To the Editor:
Right ventricular (RV) fibrosis is a histological hallmark of RV failure and has been difficult to assess using noninvasive imaging. However, novel methods in cardiac magnetic resonance imaging (CMR) now allow for the quantification of myocardial extracellular volume (ECV), a marker of diffuse interstitial fibrosis that has been histologically validated (1–3). This measure of fibrosis was recently applied to the right ventricle (4). In the current study, we set out to examine the relationship between pulmonary artery (PA) stiffness, an important contributor to RV dysfunction, and RV fibrosis by using noninvasive CMR methods to better understand the maladaptive RV response to PA stiffness. We hypothesized that the PA pulse wave velocity (PWV) and relative area change (RAC), measures of PA stiffness, would be associated with RV fibrosis, as assessed by the RV ECV.
Methods
This cross-sectional study was approved by the Providence VA Medical Center Institutional Review Board (2015-051). Adult men and women (>30 yr of age) with pulmonary hypertension (PH) who had been referred for a clinical CMR examination at the Providence VA Medical Center were recruited. Informed consent was obtained from all participants.
The study participants underwent measurements of resting blood pressure, height, and weight, and their medical charts were abstracted for demographic, historical, and right heart catheterization (RHC) data. The median (25–75%) number of days between RHC and CMR was 125 (56–365.5).
CMR
Studies were performed with a 3-T scanner (Siemens Verio, Siemens Medical Solutions). CMR with ECG gating was done using a dedicated 32-element phased-array coil, which allowed parallel imaging with integrated acquisition of coil sensitivity reference data. Left ventricular (LV) and RV volumes, mass, and function were quantified and analyzed according to the guidelines of the Society for Cardiovascular Magnetic Resonance (5, 6). Specifically, cine steady-state free precession was performed in a stack of short-axis slices to cover both the atria and the ventricles. A retrospectively gated gradient echo sequence with steady-state free precession (“trueFISP”) was used for cine CMR to acquire images for 25 phases of the cardiac cycle during breath-holding (repetition time [TR]/echo time [TE]/flip angle = 3.2/1.8 ms/45°, slice thickness = 6 mm, 192 × 170 matrix, field of view [FOV] = ∼340 × 320 mm, and iPat factor = 2). LV and RV endocardial and epicardial borders on all short-axis cine images at the end-diastolic and end-systolic frames were manually traced to determine the end-diastolic and end-systolic volumes, respectively. LV and RV mass measurements were calculated by subtracting the endocardial volume from the epicardial volume at end-diastole, and then multiplying by the tissue density of myocardium (1.05 g/ml). Papillary muscles were included when mass was measured (equivalent to weighing the ventricle) and excluded when volume was measured (equivalent to blood pool techniques). CVI42 (Circle) software was used for cardiac volume measurements.
PA PWV was measured using the transit time method (7), optimized for high temporal resolution. The high temporal resolution sequence was implemented twice: once at a proximal main PA cross-sectional location, and once at either a right PA or a left PA distal cross-sectional location. The decision to image either the right PA or the left PA was made randomly in case both branches were well defined in the scouting images. Otherwise, the branch that appeared better in the scouting images was chosen for imaging. The imaging parameters of the high-temporal resolution imaging sequences were as follows: slice thickness = 8 mm, TR = 7 ms, TE = 4 ms, number of averages = 1, number of phases = 128, velocity encoding = 150 cm/s, bandwidth/pixel = 340 Hz, flip angle = 15°, FOV = 320 × 320 mm2, matrix = 256 × 256, and imaging time ≈ 2 minutes (depending on heart rate). PA RAC was calculated as (maximal area of the PA − minimal area of the PA)/(minimal area of the PA). The RAC was measured in the right PA in all cases.
Myocardial ECV/fibrosis assessment (T1 mapping)
High-resolution T1 measurements of the RV free wall in short-axis orientation at the mid-ventricle at end-systole were performed once before and four times after contrast with a breath-hold, ECG-gated Look-Locker technique. Using a radial acquisition, we were able to achieve an in-plane resolution of approximately 1.2 mm × 1.2 mm. Additional imaging parameters of the high-temporal resolution imaging sequences were as follows: slice thickness = 8 mm, TR = 4 ms, TE = 1.9 ms, bandwidth/pixel = 500 Hz, flip angle = 7°, and FOV = 300 × 300 mm2. The matrix size was reconstructed to 128 × 128 pixels and then zero-filled to 256 × 256 pixels. The segmented Look-Locker sequence with spoiled gradient echo readout, and a temporal resolution of 87 ms for precontrast T1 measurements and 55 ms for postcontrast T1 measurements were used. ECV was calculated as (1 − hematocrit) × [(1/T1myopost − 1/T1myopre)/(1/T1bloodpost − 1/T1bloodpre)]. Hematocrit was obtained on the day of the MRI exam.
