Abstract
Background and Aim:
Different adjuncts have been utilized to promote the quality and prolong the duration of local anesthetics for a variety of regional block techniques. This study aimed to assess the effects of midazolam coadministered with bupivacaine in transversus abdominis plane (TAP) block on the 24-h morphine consumption, the postoperative analgesia duration and adverse effects.
Settings and Design:
A prospective, randomized, controlled double-blind trial that was carried out at a university hospital.
Patients and Methods:
Eighty-two females subjected to open total abdominal hysterectomy under general anesthesia were involved in this trial. Participants were allocated randomly to either of two groups (41 each). Control group: received TAP block with 20 mL of 0.25% bupivacaine or midazolam group: received TAP block using the same volume of bupivacaine plus 50 μg/kg midazolam/side. Postoperative cumulative 24-h morphine consumption, analgesia duration, pain score, sedation score, and adverse events were recorded.
Statistical Analysis:
Student's t-test, Mann–Whitney U-test, and Chi-square test were used.
Results:
Patients in the midazolam group had a lower cumulative 24-h morphine consumption [median doses (interquartile range): 15 (10–19.50) mg compared to 25 (17.50–37) mg, P < 0.001], lower postoperative pain score at rest at the 4th, 6th, and 12th h (P = 0.01, 0.02, and 0.02, respectively) and on movement at 2, 4, 6, and 12 h (P < 0.001), longer time till the first postoperative demand for rescue analgesia (430.11 ± 63.02 min) compared to 327.78 ± 61.99 min (P < 0.001), and less sedation, nausea and/or vomiting, and pruritus.
Conclusions:
Adding midazolam as a bupivacaine adjuvant for TAP block reduces the 24-h morphine consumption, extends the postoperative analgesia duration, and decreases the incidence of adverse effects following abdominal hysterectomy.
Keywords: Bupivacaine, midazolam, open abdominal hysterectomy, postoperative analgesia, transversus abdominis plane block
INTRODUCTION
Total abdominal hysterectomy (TAH) is a commonly performed major gynecological surgery that is accompanied by severe postoperative pain. Inadequate control of such pain adversely impacts the patients’ outcome.[1]
Transversus abdominis plane (TAP) block is a regional analgesic technique that provides somatic analgesia for the anterolateral aspect of the abdominal wall through blockade of the neural afferents from T7 to L1 segments. A recent meta-analysis has proved an analgesic-opioid-sparing efficacy of TAP block in patients subjected to open abdominal hysterectomy.[2]
Unfortunately, the analgesia produced by a single-injection TAP block is limited by the short duration of action of the used local anesthetic (LA).[3]
Different additives were used aiming at increasing the duration of the TAP analgesia as dexamethasone, dexmedetomidine, and magnesium sulfate with variable noted advantages and side effects.[4,5,6]
Midazolam is a short-acting benzodiazepine that has been shown to produce antinociception and a LA enhancing effect when given during central neuraxial block by acting on gamma-aminobutyric acid-A (GABA-A) receptors.[7]
GABA receptors have been also found on the myelinated axons of the peripheral nerves.[8,9]
The current trial was accomplished aiming to assess the effect of adding midazolam to bupivacaine for TAP block on the postoperative morphine consumption, duration of analgesia, and incidence of adverse events following TAH under general anesthesia.
PATIENTS AND METHODS
Following the Institutional Review Board approval of the Faculty of Medicine (N° R. 19.04.484), the current prospective, controlled, randomized, double-blind study was conducted on 82 adult females who were scheduled for an open TAH at a university hospital during the period from May 2018 to April 2019. All participants have signed written informed consent. Adult females with the American Society of Anesthesiologists (ASA) physical status Classes I–III, who were scheduled for open TAH under general anesthesia were included in this study. Patients were excluded if refusing to participate, were morbidly obese (body mass index ≥35 kg/m2), had a known allergy to the study drugs, had bleeding or psychiatric disorders, had local infection at the injection site, were on chronic opioid or pain medication therapy for the past 2 weeks or were drug abusers.
