risks of hypoxia and apnea with benzodiazepines, propofol, and opioids, especially if used in combinations.[4,5] Benzodiazepines are commonly used for its anxiolytic, amnestic, and sedative properties, but it also has cardiorespiratory depressant effects.[2,5] The selective α2 adrenergic agonist has recently gained popularity for AFOI because of its sympatholytic, analgesic, anxiolytic, and sedative effects.[6,7,8]
Although several studies and systematic reviews are carried out to compare α2 agonists for short- and long-duration sedation in intensive care unit in terms of target sedation level achieved, no study have been conducted to compare the efficacy of α2 agonists, i.e., dexmedetomidine and clonidine along with traditional sedative such as midazolam, to provide conscious sedation for short procedures such as AFOI in terms of intubation and comfort scores as well as patient tolerability. To fill this lacuna, our study has been aimed to recognize the characteristics of dexmedetomidine, clonidine, and midazolam and to compare their efficacy in providing optimal intubating conditions as well as hemodynamic stability during AFOI.
MATERIALS AND METHODS
This double-blind prospective randomized study has been conducted in a tertiary care hospital for a period of 15 months from January 2016 to March 2017. After obtaining institutional Ethical Committee approval (E.C. No. 2017/119), sixty adult patients of age group 18–60 years, American Society of Anesthesiologists (ASA) physical status Classes I and II, with anticipated difficult airway, posted for elective surgery under general anesthesia were enrolled for the study. They were allocated into three groups with 20 patients in each group, based on a computer-generated randomization program. Randomization code was handed over in a sealed envelope, by a senior anesthesiologist who was not taking part in the study.
Patients with allergy to the study drugs, morbid obesity (body mass index >40 kg/m2), pregnancy,heart blocks and dysrhythmia, neurological diseases, coagulopathy, patients on antiarrhythmic, beta-blockers or long-term opioid and sedative medications and known alcohol or drug abuser were excluded from the study. After obtaining informed written consent, patients were kept fasting for 6 h. The study was conducted by two consultant anesthetists. One was responsible for carrying out the procedure and other for observation and data collection. Both the anesthetists as well as the patients were blinded to the group allocation. The drug infusions were prepared according to group allotment, by a resident anesthetist, who was not taking part in the study.
Drug preparation
Two infusions of 50 ml syringes were prepared for each group, one for loading dose infusion for 10 min, and another for maintenance dose infusion till completion of ET intubation. These infusions were prepared in such a way to keep infusion volume and delivery rate same for loading as well as maintenance infusion of drugs for all the three groups, while delivering appropriate dosage of the study drugs. The concentration of loading infusion was 70 μg/ml for Group M, 8 μg/ml for Group D, and 15 μg/ml for Group C and of maintenance infusion was 400 μg/ml for Group M, 4 μg/ml for Group D, and 15 μg/ml for Group C.
Patient preparation
Injection lignocaine up to maximum dose of 5 mg/kg was used for topicalization of airways. Depending on the weight of the patients, 5 ml of 4% lignocaine was used for nebulization. The nostrils were prepared using two drops of 0.1% xylometazoline followed by 2% lignocaine-soaked pledgets kept for 10 min. All patients were premedicated with tablet ranitidine 150 mg and tablet ondansetron 4 mg, 2 h before the surgery with a sip of water.
Anesthesia and awake fiberoptic procedure
On arrival into the operation theater, all the standard monitoring were established including noninvasive blood pressure, electrocardiography, and pulse oximetry, and baseline readings were recorded. Two 20G intravenous cannula were secured, and injection glycopyrrolate 0.2 mg was given. Two to three puffs of 10% lignocaine were sprayed over the base of the tongue and oropharynx. All patients received the loading infusion of study drugs at 36 mL/h for 10 min and then maintenance infusion was started at 6 mL/h through syringe pump based on the groups to which they were randomized, along with oxygenation.
Group M patients received injection midazolam 0.04 mg/kg intravenously stat followed by loading infusion of injection midazolam at 36 mL/h (0.05 mg/kg) over 10 min then maintenance infusion at 6 mL/h (0.05 mg/kg/h).
Group D patients received injection midazolam 0.04 mg/kg intravenously stat followed by injection dexmedetomidine at 36 mL/h (1 μg/kg) over 10 min then maintenance infusion at 6 mL/h (0.5 μg/kg/h).