Statistical analysis
Continuous variables were described as mean ± SD in the study population. Categorical variables were recorded as frequency and percent. Pairwise correlations between the RV ECV fraction and continuous demographic, hemodynamic, and CMR variables were assessed by calculating the Pearson correlation coefficients. Scatterplots of pairwise relationships were reviewed. Regression modeling was used to further explore significant relationships on correlation testing. The statistical significance level was taken as a P value < 0.05. All analyses were performed with StataSE version 14 (StataCorp).
Results
Nineteen patients who had been referred for CMR consented to participate in the study. Two patients did not complete the CMR and one patient did not have RHC data, resulting in an analytical dataset for 16 patients. The baseline characteristics of the subjects are recorded in Table 1. The participants had a mean age of 70.2 ± 8.7 years, were predominantly male (in keeping with the population of veterans studied), and were obese (mean body mass index [BMI], 31.4 ± 8.0 kg/m2). Common comorbidities included systemic hypertension, chronic obstructive pulmonary disease, and diabetes. All of the patients had PH, and mean PA pressures ranged from 22 to 45 mm Hg, with elevated pulmonary vascular resistance (PVR, 3.1 ± 1.3 Wood units). On CMR, the cohort had preserved LV ejection fraction (LVEF) (mean LVEF 58% ± 7%), with lower RVEF (mean RVEF 46% ± 12%; normal range 47–68% from Reference 8) and frequent RV hypertrophy (RV mass: 50.7 ± 16.7 g, median 58 g; upper limit of normal 57 g from Reference 9). On average, the cohort had increased PA stiffness (7, 10) (PA PWV: 3.31 ± 1.00 m/s and PA RAC: 0.17 ± 0.10). RV ECV was significantly elevated at 0.38 ± 0.07, which is consistent with previous reports of RV ECV in subjects with PH (4).
Table 1.
Baseline Characteristics of the Participants
| Characteristics | All Participants |
|---|---|
| Age, yr | 70.2 ± 8.7 |
| Sex, male, n (%) | 15 of 16 (93.8) |
| Race, n | |
| White | 14 |
| Black | 2 |
| Ethnicity, n | |
| Non-Hispanic | 14 |
| Hispanic | 2 |
| Height, cm | 175 ± 8 |
| Weight, kg | 97.8 ± 29.6 |
| BSA, m2 | 2.16 ± 0.37 |
| Body mass index, kg/m2 | 31.4 ± 8.0 |
| Systolic blood pressure, mm Hg | 130 ± 24 |
| Diastolic blood pressure, mm Hg | 71 ± 7 |
| Heart rate, bpm | 81 ± 13 |
| Pulmonary hypertension group, n | |
| Group 1 | 2 |
| Group 2 | 6 |
| Group 3 | 6 |
| Group 4 | 1 |
| Group 5 | 0 |
| Uncertain | 1 |
| Type 2 diabetes mellitus, n (%) | 9 of 16 (56.2) |
| Coronary artery disease, n (%) | 4 of 16 (25.0) |
| Congestive heart failure, n (%) | 5 of 16 (31.2) |
| Obstructive sleep apnea, n (%) | 7 of 16 (43.8) |
| Systemic hypertension, n (%) | 12 of 16 (75.0) |