Patients were allocated in a random manner using a computer-generated randomization schedule that was kept in sealed opaque envelopes to either of two groups. Control group (n = 41) who received bilateral TAP block with 20 mL of 0.25% bupivacaine + 2 mL saline 0.9% per side or midazolam group (n = 41) who received TAP block with 20 mL of 0.25% bupivacaine plus 2 mL saline containing 50 μg/kg midazolam (Midathetic; Amoun Pharmaceutical S.A.E, Cairo, Egypt)/side.
The study injectate in both groups was prepared by an independent anesthetist who was not a participant in the study and looked identical to each other. The patients, the anesthesia providers, and postoperative care providing staffs were unaware of group assignment and were not involved in the data collection.
Preoperative management
The use of visual analog scale (VAS) for pain rating, a 10 cm horizontal line where 0 cm for pain-free and 10 cm for worst imaginable pain, and the use of patient-controlled analgesia (PCA) device for postoperative pain control were explained to all patients.
In the operative theater
Patients monitoring was achieved using electrocardiogram, noninvasive arterial blood pressure, peripheral pulse oximeter, and capnography.
Patients in both groups were subjected to a standardized general anesthesia technique. Anesthesia was induced using intravenous (i.v.) fentanyl 2 μg/kg, propofol 1.5–2.5 mg/kg, and atracurium 0.5 to facilitate intubation of the trachea. Anesthesia was maintained with isoflurane (0.7–1.5 MAC) in air–oxygen mixture (FiO2 40%) and i.v. fentanyl in increments of 50 μg to keep the hemodynamics, heart rate and mean arterial blood pressure, within 20% of their basal values. Atracurium 0.1 mg/kg was administered to maintain suppression of the second train of four twitch.
After induction of general anesthesia while the patient was supine and under complete asepsis, a bilateral single-injection TAP block was performed under ultrasound guidance in both groups. A sterile draped ultrasound probe (linear 4–12 MHz of Philips Clear Vue 350, Japan), was positioned transversally in the midaxillary line midway between the costal margin and the iliac crest. The needle was then inserted in-plane to the probe from lateral to medial and then was advanced till its tip was in the TAP plane (between the internal oblique and transversus abdominis muscles). On reaching this plane, the study solution was injected in 5 ml aliquots after careful aspiration expanding the TAP plane that appeared as a hypoechoic space.
On completion of the surgery, neostigmine 50 μg/kg and atropine 20 μg/kg were administered to antagonize residual neuromuscular blockade and the trachea was extubated on fulfilling the extubation criteria.
Postoperative analgesic regimen during the first 24 postoperative hours was standardized for all patients. It was comprised of intramuscular diclofenac 75 mg every 12 h, 6-hourly paracetamol 1 g i.v. infusion, started at the end of surgery combined with intravenous PCA morphine (1 mg bolus, 5-min lockout period and a maximum 4-h dose of 20 mg with no background infusion) that was started on admission to the postanesthesia care unit (PACU) and continued for 24 h.
Patients who reported postoperative nausea and/or vomiting score ≥1 (0: no nausea; 1: nausea no vomiting; and 2: nausea and vomiting) were given metoclopramide 10 mg i.v. Pruritus was managed with diphenhydramine 25 mg as needed by the patients.
The primary outcome of the study was the cumulative 24-h morphine consumption after surgery. Secondary outcomes included resting and moving pain scores assessed using VAS score at PACU, 2, 4, 6, 12, and 24 h after surgery, the duration of analgesia, sedation, nausea, vomiting, pruritus, needle trauma, accidental intraperitoneal or intravascular injections, and signs of LA toxicity. Sedation, nausea and/or vomiting, and pruritus were evaluated 4 hourly up to 24 h postoperatively.
The analgesia duration was considered as the time elapsed from the end of LA injection in the TAP plane to the first administered morphine analgesia. The presence and intensity of pain were evaluated by an investigator who was not aware of group allocation using VAS. Sedation scores were evaluated using a sedation scale (0: awake; 1: drowsy; 2: asleep but arousable; 3: deeply asleep). Patients were considered sedated if they had a sedation score of more than 0 at any time during the first 24 h after surgery.