Group C patients received injection midazolam 0.04 mg/kg intravenously stat followed by loading infusion of injection clonidine at 36 ml/h (2 μg/kg) over 10 min then maintenance infusion at 6 mL/h (2 μg/kg/h).
Level of sedation assessed by Ramsay Sedation Score (RSS) (1 – anxious and agitated, 2 – cooperative and oriented, 3 – respond to commands only, 4 – exhibit brisk response to light glabella tap or loud auditory stimulus, 5 – exhibit sluggish response to light glabella tap or loud auditory stimulus, and 6 – unresponsive). Once the RSS ≥2 was achieved, the fiberoptic bronchoscope (Pentax FI 13 RBS, 4.2 mm, Tokyo, Japan) loaded with appropriate size ETT for patients was inserted. This time was taken as zero, to calculate various outcomes. Topical anesthesia was performed with “spray as you go” technique using 2 ml 2% lignocaine at 15–30 s interval, via working channel of fiberoptic bronchoscope until carina was reached. The ETT was slided over the bronchoscope, inserted into trachea, fixed, and general anesthesia was induced.
The primary outcome measures the scores observed during sedation, fiberoptic bronchoscopy (FOB), intubation, and postintubation. These include the time to achieve RSS ≥2, time taken in intubation from zero time to confirmation of ET intubation, intubation score, comfort score, and patient tolerance.[9,10]
The intubation score was measured by addition of three variables:
Vocal cord movements: 1 – open, 2 – moving, 3 – closing, and 4 – closed
Coughing: 1 – none, 2 – <2 cough in sequence, 3 – 3–5 coughs in sequence, and 4 – >5 coughs in sequence
Limb movements: 1 – none, 2 – slight, 3 – moderate, and 4 – severe.
The comfort score for fiberoptic insertion and intubation was graded as:
1 – No reaction, 2 – slight grimacing, 3 – heavy grimacing, 4 – verbal objection, and 5 – defensive movement of head or hands.
The patient tolerance after intubation was assessed as:
1 – Cooperative, 2 – restless/minimal resistance, and 3 – severe resistance.
The secondary outcome includes changes in heart rate (HR), mean arterial pressure (MAP), oxygen saturation (SpO2), and respiratory rate (RR) during the procedure. The vital signs are recorded at baseline, during drug administration (1, 3, 5 min), FOB (1, 2, 3, 4, 5, 6 min), and after ET intubation (1, 2, 3, 5, 10, 15 min). Hypotension was defined as systolic blood pressure (SBP) <80 mmHg or decrease in SBP <30% below baseline. Bradycardia was defined as HR <50 beats/min or decrease to 30% below baseline. Respiratory depression was defined as RR <8 breaths/min or decrease to <25% below baseline, and hypoxia was defined as fall in Spo2 <90% or decrease to 10% below baseline. The incident of hypotension, bradycardia, hypoxia (RR <10 breaths/min or SpO2 <92%), and apnea were also recorded and were treated with intravenous fluid, injection phenylephrine 50 μg bolus, injection atropine 0.6 mg, and oxygen supplementation.
Statistical analysis
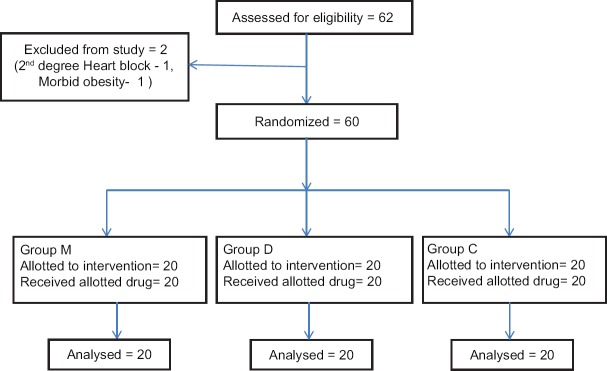
Sample size was calculated with a power of 0.8 and type one error of 0.05 so that 20 patients per group were required to demonstrate 30% difference in intubation scores [Figure 1]. All data were recorded, summarized, tabulated, and statistically analyzed using SPSS 16.0 version (Chicago, Inc., USA). The data were presented in mean ± standard deviation and analyzed using Chi-square test, one-way analysis of variance (ANOVA), and Tukey's post hoc tests. The repeated measures of ANOVA were used to compare the effect of time and time to group interaction on continuous variables. P < 0.05 was as considered statistically significant.
Figure 1.