| Chronic obstructive pulmonary disease, n (%) | 8 of 16 (50) |
| Pulmonary fibrosis, n (%) | 1 of 16 (6.2) |
| Cirrhosis, n (%) | 3 of 16 (18.8) |
| Creatinine, mg/dl | 0.96 ± 0.21 |
| Estimated glomerular filtration rate, ml/min | 58.1 ± 4.6 |
| Tricuspid annular plane systolic excursion, cm | 2.1 ± 0.05 |
| Right atrial pressure, mm Hg | 10 ± 4 |
| Pulmonary artery pressure, mm Hg | 31 ± 7 |
| Pulmonary artery pressure, mm Hg, mean range | 22–45 |
| Pulmonary artery occlusion pressure, mm Hg | 15 ± 3 |
| Cardiac index, L/min/m2 | 2.4 ± 0.6 |
| Pulmonary vascular resistance, Wood units | 3.1 ± 1.3 |
| Systemic vascular resistance, dyn · s/cm−5 | 1,593 ± 656 |
| LV end-systolic volume indexed to BSA, ml/m2 | 23.8 ± 7.7 |
| LV end-diastolic volume indexed to BSA, ml/m2 | 57.2 ± 17.7 |
| LV ejection fraction, % | 58 ± 7 |
| LV mass, g | 152 ± 41 (n = 15) |
| LV mass indexed to height, g/m | 85.9 ± 20.8 (n = 15) |
| Pulmonary artery pulse wave velocity, m/s | 3.31 ± 1.00 |
| Pulmonary artery stroke volume, ml | 119.6 ± 57.2 |
| Pulmonary artery relative area change | 0.17 ± 0.10 |
| RV end-systolic volume indexed to BSA, ml/m2 | 35.9 ± 17.4 |
| RV end-diastolic volume indexed to BSA, ml/m2 | 65.9 ± 22.0 |
| RV ejection fraction, % | 46 ± 12 |
| RV mass, g | 50.7 ± 16.7 (n = 13) |
| RV mass indexed to height, g/m | 28.8 ± 8.8 (n = 13) |
| RV extracellular volume fraction | 0.38 ± 0.07 |
Definition of abbreviations: BSA = body surface area; LV = left ventricular; RV = right ventricular.
Data are shown as mean ± SD unless otherwise specified. n = 16 unless otherwise specified.
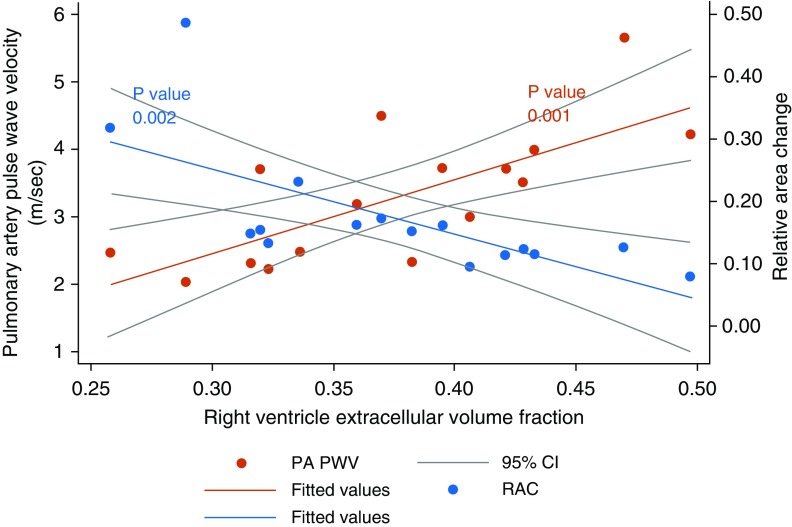
RV ECV was significantly correlated with PA PWV (0.73, P = 0.001) and was negatively correlated with PA RAC (−0.69, P = 0.003), but it was not significantly correlated with age, BMI, mean PA pressure, PVR, LV or RV mass, or EF. Scatterplots with fitted lines for the linear relationships between RV ECV and PA PWV or PA RAC are illustrated in Figure 1.
Figure 1.
Scatterplots with fitted lines and 95% confidence intervals (CIs) demonstrating a linear relationship between the pulmonary artery pulse wave velocity (PA PWV) and right ventricle extracellular volume fraction, and an inverse linear relationship between the pulmonary artery relative area change (RAC) and right ventricle extracellular volume fraction.
On univariate regression, about half of the variance in RV ECV could be explained by measures of PA stiffness (53% by PA PWV or 47% by PA RAC). The relationship between RV ECV and either PWV or RAC remained significant and unchanged or stronger in regression models adjusting for age, BMI, LVEF, RVEF, LV mass, RV mass, mean PA pressure, or PVR (β-coefficient for PA PWV ≥ 0.048/β-coefficient for RAC ≤ −0.42 in all models).