Sample size
An a priori sample size calculation was done using the G*Power software version 3.0.10 (Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany, Released 24 January 2008). The calculation has relied on the results of an initial pilot study conducted on 10 participants in each group using the cumulative morphine consumption during the 24 h following surgery as the study primary outcome. This study revealed that the mean amount of consumed morphine (mg) during the first 24 postoperative hours was 29.8 ± 9.1 in the control group and 23.2 ± 7.9 in the midazolam group. Group sample sizes of 37 participants in each group achieve 90% power with a type I (α) error of 0.05, using a two-sided two-sample t-test. To compensate for possible patient dropouts, 10% more participants were added giving a net sample size of 82 patients.
Statistical analysis
For statistical analysis of the data, IBM SPSS software was used (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. IBM Corp., Armonk, NY, USA) A data normality test was done using the Kolmogorov–Smirnov test. Data were considered as normally distributed if P > 0.050. Unpaired Student's t-test was used for intergroup comparison of normally distributed continuous data that were expressed as a mean (standard deviation). The Mann–Whitney U-test was used to analyze nonnormally distributed continuous data and they were presented as median (interquartile range), whereas the intergroup comparison of categorical data was made using the Chi-square test and they were presented as number (percentage). A value of P < 0.05 at confidence interval 95% implies a statistical significance. Figure was generated using Microsoft Excel 2010.
RESULTS
Ninety-five females were assessed for eligibility; thirteen participants were excluded from the trial. Eighty-two participants that matched the inclusion criteria were allocated randomly into either of the study groups. All the allocated patients who were subjected to open TAH under general anesthesia completed the study [Figure 1].
Figure 1.
Study flow diagram
Patients’ age, weight, height, ASA physical status, and duration of anesthesia and surgery were similar in both groups [Table 1].
Table 1.
Patients’ characteristics and operative data
| Control (n=41) | Midazolam (n=41) | P | |
|---|---|---|---|
| Age (years) | 49.16±6.158 | 51.38±6.555 | 0.14 |
| Weight (kg) | 74.35±10.07 | 71.41±10.49 | 0.22 |
| Height (cm) | 162.05±4.39 | 160.57±3.70 | 0.12 |
| ASA (I, II, III) | 29/9/3 | 26/11/4 | 0.77 |
| Surgery duration (min) | 83.11±10.98 | 86.49±12.29 | 0.21 |
| Anesthesia duration (min) | 102.51±10.83 | 104.19±12.56 | 0.54 |
Data are means±SD or n. P < 0.05 implies a statistical significance. ASA=American Society of Anesthesiologists, SD=Standard deviation
Compared to the control group, patients in the midazolam group had statistically significantly less cumulative 24-h morphine consumption [median (interquartile range) doses: [15 (10–19.50)] mg compared to [25 (17.50–37)] mg, P < 0.001], longer time to the first postoperative analgesic request (430.11 ± 63.02 min compared to 327.78 ± 61.99 min, P < 0.001), and less sedation (P = 0. 03), nausea and/or vomiting (P = 0.01), and pruritus (P = 0.04) [Table 2].
Table 2.
Duration of postoperative analgesia, cumulative 24 h morphine consumption, and side effects
| Control (n=41) | Midazolam (n=41) | P | |
|---|---|---|---|
| Duration of analgesia (min) | 327.78±61.99 | 430.11±63.02 | <0.001* |
| 24 h morphine consumption (mg) | 25 (17.50-37) | 15 (10-19.50) | <0.001* |
| Sedation score, n (%) | |||
| 0 | 23 (56.1) | 34 (82.9) | 0.031* |
| 1 | 15 (36.5) | 6 (14.6) | |
| 2 | 3 (7.3) | 1 (2.4) | |
| 3 | 0 | 0 | |
| Postoperative nausea and vomiting, n (%) | |||
| 0 | 16 (39.02) | 29 (70.7) | 0.015* |
| 1 | 15 (36.5) | 8 (19.5) | |
| 2 | 10 (24.3) | 4 (9.7) | |
| Pruritus, n (%) | 15 (36.5) | 7 (17.07) | 0.04* |
*Significant compared to the control group. Data are means±SD, median (interquartile range), or n (%). P < 0.05 implies a statistical significance. SD=Standard deviation
Midazolam group patients’ showed significantly lower pain scores at rest at 4, 6, and 12 h (P = 0.01, 0.02, and 0.02, respectively) and on movement at 2, 4, 6, and 12 postoperative hours (P < 0.001) [Figure 2a and b].
