Abstract
Rationale: House endotoxin and ambient air pollution are risk factors for asthma; however, the effects of their coexposure on asthma are not well characterized.
Objectives: To examine potential synergistic associations of coexposure to house dust endotoxin and ambient air pollutants with asthma outcomes.
Methods: We analyzed data of 6,488 participants in the National Health and Nutrition Examination Survey 2005–2006. Dust from bedding and bedroom floor was analyzed for endotoxin content. The Community Multiscale Air Quality Modeling System (CMAQ) and Downscaler Model data were used to determine annual average particulate matter ≤2.5 μm in aerodynamic diameter (PM2.5), ozone (O3), and nitrogen dioxide (NO2) exposures at participants’ residential locations. The associations of the coexposures with asthma outcomes were assessed and tested for synergistic interaction.
Measurements and Main Results: In adjusted analysis, PM2.5 (CMAQ) (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.07–1.18), O3 (Downscaler Model) (OR, 1.07; 95% CI, 1.02–1.13), and log10 NO2 (CMAQ) (OR, 3.15; 95% CI, 1.33–7.45) were positively associated with emergency room visits for asthma in the past 12 months. Coexposure to elevated concentrations of house dust endotoxin and PM2.5 (CMAQ) was synergistically associated with the outcome, increasing the odds by fivefold (OR, 5.01; 95% CI, 2.54–9.87). A synergistic association was also found for coexposure to higher concentrations of endotoxin and NO2 in children (OR, 3.45; 95% CI, 1.65–7.18).
Conclusions: Coexposure to elevated concentrations of residential endotoxin and ambient PM2.5 in all participants and NO2 in children is synergistically associated with increased emergency room visits for asthma. Therefore, decreasing exposure to both endotoxin and air pollution may help reduce asthma morbidity.
Keywords: air pollution, asthma, endotoxin, particulate matter, wheeze
At a Glance Commentary
Scientific Knowledge on the Subject
Endotoxin is an LPS on the cell wall of gram-negative bacteria known to cause bronchial asthma and asthma-like symptoms. Ambient air pollutants exacerbate existing asthma and may contribute to causing the disease. In vitro and animal studies suggest that coexposure to residential endotoxin and ambient air pollutants may have effects on the respiratory system worse than the sum of the individual exposures’ effects.
What This Study Adds to the Field
We examined the synergistic association of coexposure to house dust endotoxin and ambient air pollution with asthma outcomes for the first time, to our knowledge, in a nationwide study including both children and adults. We demonstrated that coexposure to elevated levels of endotoxin and particulate matter ≤2.5 μm in aerodynamic diameter was synergistically associated with more emergency room visits for asthma in the past 12 months in all participants, especially children, and in individuals sensitized to inhalant allergens. In children, coexposure to higher concentrations of endotoxin and nitrogen dioxide was also synergistically associated with the outcome. Therefore, comprehensive measures to decrease both residential endotoxin and ambient air pollution exposures might be more effective than interventions targeting a single exposure for reducing asthma morbidity.
Both house dust endotoxin and ambient air pollutants are ubiquitous environmental contaminants to which humans are commonly exposed (1, 2). Ambient air pollutants are well known to exacerbate preexisting asthma, and recent evidence indicates that they may contribute to the development of the disease (3, 4). In addition to causing oxidative stress, damaging airway mucosa, and impairing mucociliary clearance, they have been reported to enhance the effects of inhaled allergens and endotoxin in inducing airway sensitization and asthma (4, 5). Endotoxin is an LPS from the cell wall of gram-negative bacteria released into the environment during bacterial growth and lysis (6). It is a component of bioaerosols found in residential, instructional, and occupational settings. Because of its small size, endotoxin can remain airborne for extended periods of time and may be resuspended from settled dust by human activities and environmental factors (7, 8). It often binds to particulate matter in indoor and outdoor environments and plays a role in the inflammatory effects of particles (7). Endotoxin is associated with bronchial asthma and asthma-like symptoms as well as with chronic bronchitis and emphysema in both occupational and domestic settings through the production of proinflammatory cytokines (9–12). There are, however, reports that childhood exposure to low doses of endotoxin during a narrow window of opportunity may protect against the development of atopy and atopic asthma (13–15). The mechanism for this is unclear but seems to involve the downregulation of the T-helper cell type 2–mediated immune system and the inhibition of inflammatory cytokines (10, 14).
Exposure to multiple toxicants may have effects that cannot be predicted by separately evaluating the individual exposures. Animal and human studies suggest that coexposure to house dust endotoxin and traffic-related particulate matter may potentiate their effects on the respiratory system (16–20). In animal models, diesel exhaust particles (DEP) have been reported to synergistically exacerbate acute lung injury caused by LPS by amplifying the expression of proinflammatory cytokines and to abrogate any possible protective effect of endotoxin against atopy (16–18). In humans, the only two studies that have investigated coexposure to endotoxin and air pollutants were limited to urban children (19, 20). One suggested that coexposure to endotoxin and traffic air particles might be synergistically associated with an increased risk of persistent wheezing in toddlers (19). The other found that indoor nitrogen dioxide (NO2) and air nicotine modify the effect of endotoxin on childhood asthma (20). However, neither prior study examined the effect of coexposure to endotoxin and pollutants among adults, and neither included children living outside urban centers. To address this gap in the literature, we examined the possible synergistic association of coexposure to house dust endotoxin and ambient air pollutants with asthma outcomes in a nationally representative sample of both children and adults in the United States. Our analysis included sensitization status to inhalant allergens and air pollutants such as ozone (O3) not studied in the previous human reports.
Methods
Data Source and Study Design
We used data from the 2005 to 2006 cycle of the National Health and Nutrition Examination Survey (NHANES). The NHANES is a continuous cross-sectional survey of the U.S. noninstitutionalized civilian population conducted by the National Center for Health Statistics (NCHS) of the CDC. It uses a complex multistage sampling design to derive a sample representative of the U.S. population and collects data on the health status of participants through interviews, physical examinations, and laboratory tests (21).
Among the 10,348 participants in the 2005–2006 NHANES, 6,963 had data on endotoxin, and 6,488 of them (93.2%) were linked spatially and temporally to air pollution data. The 475 participants who did not have air pollution data were those whose geocoded address fell outside the contiguous United States (Hawaii, Alaska, or U.S. territories). Compared with the 10,348 NHANES 2005–2006 participants, the 6,488 individuals with data on endotoxin and ambient air pollutants were more heavily represented by those residing in the Midwest of the United States (Table E1 in the online supplement). NHANES protocols were approved by the institutional review boards of the NCHS and CDC, and informed consent was obtained from all participants (21).