Consort diagram: Flow of patients in the trial
RESULTS
All the three groups were comparable in demographic data (P > 0.05) [Table 1]. The mean time to achieve RSS ≥2 was significantly higher in Group M than Groups D and C (P < 0.001), although no significant difference is seen between Group D and C (P = 0.132). Among the groups, patients in Group D took least time (5.53 ± 0.74) to achieve desired sedation level [Table 2]. Similarly, mean time taken in intubation was much lesser in Group D (4.53 ± 0.91) as compared to Groups M and C and was statistically significant (P < 0.001) [Table 3].
Table 1.
Distribution of demographic parameters among groups
| Parameters | Group M (n=15) | Group D (n=15) | Group C (n=15) | P (X/Y/Z)* | |
|---|---|---|---|---|---|
| Age (years) | 40.47±10.87 | 36.80±12.72 | 37.80±10.62 | 0.66† | X=0.658, Y=0.800, Z=0.969 |
| Sex (male/female) | 9/6 | 7/8 | 8/7 | 0.76† | - |
| Weight (kg) | 54.33±6.52 | 54.27±5.50 | 56.87±4.94 | 0.37† | X=0.999, Y=0.449, Z=0.431 |
| Height (cm) | 157.33±8.20 | 156.73±6.27 | 158.13±4.64 | 0.84† | X=0.966, Y=0.940, Z=0.828 |
| BMI (kg/m2) | 21.74±1.98 | 22.05±1.69 | 21.97±1.60 | 0.88† | X=0.879, Y=0.931, Z=0.646 |
*X=Group M and D, Y=Group M and C, Z=Group D and C. BMI=Body mass index. †All the groups were comparable in terms of demographic parameters as denoted by overall insignificant P-value, as well as in intergroup analysis denoted by X/Y/Z
Table 2.
Time to achieve Ramsay Sedation Score ≥2 among groups
| Groups | Time (min) |
|---|---|
| Group M (n=15) | 6.87±0.91 |
| Group D (n=15) | 5.53±0.74 |
| Group C (n=15) | 6.13±0.83 |
| P* (X/Y/Z)† | 0.0001* X=0.0001, Y=0.052, Z=0.132 |
*P=Significant, †X=Group M and D, Y=Group M and C, Z=Group D and C
Table 3.
Intergroup comparison in time taken in intubation
| Groups | Time (min) |
|---|---|
| Group M (n=15) | 6.40±0.50 |
| Group D (n=15) | 4.53±0.91 |
| Group C (n=15) | 5.53±0.74 |
| P* (X/Y/Z)† | 0.0001* X=0.0001, Y=0.007, Z=0.002 |
*P=Significant. †X=Group M and D, Y=Group M and C, Z=Group D and C
The overall intubation score was significantly more in Group M compared to Groups D and C during the procedure [Table 4]. Although the vocal cords were either open or moving in all the three groups, Group D had highest number of patients with open vocal cords during procedure followed by Groups C and M. The difference among the groups was not statistically significant (P = 0.34). The incidence of coughing was comparable in Groups M and C (P = 0.175) and Groups D and C (P = 0.321), but statistically significant between Groups M and D (P = 0.006). Among the groups, Group D has least incidence of coughing. Limb movements were significantly less in Group D and C as compared to Group M (P < 0.001), but comparable between Group D and C (P = 0.473). Both the comfort score and patient's tolerance score were significantly lesser in Group D and C as compared to Group M (P < 0.001). No significant difference is seen between Group D and C (P = 0.951). Among the groups, least scores were achieved by patients in Group D (2.53 ± 0.74) [Table 5].
Table 4.