Discussion
In the current study, we report for the first time that RV ECV, a measure of RV fibrosis, was significantly correlated with noninvasive measures of PA stiffness (PWV and RAC) in a group of patients with PH undergoing CMR. We did not find a significant relationship between RV ECV and hemodynamic measurements, including PVR. PA stiffness measures relate to the pulsatile/oscillatory component of RV afterload, whereas PVR reflects the steady afterload component (11). PA stiffness measures have been related to decreased RV function, increased RV hypertrophy, and higher RV workload (12). We extend those findings to demonstrate, noninvasively, a relationship between PA stiffness and RV fibrosis that may reflect deleterious RV and PA remodeling. Limitations of our study include the small sample size; the recruitment of a referral population, which limits generalizability; the fact that we studied a population of veterans, resulting in an older, almost entirely male population, and thus the observed relationship between PA stiffness and RV fibrosis may not pertain to women; the fact that RHCs had already been performed and were not done on the same day as CMR; and the lack of histologic sampling of the PA or right ventricle. A prior study of late gadolinium enhancement in the interventricular septum in patients with PH showed that this was a significant predictor of mortality in univariate, but not multivariate, analysis (13); however, an examination of the effects of RV free-wall fibrosis on survival was not possible in the current cross-sectional study. In conclusion, our study shows a relationship between RV fibrosis and PA stiffness on CMR. Given the cross-sectional nature of this study, we cannot infer a causal relationship between RV fibrosis and PA stiffness, and the nature of this interaction requires further exploration. Noninvasive assessments of RV fibrosis on CMR will allow further longitudinal explorations of the relationship between RV remodeling, central artery stiffening, and clinical outcomes in patients with PH.
Supplementary Material
Footnotes
Supported by the CardioPulmonary Vascular Biology Center of Biomedical Research Excellence (National Institute for General Medical Sciences grant P20GM103652 [S.A.A.]) and NIH grant R01HL128661 (G.C.). The views expressed in this manuscript are those of the authors and do not necessarily reflect those of the Department of Veterans Affairs or the United States government.
Originally Published in Press as DOI: 10.1164/rccm.201903-0580LE on May 30, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Neilan TG, Coelho-Filho OR, Shah RV, Abbasi SA, Heydari B, Watanabe E, et al. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging. 2013;6:672–683. doi: 10.1016/j.jcmg.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kockova R, Kacer P, Pirk J, Maly J, Sukupova L, Sikula V, et al. Native T1 relaxation time and extracellular volume fraction as accurate markers of diffuse myocardial fibrosis in heart valve disease—comparison with targeted left ventricular myocardial biopsy. Circ J. 2016;80:1202–1209. doi: 10.1253/circj.CJ-15-1309. [DOI] [PubMed] [Google Scholar]
- 3.de Meester de Ravenstein C, Bouzin C, Lazam S, Boulif J, Amzulescu M, Melchior J, et al. Histological validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from modified Look-Locker imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson. 2015;17:48. doi: 10.1186/s12968-015-0150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta BB, Auger DA, Gonzalez JA, Workman V, Chen X, Chow K, et al. Detection of elevated right ventricular extracellular volume in pulmonary hypertension using accelerated and navigator-gated Look-Locker imaging for cardiac T1 estimation (ANGIE) cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2015;17:110. doi: 10.1186/s12968-015-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. doi: 10.1186/1532-429X-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradlow WM, Gatehouse PD, Hughes RL, O’Brien AB, Gibbs JS, Firmin DN, et al. Assessing normal pulse wave velocity in the proximal pulmonary arteries using transit time: a feasibility, repeatability, and observer reproducibility study by cardiovascular magnetic resonance. J Magn Reson Imaging. 2007;25:974–981. doi: 10.1002/jmri.20888. [DOI] [PubMed] [Google Scholar]
- 8.Petersen SE, Aung N, Sanghvi MM, Zemrak F, Fung K, Paiva JM, et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J Cardiovasc Magn Reson. 2017;19:18. doi: 10.1186/s12968-017-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gan CT, Lankhaar JW, Westerhof N, Marcus JT, Becker A, Twisk JW, et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest. 2007;132:1906–1912. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 11.Tan W, Madhavan K, Hunter KS, Park D, Stenmark KR. Vascular stiffening in pulmonary hypertension: cause or consequence? (2013 Grover Conference series) Pulm Circ. 2014;4:560–580. doi: 10.1086/677370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens GR, Garcia-Alvarez A, Sahni S, Garcia MJ, Fuster V, Sanz J. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging. 2012;5:378–387. doi: 10.1016/j.jcmg.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Swift AJ, Rajaram S, Capener D, Elliot C, Condliffe R, Wild JM, et al. LGE patterns in pulmonary hypertension do not impact overall mortality. JACC Cardiovasc Imaging. 2014;7:1209–1217. doi: 10.1016/j.jcmg.2014.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.