Endotoxin Analysis
Combined bed and bedroom floor dust was sampled in the homes of the NHANES participants using a Sanitaire Model 3683 vacuum cleaner and a Mitest Dust Collector (Indoor Biotechnologies, Inc.) to vacuum 1 sq. yd. of the bed surface and the adjacent floor for 2 minutes. Sieved dust was frozen and shipped to our laboratory at the University of Iowa for extraction and analysis of endotoxin employing multiple levels of quality assurance. The dust was extracted with 1 ml of sterile pyrogen-free Limulus amoebocyte lysate water plus 0.05% Tween 20 to a final concentration of 50 mg/ml. Dust was shaken for 1 hour at 22°C before being centrifuged at 4°C to remove large insoluble particles, and the supernatant was assayed. Endotoxin was measured using a kinetic chromogenic Limulus amoebocyte lysate assay. The lower limit of detection was 0.000488 endotoxin units (EU) per milligram of house dust (22).
Air Pollution
The U.S. Environmental Protection Agency (EPA) Community Multiscale Air Quality Modeling System (CMAQ) model data were used to estimate the annual average of particulate matter ≤2.5 μm in aerodynamic diameter (PM2.5), the annual average NO2, and the mean 8-hour summer maximum O3 in the continental United States on 36 × 36-km grids. The CMAQ estimates were modeled using meteorological data and chemical reaction kinetics (23). A secondary measurement of the annual averages of PM2.5 and mean 8-hour summer maximum O3 was obtained from the Downscaler Model (DS), which combines CMAQ model estimates on 12 × 12-km grids, and was used to monitor air pollution data to provide air pollutant concentrations at local and community scales. The DS is a Bayesian space–time model developed by the EPA that estimates daily concentrations of air pollutants for each census tract centroid in the contiguous United States (24).
The ambient air pollution data were generated for the years 2004–2006 and linked to the 2005–2006 NHANES using participants’ geocoded addresses. The air pollution averages were estimated for each participant for the year period before his/her examination date. Because these data contain identifiable geographic information, the data are restricted from public use. Therefore, a proposal detailing our data request and analysis plan was submitted to the Research Data Center of the CDC with confidentiality assurances. After the proposal was approved, the data analysis was performed on-site at the CDC.
Wheeze and Asthma Outcomes and Sensitization to Inhalant Allergens
Wheeze and asthma outcomes were assessed using questionnaires. Wheeze outcomes included any wheeze and physician/emergency room (ER) visits for wheeze in the 12 months before examination. Asthma outcomes included current asthma and ER visits for asthma in the past 12 months. We also included a combined outcome of current asthma or wheeze in the past 12 months. Sensitization status was defined as serum IgE specific to any inhalant allergen greater than or equal to 0.35 kU/L. The tested allergens were Dermatophagoides farinae, Dermatophagoides pteronyssinus, cat, dog, cockroach, Alternaria alternata, ragweed, rye grass, Bermuda grass, oak, birch, Aspergillus fumigatus, thistle, mouse, and rat. The serum IgE concentrations specific to these allergens were measured using the Pharmacia Diagnostics ImmunoCAP 1000 System, now known as ImmunoCAP Specific IgE (Thermo Fisher Scientific).
Covariates
Data on age, sex, race/ethnicity, presence of a smoker in the home, and annual household income were collected using questionnaires. Poverty/income ratio (PIR) was calculated using guidelines and adjustment for family size, year, and state. It was dichotomized into levels below and above 1.85, the cutoff for eligibility for the Supplemental Nutrition Assistance Program in the United States (25). Body mass index was calculated as weight in kilograms divided by height in meters squared and was categorized into levels less than 25 kg/m2 (normal), from 25 to less than 30 kg/m2 (overweight), and greater than or equal to 30 kg/m2 (obese). Area of residence was characterized by U.S. Census regions (Northeast, South, Midwest, and West) and degree of urbanization (metropolitan vs. nonmetropolitan).
Analysis
Descriptive analyses were performed to examine the central tendency and variability of house dust endotoxin and ambient air pollutants as well as their intercorrelations. Because of their significant skewness, endotoxin and NO2 were log10 transformed to improve the normality of their distribution. Coexposure to house dust endotoxin and each of the ambient air pollutants was defined using the following combination groups: low endotoxin/low pollutant (reference category), low endotoxin/high pollutant, high endotoxin/low pollutant, and high endotoxin/high pollutant. Low and high concentrations of house dust endotoxin and ambient air pollutant concentrations were defined by exposures to levels less than versus greater than or equal to the 75th percentile. Given the very low levels of the exposures and based on the literature, the 75th percentile was used as a threshold. This cutoff has also been used in a similar report on endotoxin coexposure with air pollutants in children from the Cincinnati Childhood Allergy and Air Pollution Study (19). Multivariable logistic regression was used to assess the associations of each ambient air pollutant and of the coexposure to house dust endotoxin and ambient air pollutants with asthma and wheeze outcomes as well as with sensitization to any inhalant allergen. The models were adjusted for age, sex, race/ethnicity, PIR, the presence of a smoker in the household, body mass index, census region, and degree of urbanization. Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were reported for the associations. Additive interaction was evaluated by calculating the attributable proportion (AP) due to interaction using the methods described by Andersson and colleagues (26). The AP quantifies the proportion of asthma outcomes in participants coexposed to house dust endotoxin and the ambient air pollutants that is due to the interaction between the single exposures. It suggests that an interaction is synergistic when positive or antagonistic when negative (27). All analyses were performed using SAS software (version 9.4; SAS Institute), accounting for the NHANES sampling weights and complex survey design to obtain national estimates. P values less than 0.05 were considered statistically significant.
Results
Descriptive Analysis
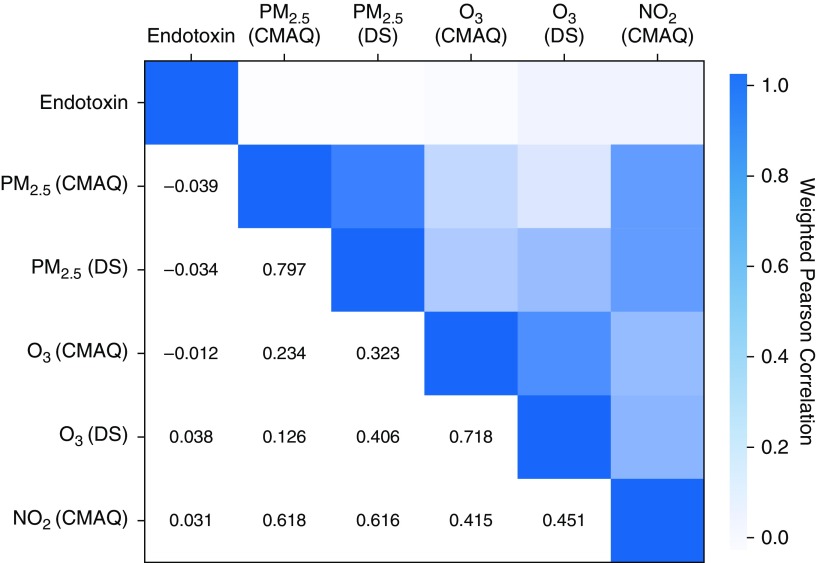
Among the 6,488 NHANES participants included in our study, the geometric mean (SE) of house dust endotoxin was 15.33 (0.59) EU/mg. For the ambient air pollutants, the geometric means (SEs) were 11.06 (0.76) μg/m3 (CMAQ) and 11.69 (0.51) μg/m3 (DS) for PM2.5, 54.34 (1.12) ppb (CMAQ) and 49.25 (1.05) ppb (DS) for O3, and 6.98 (1.03) ppb for NO2 (CMAQ). These concentrations were well below the EPA National Ambient Air Quality Standards for the annual averages of PM2.5 (15.0 μg/m3), O3 (70 ppb), and NO2 (53 ppb) (www.epa.gov/criteria-air-pollutants/naaqs-table). The medians and variability of these exposures are reported in Table 1. As expected, Figure 1 shows that CMAQ and DS estimates were strongly correlated, and the ambient air pollutants were significantly interrelated. Endotoxin had significant but weak negative correlations with PM2.5 (CMAQ) and PM2.5 (DS) and weak positive correlations with O3 (DS) and NO2.