Figure 2.
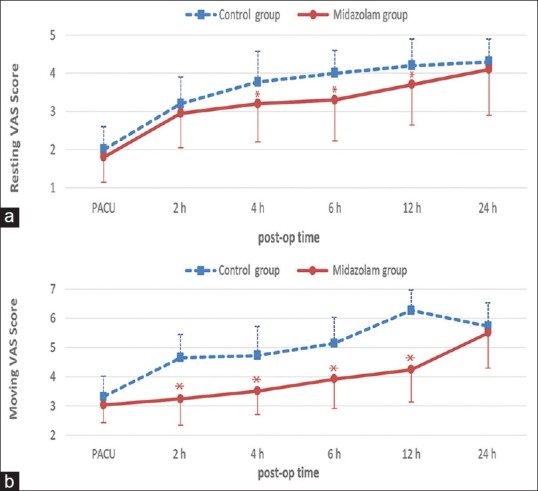
(a) Postoperative pain visual analog scale score at rest. Data are mean ± standard deviation. (b) Postoperative pain visual analog scale score on movement. Data are mean ± standard deviation
No block-related complications were recorded in both groups.
DISCUSSION
In the current clinical trial, midazolam used as a bupivacaine adjunct for TAP block had shown to reduce the cumulative 24 postoperative hour's morphine consumption and pain scores at rest and on movement, prolong the duration of postoperative analgesia and lower the incidence of postoperative nausea, vomiting, sedation, and pruritus in subjects underwent TAH under general anesthesia.
The greater concern about the use of midazolam as LA adjuvant is the potential neurotoxicity. However, previous animal studies demonstrated a lack of neurotoxicity of intrathecally administered midazolam.[10,11,12] The dose of midazolam adopted in this study was chosen because it was proved to be safe when administered neuraxially in previous studies.[13,14]
Numerous studies had revealed the analgesic enhancing properties of midazolam coadministered with LAs during the neuraxial blockade in comparison to LA alone. Similar to our results, Ghai et al.,[15] in their study, on pediatric patients subjected to upper abdominal and flank surgeries, demonstrated a reduced need for rescue analgesia with the use of midazolam (20 μg/kg/h) as an adjuvant to 0.125% bupivacaine in continuous epidural infusion for postoperative analgesia. In agreement with current trial results, other investigators[16] have reported improved analgesic profile when adding midazolam to bupivacaine in continuous postoperative epidural analgesia. Another study showed that caudally administered midazolam (50 μg/kg) gave equal analgesia to 0.25% bupivacaine in pediatrics.[17] Furthermore, using 2 mg of midazolam to bupivacaine intrathecally during cesarean delivery had improved the quality and prolonged the duration of postoperative analgesia without side effects.[18]
Midazolam exerts it's analgesic and LAs additive effects during different central neuraxial blockade through acting on spinal cord central GABA-A receptors.[7] The existence of peripherally located GABA receptors has been proved by different studies.[19,20]
Jarbo et al.[21] have used midazolam (50 μg/kg) added to bupivacaine in supraclavicular brachial plexus block and drawn a conclusion that midazolam as an adjuvant to bupivacaine enhanced the onset of sensory and motor blockade and lowered the pain scores.
A recent trial by Ammar et al.[22] has reached a conclusion that midazolam added to bupivacaine for rectus sheath blockade resulted in better postoperative analgesia evidenced by a lesser amount of consumed morphine, a longer analgesic duration, a lower pain VAS score, and reduced sedation and postoperative nausea and vomiting.
In contrast to our finding, other investigators studied the effect of using midazolam in conjunction to bupivacaine for the caudal block during inguinal hernia repair in children and demonstrated that the addition of midazolam to bupivacaine did not result in better analgesia compared to bupivacaine alone for children.[23] However, they performed their study on pediatric patients subjected to a less painful surgery (inguinal herniorrhaphy) for which bupivacaine alone may provide sufficient analgesia in addition to the known difficulty in reporting and interpreting children's pain.