Intubation scores among the groups
| Parameters | Group M (n=15) | Group D (n=15) | Group C (n=15) | P*(X/Y/Z)† | |
|---|---|---|---|---|---|
| Vocal cord movements | |||||
| (1/2/3/4)α | 7/8/0/0 | 11/4/0/0 | 9/6/0/0 | 0.34 | X=0.312, Y=0.742, Z=0.742 |
| Mean±SD | 1.53±0.51 | 1.27±0.45 | 1.40±0.50 | ||
| Coughing | |||||
| (1/2/3/4)β | 4/10/1/0 | 12/3/0/0 | 8/7/0/0 | 0.008* | X=0.006, Y=0.175, Z=0.321 |
| Mean±SD | 1.80±0.56 | 1.20±0.41 | 1.47±0.51 | ||
| Limb movements | |||||
| (1/2/3/4)γ | 0/12/3/0 | 10/5/0/0 | 10/5/0/0 | ||
| Mean±SD | 2.20±0.41 | 1.33±0.48 | 1.33±0.48 | 0.0001* | X=0.0001, Y=0.0001, Z=1.00 |
| Total score | 5.40±0.98 | 3.80±0.67 | 4.20±1.08 | 0.0001* | X=0.0001, Y=0.003, Z=0.473 |
*P=Significant. †X=Group M and D, Y=Group M and C, Z=Group D and C, α1=Open, 2=Moving, 3=Closing, 4=Closed, β1=None, 2=Slight, 3=Moderate, 4=Severe, γ1=None, 2=Slight, 3=Moderate, 4=Severe. SD=Standard deviation
Table 5.
Patient’s comfort score and tolerance score among groups
| Parameters | Group M (n=15) | Group D (n=15) | Group C (n=15) | P* (X/Y/Z)† | |
|---|---|---|---|---|---|
| FOI comfort score | |||||
| (1/2/3/4/5)α | 0/9/4/2/0 | 9/6/0/0/0 | 8/7/0/0/0 | 0.0001* | X=0.0001, Y=0.0001, Z=0.952 |
| Mean±SD | 2.53±0.74 | 1.40±0.50 | 1.47±0.51 | ||
| After intubation | |||||
| (1/2/3)β | 1/12/2 | 13/2/0 | 12/3/0 | 0.0001* | X=0.0001, Y=0.0001, Z=0.897 |
| Mean±SD | 2.07±0.45 | 1.13±0.35 | 1.20±0.41 | ||
| Total score | 4.60±1.12 | 2.53±0.74 | 2.67±0.81 | 0.0001* | X=0.0001, Y=0.0001, Z=0.915 |
*P=Significant. †X=Group M and D, Y=Group M and C, Z=Group D and C. α1=No reaction, 2=Slight reaction, 3=Heavy reaction, 4=Verbal objection, 5=Defensive movement of hand and head, β1=Cooperative, 2=Restless/minimal resistance, 3=Severe resistance. SD=Standard deviation
HR was comparable in all the groups preoperatively but was significantly (P < 0.001) higher in Group M as compared to Groups D and C at most of the time periods. The difference between the Group D and C was statistically significant during FOB insertion (1, 2, 3 min) and after intubation (2, 3 min) [Figure 2]. Similarly, MAP was significantly higher in Group M as compared to Group D and C at all times, except drug administration. Intergroup analysis between Group D and C showed significant difference in MAP during FOB (1, 5, 6 min), but comparable after intubation [Figure 3].
Figure 2.
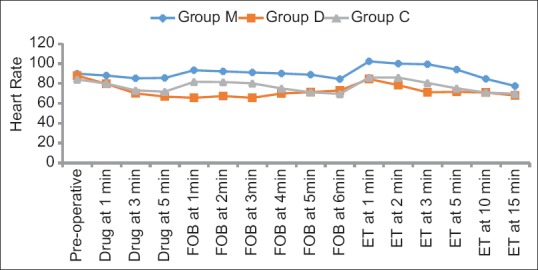
Intergroup comparison of changes in heart rate across the time periods
Figure 3.
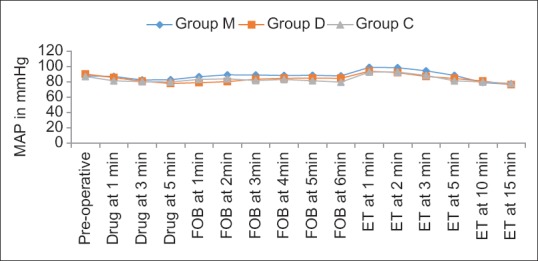
Intergroup comparison of changes in mean atrial pressure across the time periods
There was no significant difference in RR among the groups during drug administration, FOB, and ET insertion at all the time periods. Spo2 was found to be comparable between M and D groups at all time periods [Figure 4]. The incidence of desaturation was found to be significant in Group C as compared to Groups M and D, during FOB (at 3 min) and intubation (at 5 min) (P < 0.001).
Figure 4.