Table 1.
Concentrations of House Dust Endotoxin and Air Pollutants, National Health and Nutrition Examination Survey 2005–2006
| Exposure | GM (SE) | Median (IQR) | 5th–95th Percentiles |
|---|---|---|---|
| Endotoxin, EU/mg dust | 15.33 (0.59) | 16.18 (7.95–34.31) | 1.41–104.95 |
| PM2.5, μg/m3 (CMAQ) | 11.06 (0.76) | 11.51 (8.22–13.81) | 6.57–18.88 |
| PM2.5, μg/m3 (DS) | 11.69 (0.51) | 12.13 (9.82–13.71) | 8.76–15.51 |
| O3, ppb (CMAQ) | 54.34 (1.12) | 56.17 (50.79–58.60) | 45.35–61.89 |
| O3, ppb (DS) | 49.25 (1.05) | 49.65 (45.71–54.10) | 39.69–57.14 |
| NO2, ppb (CMAQ) | 6.98 (1.03) | 7.37 (3.18–13.10) | 1.96–21.84 |
Definition of abbreviations: CMAQ = Community Multiscale Air Quality Modeling System; DS = Downscaler Model; EU = endotoxin units; GM = geometric mean; IQR = interquartile range; NO2 = nitrogen dioxide; O3 = ozone; PM2.5 = particulate matter ≤2.5 μm in aerodynamic diameter.
Figure 1.
Heat map for the Pearson correlation between house dust endotoxin and ambient air pollutants. Endotoxin and NO2 logarithmically transformed to improve the normality of their distribution. CMAQ = Community Multiscale Air Quality Modeling System; DS = Downscaler Model; NO2 = nitrogen dioxide; O3 = ozone; PM2.5 = particulate matter ≤2.5 μm in aerodynamic diameter.
The concentrations of house dust endotoxin and ambient air pollutants differed by participant characteristics. House dust endotoxin concentrations were higher in homes with young children (<6 yr old), with Hispanic individuals, located in the western United States, occupied by participants with a low socioeconomic status (PIR, ≤1.85), or in the homes of a smoker. O3 (CMAQ) and O3 (DS) were both higher in metropolitan areas, whereas O3 (DS) concentrations were also higher in the western United States. NO2 concentrations were higher in areas with more Hispanic participants and in the western United States. With regard to health outcomes, participants with ER visits for asthma in the past 12 months lived in homes with higher dust endotoxin concentrations and/or in areas with higher PM2.5 (CMAQ). Those with wheeze in the past 12 months or current asthma or wheeze disproportionately lived in homes with higher dust endotoxin concentrations. Participants sensitized to any inhalant allergen were more prevalent in areas with higher NO2 (Table 2).
Table 2.
Geometric Mean and SE of Dust Endotoxin and Air Pollutants, by Characteristics of Study Participants, National Health and Nutrition Examination Survey 2005–2006
| Characteristics | n (%) | Endotoxin (EU/mg) |
PM2.5 (μg/m3) (CMAQ) |
PM2.5 (μg/m3) (DS) |
O3 (ppb) (CMAQ) |
O3 (ppb) (DS) |
NO2 (ppb) (CMAQ) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM (SE) | P Value | GM (SE) | P Value | GM (SE) | P Value | GM (SE) | P Value | GM (SE) | P Value | GM (SE) | P Value | ||
| Age | <0.001 | 0.68 | 0.34 | 0.53 | 0.60 | 0.06 | |||||||
| <6 yr | 894 (7.0) | 29.3 (1.4) | 11.2 (0.9) | 11.8 (0.5) | 54.0 (1.1) | 49.2 (0.9) | 7.4 (1.1) | ||||||
| 6–17 yr | 2,019 (17.1) | 22.5 (1.5) | 11.1 (0.7) | 11.6 (0.5) | 54.4 (1.1) | 49.5 (1.1) | 7.2 (1.0) | ||||||
| ≥18 yr | 3,575 (75.9) | 13.2 (0.5) | 11.0 (0.8) | 11.7 (0.5) | 54.3 (1.1) | 49.2 (1.1) | 6.9 (1.0) | ||||||
| Sex | 0.14 | 0.14 | 0.29 | 0.38 | 0.59 | 0.33 | |||||||
| M | 3,206 (49.0) | 14.9 (0.7) | 11.0 (0.8) | 11.7 (0.5) | 54.3 (1.2) | 49.2 (1.1) | 6.9 (1.0) | ||||||
| F | 3,282 (51.0) | 15.8 (0.6) | 11.1 (0.8) | 11.7 (0.5) | 54.4 (1.1) | 49.3 (1.0) | 7.0 (1.0) | ||||||
| Race/ethnicity | <0.001 | 0.07 | 0.06 | 0.43 | 0.06 | 0.011 | |||||||
| Non-Hispanic white | 2,269 (67.7) | 14.5 (0.7) | 10.6 (0.8) | 11.3 (0.6) | 53.8 (1.4) | 48.7 (1.4) | 5.9 (1.0) | ||||||
| Non-Hispanic black | 1,922 (13.1) | 15.1 (1.4) | 13.1 (1.0) | 13.0 (0.5) | 56.2 (1.0) | 49.7 (1.0) | 8.6 (1.4) | ||||||
| Hispanic | 1,788 (9.4) | 23.3 (1.3) | 11.2 (0.7) | 12.2 (0.8) | 55.4 (0.5) | 52.1 (0.6) | 11.8 (0.9) | ||||||
| Other | 509 (9.8) | 15.4 (1.1) | 11.8 (0.8) | 12.3 (0.4) | 54.5 (0.7) | 50.0 (0.6) | 9.7 (0.9) | ||||||