The current study has some limitations. First, we did not use a continuous catheter block as we aimed to study the opioid requirement in the first 24 postoperative hours which could be masked by the continuous block. Furthermore, we wanted to assess the analgesic duration of a single injection TAP block with the addition of midazolam. Second, we didn’t include a third group who receive systemic midazolam as the observed LA enhancing effect of midazolam may be due to its systemic absorption.
Our study has some strengths; it is an original study as, till date, there is no study on the effect of midazolam co-administered with bupivacaine on the postoperative analgesic profile of the TAP block, in addition to its good sample size.
Midazolam could be a promising adjuvant as it does not alter the patient's hemodynamics, has a relatively low cost[24] hemodynamis and in a single dose, it does not cause prolonged sedation due to its high lipid solubility that leads to its rapid redistribution in addition to its rapid clearance by the liver.[25] Further researches comparing the analgesic potentiating effect of midazolam in TAP block to other adjuvants are required.
CONCLUSIONS
Adding midazolam as a bupivacaine adjuvant for TAP block reduces the 24-h morphine consumption, prolongs the duration of postoperative analgesia, and decreases the incidence of adverse effects following abdominal hysterectomy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Carney J, McDonnell JG, Ochana A, Bhinder R, Laffey JG. The transversus abdominis plane block provides effective postoperative analgesia in patients undergoing total abdominal hysterectomy. Anesth Analg. 2008;107:2056–60. doi: 10.1213/ane.0b013e3181871313. [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, Ma X, Pan J, Shuai H, Liu S, Luo X, et al. Effects of transversus abdominis plane blocks after hysterectomy: A meta-analysis of randomized controlled trials. J Pain Res. 2018;11:2477–89. doi: 10.2147/JPR.S172828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdallah FW, Laffey JG, Halpern SH, Brull R. Duration of analgesic effectiveness after the posterior and lateral transversus abdominis plane block techniques for transverse lower abdominal incisions: A meta-analysis. Br J Anaesth. 2013;111:721–35. doi: 10.1093/bja/aet214. [DOI] [PubMed] [Google Scholar]
- 4.Ammar AS, Mahmoud KM. Effect of adding dexamethasone to bupivacaine on transversus abdominis plane block for abdominal hysterectomy: A prospective randomized controlled trial. Saudi J Anaesth. 2012;6:229–33. doi: 10.4103/1658-354X.101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almarakbi WA, Kaki AM. Addition of dexmedetomidine to bupivacaine in transversus abdominis plane block potentiates post-operative pain relief among abdominal hysterectomy patients: A prospective randomized controlled trial. Saudi J Anaesth. 2014;8:161–6. doi: 10.4103/1658-354X.130683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelany A, Khaled M, Montaser A, Mohamad F, Ahmad M, Moaaz M. Efficacy of magnesium sulfate added to local anesthetic in a transversus abdominis plane block for analgesia following total abdominalhysterectomy: A randomized trial. Pain Physician. 2017;20:641–7. [PubMed] [Google Scholar]
- 7.Edwards M, Serrao JM, Gent JP, Goodchild CS. On the mechanism by which midazolam causes spinally mediated analgesia. Anesthesiology. 1990;73:273–7. doi: 10.1097/00000542-199008000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Bhisitkul RB, Villa JE, Kocsis JD. Axonal GABA receptors are selectively present on normal and regenerated sensory fibers in rat peripheral nerve. Exp Brain Res. 1987;66:659–63. doi: 10.1007/BF00270698. [DOI] [PubMed] [Google Scholar]
- 9.Cairns BE, Sessle BJ, Hu JW. Activation of peripheral GABAA receptors inhibits temporomandibular joint-evoked jaw muscle activity. J Neurophysiol. 1999;81:1966–9. doi: 10.1152/jn.1999.81.4.1966. [DOI] [PubMed] [Google Scholar]