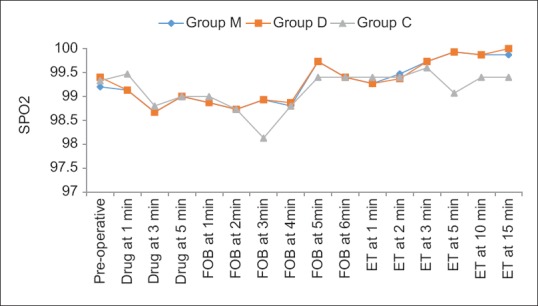
Intergroup comparison of changes in oxygen saturation across the time periods
DISCUSSION
The hypnotic effect of α2 agonists is mediated by the hyperpolarization of noradrenergic neurons in the locus ceruleus of the brain stem, which is the primary site in modulating wakefulness.[11] The hypnosis produced is similar to that found in normal sleep in that the reduction of norepinephrine release by the locus ceruleus triggers the release of γ-aminobutyric acid and galanin by the ventrolateral preoptic nucleus. These neurotransmitters further inhibit norepinephrine release by the locus ceruleus and suppress histamine secretion by the tuberomammillary nucleus. The reduced occupancy of the histamine receptors on the cells of the subcortical areas induces a hypnotic state.[12] Perhaps because of a noncortical site of action, they produce an unusually cooperative form of sedation. In this, there is easy transition from sleep to wakefulness, and task can be performed when aroused, and then, the patient goes back to sleep when not stimulated.[13] The locus ceruleus is also the site of origin for the descending medullospinal adrenergic pathway, which is known to be a key mechanism in regulating nociceptive neurotransmission.[14]
In the present study, we have compared the effect of midazolam alone and when used along with a2 agonists to provide conscious sedation for AFOI, in terms of time taken to achieve target sedation level, intubation score, comfort score, and patient tolerability score. Although dexmedetomidine and clonidine belong to the same pharmacological group, there is a significant difference in their pharmacodynamic and pharmacokinetic profile such as higher selectivity of dexmedetomidine for α2 receptors (8:1) and shorter distribution and elimination of half-life, as compared to clonidine.[11] Such differences has led us to assess and compare the effect of these two drugs in providing optimal sedation in terms of quantitative scores along with maintenance of hemodynamic stability. Few studies and systematic reviews have been carried out to compare the efficacy of α2 agonists either individually or in combination with other sedative agents for providing short- or long-term sedation in critically ill patients and to assess the hemodynamic pressor response to intubation.[15,16] Systematic review done by Cruickshank et al. compared α2 agonists for sedation in mechanically ventilated patients, but they found only one study which compared clonidine and dexmedetomidine for target sedation level while rest of the studies compared α2 agonists to benzodiazepines. In their analysis, they found dexmedetomidine to be a suitable drug to provide conscious sedation in these patients.[17] Similarly, in another Cochrane meta-analysis done by Chen et al., no eligible study for clonidine was found and all the studies compared dexmedetomidine with traditional sedatives.[18] However, there is one recent meta-analysis by Zhou et al., in which dexmedetomidine is assessed in terms of level of sedation achieved as well as intubation time and score. It concluded that it is a safe and effective agent for awake fiberoptic intubation as compared to other sedative agents.[19] Similar results have been achieved in our study and are in favor of dexmedetomidine in comparison to clonidine and midazolam.
In our study, injection midazolam was given in a dose of 0.04 mg/kg bolus followed by continuous infusion at 0.05 mg/kg/h. Injection midazolam has been used in a dose range of 0.02–0.05 mg/kg in several studies, both as intravenous bolus and infusion.[5,20] The dose of injection dexmedetomidine as loading infusion of 1 μg/kg and maintenance infusion of 0.2–0.7 μg/kg/h is quite established. We used loading dose of 1 μg/kg and maintenance dose of 0.5 μg/kg/h in our study.[5,7,8,10,20,21,22] Injection clonidine is mostly used as intravenous bolus but can also be used as infusion. Srivastava et al. have used injection clonidine in a dose of 1-2 μg/kg/h in their study.[16] We have given the loading dose of 2 μg/kg over 10 min followed by maintenance infusion at 2 μg/kg/h.[4,23] For all the three drugs, the rate of loading and maintenance infusion was kept similar for the purpose of blinding.