| BMI | <0.001 | 0.96 | 0.61 | 0.25 | 0.10 | 0.042 | |||||||
| Underweight or normal | 3,334 (44.2) | 16.9 (0.7) | 11.1 (0.8) | 11.7 (0.5) | 54.4 (1.1) | 49.4 (1.0) | 7.4 (1.1) | ||||||
| Overweight | 1,415 (25.4) | 13.7 (0.6) | 11.1 (0.8) | 11.7 (0.5) | 54.6 (1.0) | 49.5 (1.0) | 7.1 (1.0) | ||||||
| Obese | 1,425 (27.5) | 13.9 (1.0) | 11.1 (0.7) | 11.6 (0.5) | 54.1 (1.2) | 48.9 (1.2) | 6.2 (1.0) | ||||||
| Missing | 314 (2.9) | 24.3 (1.8) | 10.9 (0.8) | 11.6 (0.5) | 53.3 (1.3) | 48.7 (1.2) | 6.7 (1.1) | ||||||
| Census region | 0.025 | 0.24 | 0.62 | 0.24 | 0.001 | 0.003 | |||||||
| Northeast | 865 (15.9) | 14.5 (1.6) | 11.2 (3.3) | 10.6 (1.6) | 49.4 (3.5) | 42.1 (2.2) | 5.2 (3.6) | ||||||
| Midwest | 1,406 (27.8) | 14.7 (1.2) | 12.3 (1.3) | 12.0 (1.0) | 53.5 (2.3) | 49.5 (2.5) | 6.5 (1.6) | ||||||
| South | 2,343 (30.4) | 13.8 (1.0) | 12.1 (1.1) | 12.4 (0.7) | 56.2 (1.3) | 49.1 (1.1) | 5.6 (0.9) | ||||||
| West | 1,874 (25.9) | 18.8 (1.2) | 8.8 (1.2) | 11.2 (0.9) | 56.3 (0.5) | 54.1 (0.7) | 11.6 (0.8) | ||||||
| Urbanicity | 0.21 | 0.31 | 0.13 | 0.009 | 0.007 | <0.001 | |||||||
| Metropolitan | 3,988 (54.4) | 16.1 (0.8) | 11.7 (1.1) | 12.3 (0.6) | 57.0 (0.5) | 52.3 (0.9) | 11.4 (1.2) | ||||||
| Nonmetropolitan | 2,500 (45.6) | 14.5 (0.9) | 10.4 (0.9) | 10.9 (0.7) | 51.3 (1.6) | 45.8 (1.4) | 3.9 (0.7) | ||||||
| PIR | <0.001 | 0.63 | 0.38 | 0.49 | 0.13 | 0.37 | |||||||
| ≤1.85 | 3,114 (32.7) | 20.1 (1.0) | 10.9 (0.7) | 11.5 (0.5) | 54.1 (1.2) | 48.7 (1.0) | 6.7 (1.0) | ||||||
| >1.85 | 3,091 (67.3) | 13.3 (0.6) | 11.1 (0.8) | 11.7 (0.5) | 54.5 (1.1) | 49.5 (1.1) | 7.0 (1.1) | ||||||
| Smoker in household | 0.003 | 0.53 | 0.60 | 0.08 | 0.028 | 0.014 | |||||||
| Yes | 1,261 (20.6) | 17.8 (1.0) | 11.3 (0.9) | 11.6 (0.5) | 53.7 (1.3) | 48.3 (1.2) | 6.1 (1.1) | ||||||
| No | 5,181 (79.4) | 14.8 (0.6) | 11.0 (0.8) | 11.7 (0.5) | 54.5 (1.1) | 49.5 (1.0) | 7.2 (1.0) | ||||||
| Sensitization to inhalant allergens | 0.72 | 0.66 | 0.23 | 0.11 | 0.15 | 0.014 | |||||||
| Yes | 2,522 (44.2) | 14.8 (0.6) | 11.1 (0.8) | 11.8 (0.5) | 54.7 (1.0) | 49.6 (0.9) | 7.4 (1.0) | ||||||
| No | 2,705 (55.8) | 14.6 (0.8) | 11.0 (0.8) | 11.6 (0.5) | 54.0 (1.2) | 48.9 (1.2) | 6.6 (1.0) | ||||||
| Any wheeze | 0.049 | 0.30 | 0.22 | 0.82 | 0.27 | 0.23 | |||||||
| Yes | 899 (15.9) | 17.6 (1.4) | 10.9 (0.7) | 11.6 (0.5) | 54.3 (1.1) | 48.9 (1.0) | 6.7 (1.0) | ||||||
| No | 5,586 (84.1) | 14.9 (0.6) | 11.1 (0.8) | 11.7 (0.5) | 54.4 (1.1) | 49.3 (1.1) | 7.0 (1.0) | ||||||
| Doctor/ER visits for wheeze | 0.10 | 0.65 | 0.65 | 0.77 | 0.60 | 0.75 | |||||||
| Yes | 438 (7.1) | 18.7 (2.4) | 11.2 (0.9) | 11.8 (0.5) | 54.5 (1.1) | 48.9 (0.9) | 7.1 (1.2) | ||||||
| No | 6,047 (92.9) | 15.1 (0.6) | 11.1 (0.8) | 11.7 (0.5) | 54.3 (1.1) | 49.3 (1.1) | 7.0 (1.0) | ||||||
| Current asthma | 0.14 | 0.22 | 0.21 | 0.74 | 0.51 | 0.32 | |||||||
| Yes | 602 (8.8) | 17.4 (1.7) | 10.8 (0.8) | 11.5 (0.5) | 54.2 (1.1) | 48.9 (1.0) | 7.3 (1.2) | ||||||
| No | 5,861 (91.2) | 15.2 (0.6) | 11.1 (0.8) | 11.7 (0.5) | 54.4 (1.1) | 49.3 (1.1) | 6.9 (1.0) | ||||||
| ER visits for asthma in past 12 mo | 0.021 | 0.046 | 0.20 | 0.42 | 0.90 | 0.30 | |||||||
| Yes | 114 (1.7) | 21.4 (3.2) | 12.3 (1.1) | 12.1 (0.6) | 54.9 (1.3) | 49.1 (1.2) | 7.7 (1.6) | ||||||
| No | 5,740 (98.3) | 15.1 (0.6) | 11.1 (0.8) | 11.7 (0.5) | 54.3 (1.1) | 49.2 (1.1) | 6.9 (1.0) | ||||||
| Current asthma or wheeze | 0.038 | 0.35 | 0.27 | 0.89 | 0.30 | 0.30 | |||||||
| Yes | 940 (16.5) | 17.6 (1.4) | 10.9 (0.8) | 11.6 (0.5) | 54.3 (1.1) | 48.9 (1.0) | 6.7 (1.0) | ||||||
| No | 5,530 (83.5) | 14.9 (0.6) | 11.1 (0.8) | 11.7 (0.5) | 54.4 (1.1) | 49.3 (1.1) | 7.0 (1.0) | ||||||
Definition of abbreviations: BMI = body mass index; CMAQ = Community Multiscale Air Quality Modeling System; DS = Downscaler Model; ER = emergency room; EU = endotoxin units; GM = geometric mean; NO2 = nitrogen dioxide; O3 = ozone; PIR = poverty/income ratio; PM2.5 = particulate matter ≤2.5 μm in aerodynamic diameter.