- 10.Serrao JM, MacKenzie JM, Goodchild CS, Gent JP. Intrathecal midazolam in the rat: An investigation of possible neurotoxic effects. Eur J Anaesthesiol. 1990;7:115–22. [Google Scholar]
- 11.Nishiyama T, Matsukawa T, Hanaoka K. Acute phase histopathological study of spinally administered midazolam in cats. Anesth Analg. 1999;89:717–20. doi: 10.1097/00000539-199909000-00035. [DOI] [PubMed] [Google Scholar]
- 12.Schwieger IM, Jorge-Costa M, Pizzolato GP, Forster A, Morel DR. Intrathecal midazolam reduces isoflurane MAC and increases the apnoeic threshold in rats. Can J Anaesth. 1994;41:144–8. doi: 10.1007/BF03009809. [DOI] [PubMed] [Google Scholar]
- 13.Güleç S, Büyükkidan B, Oral N, Ozcan N, Tanriverdi B. Comparison of caudal bupivacaine, bupivacaine-morphine and bupivacaine-midazolam mixtures for post-operative analgesia in children. Eur J Anaesthesiol. 1998;15:161–5. [PubMed] [Google Scholar]
- 14.Batra YK, Jain K, Chari P, Dhillon MS, Shaheen B, Reddy GM, et al. Addition of intrathecal midazolam to bupivacaine produces better post-operative analgesia without prolonging recovery. Int J Clin Pharmacol Ther. 1999;37:519–23. [PubMed] [Google Scholar]
- 15.Ghai B, Makkar JK, Chari P, Rao KL. Addition of midazolam to continuous postoperative epidural bupivacaine infusion reduces requirement for rescue analgesia in children undergoing upper abdominal and flank surgery. J Clin Anesth. 2009;21:113–9. doi: 10.1016/j.jclinane.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 16.Nishiyama T, Matsukawa T, Hanaoka K. Effects of adding midazolam on the postoperative epidural analgesia with two different doses of bupivacaine. J Clin Anesth. 2002;14:92–7. doi: 10.1016/s0952-8180(01)00347-6. [DOI] [PubMed] [Google Scholar]
- 17.Pradhan B, Bajracharya GR. Midazolam for caudal analgesia in children: Comparison with caudal bupivacaine. Kathmandu Univ Med J (KUMJ) 2008;6:166–72. [PubMed] [Google Scholar]
- 18.Dodawad R, Sumalatha GB, Pandarpurkar S, Jajee P. Intrathecal midazolam as an adjuvant in pregnancy-induced hypertensive patients undergoing an elective caesarean section: A clinical comparative study. Anesth Pain Med. 2016;6:e38550. doi: 10.5812/aapm.38550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris ME, Di Costanzo GA, Fox S, Werman R. Depolarizing action of GABA (gamma-aminobutyric acid) on myelinated fibers of peripheral nerves. Brain Res. 1983;278:117–26. doi: 10.1016/0006-8993(83)90230-5. [DOI] [PubMed] [Google Scholar]
- 20.Liske S, Morris ME. Extrasynaptic effects of GABA (gamma-aminobutyric acid) agonists on myelinated axons of peripheral nerve. Can J Physiol Pharmacol. 1994;72:368–74. doi: 10.1139/y94-054. [DOI] [PubMed] [Google Scholar]
- 21.Jarbo K, Batra YK, Panda NB. Brachial plexus block with midazolam and bupivacaine improves analgesia. Can J Anaesth. 2005;52:822–6. doi: 10.1007/BF03021776. [DOI] [PubMed] [Google Scholar]
- 22.Ammar A, Mahmoud K, Kasemy Z. Effect of adding midazolam to bupivacaine during rectus sheath block: A randomised controlled trial. Acta Anaesthesiol Scand. 2018;62:857–62. doi: 10.1111/aas.13090. [DOI] [PubMed] [Google Scholar]
- 23.Baris S, Karakaya D, Kelsaka E, Güldogus F, Ariturk E, Tür A. Comparison of fentanyl-bupivacaine or midazolam-bupivacaine mixtures with plain bupivacaine for caudal anaesthesia in children. Paediatr Anaesth. 2003;13:126–31. doi: 10.1046/j.1460-9592.2003.00979.x. [DOI] [PubMed] [Google Scholar]
- 24.Frölich MA, Arabshahi A, Katholi C, Prasain J, Barnes S. Hemodynamic characteristics of midazolam, propofol, and dexmedetomidine in healthy volunteers. J Clin Anesth. 2011;23:218–23. doi: 10.1016/j.jclinane.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: Pharmacology and uses. Anesthesiology. 1985;62:310–24. [PubMed] [Google Scholar]