The primary outcome of the present study depicted that the patients receiving α2 agonists were more calm and cooperative with less pain and discomfort than patient receiving midazolam alone.[7] Both the Group D and C patients took significantly lesser time to achieve desired sedation level (RSS ≥2) than Group M (P < 0.001). Similar result was obtained in the study conducted by Patel et al.[6,23,24] However, in another two studies, midazolam group achieved desired RSS (3–4) significantly earlier than patients in dexmedetomidine group, while no difference is seen between clonidine and midazolam group.[25,26] We have used bolus dose (0.04 mg/kg) of midazolam in all the three groups, which could have acted synergistically with dexmedetomidine and clonidine to achieve the desired RSS faster. In our study, the time to achieve RSS ≥2 by Group D was 5.53 ± 0.74, which is similar to the result obtained by Cattano et al.[2] Among α2 agonist, Group D took lesser time to achieve target RSS as compared to Group C, as dexmedetomidine is 8 times more specific for α2 receptors, especially for 2A subtype, thus making it a more effective sedative agent as compared to clonidine.[11] The time to achieve ET intubation was also least in Group D (4.53 ± 0.9) among all the three groups. Similar result was obtained in a study conducted by Li et al., where the mean intubation time in dexmedetomidine group was 4.6 ± 1.4.[23] In a study done by Tsai et al., this time is even lesser (3.8 min).[9] This probably is because Tsai et al. started the procedure after completion of loading dose of dexmedetomidine, which made patients achieve deeper levels of sedation during intubation. However, in our study, FOB insertion was started during the loading infusion as soon as the patient achieved RSS ≥2, thus prolonging the mean intubation time.
The Group D and Group C patients were found to be more sedated with lesser reaction to FOB insertion and ET intubation leading to more comfort and better patient tolerability.[7] This was represented by significantly lesser intubation score, comfort score, and better patient tolerance after intubation. In our study, the overall intubation scores were significantly lesser with dexmedetomidine and clonidine as compared to midazolam (P < 0.001). Although the vocal cords movement is comparable in all the three groups, Group D had maximum number of patients with open vocal cords with least incidence of coughing and limb movement.[21,27] This finding can explain the better ability to maintain spontaneous ventilation and SpO2 with dexmedetomidine as compared to midazolam and clonidine. Comfort score and patient tolerance score were also significantly lower in Groups D and C when compared to Group M (P < 0.001), also seen in the study done by Singh et al.[3] Li et al. and Bergese et al. found similar response to FOB insertion and intubation.[23,28]
Cardiovascular response to the procedure and intubation was more favorable in Groups D and C as compared to Group M. We found consistent decrease in HR and MAP during a bolus infusion of 10 min and less intubation response.[7,10,26,29] The lower HR in Group D and Group C than Group M can be explained on the basis of hemodynamic effects of dexmedetomidine and clonidine as these drugs cause a decrease in noradrenaline release and diminish centrally mediated sympathetic tone.[30] Intubation stress response was also diminished in Group D and C, which is explained by the property of attenuation of sympathetic response of intubation by α2 agonists.[15] After the intubation, responses due to FOB and intubation might be masked due to the added hemodynamic changes brought about by the induction of general anesthesia once the ETT position was confirmed.[10,26] The respiratory parameters were comparable among the three groups.[4,7] The event of desaturation was more in Group C when compared to Groups M and D, with maximum fall between 3 and 4 min of bronchoscopy. This finding can be explained by the fact that although a2 agonist cause very little respiratory depression, but when combined with other sedative drugs can lead to statistically significant fall in SpO2, which although is clinically insignificant.[11,31] This effect was not seen in Group D which can be explained by shorter distribution (6 min) and elimination half-life of dexmedetomidine (2 h) as compared to clonidine.[11,32]
In our study, none of the patients in Groups M, D, or C experienced bradycardia, hypotension, or transient hypertension (commonly seen with α2 agonists) requiring intervention. A meta-analysis done by Tan and Ho observed that the hemodynamic instability requiring intervention is increased in studies that used higher loading and maintenance doses of dexmedetomidine (>0.7 μg/kg/h) and clonidine (>2 μg/kg/h).[33] Transient hypertensive response by clonidine is observed with high doses due to initial stimulation of α2B receptors present in vascular smooth muscles.
Limitations of the study
The patient population was small, and level of recall was not assessed for evaluation of amnestic properties of the study drugs. The various scores used by the researchers are based on the subjective responses of the individuals which could be variable and cannot be standardized. Since, we have included only ASA physical status Classes I and II patients, further trials are needed to assess the effect of α2 agonists in patients with difficult airway having significant comorbidities, where better hemodynamic profile provided by α2 agonists is more valuable.