P values indicate significance of differences in endotoxin and air pollutant levels by characteristics of participants. Bold indicates significant differences in endotoxin and ambient air pollutant levels by characteristics of study participants.
Association of Endotoxin and Air Ambient Pollutants with Health Outcomes
In logistic regression adjusted for covariates, PM2.5 (CMAQ) (OR, 1.12; 95% CI, 1.07–1.18), O3 (DS) (OR, 1.07; 95% CI, 1.02–1.13), and log10 NO2 (OR, 3.15; 95% CI, 1.33–7.45) were associated with significantly higher ER visits for asthma in the past 12 months. Elevated NO2 exposure was also associated with a higher prevalence of current asthma or wheeze in the past 12 months (OR, 1.57; 95% CI, 1.07–2.31) (Table 3).
Table 3.
Odds Ratios and 95% Confidence Intervals for Association of Air Pollutants with Wheeze and Asthma Outcomes and Sensitization to Inhalant Allergens, National Health and Nutrition Examination Survey 2005–2006
| Air Pollutants | Wheeze in Past 12 mo |
Asthma Outcomes |
Current Asthma or Wheeze | Sensitization to Inhalant Allergens | ||
|---|---|---|---|---|---|---|
| Any Wheeze | Doctor/ER Visits for Wheeze | Current Asthma | ER Visits for Asthma | |||
| PM2.5 (CMAQ) | 1.01 (0.98–1.04) | 1.03 (0.98–1.08) | 0.99 (0.96–1.03) | 1.12 (1.07–1.18)* | 1.01 (0.99–1.04) | 1.01 (1.00–1.03) |
| PM2.5 (DS) | 1.01 (0.97–1.06) | 1.04 (0.97–1.12) | 0.98 (0.94–1.03) | 1.13 (0.99–1.30) | 1.02 (0.98–1.06) | 1.02 (1.00–1.04) |
| O3 (CMAQ) | 1.00 (0.97–1.03) | 0.99 (0.95–1.04) | 0.99 (0.96–1.02) | 1.06 (1.00–1.12) | 1.00 (0.98–1.03) | 1.01 (1.00–1.01) |
| O3 (DS) | 0.98 (0.95–1.01) | 0.98 (0.93–1.04) | 0.97 (0.93–1.01) | 1.07 (1.02–1.13)† | 0.98 (0.96–1.01) | 1.01 (1.00–1.02) |
| Log-NO2 (CMAQ) | 1.44 (0.98–2.12) | 1.53 (0.84–2.77) | 1.57 (0.82–3.02) | 3.15 (1.33–7.45)† | 1.57 (1.07–2.31)‡ | 1.29 (0.99–1.69) |
Definition of abbreviations: CMAQ = Community Multiscale Air Quality Modeling System; DS = Downscaler Model; ER = emergency room; NO2 = nitrogen dioxide; O3 = ozone; PM2.5 = particulate matter ≤2.5 μm in aerodynamic diameter.
Model adjusted for age, sex, race/ethnicity, presence of a smoker in the house, poverty/income ratio, body mass index, census region, and level of urbanization. Bold indicates significant associations of ambient air pollutants with asthma and wheeze outcomes.
P < 0.001.
P < 0.01.
P < 0.05.
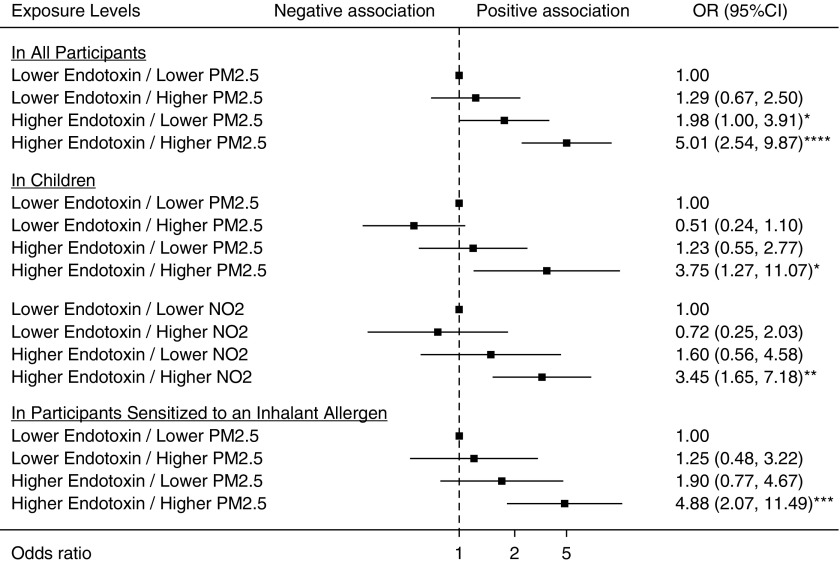
Coexposure to house dust endotoxin and ambient PM2.5 (CMAQ) was synergistically associated with ER visits for asthma in the past 12 months in all participants (AP, 0.55; P = 0.0055). The association of the coexposure with the outcome (OR, 5.01; 95% CI, 2.54–9.87) was significantly higher than with the sum of the associations for the individual exposures (for endotoxin alone, OR, 1.98; 95% CI, 1.00–3.91; for PM2.5 [CMAQ] alone, OR, 1.29; 95% CI, 0.67–2.50). In subgroup analysis by age and sensitization status to inhalant allergens, the synergistic association remained significant in children (AP, 0.80; P < 0.0001) and in participants sensitized to any inhalant allergen (AP, 0.56; P = 0.022) (Figure 2).
Figure 2.
Forest plots for coexposures to endotoxin and air pollutants synergistically associated with emergency room visits for asthma in the past 12 months. Air pollutant data were obtained using the Community Multiscale Air Quality Modeling System. The models were adjusted for age, sex, race/ethnicity, smoker in household, poverty/income ratio, body mass index, census region, and level of urbanization. The squares indicate the odds ratios, and the lines indicate the 95% confidence intervals (CIs) for the odds ratios (ORs). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. NO2 = nitrogen dioxide; PM2.5 = particulate matter ≤2.5 μm in aerodynamic diameter.
In children, coexposure to house dust endotoxin and ambient NO2 was also synergistically associated with ER visits for asthma in the past 12 months (AP, 0.62; P = 0.011). The relationship of the coexposure with the outcome (OR, 3.45; 95% CI, 1.65–7.18) was significantly higher than the sum of the associations for the single exposures (for endotoxin alone, OR, 1.60; 95% CI, 0.56–4.58; for NO2 alone, OR, 0.72; 95% CI, 0.25–2.03) (Figure 2). The departure from additivity for the coexposures of house dust endotoxin with the CMAQ estimates of ambient PM2.5 and NO2 in relationship to ER visits for asthma in the past 12 months is graphically illustrated in Figure 2.
Although coexposures of house dust endotoxin with other ambient air pollutants (PM2.5 [DS], O3 [CMAQ], and NO2 [CMAQ]) were all positively associated with ER visits for asthma in the past 12 months, the additive interaction testing for these associations failed to reach statistical significance (Tables E2–E4). We found no synergistic relationships for the association of coexposure to house dust endotoxin and ambient air pollutants with the other asthma and wheeze outcomes or with sensitization to any inhalant allergen (Tables E5–E9).
Discussion
In the present study, conducted in a large sample representative of the U.S. population, coexposure to elevated concentrations of endotoxin and PM2.5 had a synergistic positive association with ER visits for asthma in the past 12 months. In children, coexposure to endotoxin and NO2 was synergistically associated with a higher prevalence of ER visits for asthma in the past 12 months. To our knowledge, this is the first nationwide study on the synergistic association of coexposure to house dust endotoxin and ambient air pollution with asthma outcomes. It includes both children and adults as well as pollutants such as O3 not previously investigated in epidemiological studies.
Interactions between endotoxin and air pollutants have been investigated in in vitro studies. LPS has been found to enhance the production by alveolar macrophages of inflammatory cytokines in the presence of PM2.5 and airborne particles (28–30). Animal studies have shown that exposure to DEP, a major constituent of PM2.5 in urban areas, interacts with endotoxin to exacerbate neutrophilic lung inflammation (16, 17). In humans, only two studies on the synergistic effect of endotoxin and air pollutants in children have been conducted. In a birth cohort of 762 children from the Cincinnati Childhood Allergy and Air Pollution Study, the association of the coexposure to house dust endotoxin and traffic-related particles with the risk of persistent wheeze at the age of 36 months was examined. Daily elemental carbon attributable to traffic was used as a marker of exposure to traffic-related particles and was found to interact with house dust endotoxin to enhance the risk of recurrent wheeze (19). In a smaller sample of 146 children aged 5–17 years, Matsui and colleagues examined whether indoor concentrations of particulate matter, nicotine and NO2 modified the relationship between airborne endotoxin and asthma outcomes (20). Although they found significant effect modification by nicotine and NO2, their results did not appear to suggest that the coexposures to endotoxin and nicotine or NO2 were synergistically associated with worse asthma outcomes. They reported a positive association of endotoxin with asthma symptoms and acute visits in homes with lower indoor NO2 concentrations, whereas in homes with higher NO2 concentrations, the relationship was inverse (20).
In our stratified analyses, the synergistic associations of the coexposure to endotoxin and PM2.5 persisted in children and in participants sensitized to any inhalant allergens. To our knowledge, this study is the first to show a synergistic association of the coexposure to endotoxin and NO2 with ER visits for asthma in children. Although no previous study has reported a synergistic interaction between endotoxin and NO2 in relation to asthma, NO2 has been found to be associated with higher asthma prevalence and severity as well as with sensitization to inhalant allergens (4, 31). In the two nationwide studies of endotoxin performed in the United States, we have shown that regardless of age and sensitization status, endotoxin is positively associated with asthma symptoms and severity (11, 12).
Yet, there are reports that early-life exposure to low doses of endotoxin might be protective against allergies and allergic asthma through mechanisms not fully understood (14). In mice, this potentially protective effect of perinatal endotoxin exposure against the development of atopic asthma was found to be inhibited by DEP (18). The study concluded that endotoxin by itself might be protective against atopy, whereas the coexposure to endotoxin and DEP might be a cause of allergic asthma (18). Inconsistent with our finding of a synergistic effect found in participants sensitized to inhalant allergens, Ryan and colleagues report that the synergistic effect of coexposure to endotoxin and DEP on recurrent wheeze was mainly observed in participants not sensitized (19). These results reported by Ryan and colleagues also contradicted previous reports that exposure to endotoxin is associated with worse inflammation in rats sensitized to inhalant allergens (32).
The mechanisms by which house dust endotoxin and ambient air pollutants might synergistically affect asthma outcomes are multiple. Simultaneous exposure to endotoxin and ambient air pollutants has been reported to synergistically produce oxygen free radicals in the lung through the activation of xanthine oxidase. This can cause acute lung injury with neutrophil influx and increased production of inflammatory cytokines (33). In rats, preexposure to endotoxin increased the number of neutrophils in lavage fluid and the secretion of mucus into airways during exposure to ambient air pollutants such as O3 (34). Postexposure to ambient air pollutants may also increase endotoxin-induced production and storage of mucin glycoproteins and endotoxin-induced metaplasia (34). Likewise, initial exposure to ambient air pollutants can cause acute airway and lung injury and secondarily enable endotoxin to cross the epithelial barrier and exert toxic effects, causing the epithelial cells to produce inflammatory cytokines (35, 36). Consistent with our results of a persistent significant synergistic effect in children, children have been reported to be particularly vulnerable to the respiratory effects of air pollutants because of the immaturity of their lungs and their narrower airways, as well as their ability to inhale more air per body weight than adults (37).
Our study has major strengths. To date, it is the largest study to investigate coexposure to residential endotoxin and ambient air pollution in relation to respiratory outcomes. The study sample is representative of the U.S. population and includes both children and adults. Exposure to ambient air pollutants was estimated using both the CMAQ and DS data. This is the only epidemiologic study on coexposure to endotoxin and air pollutants to quantify an additive interaction that is an indicator of biological synergism (38). However, our report has limitations. Owing to the cross-sectional design of the study, temporality between the exposures and the outcomes could not be established. The asthma outcomes were self-reported and could not be confirmed. However, ER visits for asthma in the past year may be more memorable events that could be reported more accurately than the other asthma outcomes. The number of outcome events for ER visits for asthma in the past 12 months was limited in the subgroup analysis. Overall, 114 reported the outcome; in the logistic regression analysis, the outcome events were reduced to 103 because of missing covariate data. In the subgroup analyses by age and sensitization status, the number of outcome events ranged from 40 to 63. In our study, concentrations of air pollutants were very low. Although the concentrations were comparable to those previously reported in the United States (39, 40), many other countries have reported higher air pollution concentrations during the same period (41–45). Our statistical analysis included multiple testing, which might have increased the probability of finding significant results at P < 0.05 across multiple asthma outcomes. However, most of our results on the association of the coexposures to endotoxin and ambient air pollutants remained significant, even after adjusting P values for multiple comparisons and considering the more stringent significance level of P < 0.01. (The adjusted P value was calculated with the Bonferroni correction by dividing the traditional significance level of 0.05 by 5, which corresponds to the number of asthma outcomes included in the study.) The synergistic association of coexposure to endotoxin and PM2.5 with ER visits for asthma was observed only when the pollutant was measured with the CMAQ but not with the DS, although PM2.5 (CMAQ) and PM2.5 (DS) were highly correlated. The exact reason for this is unclear. The DS combines monitoring and CMAQ data and adjusts for potential biases using studies on the health effects of air pollution. However, its performance in nonurban areas where monitoring data are scarce has not been adequately described. Therefore, the association between the DS estimates and health effects in nonurban locations should be interpreted cautiously (46, 47). The CMAQ, on the other hand, has been widely evaluated but also has limitations. It uses a coarse grid and is vulnerable to biases from meteorological input and emissions inventory (46, 48).