CONCLUSION
The α2 agonists when compared to midazolam provide unique form of sedation in which patient appears to be sleepy, but easily arousable, cooperative, communicative when stimulated, along with analgesia and antisialogogue effect. They show lesser response to fiberoptic bronchoscope insertion and ET intubation with better hemodynamic stability. Dexmedetomidine seems to be a better choice over clonidine as it provides better respiratory stability with maintenance of spontaneous respiration when combined with other sedative agents.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Johnston KD, Rai MR. Conscious sedation for awake fibreoptic intubation: A review of the literature. Can J Anaesth. 2013;60:584–99. doi: 10.1007/s12630-013-9915-9. [DOI] [PubMed] [Google Scholar]
- 2.Cattano D, Lam NC, Ferrario L, Seitan C, Vadhat K, Wilcox DW, et al. A comparative study of dexmedetomidine and remifentanil for sedation during awake fiberoptic intubation. Anesthesiol Res Pract. 2012;2012:753107. doi: 10.1155/2012/753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh P, Punia TS, Kaur B, Ramachandriah P, Kaur J. A randomised comparative study of dexmedetomidine and midazolam for sedation during awake fiberoptic intubation in laparoscopic cholecystectomy patients. Int J Clin Trials. 2015;2:1–9. [Google Scholar]
- 4.Collins SR, Blank RS. Fiberoptic intubation: An overview and update. Respir Care. 2014;59:865–78. doi: 10.4187/respcare.03012. [DOI] [PubMed] [Google Scholar]
- 5.Bailey PL, Pace NL, Ashburn MA, Moll JW, East KA, Stanley TH. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesiology. 1990;73:826–30. doi: 10.1097/00000542-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Chopra P, Dixit MB, Dang A, Gupta V. Dexmedetomidine provides optimum conditions during awake fiberoptic intubation in simulated cervical spine injury patients. J Anaesthesiol Clin Pharmacol. 2016;32:54–8. doi: 10.4103/0970-9185.175666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelmalak B, Makary L, Hoban J, Doyle DJ. Dexmedetomidine as sole sedative for awake intubation in management of the critical airway. J Clin Anesth. 2007;19:370–3. doi: 10.1016/j.jclinane.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. Br J Anaesth. 2001;86:5–11. doi: 10.1093/bja/86.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Tsai CJ, Chu KS, Chen TI, Lu DV, Wang HM, Lu IC. A comparison of the effectiveness of dexmedetomidine versus propofol target-controlled infusion for sedation during fibreoptic nasotracheal intubation. Anaesthesia. 2010;65:254–9. doi: 10.1111/j.1365-2044.2009.06226.x. [DOI] [PubMed] [Google Scholar]
- 10.Ramkumar V. Preparation of the patient and the airway for awake intubation. Indian J Anaesth. 2011;55:442–7. doi: 10.4103/0019-5049.89863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan ZP, Ferguson CN, Jones RM. Alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–65. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 12.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–36. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Martin E, Ramsay G, Mantz J, Sum-Ping ST. The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J Intensive Care Med. 2003;18:29–41. doi: 10.1177/0885066602239122. [DOI] [PubMed] [Google Scholar]
- 14.Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000;93:1345–9. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar A, Tripathi RK, Choubey S, Singh RB, Awasthi S. Comparison of effects of intravenous clonidine and dexmedetomidine for blunting pressor response during laryngoscopy and tracheal intubation: A randomized control study. Anesth Essays Res. 2014;8:361–6. doi: 10.4103/0259-1162.143144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava U, Sarkar ME, Kumar A, Gupta A, Agarwal A, Singh TK. Comparison of clonidine and dexmedetomidine for short-term sedation of intensive care unit patients. Indian J Crit Care Med. 2014;18:431–6. doi: 10.4103/0972-5229.136071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruickshank M, Henderson L, MacLennan G, Fraser C, Campbell M, Blackwood B, et al. Alpha-2 agonists for sedation of mechanically ventilated adults in intensive care units: A systematic review. Health Technol Assess. 2016;20:v–xx. doi: 10.3310/hta20250. 1-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen K, Lu Z, Xin YC, Cai Y, Chen Y, Pan SM. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev. 2015;1:CD010269. doi: 10.1002/14651858.CD010269.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou LJ, Fang XZ, Gao J, Zhangm Y, Tao LJ. Safety and efficacy of dexmedetomidine as a sedative agent for performing awake intubation: A Meta-analysis. Am J Ther. 2016;23:e1788–800. doi: 10.1097/MJT.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 20.Yousuf A, Ahad B, Mir AH, Mir AW, Wani JG, Hussain SQ. Evaluation of effectiveness of dexmedetomidine and fentanyl-midazolam combination on sedation and safety during awake fiberoptic intubation: A Randomized comparative study. Anesth Essays Res. 2017;11:998–1003. doi: 10.4103/aer.AER_150_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niyogi S, Basak S, Acharjee A, Chakraborty I. Efficacy of intravenous dexmedetomidine on patient's satisfaction, comfort and sedation during awake fibre-optic intubation in patients with cervical spondylotic myelopathy posted for elective cervical fixation. Indian J Anaesth. 2017;61:137–43. doi: 10.4103/0019-5049.199856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf A, McKay A, Spowart C, Granville H, Boland A, Petrou S, et al. Prospective multicentre randomised, double-blind, equivalence study comparing clonidine and midazolam as intravenous sedative agents in critically ill children: The SLEEPS (Safety profiLe, efficacy and equivalence in paediatric intensive care sedation) study. Health Technol Assess. 2014;18:1–212. doi: 10.3310/hta18710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li CW, Li YD, Tian HT, Kong XG, Chen K. Dexmedetomidine-midazolam versus sufentanil-midazolam for awake fiberoptic nasotracheal intubation: A Randomized double-blind study. Chin Med J (Engl) 2015;128:3143–8. doi: 10.4103/0366-6999.170260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel VH, Patel HR. A comparison between dexmedetomidine and midazolam infusion on characteristic of spinal anesthesia. Int J Med Sci Public Health. 2016;5:906–10. [Google Scholar]
- 25.Sethi P, Mohammed S, Bhatia PK, Gupta N. Dexmedetomidine versus midazolam for conscious sedation in endoscopic retrograde cholangiopancreatography: An open-label randomised controlled trial. Indian J Anaesth. 2014;58:18–24. doi: 10.4103/0019-5049.126782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wawrzyniak K, Kusza K, Cywinski JB. Comparison of clonidine and midazolam premedication before endoscopic sinus surgery: Results of clinical trial. Clin Exp Otorhinolaryngol. 2014;7:307–11. doi: 10.3342/ceo.2014.7.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah BK, Thosani RM, Trivedi VC, Shah CD, Prajapati MM, Sharathkumar K, et al. A comparison of the effectiveness of dexmedetomidine infusion and midazolam for sedating cardiac patients undergoing awake fibreoptic nasal intubation. Indian J Appl Basic Med Sci. 2013;15:96–107. [Google Scholar]
- 28.Bergese SD, Candiotti KA, Bokesch PM, Zura A, Wisemandle W, Bekker AY. A phase IIIb, randomized, double-blind, placebo-controlled, multicenter study evaluating the safety and efficacy of dexmedetomidine for sedation during awake fiberoptic intubation. Am J Ther. 2010;17:586–95. doi: 10.1097/MJT.0b013e3181d69072. [DOI] [PubMed] [Google Scholar]
- 29.Cheung CW, Ying CL, Chiu WK, Wong GT, Ng KF, Irwin MG, et al. A comparison of dexmedetomidine and midazolam for sedation in third molar surgery. Anaesthesia. 2007;62:1132–8. doi: 10.1111/j.1365-2044.2007.05230.x. [DOI] [PubMed] [Google Scholar]
- 30.Afonso J, Reis F. Dexmedetomidine: Current role in anesthesia and intensive care. Rev Bras Anestesiol. 2012;62:118–33. doi: 10.1016/S0034-7094(12)70110-1. [DOI] [PubMed] [Google Scholar]
- 31.Bailey PL, Sperry RJ, Johnson GK, Eldredge SJ, East KA, East TD, et al. Respiratory effects of clonidine alone and combined with morphine, in humans. Anesthesiology. 1991;74:43–8. doi: 10.1097/00000542-199101000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Venn RM, Karol MD, Grounds RM. Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive caret. Br J Anaesth. 2002;88:669–75. doi: 10.1093/bja/88.5.669. [DOI] [PubMed] [Google Scholar]
- 33.Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: A meta-analysis. Intensive Care Med. 2010;36:926–39. doi: 10.1007/s00134-010-1877-6. [DOI] [PubMed] [Google Scholar]