In conclusion, coexposure to house dust endotoxin and PM2.5 measured using the CMAQ was synergistically associated with ER visits for asthma. A synergistic association of coexposure to endotoxin and NO2 with ER visits for asthma was also observed in children. Prospective studies are needed to confirm these findings and examine the association of the coexposure to domestic endotoxin and ambient air pollutants with other respiratory outcomes such as chronic obstructive pulmonary disease. If the observed associations are indeed causal, comprehensive public health measures to reduce asthma morbidity and associated healthcare costs that target both residential endotoxin and air pollution exposure may be more effective than interventions targeting individual exposures.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Frances McCarty and Jaylan Richardson at the NCHS Research Data Center for their assistance with the data linkage and analysis. The authors also thank Dr. Ambarish Vaidyanathan at the CDC National Center for Environmental Health for his help with the Downscaler Model data linkage. The authors acknowledge Dr. Nervana Metwali for performing the endotoxin analysis of the NHANES dust samples and Dr. Richard D. Cohn for sage advice on biostatistical analyses.
Footnotes
Sample extraction and endotoxin analysis performed at the University of Iowa was funded by the CDC/National Center for Health Statistics (200-2010-34238 NCE1). Data analysis was funded through a grant to the University of Iowa Environmental Health Sciences Research Center (NIH P30 ES005605 [P.S.T.]), by the University of Iowa Center for Health Effects of Environmental Contamination, and through a contract to Social and Scientific Systems, Inc. (HHSN273201600002). This work was also funded in part by the NIH Intramural Research Program through the National Institute of Environmental Health Sciences (NIH Z01 ES025041 [D.C.Z.]). The findings and conclusions reported in this paper are those of the authors and do not necessarily represent the views of the Research Data Center, the National Center for Health Statistics, or the CDC.
Author Contributions: A.M. assisted with development of the research questions and data analysis and was principal author of the manuscript. P.S.T. directed the endotoxin analysis of the National Health and Nutrition Examination Survey samples, contributed to the study design, collaborated on development of the research questions, and edited the manuscript. P.M.S. and D.C.Z. contributed to the conception, hypothesis delineation, and design of this component of the National Health and Nutrition Examination Survey study. C.H.W. contributed to the production of the air pollution data. J.W. and L.F. contributed to the statistical analysis and interpretation of the data. All authors provided editing of and comments on the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201809-1733OC on April 9, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Thorne PS, Cohn RD, Mav D, Arbes SJ, Zeldin DC. Predictors of endotoxin levels in U.S. housing. Environ Health Perspect. 2009;117:763–771. doi: 10.1289/ehp.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katsouyanni K. Ambient air pollution and health. Br Med Bull. 2003;68:143–156. doi: 10.1093/bmb/ldg028. [DOI] [PubMed] [Google Scholar]
- 3.North ML, Alexis NE, Ellis AK, Carlsten C. Air pollution and asthma: how can a public health concern inform the care of individual patients? Ann Allergy Asthma Immunol. 2014;113:343–346. doi: 10.1016/j.anai.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Gasana J, Dillikar D, Mendy A, Forno E, Ramos Vieira E. Motor vehicle air pollution and asthma in children: a meta-analysis. Environ Res. 2012;117:36–45. doi: 10.1016/j.envres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383:1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 7.Pavilonis BT, Anthony TRT, O’Shaughnessy PT, Humann MJ, Merchant JA, Moore G, et al. Indoor and outdoor particulate matter and endotoxin concentrations in an intensely agricultural county. J Expo Sci Environ Epidemiol. 2013;23:299–305. doi: 10.1038/jes.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raja S, Xu Y, Ferro AR, Jaques PA, Hopke PK. Resuspension of indoor aeroallergens and relationship to lung inflammation in asthmatic children. Environ Int. 2010;36:8–14. doi: 10.1016/j.envint.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Mendy A, Salo PM, Cohn RD, Wilkerson J, Zeldin DC, Thorne PS. House dust endotoxin association with chronic bronchitis and emphysema. Environ Health Perspect. 2018;126:037007. doi: 10.1289/EHP2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radon K. The two sides of the “endotoxin coin”. Occup Environ Med. 2006;63:73–78. doi: 10.1136/oem.2004.017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorne PS, Kulhánková K, Yin M, Cohn R, Arbes SJ, Jr, Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med. 2005;172:1371–1377. doi: 10.1164/rccm.200505-758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorne PS, Mendy A, Metwali N, Salo P, Co C, Jaramillo R, et al. Endotoxin exposure: predictors and prevalence of associated asthma outcomes in the United States. Am J Respir Crit Care Med. 2015;192:1287–1297. doi: 10.1164/rccm.201502-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016;375:411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Allergy and Endotoxin Study Team. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 15.Mendy A, Gasana J, Vieira ER, Forno E, Patel J, Kadam P, et al. Endotoxin exposure and childhood wheeze and asthma: a meta-analysis of observational studies. J Asthma. 2011;48:685–693. doi: 10.3109/02770903.2011.594140. [DOI] [PubMed] [Google Scholar]
- 16.Yanagisawa R, Takano H, Inoue K, Ichinose T, Sadakane K, Yoshino S, et al. Enhancement of acute lung injury related to bacterial endotoxin by components of diesel exhaust particles. Thorax. 2003;58:605–612. doi: 10.1136/thorax.58.7.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takano H, Yanagisawa R, Ichinose T, Sadakane K, Yoshino S, Yoshikawa T, et al. Diesel exhaust particles enhance lung injury related to bacterial endotoxin through expression of proinflammatory cytokines, chemokines, and intercellular adhesion molecule-1. Am J Respir Crit Care Med. 2002;165:1329–1335. doi: 10.1164/rccm.2108122. [DOI] [PubMed] [Google Scholar]
- 18.Reiprich M, Rudzok S, Schütze N, Simon JC, Lehmann I, Trump S, et al. Inhibition of endotoxin-induced perinatal asthma protection by pollutants in an experimental mouse model. Allergy. 2013;68:481–489. doi: 10.1111/all.12121. [DOI] [PubMed] [Google Scholar]
- 19.Ryan PH, Bernstein DI, Lockey J, Reponen T, Levin L, Grinshpun S, et al. Exposure to traffic-related particles and endotoxin during infancy is associated with wheezing at age 3 years. Am J Respir Crit Care Med. 2009;180:1068–1075. doi: 10.1164/rccm.200808-1307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsui EC, Hansel NN, Aloe C, Schiltz AM, Peng RD, Rabinovitch N, et al. Indoor pollutant exposures modify the effect of airborne endotoxin on asthma in urban children. Am J Respir Crit Care Med. 2013;188:1210–1215. doi: 10.1164/rccm.201305-0889OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services (DHHS); CDC; National Center for Health Statistics (NCHS) Hyattsville, MD: NCHS; 2005. National Health and Nutrition Examination Survey. [accessed 2018 Jul 18]. Available from: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. [Google Scholar]
- 22.Thorne P. Endotoxin in dust - laboratory procedure manual. NHANES 2005-2006. [accessed 2018 Jul 18]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2005-2006/labmethods/aldust_d_met_endotoxin.pdf.
- 23.Foley KM, Roselle SJ, Appel KW, Bhave PV, Pleim JE, Otte TL, et al. Incremental testing of the Community Multiscale Air Quality (CMAQ) modeling system version 4.7. Geosci Model Dev. 2010;3:205–226. [Google Scholar]
- 24.Berrocal VJ, Gelfand AE, Holland DM. A spatio-temporal downscaler for output from numerical models. J Agric Biol Environ Stat. 2010;15:176–197. doi: 10.1007/s13253-009-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacEwan JP, Smith A, Alston JM. The supplemental nutrition assistance program, energy balance, and weight gain. Food Policy. 2016;61:103–120. [Google Scholar]
- 26.Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 27.VanderWeele TJ. Reconsidering the denominator of the attributable proportion for interaction. Eur J Epidemiol. 2013;28:779–784. doi: 10.1007/s10654-013-9843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long CM, Suh HH, Kobzik L, Catalano PJ, Ning YY, Koutrakis P. A pilot investigation of the relative toxicity of indoor and outdoor fine particles: in vitro effects of endotoxin and other particulate properties. Environ Health Perspect. 2001;109:1019–1026. doi: 10.1289/ehp.011091019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ning Y, Imrich A, Goldsmith CA, Qin G, Kobzik L. Alveolar macrophage cytokine production in response to air particles in vitro: role of endotoxin. J Toxicol Environ Health A. 2000;59:165–180. doi: 10.1080/009841000156952. [DOI] [PubMed] [Google Scholar]
- 30.Imrich A, Ning YY, Koziel H, Coull B, Kobzik L. Lipopolysaccharide priming amplifies lung macrophage tumor necrosis factor production in response to air particles. Toxicol Appl Pharmacol. 1999;159:117–124. doi: 10.1006/taap.1999.8731. [DOI] [PubMed] [Google Scholar]
- 31.Weir CH, Yeatts KB, Sarnat JA, Vizuete W, Salo PM, Jaramillo R, et al. Nitrogen dioxide and allergic sensitization in the 2005-2006 National Health and Nutrition Examination Survey. Respir Med. 2013;107:1763–1772. doi: 10.1016/j.rmed.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tulić MK, Wale JL, Holt PG, Sly PD. Modification of the inflammatory response to allergen challenge after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 2000;22:604–612. doi: 10.1165/ajrcmb.22.5.3710. [DOI] [PubMed] [Google Scholar]
- 33.Arimoto T, Kadiiska MB, Sato K, Corbett J, Mason RP. Synergistic production of lung free radicals by diesel exhaust particles and endotoxin. Am J Respir Crit Care Med. 2005;171:379–387. doi: 10.1164/rccm.200402-248OC. [DOI] [PubMed] [Google Scholar]
- 34.Wagner JG, Hotchkiss JA, Harkema JR. Effects of ozone and endotoxin coexposure on rat airway epithelium: potentiation of toxicant-induced alterations. Environ Health Perspect. 2001;109:591–598. doi: 10.1289/ehp.01109s4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leikauf GD, McDowell SA, Wesselkamvdie WD, Gammon K, et al. Pathogenomic mechanisms for particulate matter induction of acute lung injury and inflammation in mice. Res Rep Health Eff Inst. 2001;105:5–58. [Discussion, pp. 59–71.] [PubMed] [Google Scholar]
- 36.Dye JA, Lehmann JR, McGee JK, Winsett DW, Ledbetter AD, Everitt JI, et al. Acute pulmonary toxicity of particulate matter filter extracts in rats: coherence with epidemiologic studies in Utah Valley residents. Environ Health Perspect. 2001;109:395–403. doi: 10.1289/ehp.01109s3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makri A, Stilianakis NI. Vulnerability to air pollution health effects. Int J Hyg Environ Health. 2008;211:326–336. doi: 10.1016/j.ijheh.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 39.Young MT, Sandler DP, DeRoo LA, Vedal S, Kaufman JD, London SJ. Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. women. Am J Respir Crit Care Med. 2014;190:914–921. doi: 10.1164/rccm.201403-0525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker JD, Akinbami LJ, Woodruff TJ. Air pollution and childhood respiratory allergies in the United States. Environ Health Perspect. 2009;117:140–147. doi: 10.1289/ehp.11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin XD, Qian Z, Vaughn MG, Trevathan E, Emo B, Paul G, et al. Gender-specific differences of interaction between obesity and air pollution on stroke and cardiovascular diseases in Chinese adults from a high pollution range area: a large population based cross sectional study. Sci Total Environ. 2015;529:243–248. doi: 10.1016/j.scitotenv.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Schleicher N, Fricker M, Cen K, Liu XL, Kaminski U, et al. Long-term variation of black carbon and PM2.5 in Beijing, China with respect to meteorological conditions and governmental measures. Environ Pollut. 2016;212:269–278. doi: 10.1016/j.envpol.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Doiron D, de Hoogh K, Probst-Hensch N, Mbatchou S, Eeftens M, Cai Y, et al. Residential air pollution and associations with wheeze and shortness of breath in adults: a combined analysis of cross-sectional data from two large European cohorts. Environ Health Perspect. 2017;125:097025. doi: 10.1289/EHP1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith RB, Fecht D, Gulliver J, Beevers SD, Dajnak D, Blangiardo M, et al. Impact of London’s road traffic air and noise pollution on birth weight: retrospective population based cohort study. BMJ. 2017;359:j5299. doi: 10.1136/bmj.j5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orioli R, Cremona G, Ciancarella L, Solimini AG. Association between PM10, PM2.5, NO2, O3 and self-reported diabetes in Italy: a cross-sectional, ecological study. PLoS One. 2018;13:e0191112. doi: 10.1371/journal.pone.0191112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sacks JD, Rappold AG, Davis JA, Jr, Richardson DB, Waller AE, Luben TJ. Influence of urbanicity and county characteristics on the association between ozone and asthma emergency department visits in North Carolina. Environ Health Perspect. 2014;122:506–512. doi: 10.1289/ehp.1306940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bravo MA, Ebisu K, Dominici F, Wang Y, Peng RD, Bell ML. Airborne fine particles and risk of hospital admissions for understudied populations: effects by urbanicity and short-term cumulative exposures in 708 U.S. counties. Environ Health Perspect. 2017;125:594–601. doi: 10.1289/EHP257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crooks JL, Özkaynak H. Simultaneous statistical bias correction of multiple PM2.5 species from a regional photochemical grid model. Atmos Environ. 2014;95:126–141. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.